Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding
Abstract
:1. Introduction
2. Results and Discussion
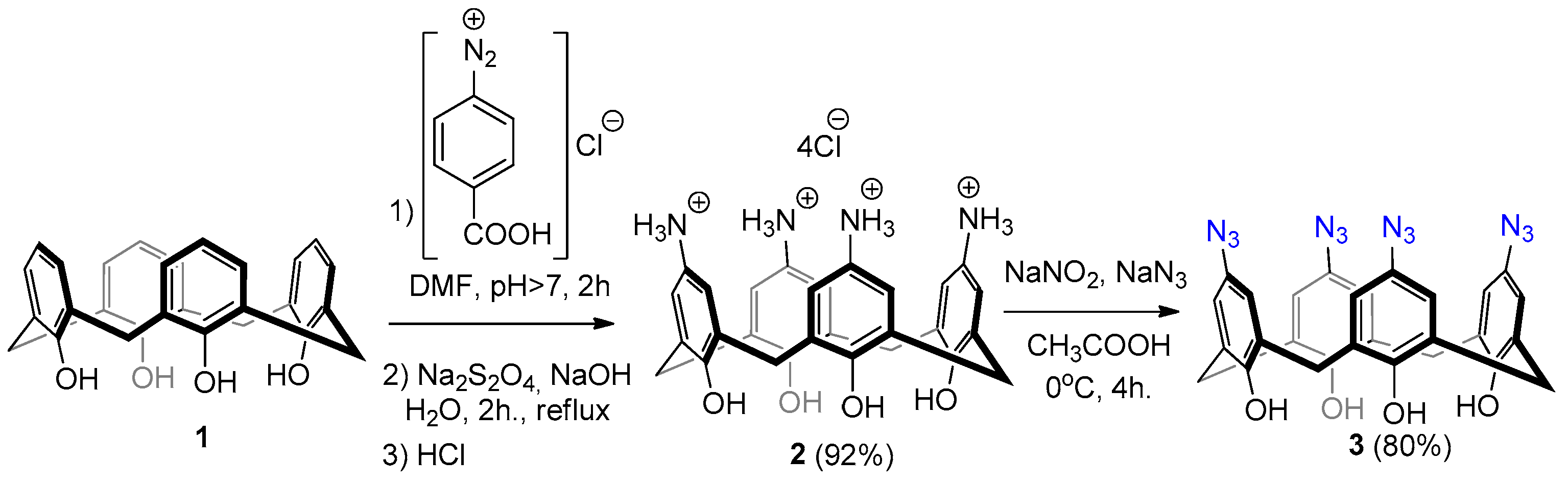
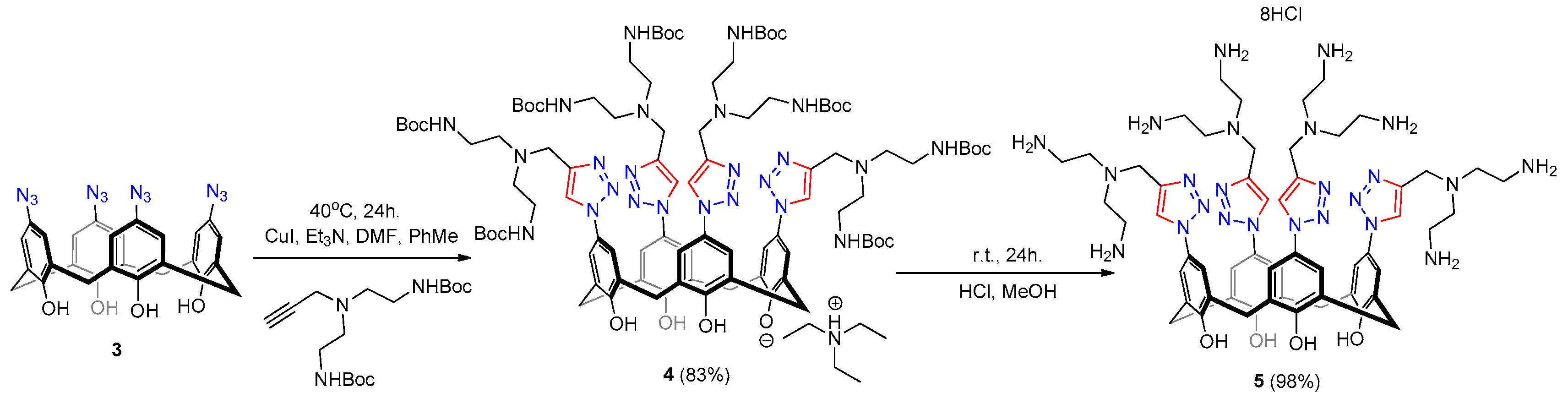
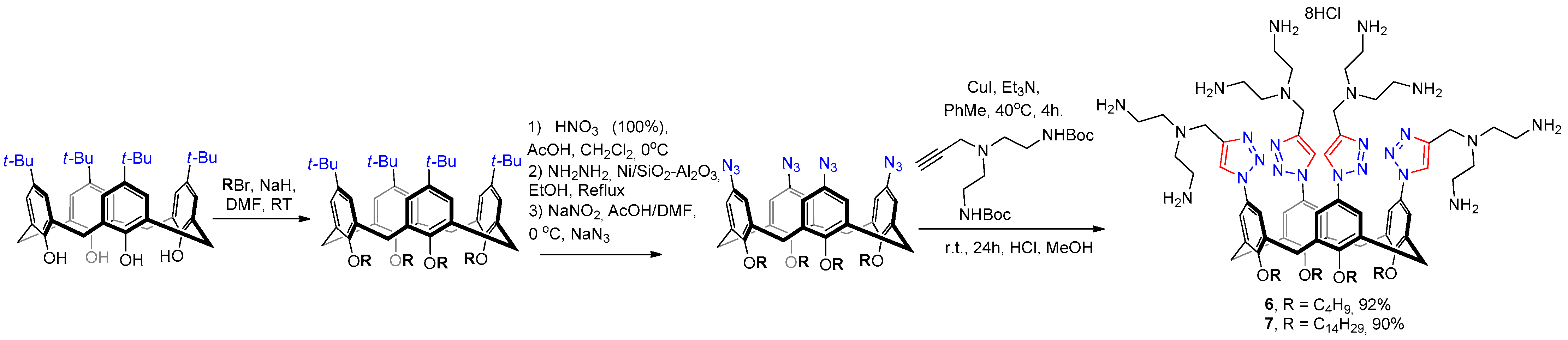
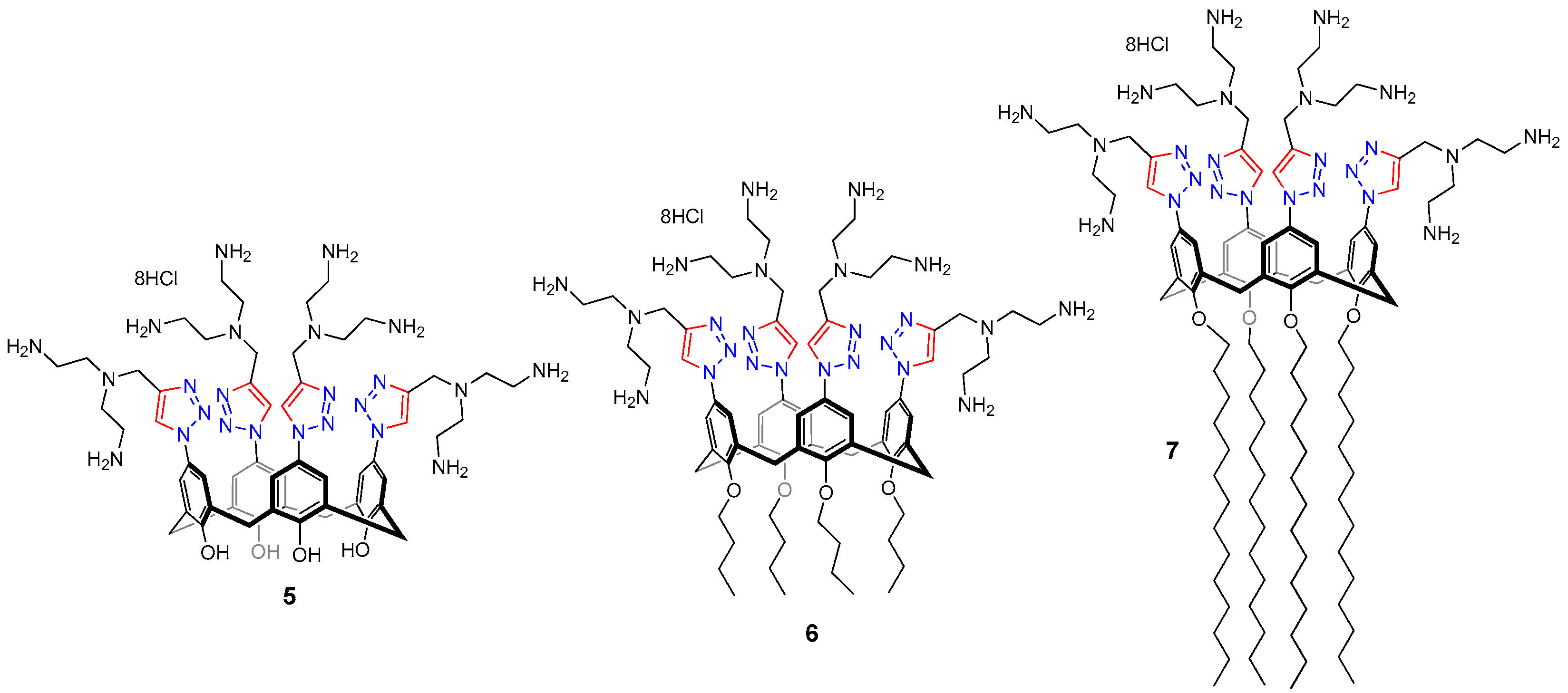
2.1. Synthesis
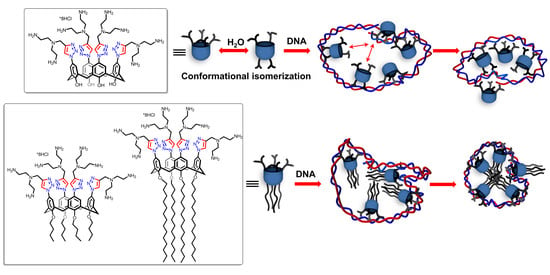
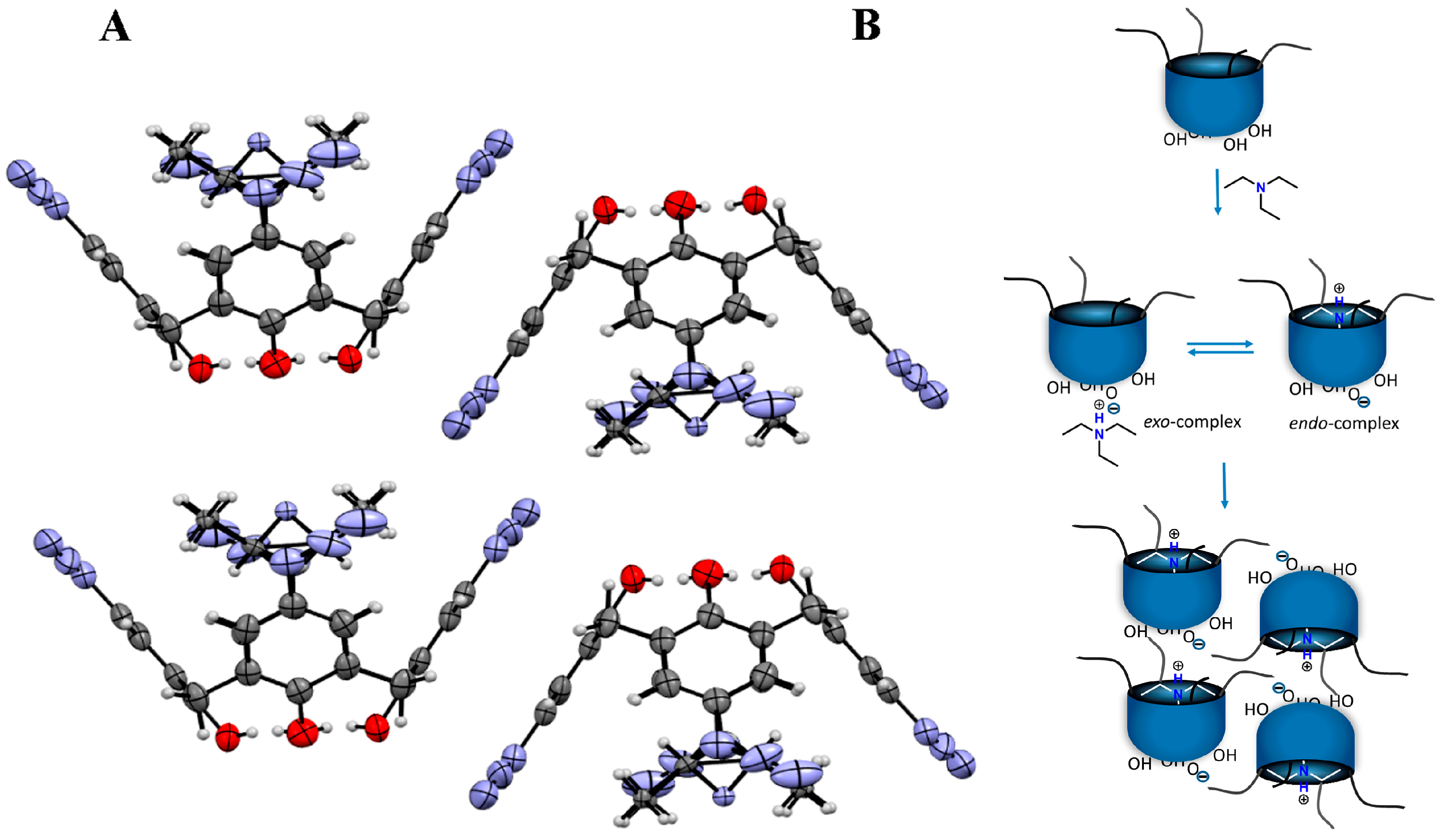
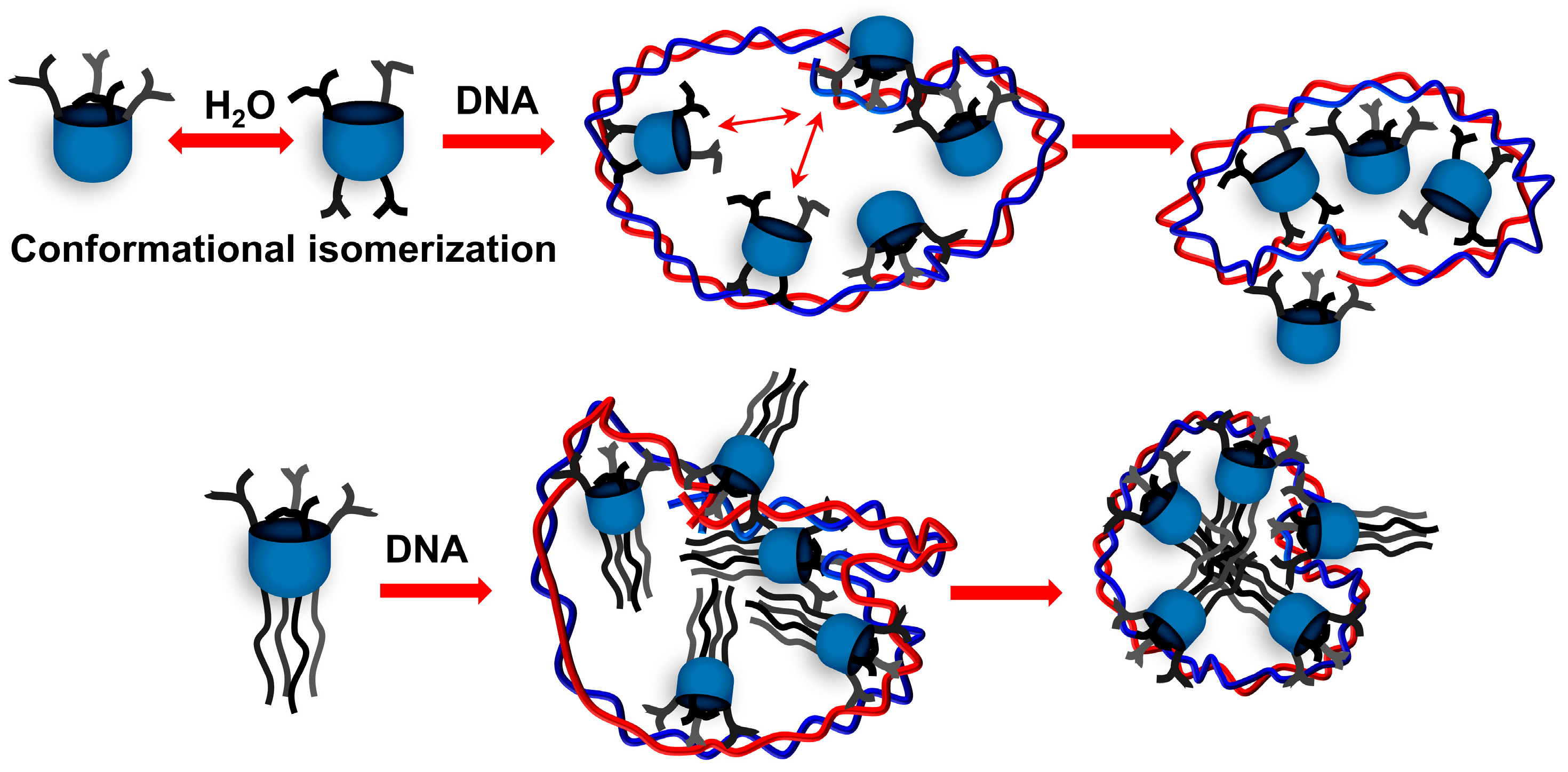
2.2. Aggregation Behavior
2.3. CT DNA Binding Abilities
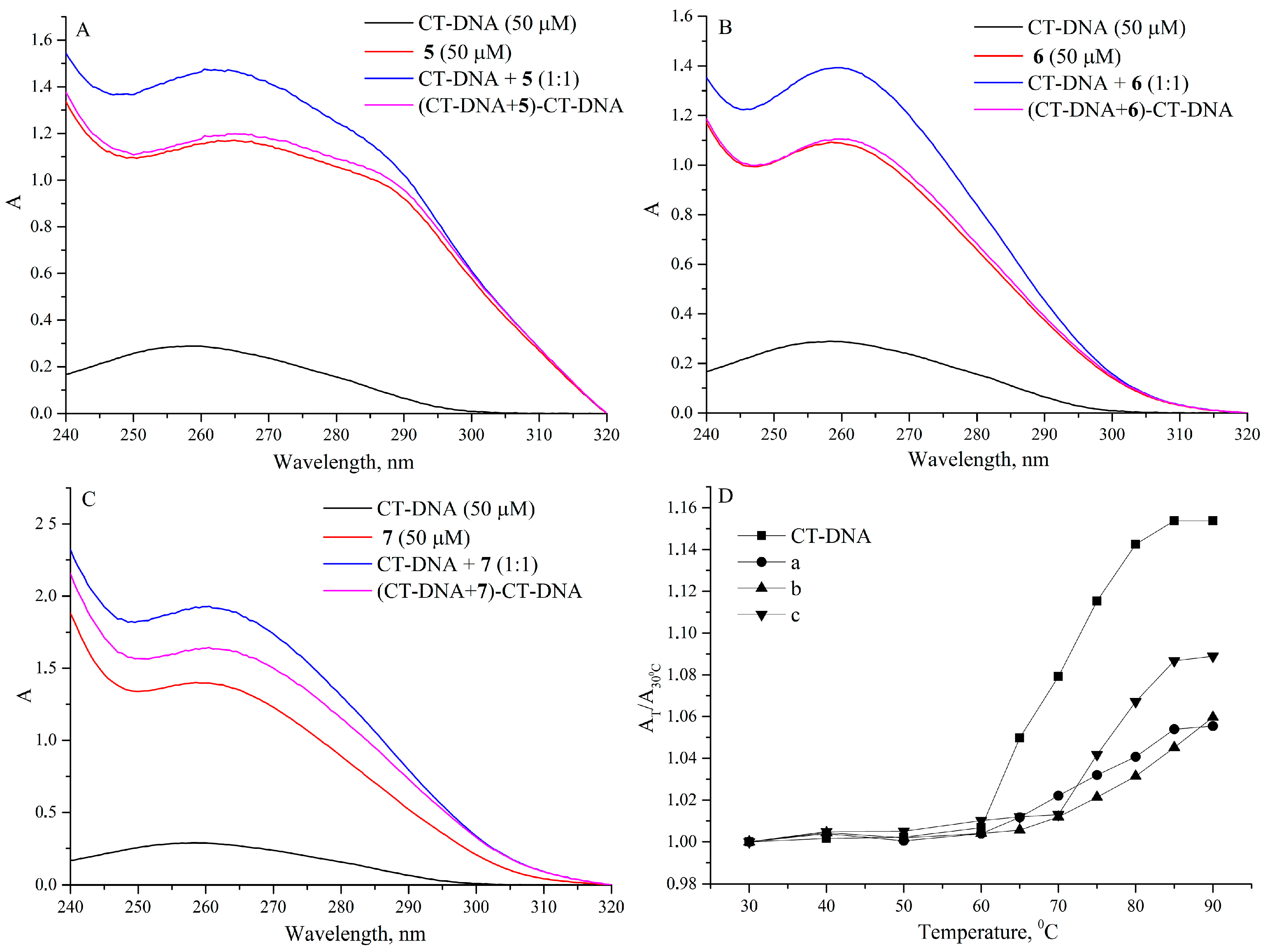
2.3.1. UV Absorption Spectroscopic Study
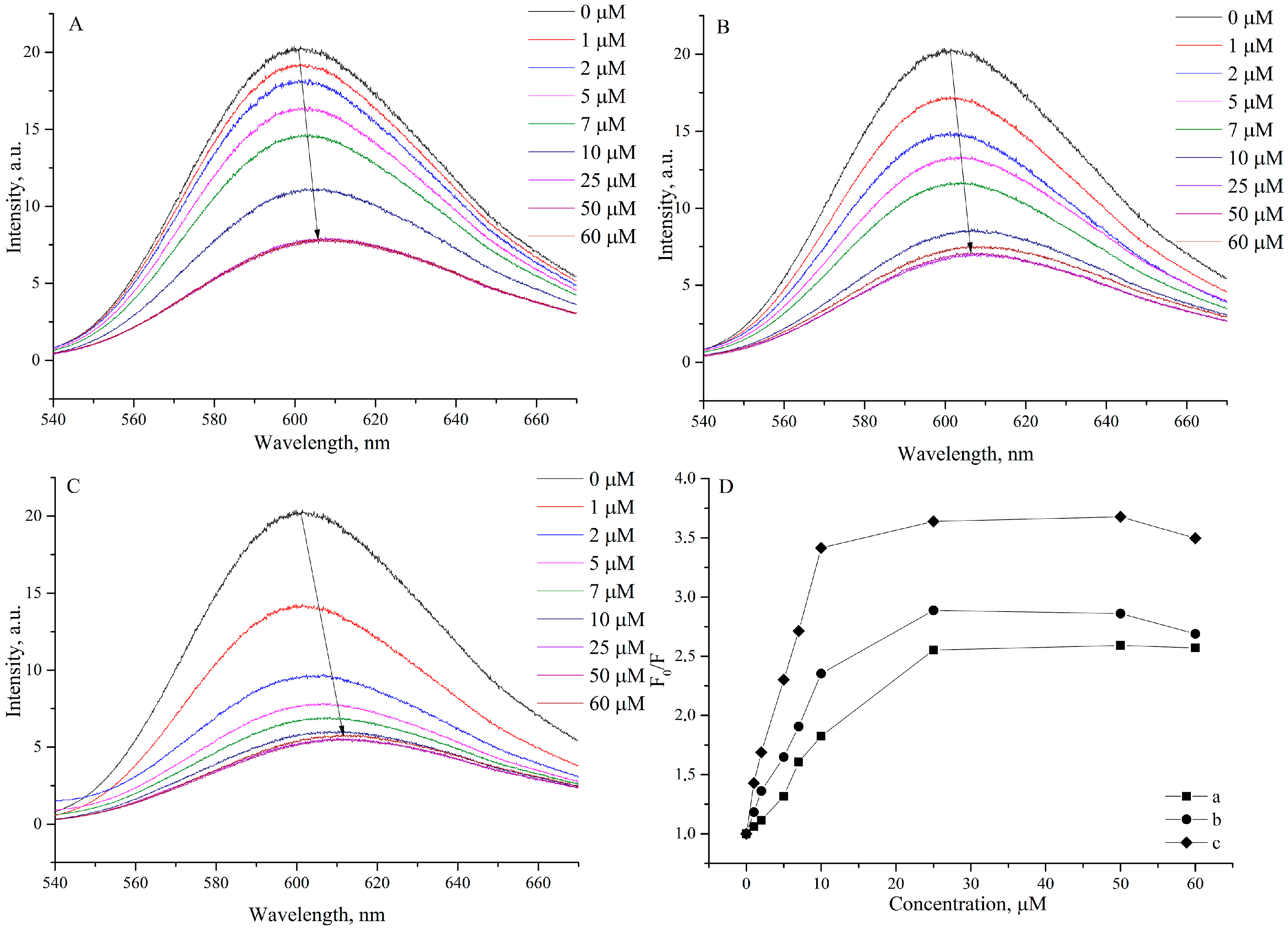
2.3.2. Fluorescence Spectroscopic Studies
2.3.3. Circular Dichroism Spectral Studies
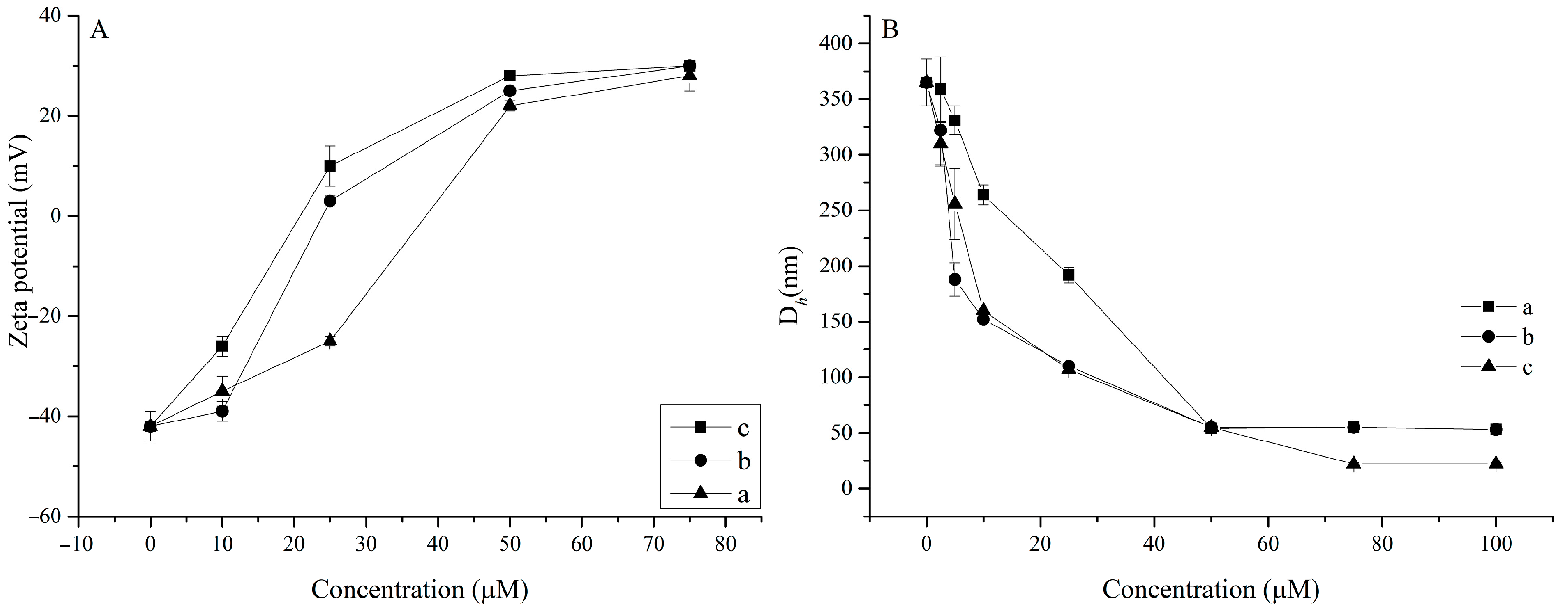
2.3.4. Particle Size and Zeta Potential Measurements
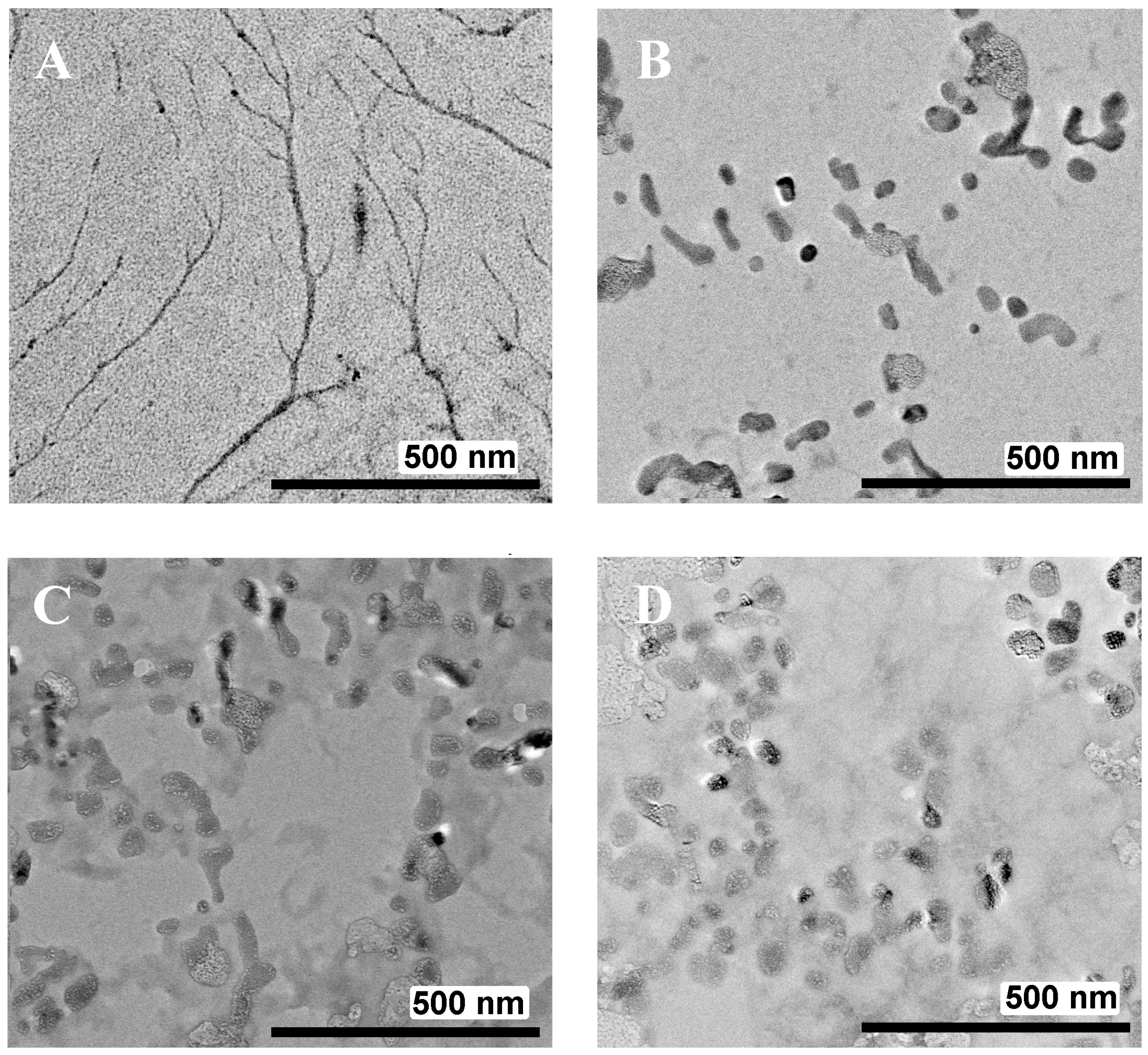
2.3.5. Transmission Electron Microscopy (TEM)
3. Materials and Methods
3.1. Characterisation Methods
3.2. Reagents
3.3. Sample Preparation
3.4. Instrumentations
3.5. Dynamic and Electrophoretic Light Scattering Study
3.6. UV Absorption Spectroscopic Study
3.7. Steady-State Fluorescence Study
3.8. Circular Dichroism (CD) Study
3.9. Transition Electron Microscopy (TEM) Study
