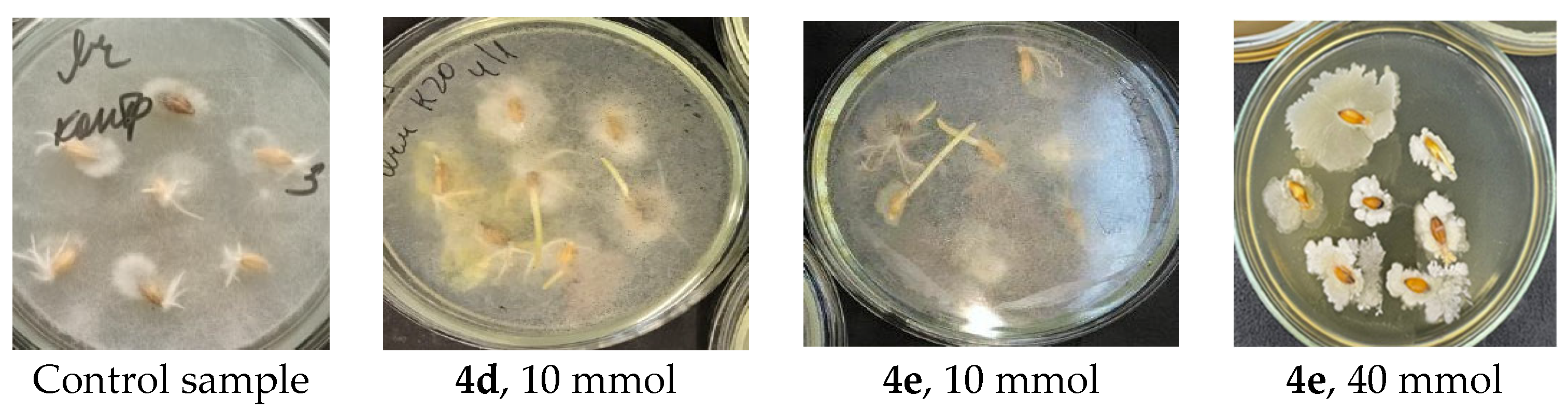
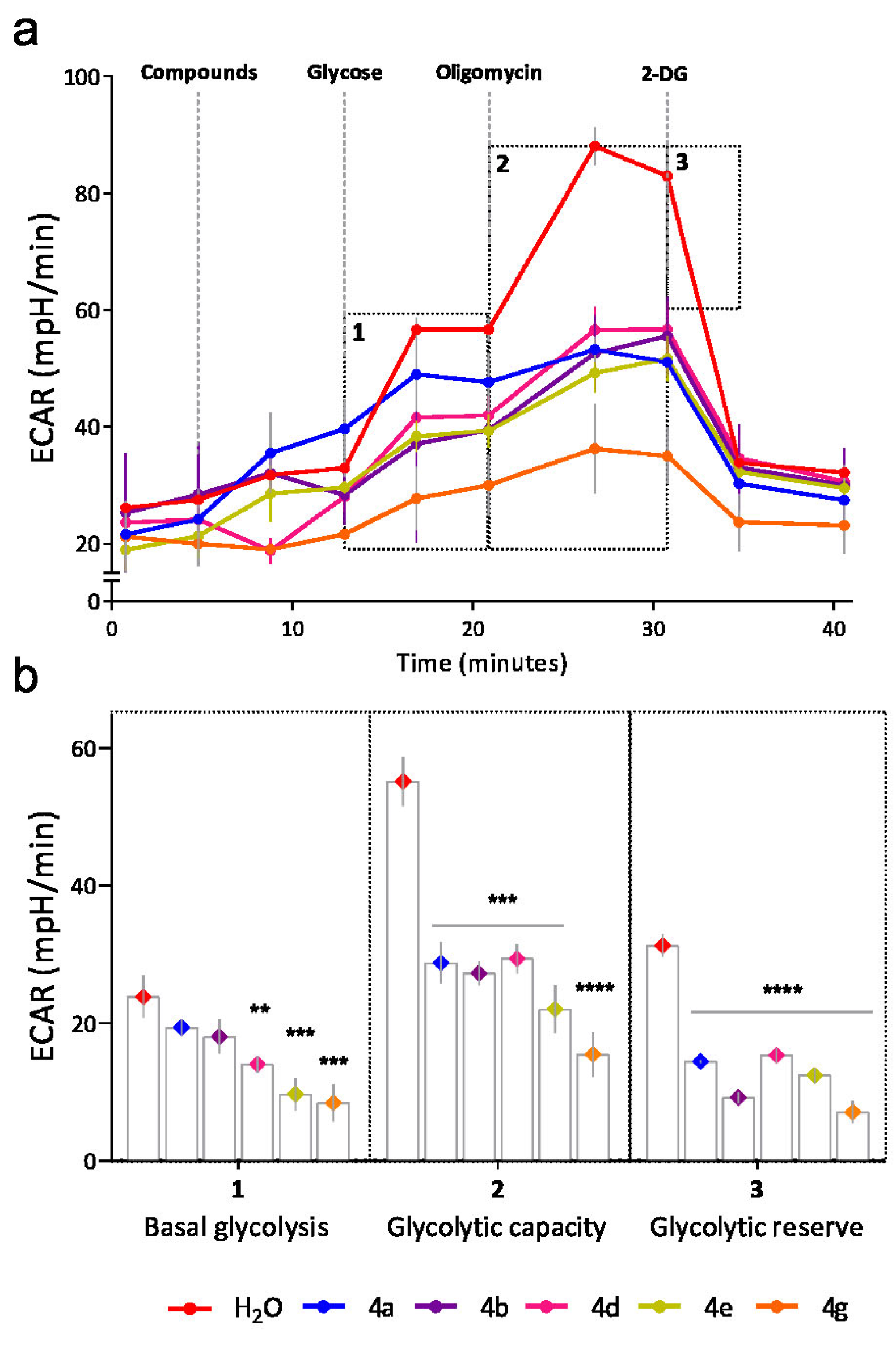
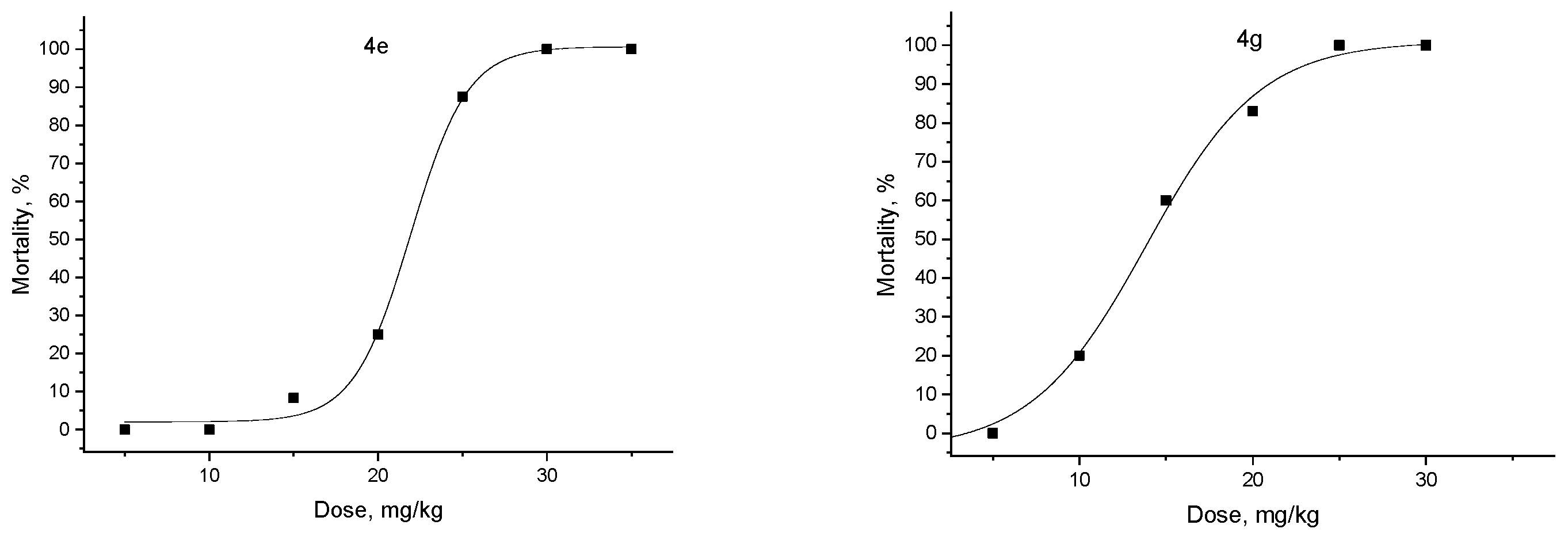
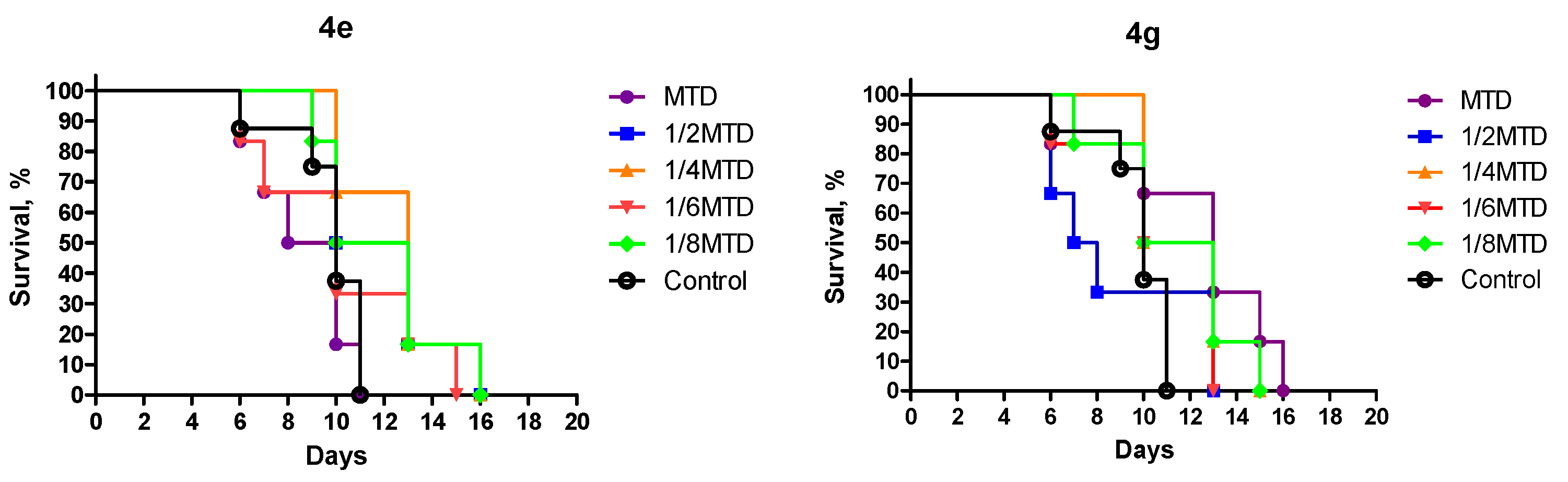
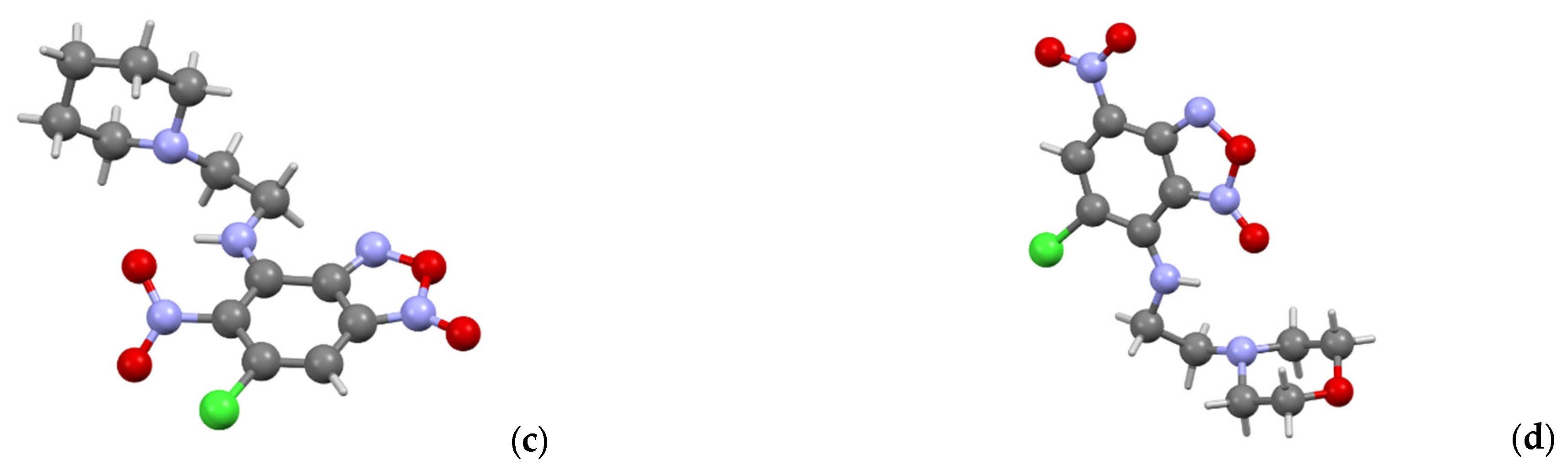3.1. Chemistry
IR spectra were recorded on an IR Fourier spectrometer Tensor 37 (Bruker Optik GmbH, Germany) in the 400–3600 cm
−1 range in KBr. The
1H- and
13C-NMR spectra were recorded on a Bruker AVANCE 400 spectrometer (Bruker BioSpin, Rheinstetten, Germany) operating at 400 MHz (for
1H NMR) and 101 MHz (for
13C NMR), Brucker spectrometers AVANCE
III-500 (Bruker BioSpin, Rheinstetten, Germany) operating at 500 MHz (for
1H NMR) and 126 MHz (for
13C MMR) and Bruker Avance 600 spectrometer (Bruker BioSpin, Rheinstetten, Germany) operating at 600 MHz (for
1H NMR) and 151 MHz (for
13C NMR) (
Figures S1–S23, Supplementary Materials). Chemical shifts were measured in δ (ppm) with reference to the solvent (δ = 7.27 ppm and 77.00 ppm for CDCl
3, δ = 2.06 ppm and 28.94 ppm for (CD
3)
2CO, δ = 4.79 ppm for D
2O for
1H and
13C NMR, respectively). Elemental analysis was performed on a CHNS-O Elemental Analyser EuroEA3028-HT-OM (EuroVector S.p.A., Milan, Italy). The melting points were determined on JK-MAM-4 Melting-point Apparatus with Microscope (JINGKE SCIENTIFIC INSTRUMENT CO, Shanghai, China).
