What Exactly Is Inflammation (and What Is It Not?) †
Abstract
1. Introduction—An Analogy with Fire
2. The First Clinical Definition of Inflammation—A Fire Alarm
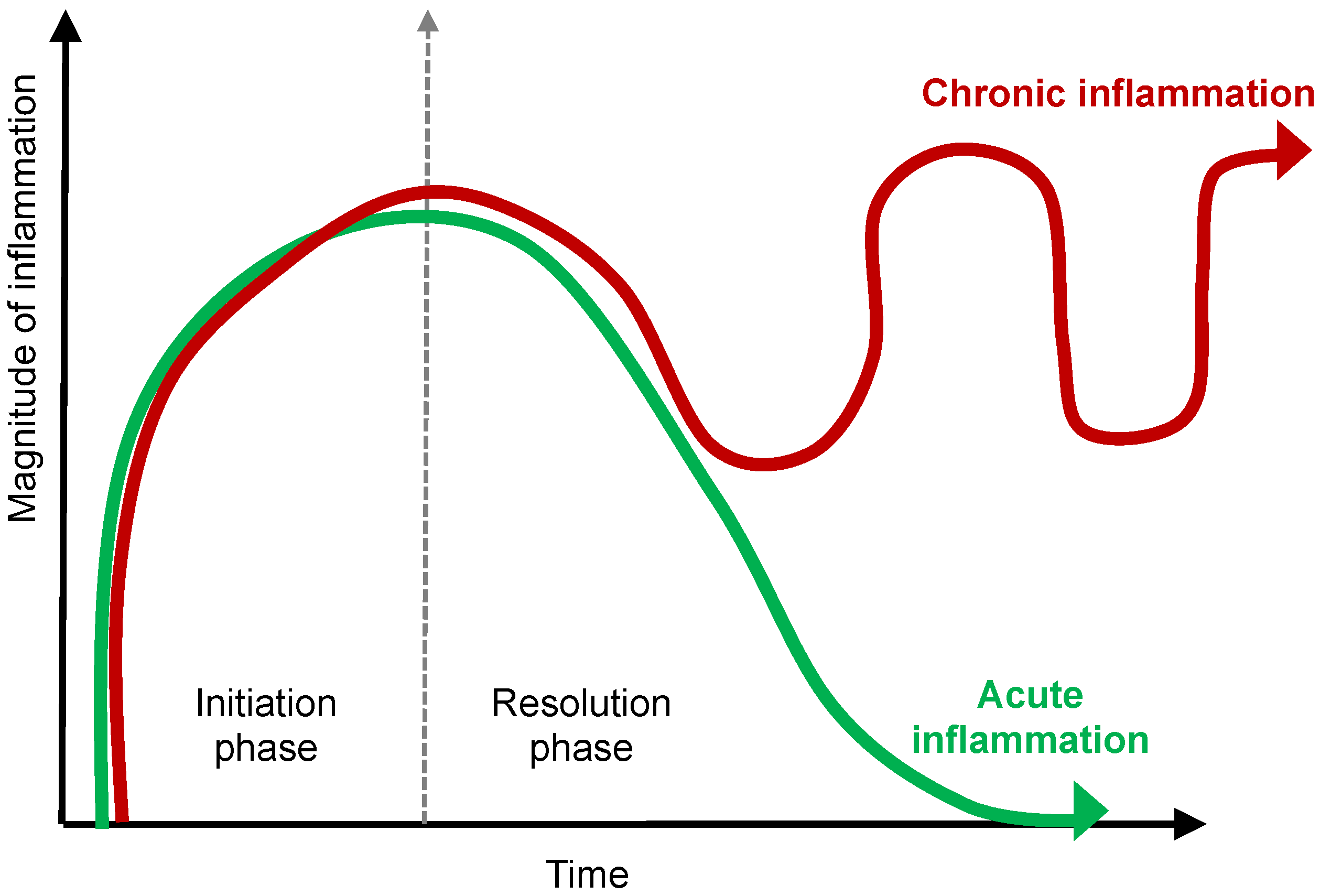
3. Modern Definition of Inflammation—Good Fire vs. Bad Fire
4. The Arsonists and the Firefighters
4.1. The Arsonists—Neutrophils, Macrophages, T Cells, and Cytokines
4.2. The Firefighters—M2 Macrophages, Tregs, and Pro-Resolving Mediators
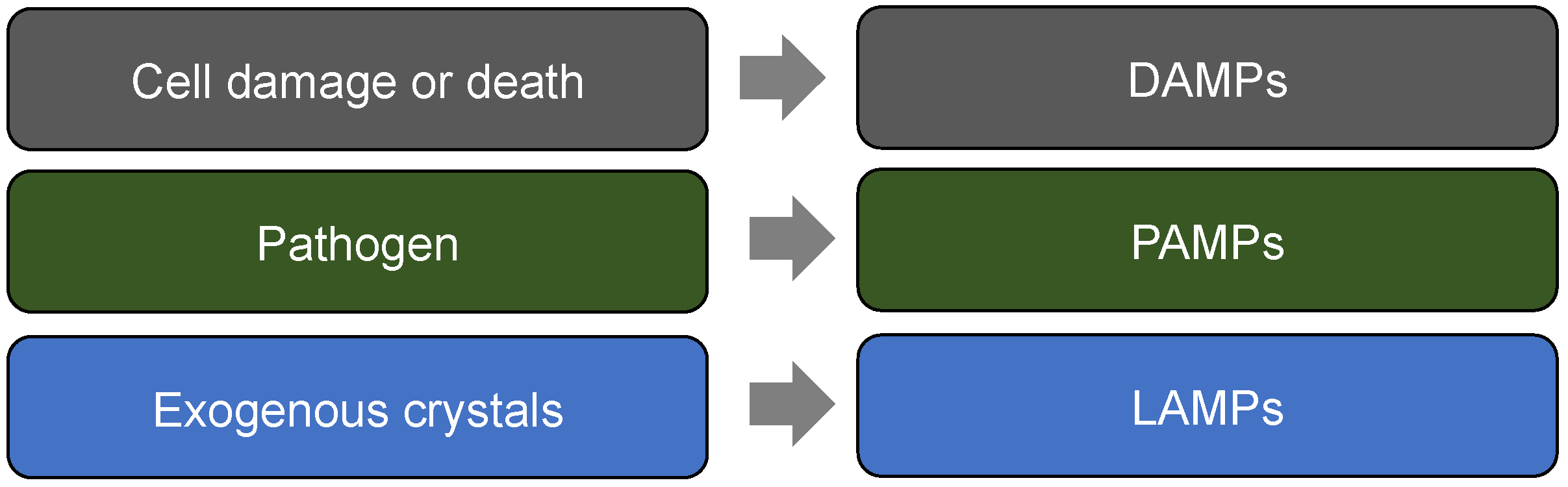
5. Fire Accelerants—DAMPs, PAMPs and LAMPs
6. Fire Retardants—SAMPs
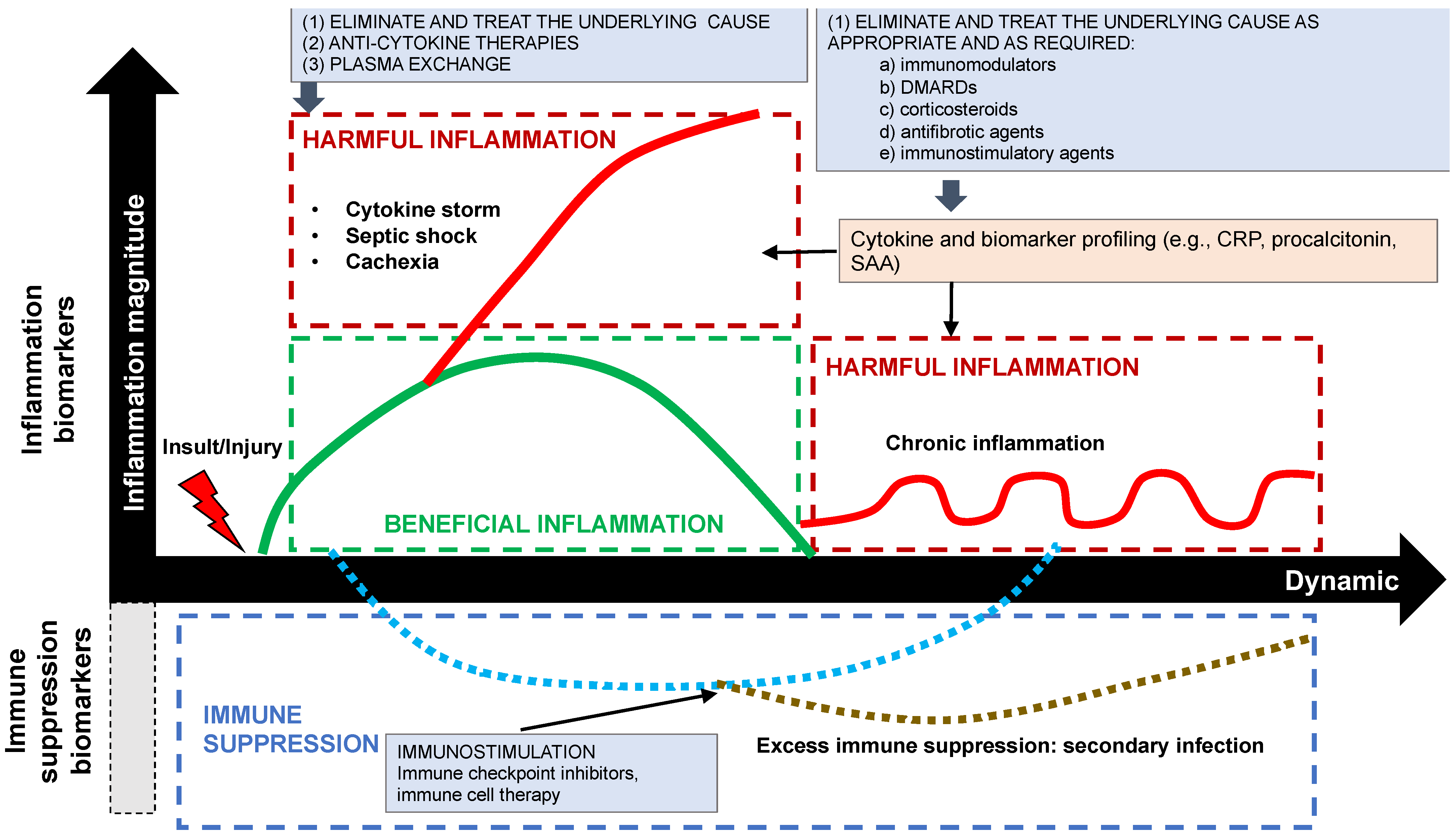
7. Management of Inflammation: Fan the Flames, Let It Take Its Course, Let It Burn, or Prevent It
7.1. Fan the Flames
7.2. Let It Flame Out, i.e., Let It Take Its Course or If in Doubt, Do Not Put It Out
7.3. Put It Out or Suppress It
8. Prevent It
9. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Reeves, G.; Billman, G.E.; Sturmberg, J.P. Inflammation-Nature’s Way to Efficiently Respond to All Types of Challenges: Implications for Understanding and Managing “the Epidemic” of Chronic Diseases. Front. Med. 2018, 5, 316. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.M. Once upon a time, inflammation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, e20200147. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kalbasi, A.; Beatty, G.L. Functio Laesa: Cancer Inflammation and Therapeutic Resistance. J. Oncol. Pract. 2017, 13, 173–180. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hannoodee, S.; Nasuruddin, D.N. Acute Inflammatory Response. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2020, 191, 4–17. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of Inflammation: What Controls Its Onset? Front. Immunol. 2016, 7, 160. [Google Scholar]