3.10. X-ray Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boto, L. Horizontal Gene Transfer in Evolution: Facts and Challenges. Proc. R. Soc. 2009, 277, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Husnik, F.; McCutcheon, J.P. Functional Horizontal Gene Transfer from Bacteria to Eukaryotes. Nat. Rev. Microbiol. 2018, 16, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery Technologies for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the Delivery of RNA Therapeutics: From Concept to Clinical Reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Setten, R.L.; Rossi, J.J.; Han, S.P. The Current State and Future Directions of RNAi-based Therapeutics. Nat. Rev. Drug. Discov. 2019, 18, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug. Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Dominska, M.; Dykxhoorn, D.M. Breaking Down the Barriers: siRNA Delivery and Endosome Escape. J. Cell. Sci. 2010, 123, 1183–1189. [Google Scholar] [CrossRef]
- Nishikawa, M.; Huang, L. Nonviral Vectors in the New Millennium: Delivery Barriers in Gene Transfer. Hum. Gene Ther. 2001, 12, 861–870. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, R.J.; Anderson, D.G. Non-viral Vectors for Gene-based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Felgner, P.L. Nonviral Strategies for Gene Therapy. Sci. Am. 1997, 276, 102–106. [Google Scholar] [CrossRef]
- Fraley, R.; Subramani, S.; Berg, P.; Papahadjopoulos, D. Introduction of Liposome-encapsulated SV40 DNA into Cells. J. Biol. Chem. 1980, 255, 10431–10435. [Google Scholar] [CrossRef]
- Wu, G.Y.; Wu, C.H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 1987, 262, 4429–4432. [Google Scholar] [CrossRef]
- Geng, W.C.; Huang, Q.; Xu, Z.; Wang, R.; Guo, D.S. Gene Delivery Based on Macrocyclic Amphiphiles. Theranostics 2019, 9, 3094–3106. [Google Scholar] [CrossRef] [PubMed]
- Ranamalla, S.R.; Porfire, A.S.; Tomuță, I.; Banciu, M. An Overview of the Supramolecular Systems for Gene and Drug Delivery in Tissue Regeneration. Pharmaceutics 2022, 14, 1733. [Google Scholar] [CrossRef] [PubMed]
- Rodik, R.; Poberezhnyk, M.; Kalchenko, V. Calixarene Derivatives for (Nano)Biotechnologies. Macroheterocycles 2017, 10, 421–431. [Google Scholar] [CrossRef]
- Jiménez Blanco, J.L.; Benito, J.M.; Ortiz Mellet, C.; García Fernández, J.M. Molecular nanoparticle-based gene delivery systems. J. Drug Deliv. Sci. Technol. 2017, 42, 18–37. [Google Scholar] [CrossRef]