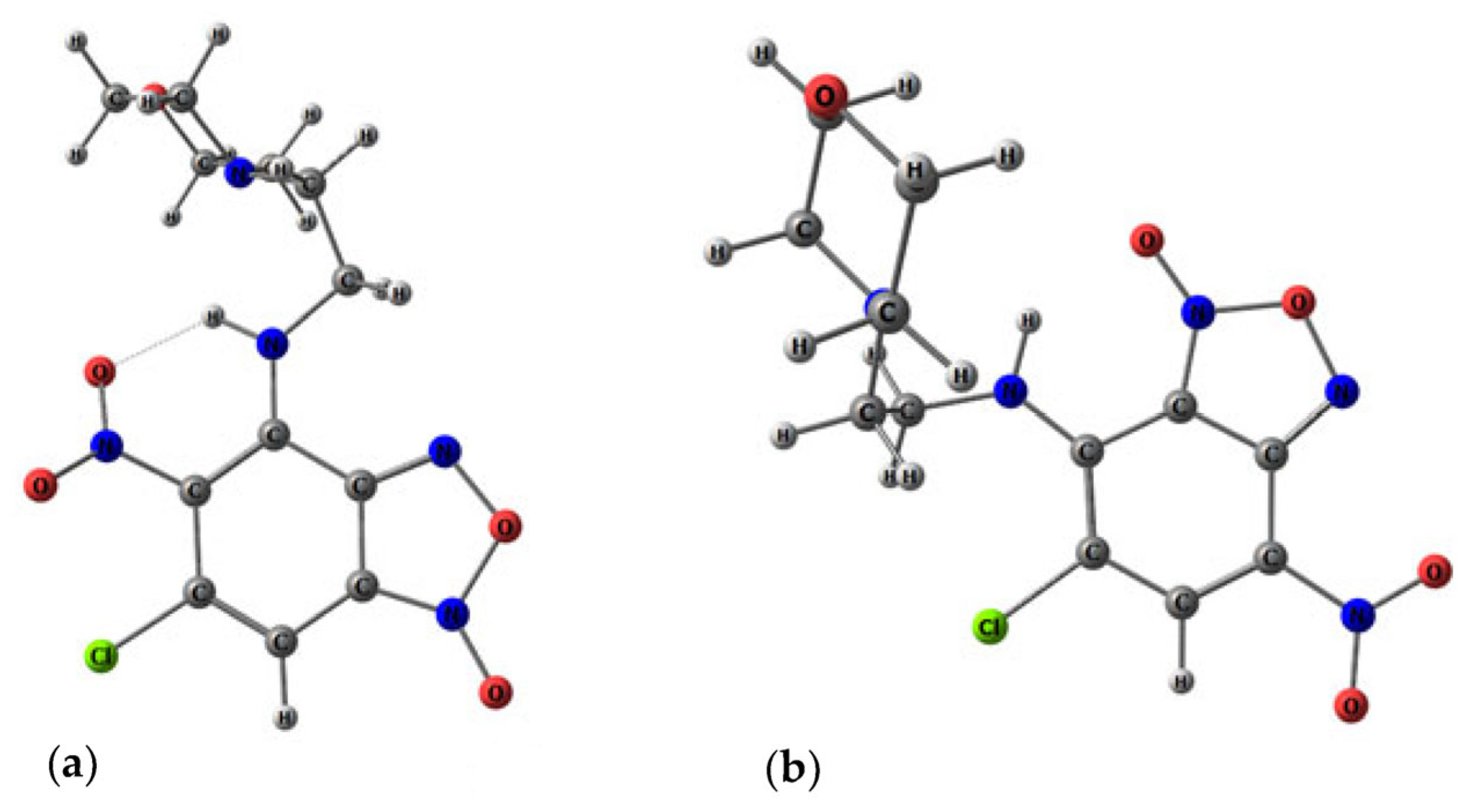
X-ray crystallography data. Data of
3a,
3d,
3f and
5d were collected on a Bruker D8 QUEST with PHOTON II CCD diffractometer (Bruker AXS, Ettlingen, Germany), using graphite monochromatedMoKα (λ = 0.71073 Å) radiation and ω-scan rotation. Data collection images were indexed, integrated, and scaled using the APEX3 [
51] data reduction package
70 and corrected for absorption using SADABS [
52]. The structure was solved by direct methods and refined using SHELX program [
53]. All non-hydrogen atoms were refined anisotropically. H atoms were calculated on idealized positions and refined as riding atoms. Crystal Data and Refinement Details are presented in
Tables S1 and S2. The X-ray analysis was performed on the equipment of Spectral-Analytical Center of FRC Kazan Scientific Center of RAS.
CCDC 2220082–2220085 (
3a,
3d,
3f and
5d) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via
www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 26 October 2022) or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223-336-033; or deposit@ccdc.cam.uk).