- Jentho, E.; Weis, S. DAMPs and Innate Immune Training. Front. Immunol. 2021, 12, 699563. [Google Scholar] [CrossRef]
- Land, W.G. The Role of Damage-Associated Molecular Patterns in Human Diseases: Part I-Promoting inflammation and immunity. Sultan. Qaboos. Univ. Med. J. [SQUMJ] 2015, 15, e9–e21. [Google Scholar]
- Dalli, J.; Serhan, C. Macrophage Proresolving Mediators—the When and Where. Microbiol. Spectr. 2016, 4, 367–383. [Google Scholar] [CrossRef]
- Kyi, C.; Postow, M.A. Immune checkpoint inhibitor combinations in solid tumors: Opportunities and challenges. Immunotherapy 2016, 8, 821–837. [Google Scholar] [CrossRef] [PubMed]
- Osuchowski, M.F.; Craciun, F.; Weixelbaumer, K.M.; Duffy, E.R.; Remick, D.G. Sepsis chronically in MARS: Systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J. Immunol. 2012, 189, 4648–4656. [Google Scholar] [CrossRef] [PubMed]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Ray, J.J.; Schulman, C.I. Fever: Suppress or let it ride? J. Thorac. Dis. 2015, 7, E633–E636. [Google Scholar] [PubMed]
- Hébert, J.R.; Hofseth, L.J. Chapter 17—Following the long arc of history: Where do we go from here? In Diet, Inflammation, and Health; Hébert, J.R., Hofseth, L.J., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 819–875. ISBN 9780128221303. [Google Scholar]
- Patel, P.H.; Hashmi, M.F. Macrolides. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551495/ (accessed on 1 November 2022).