- Peters, M.S.; Schrader, T. Calix[n]arenes and Nucleic Acids. In Calixarenes and Beyond; Neri, P., Sessler, J., Wang, M.X., Eds.; Springer: Cham, Switzerland, 2016; pp. 627–669. [Google Scholar] [CrossRef]
- Böhmer, V. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew. Chem. Int. Ed. Engl. 1995, 34, 713–745. [Google Scholar] [CrossRef]
- Zadmard, R.; Schrader, T. DNA Recognition with Large Calixarene Dimers. Angew. Chem. Int. Ed. 2006, 45, 2703–2706. [Google Scholar] [CrossRef]
- Lalor, R.; DiGesso, J.L.; Mueller, A.; Matthews, S.E. Efficient Gene Transfection with Functionalized Multicalixarenes. Chem. Commun. 2007, 46, 4907–4909. [Google Scholar] [CrossRef]
- Mostovaya, O.; Padnya, P.; Shiabiev, I.; Mukhametzyanov, T.; Stoikov, I. PAMAM-calix-dendrimers: Synthesis and Thiacalixarene Conformation Effect on DNA Binding. Int. J. Mol. Sci. 2021, 22, 11901. [Google Scholar] [CrossRef]
- Samanta, K.; Ranade, D.S.; Upadhyay, A.; Kulkarni, P.P.; Rao, C.P. A Bimodal, Cationic, and Water-Soluble Calix [4]arene Conjugate: Design, Synthesis, Characterization, and Transfection of Red Fluorescent Protein Encoded Plasmid in Cancer Cells. ACS Appl. Mater. Interfaces 2017, 9, 5109–5117. [Google Scholar] [CrossRef]