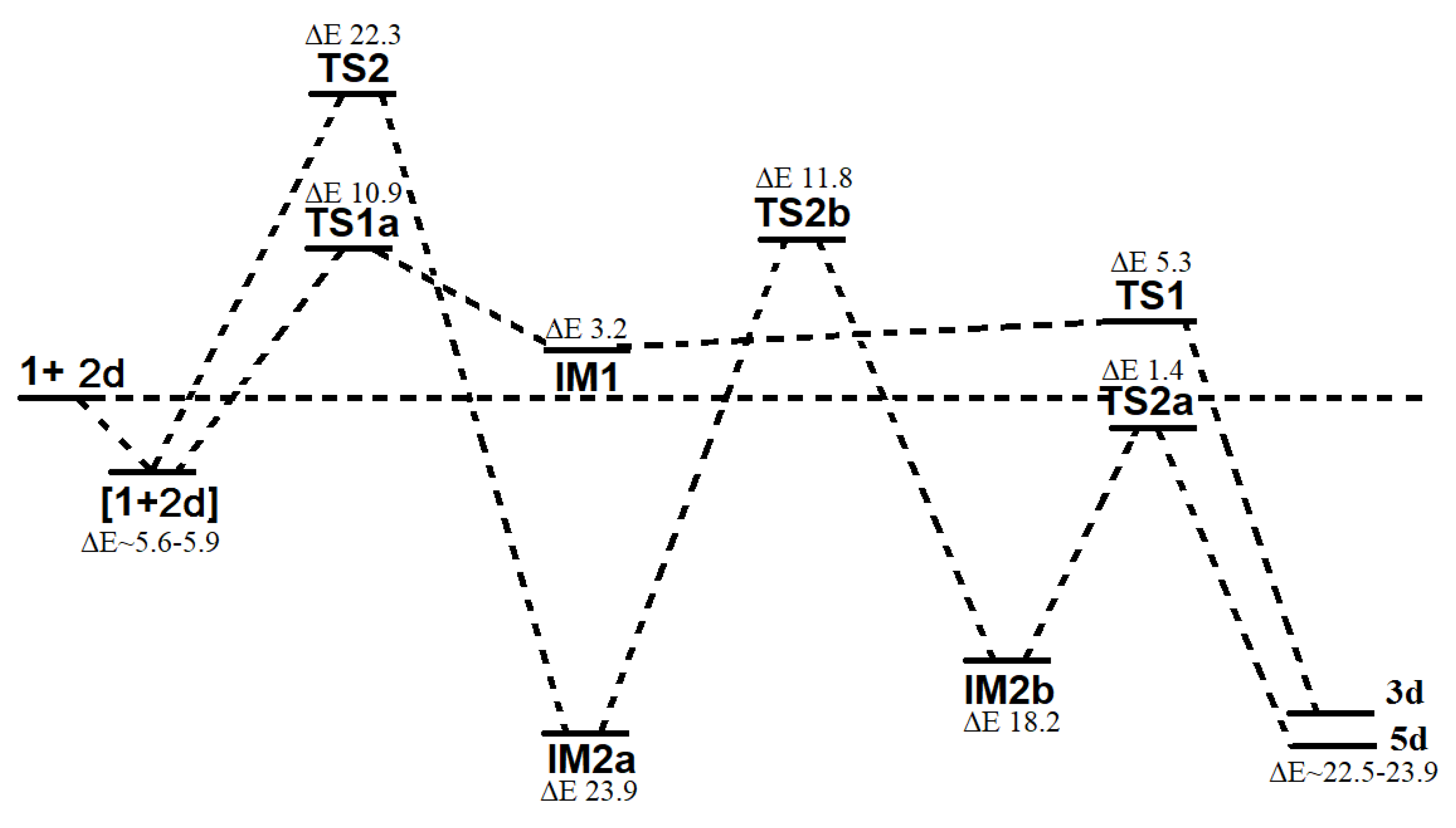
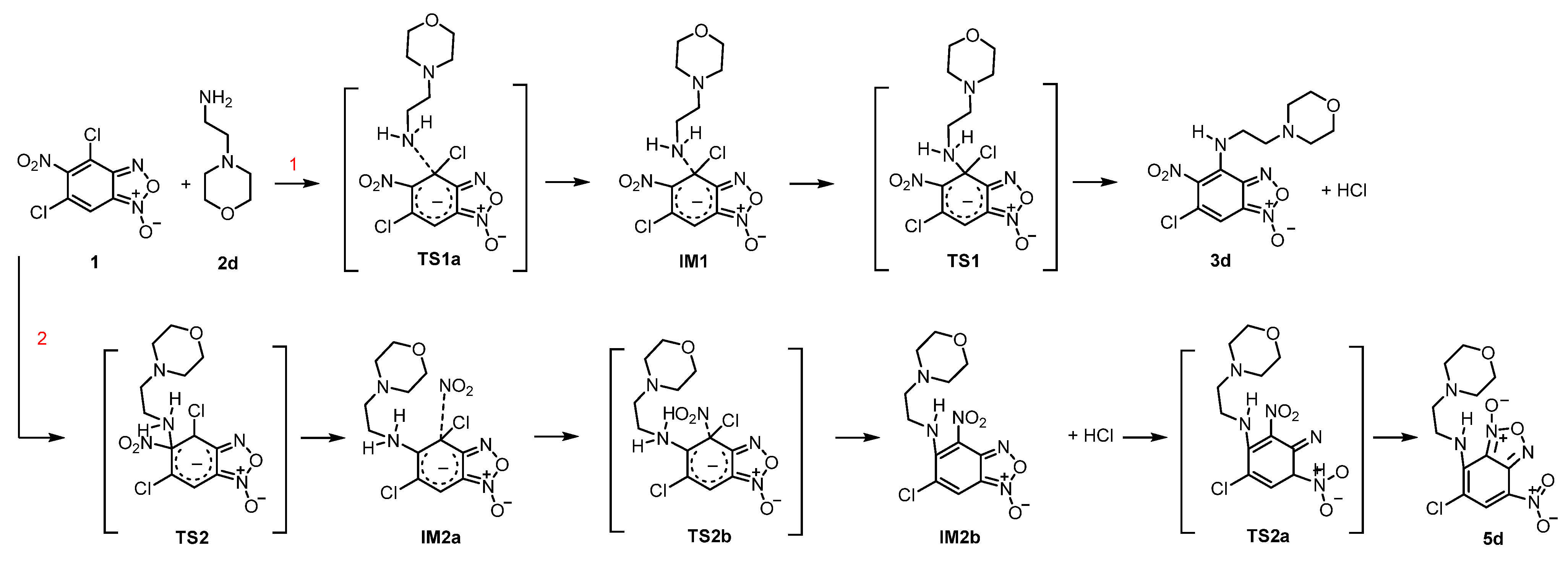
Quantum-chemical computations. All quantum-chemical computations were carried out with the use of Gaussian 16 suite of programs [
54]. Calculations were performed with Becke’s three parameter hybrid exchange functional [
29] and the gradient-corrected nonlocal correlation functional of Lee et al. [
30] (B3LYP) in combination with standard 6–31+G* basis set [
31,
32,
55]. For all compounds, geometry optimization of structures was performed without symmetry constraints. To ensure the calculated structures of reagents and products were indeed minima, vibrational analyses were performed using the same methods and were proved by all positive eigenvalues of Hessian matrix. The transition states were confirmed by the presence of one negative eigenvalue in the Hessian matrix of the second derivatives. Additionally, intrinsic reaction coordinate (IRC) was performed to examine connections between all the species involved in the reaction. All calculations were performed for a singlet surface and the solutions found were tested for stability against perturbations imposed on the wave function using the Stable procedure.