- Heard, K.J.; Ries, N.L.; Dart, R.C.; Bogdan, G.M.; Zallen, R.D.; Daly, F. Overuse of non-prescription analgesics by dental clinic patients. BMC Oral Health 2008, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Nirk, E.L.; Reggiori, F.; Mauthe, M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol. Med. 2020, 12, e12476. [Google Scholar] [CrossRef]
- Klebes, M.; Wutte, N.; Aberer, E. Dapsone as Second-Line Treatment for Cutaneous Lupus Erythematosus? A Retrospective Analysis of 34 Patients and a Review of the Literature. Dermatology 2016, 232, 91–96. [Google Scholar] [CrossRef]
- Tran, S.; Ksajikian, A.; Overbey, J.; Li, P.; Li, Y. Pathophysiology of Pulmonary Fibrosis in the Context of COVID-19 and Implications for Treatment: A Narrative Review. Cells 2022, 11, 2489. [Google Scholar] [CrossRef]
- Noreen, S.; Maqbool IMadni, A. Dexamethasone: Therapeutic potential, risks, and future projection during COVID-19 pandemic. Eur. J. Pharmacol. 2021, 894, 173854. [Google Scholar] [CrossRef]
- Chavarria-Avila, E.; Vazquez-Del Mercado, M.; Pizano-Martínez, O.; Roman-Lugo, G.; Arrona-Rios, K.; Perez-Vazquez, F.; De-La-Cruz, J.P.; Calderon-Espinoza, I.; Aguilar-Vazquez, A.; Esesarte-Rodriguez, M.; et al. Going Further: Comprehensive Disease Control of Rheumatoid Arthritis, Targeting Cytokines and Chemokines. J. Clin. Rheumatol. Pract. Rep. Rheum. Musculoskelet. Dis. 2021, 27, e432–e439. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Rubio-Arrellano, E.D.; Duran-Barragan, S. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Tomfohr, L.M.; Edwards, K.M.; Madsen, J.W.; Mills, P.J. Social support moderates the relationship between sleep and inflammation in a population at high risk for developing cardiovascular disease. Psychophysiology 2015, 52, 1689–1697. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the Immune System and Reducing Inflammation and Oxidative Stress through Diet and Nutrition: Considerations during the COVID-19 Crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef]