- Bagnacani, V.; Franceschi, V.; Fantuzzi, L.; Casnati, A.; Donofrio, G.; Sansone, F.; Ungaro, R. Lower Rim Guanidinocalix [4]arenes: Macrocyclic Nonviral Vectors for Cell Transfection. Bioconjug. Chem. 2012, 23, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Galukhin, A.; Imatdinov, I.; Osin, Y. p-tert-Butylthiacalix [4]arenes Equipped with Guanidinium Fragments: Aggregation, Cytotoxicity, and DNA Binding Abilities. RSC Adv. 2016, 6, 32722–32726. [Google Scholar] [CrossRef]
- Bagnacani, V.; Franceschi, V.; Bassi, M.; Lomazzi, M.; Donofrio, G.; Sansone, F.; Casnati, A.; Ungaro, R. Arginine clustering on calix [4]arene macrocycles for improved cell penetration and DNA delivery. Nat. Commun. 2013, 4, 1721. [Google Scholar] [CrossRef] [PubMed]
- Lebron, J.A.; Lopez-Lopez, M.; Garcia-Calderon, C.B.; Rosado, I.V.; Balestra, F.R.; Huertas, P.; Rodik, R.V.; Kalchenko, V.I.; Bernal, E.; Moya, M.L.; et al. Multivalent Calixarene-Based Liposomes as Platforms for Gene and Drug Delivery. Pharmaceutics 2021, 13, 1250. [Google Scholar] [CrossRef]