The completeness of the reactions and the purity of the synthesized compounds were monitored by thin layer chromatography (TLC) on Sorbfil PTSH-AF-A-UF plates (Sorbpolimer, Krasnodar, Russia), UV light was used as a developer.
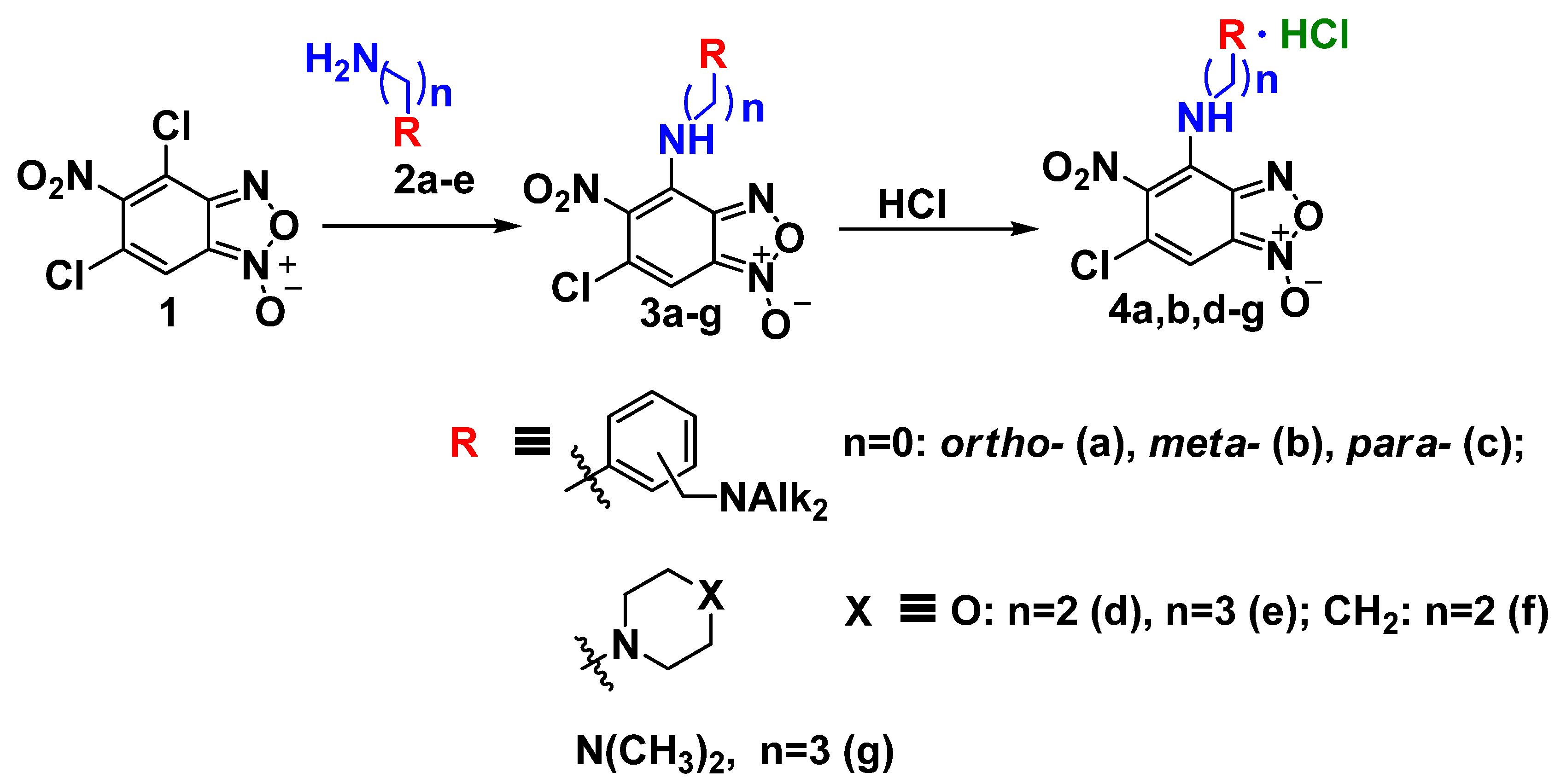
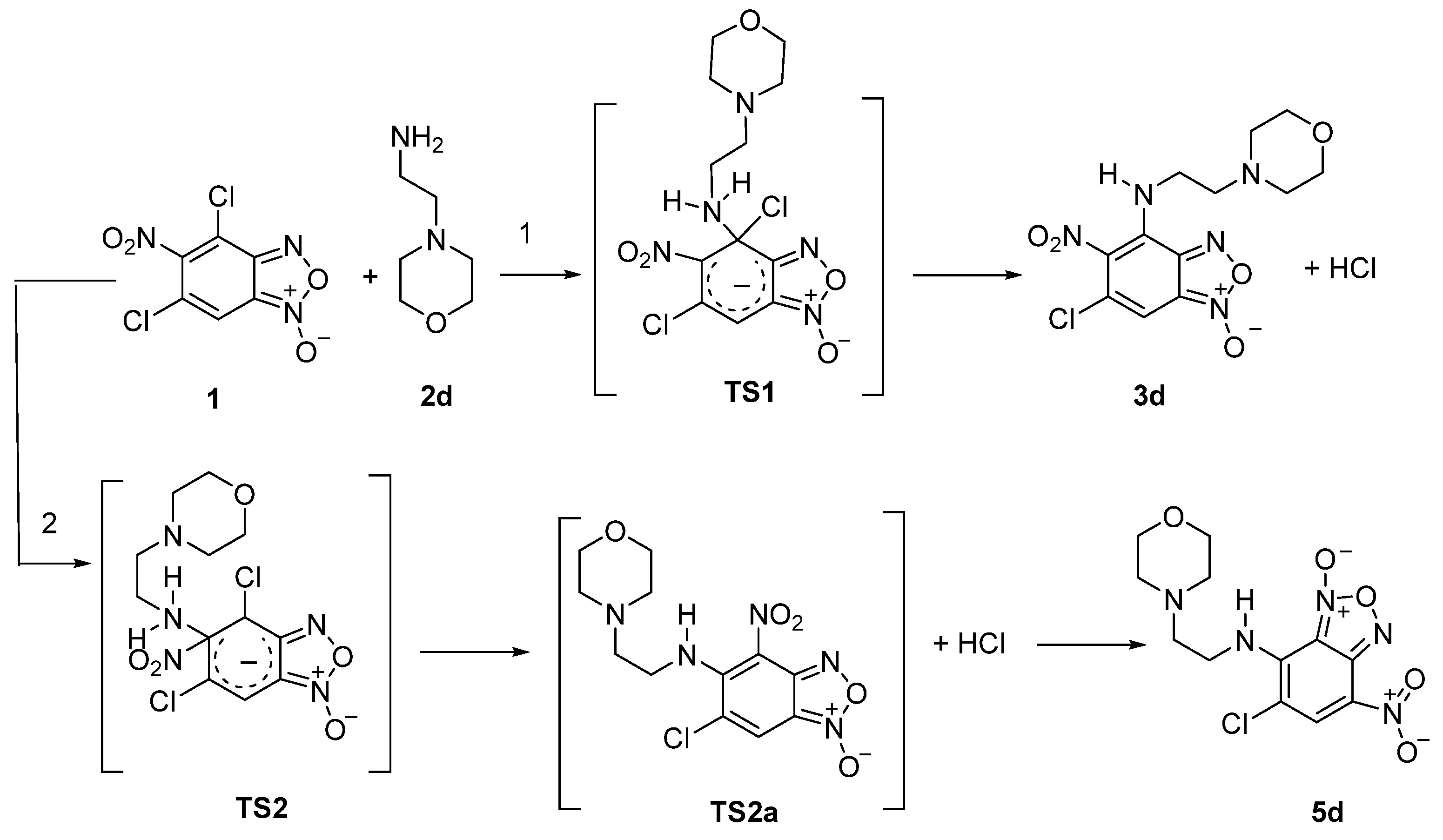
The following compounds were prepared following the literature procedures indicated: 4,6-dichloro-5-nitrobenzofuroxan
1 [
56], 6-chloro-4-(3-morpholinoprophylamino)-5-nitrobenzo[
c][1,2,5]oxadiazole 1-oxide
3e [
28].
Synthesis of amine-containing benzofuroxans 3a-f (general method). A solution of amine (1.6 mmol) in chloroform (2 mL) was added to a solution of benzofuroxan1 (0.8 mmol) in chloroform (2 mL) at room temperature with stirring. The reaction mixture was kept for 2 h at room temperature with constant stirring (the reaction progress and the purity of the obtained products were monitored by TLC, eluent toluene:ethyl acetate, 