- Datar, G.K.; Goodell, M.A. Where There’s Smoke, There’s Fire: Inflammation Drives MDS. Trends Immunol. 2020, 41, 558–560. [Google Scholar] [CrossRef]
- Kim, G.; Kronenberg, M. Cooling the fires of inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 16493–16494. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Yao, Y.; Cao, W.; Geisslinger, E.D.; Boer, S. The Dynamic Inflammatory Tissue Microenvironment: Signality and Disease Therapy by Biomaterials. Research 2021, 2021, 4189516. [Google Scholar] [CrossRef] [PubMed]
- Rock, K.L.; Kono, H. The inflammatory response to cell death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of Sterile Inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef]
- Germain, R.N. Maintaining system homeostasis: The third law of Newtonian immunology. Nat. Immunol. 2012, 13, 902–906. [Google Scholar] [CrossRef]
- Hanke, T.; Merk, D.; Steinhilber, D.; Geisslinger, G.; Schubert-Zsilavecz, M. Small molecules with anti-inflammatory properties in clinical development. Pharmacol. Ther. 2015, 157, 163–187. [Google Scholar] [CrossRef]
- Genuis, S.J. An ounce of prevention: A pound of cure for an ailing health care system. Can. Fam. Physician 2007, 53, 597–607. [Google Scholar] [PubMed]
- Honoré, P.; Jacobs, R.; Boer, W.; Joannes-Boyau, O.; De Regt, J.; De Waele, E.; Van Gorp, V.; Collin, V.; Spapen, H. New Insights Regarding Rationale, Therapeutic Target and Dose of Hemofiltration and Hybrid Therapies in Septic Acute Kidney Injury. Blood Purif. 2011, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Slaats, J.; Ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct Inflammatory Programs in Infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar]