- Ostos, F.J.; Lebron, J.A.; Lopez-Cornejo, P.; Lopez-Lopez, M.; Garcia-Calderon, M.; Garcia-Calderon, C.B.; Rosado, I.V.; Kalchenko, V.I.; Rodik, R.V.; Moya, M.L. Self-aggregation in Aqueous Solution of Amphiphilic Cationic Calix [4]arenes. Potential Use as Vectors and Nanocarriers. J. Mol. Liq. 2020, 304, 112724. [Google Scholar] [CrossRef]
- Krosl, I.; Otkovic, E.; Niksic-Franjic, I.; Colasson, B.; Reinaud, O.; Visnjevac, A.; Piantanida, I. Impact of Positive Charge and Ring-size on the Interactions of Calixarenes with DNA, RNA and Nucleotides. New J. Chem. 2022, 46, 6860–6869. [Google Scholar] [CrossRef]
- Ibragimova, R.I.; Burilov, V.A.; Aimetdinov, A.R.; Mironova, D.A.; Evtugyn, V.G.; Osin, Y.N.; Solovieva, S.E.; Antipin, I.S. Polycationic Derivatives of p-tert-Butylthiacalix [4]arene in 1,3-alternate Stereoisomeric Form: New DNA Condensing Agents. Macroheterocycles 2016, 9, 433–441. [Google Scholar] [CrossRef]
- Burilov, V.A.; Mironova, D.A.; Grygoriev, I.A.; Valiyakhmetovaa, A.M.; Solovieva, S.E.; Antipin, I.S. Synthesis of Water-Soluble Polyammonium Thiacalix [4]arene Derivative and Its Interaction with Calf Thymus DNA. Russ. J. Gen. Chem. 2020, 90, 99–104. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic Azides: An Exploding Diversity of a Unique Class of Compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef] [PubMed]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-catalyzed Regioselective "Ligation" of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. Engl. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Neumann, S.; Biewend, M.; Rana, S.; Binder, W.H. The CuAAC: Principles, Homogeneous and Heterogeneous Catalysts, and Novel Developments and Applications. Macromol. Rapid Commun. 2020, 41, 1900359. [Google Scholar] [CrossRef]
- Burilov, V.A.; Fatikhova, G.A.; Dokuchaeva, M.N.; Nugmanov, R.I.; Mironova, D.A.; Dorovatovskii, P.V.; Khrustalev, V.N.; Solovieva, S.E.; Antipin, I.S. Synthesis of New p-tert-Butylcalix [4]arene-based Polyammonium Triazolyl Amphiphiles and Their Binding with Nucleoside Phosphates. Beilstein J. Org. Chem. 2018, 14, 1980–1993. [Google Scholar] [CrossRef]
- Wilson, L.R.B.; Coletta, M.; Evangelisiti, M.; Piligkos, S.; Dalgarno, S.J.; Brechin, E.K. The Coordination Chemistry of p-tert-Butylcalix [4]arene with Paramagnetic Transition and Lanthanide Metal Ions: An Edinburgh Perspective. Dalton Trans. 2022, 51, 4213–4226. [Google Scholar] [CrossRef] [PubMed]