2:1). At the end of the exposure, the reaction mixture was reprecipitated in hexane (10 mL), the resulting precipitate was filtered off, washed with water (100 mL), and dried in vacuum (0.06 mm Hg) at a temperature of 40 °C to constant weight.
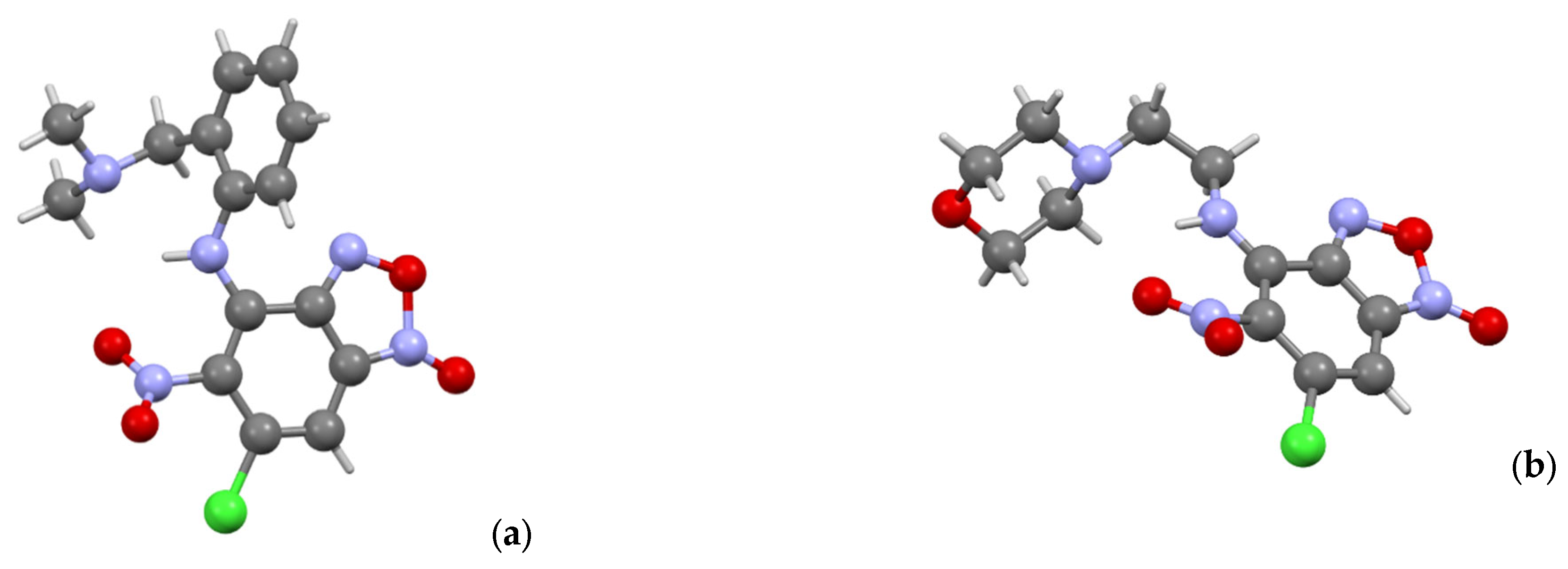
6-chloro-4-(2-((dimethylamino)methyl)phenylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide (3a). Brick red powder. Yield 276 mg (95%), m.p. = 152–153 °C with decomposition. IR spectrum, ν, cm−1: 721 (CCl), 1313 (NO2 symm), 1563 (NO2 asymm), 1624 (furoxan ring), 3084 (C7H).1H NMR (400 MHz, CDCl3) δ 7.19 (m, 2H, Ph), 7.13 (m, 1H, Ph), 7.05 (d, J = 7.9 Hz, 1H, Ph), 6.90 (s, 1H, Bf), 3.63 (s, 2H, CH2), 2.32 (s, 6H, CH3). 13C NMR (101 MHz, CDCl3) δ 147.3, 139.4, 134.8, 130.4, 129.3, 128.7, 128.2, 125.4, 121.3, 114.0, 106.7, 102.1, 63.4, 44.9. Found: C, 49.57; H, 3.89; Cl, 9.72; N, 19.21. Anal. calcd (%) for C15H14ClN5O4: C, 49.53; H, 3.88; Cl, 9.75; N, 19.25.