| Antigen-Directed Immunotherapy | Immunomodulation | Anti-Fibrotic Agents | Immunosuppression | |
|---|---|---|---|---|
| Diseases | asthma, allergic rhinitis, atopic dermatitis, anaphylaxis | SLE, RA, Sjögren’s, COPD, UC | pulmonary fibrosis | autoimmune diseases |
| Treatments | allergen immunotherapy | HCQ, sulfasalazine, dapsone, azithromycin | nintedanib, pirfenidone | corticosteroids, DMARDs, anti-cytokines |
| Treatment characteristics | potential for permanent desensitization | non-immunosuppressive | anti-fibrotic and anti-inflammatory effects especially with pirfenidone | more targeted immunosuppression with anti-cytokines |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oronsky, B.; Caroen, S.; Reid, T. What Exactly Is Inflammation (and What Is It Not?). Int. J. Mol. Sci. 2022, 23, 14905. https://doi.org/10.3390/ijms232314905
Oronsky B, Caroen S, Reid T. What Exactly Is Inflammation (and What Is It Not?). International Journal of Molecular Sciences. 2022; 23(23):14905. https://doi.org/10.3390/ijms232314905
Chicago/Turabian StyleOronsky, Bryan, Scott Caroen, and Tony Reid. 2022. "What Exactly Is Inflammation (and What Is It Not?)" International Journal of Molecular Sciences 23, no. 23: 14905. https://doi.org/10.3390/ijms232314905
APA StyleOronsky, B., Caroen, S., & Reid, T. (2022). What Exactly Is Inflammation (and What Is It Not?). International Journal of Molecular Sciences, 23(23), 14905. https://doi.org/10.3390/ijms232314905