6-chloro-4-(3-((dimethylamino)methyl)phenylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide (3b). Purple powder. Yield 220 mg (76%), m.p. = 118–119 °C. IR spectrum, ν, cm−1: 700 (CCl), 1310 (NO2 symm), 1561 (NO2 asymm), 1625 (furoxan ring).1H NMR (400 MHz, acetone-d6) δ 7.31 (m, 2H, Ph), 7.23 (m, 2H, Ph), 7.13 (s, 1H, Bf), 3.40 (s, 2H, CH2), 2.19 (s, 6H, CH3). 13C NMR (101 MHz, acetone-d6) δ 148.2, 141.0, 139.1, 133.3, 131.9, 129.2, 127.9, 127.4, 124.9, 123.2, 114.1, 102.5, 64.0, 45.2. Found: C, 49.55; H, 3.86; Cl, 9.78; N, 19.22. Anal. calcd (%) for C15H14ClN5O4: C, 49.53; H, 3.88; Cl, 9.75; N, 19.25.
6-chloro-4-(4-((dimethylamino)methyl)phenylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide (3c). Dark brown powder. Yield 247 mg (85%), m.p. > 300 °C with decomposition. IR spectrum, ν, cm−1: 722 (CCl), 1536 (NO2 asymm), 1612 (furoxan ring).1H NMR (400 MHz, CDCl3) δ 9.01 (s, 1H, NH), 7.39 (d, J = 8.4 Hz, 2H, Ph), 7.35 (s, 1H, Bf), 7.21 (d, J = 8.4 Hz, 2H, Ph), 3.52 (s, 2H, CH2), 2.30 (s, 6H, CH3). 13C NMR (101 MHz, CDCl3) δ 148.8, 144.2, 139.2, 137.3, 134.1, 132.4, 130.7, 130.4, 125.6, 106.5, 64.2, 45.8. Found: C, 49.58; H, 3.84; Cl, 9.74; N, 19.28. Anal. calcd (%) for C15H14ClN5O4: C, 49.53; H, 3.88; Cl, 9.75; N, 19.25.
6-chloro-4-(2-morpholinoethylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide (3d). Bright orange powder. Yield 266 mg (98%), m.p. = 140–141 °C. IR spectrum, ν, cm−1: 679 (CCl), 1387 (NO2 symm), 1579 (NO2 asymm), 1627 (furoxan ring), 3052 (C7H). 1H NMR (400 MHz, acetone-d6) δ 8.87 (s, 1H, NH), 6.79 (s, 1H, Bf), 4.19 (d, J = 5.6 Hz, 2H, CH2), 3.67 (s, 4H, CH2), 2.80 (s, 4H, CH2), 2.57 (m, 2H, CH2). 13C NMR (101 MHz, acetone-d6, δ, m.d.): 148.4, 138.2, 128.9, 127.3, 113.0, 98.8, 66.6, 55.9, 52.9, 43.1. Found: C, 41.96; H, 4.09; Cl, 10.33; N, 20.34. Anal. calcd (%) for C12H14ClN5O5: C, 41.93; H, 4.11; Cl, 10.31; N, 20.37.
6-chloro-5-nitro-4-(2-(piperidin-1-yl)ethylamino)benzo[c][1,2,5]oxadiazole 1-oxide (3f). Purple powder. Yield 205 mg (75%), m.p. = 88–89 °C. IR spectrum, ν, cm−1: 687 (CCl), 1366 (NO2 symm), 1561 (NO2 asymm), 1627 (furoxan ring), 3091 (C7H). 1H NMR (600 MHz, acetone-d6, δ): 8.92 (s, 1H, NH), 6.77 (s, 1H, Bf), 4.14 (m, 2H, CH2), 2.73 (m, 2H, CH2), 2.51 (m, 4H, CH2), 1.61 (m, 4H, CH2), 1.49 (m, 2H, CH2). 13C NMR (151 MHz, acetone-d6) δ 148.5, 138.1, 129.1, 127.2, 113.1, 98.6, 56.0, 53.7, 43.5, 26.0, 24.2. Found: C, 45.72; H, 4.70; Cl, 10.34; N, 20.45. Anal. calcd (%) for C13H16ClN5O4: C, 45.69; H, 4.72; Cl, 10.37; N, 20.49.
6-chloro-4-(3-(dimethylamino)propylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide (3g). Orange powder. Yield 219 mg (87%), m.p.= 55–56 °C. IR spectrum, ν, cm−1: 684 (CCl), 1348 (NO2 symm), 1536 (NO2 asymm), 1619 (furoxan ring), 3104 (C7H), 3372 (NH).1H NMR (400 MHz, CDCl3) δ 10.01 (m, 1H, NH), 6.56 (s, 1H, Bf), 4.14 (m, 2H, CH2), 2.57 (m, 6H, CH3), 2.32 (s, 2H, CH2), 1.87 (m, 2H, CH2). 13C NMR (101 MHz, CDCl3) δ 147.9, 136.6, 129.6, 125.5, 113.2, 97.4, 58.7, 48.3, 45.0, 25.0. Found: C, 41.80; H, 4.44; Cl, 11.28; N, 22.11. Anal. calcd (%) for C11H14ClN5O4: C, 41.85; H, 4.47; Cl, 11.23; N, 22.18.
Synthesis of benzofuroxans salts 4a-f (general method). Hydrochloric acid (1.0 mmol) was added dropwise to a solution of benzofuroxan3a-f (0.8 mmol) in ethyl alcohol (2 mL) at room temperature with stirring. The reaction mixture was kept overnight at room temperature with constant stirring. At the end of the exposure, the precipitate that formed was filtered off and dried in a vacuum (0.06 mm Hg) at a temperature of 40 °C to constant weight.
6-chloro-4-(2-((dimethylammonio)methyl)phenylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide chloride (4a). Orange powder. Yield 260 mg (82%), m.p. = 125–126 °C with decomposition. IR spectrum, ν, cm−1: 689 (CCl), 1348 (NO2 symm), 1555 (NO2 asymm), 1623 (furoxan ring), 2465 (NH+), 3100 (C7H).1H NMR (600 MHz, D2O) δ 7.70 (d, J = 7.3 Hz, 1H, Ph), 7.55 (m, 2H, Ph), 7.36 (d, J = 7.6 Hz, 1H, Ph), 7.04 (s, 1H, Bf), 4.52 (s, 2H, CH2), 2.96 (s, 6H, CH3). 13C NMR (101 MHz, D2O) δ 147.9, 138.4, 133.1, 132.8, 132.4, 131.8, 129.6, 128.7, 128.3, 127.2, 114.9, 102.7, 57.2, 43.3. Found: C, 44.96; H, 3.81; Cl, 17.79; N, 17.73. Anal. calcd (%) for C15H15Cl2N5O4: C, 45.02; H, 3.78; Cl, 17.72; N, 17.50.
6-chloro-4-(3-((dimethylammonio)methyl)phenylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide chloride (4b). Orange oil. Yield 310 mg (96%). IR spectrum, ν, cm−1: 699 (CCl), 1354 (NO2 symm), 1563 (NO2 asymm), 1624 (furoxan ring), 2478 (NH+), 3407 (NH).1H NMR (600 MHz, D2O) δ 7.55 (m, 3H, Ph), 7.48 (s, 1H, Ph), 7.46 (s, 1H, Bf), 4.31 (s, 2H, CH2), 2.79 (s, 6H, CH3). 13C NMR (101 MHz, D2O) δ 143.7, 132.7, 132.0, 131.9, 131.8, 131.7, 131.3, 125.8, 125.2, 124.2, 114.0, 96.9, 60.6, 42.8. Found: C, 45.01; H, 3.85; Cl, 17.81; N, 17.56. Anal. calcd (%) for C15H15Cl2N5O4: C, 45.02; H, 3.78; Cl, 17.72; N, 17.50
6-chloro-4-(2-(morpholino-4-ium)ethylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide chloride (4d). Orange powder. Yield 230 mg (76%), m.p. = 186–187 °C. IR spectrum, ν, cm−1: 688 (CCl), 1392 (NO2 symm), 1577 (NO2 asymm), 1630 (furoxan ring), 2481 (NH+), 3248 (NH).1H NMR (400 MHz, D2O) δ 6.72 (s, 1H, Bf), 4.42 (t, J = 6.7 Hz, 2H, CH2), 4.02 (br.s, 4H, CH2), 3.59 (t, J = 6.7 Hz, 4H, CH2), 3.49 (br.s, 4H, CH2). ЯMP13C (101 Hz, D2O) δ 147.9, 137.1, 129.6, 129.0, 114.6, 100.5, 64.3, 56.6, 52.9, 40.7. Found: C, 37.98; H, 3.93; Cl, 18.67; N, 18.49. Anal. calcd (%) for C12H15Cl2N5O5: C, 37.91; H, 3.98; Cl, 18.65; N, 18.42.
6-chloro-4-(3-(morpholino-4-ium)propylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide chloride (4e). Red powder. Yield 250 mg (79%), m.p.= 239–240 °C. IR spectrum, ν, cm−1:1376 (NO2 symm), 1567 (NO2 asymm), 1613 (furoxan ring), 2361 (NH+), 3197 (NH).1H NMR (400 MHz, D2O) δ 6.88 (s, 1H, Bf), 4.12 (m, 4H, CH2), 3.85 (m, 2H, CH2), 3.53 (m, 2H, CH2), 3.28 (m, 4H, CH2), 2.24 (m, 2H, CH2). 13C NMR (101 MHz, D2O) δ 147.9, 138.4, 129.8, 126.3, 114.7, 99.9, 63.9, 54.5, 51.9, 43.3, 24.3. Found: C, 39.68; H, 4.32; Cl, 17.95; N, 17.82. Anal. calcd (%) for C13H17Cl2N5O5: C, 39.61; H, 4.35; Cl, 17.99; N, 17.77.
6-chloro-5-nitro-4-(2-(piperidinium-1-yl)ethylamino)benzo[c][1,2,5]oxadiazole 1-oxide chloride (4f). Red powder. Yield 230 mg (78%), m.p. = 106–107 °C. IR spectrum, ν, cm−1:697 (CCl), 1373 (NO2 symm), 1561 (NO2 asymm), 1613 (furoxan ring), 2530 (NH+), 3099 (C7H), 3429 (NH). 1H NMR (500 MHz, D2O) δ 6.92 (s, 1H, Bf), 4.39 (m, 2H, CH2), 3.60 (d, J = 11.9 Hz, 2H, CH2), 3.48 (m, 2H, CH2), 3.03 (t, J = 12.1 Hz, 2H, CH2), 1.94 (m, 2H, CH2), 1.76 (m, 4H, CH2). 13C NMR (126 MHz, D2O) δ 147.7, 137.1, 129.3, 128.7, 114.5, 100.5, 55.64, 53.8, 40.3, 22.6, 20.9. Found: C, 41.17; H, 4.57; Cl, 18.82; N, 18.51. Anal. calcd (%) for C13H17Cl2N5O4: C, 41.28; H, 4.53; Cl, 18.75; N, 18.52.
6-chloro-4-(3-(dimethylammonio)propylamino)-5-nitrobenzo[c][1,2,5]oxadiazole 1-oxide chloride (4g). Orange powder. Yield 200 mg (73%), m.p. = 196–197 °C with decomposition. IR spectrum, ν, cm−1: 687 (CCl), 1370 (NO2 symm), 1566 (NO2 asymm), 1621 (furoxan ring), 2481 (NH+), 3100 (C7H),3342 (NH).1H NMR (400 MHz, D2O) δ 6.57 (m, 1H, Bf), 4.13 (t, J = 6.9 Hz, 2H, CH2), 3.39 (m, 2H, CH2), 3.03 (s, 6H, CH3), 2.30 (m, 2H, CH2). 13C NMR (101 MHz, D2O) δ 147.9, 138.1, 129.8, 127.5, 114.3, 99.4, 55.5, 44.0, 43.5, 25.7. Found: C, 37.62; H, 4.33; Cl, 20.25; N, 19.93. Anal. calcd (%) for C11H15Cl2N5O4: C, 37.51; H, 4.29; Cl, 20.13; N, 19.89.