- Santoro, O.; Redshaw, C. Metallocalix[n]arenes in Catalysis: A 13-year Update. Coord. Chem. Rev. 2021, 448, 214173. [Google Scholar] [CrossRef]
- Li, Y.; Hoskins, J.N.; Sreerama, S.G.; Grayson, S.M. MALDI−TOF Mass Spectral Characterization of Polymers Containing an Azide Group: Evidence of Metastable Ions. Macromolecules 2010, 43, 6225–6228. [Google Scholar] [CrossRef]
- Hanna, T.A.; Liu, L.; Angeles-Boza, A.M.; Kou, X.; Gutsche, C.D.; Ejsmont, K.; Watson, W.H.; Zakharov, L.N.; Incarvito, C.D.; Rheingold, A.L. Synthesis, Structures, and Conformational Characteristics of Calixarene Monoanions and Dianions. J. Am. Chem. Soc. 2003, 125, 6228–6238. [Google Scholar] [CrossRef] [PubMed]
- Nachtigall, F.F.; Vencato, I.; Lazzarotto, M.; Nome, F. Calix [4]arene Piperidinium Salt. Acta Crystallogr. C 1998, 54, 1007–1010. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M.; Alam, I. Calixarenes. 20. The Interaction of Calixarenes and Amines. J. Am. Chem. Soc. 1987, 109, 4314–4320. [Google Scholar] [CrossRef]
- Whiteside, R.; Gunaratne, H.Q.N.; Muzio, A.F.V.; Nockemann, P. Selective Monoalkylation of p-tert-Butylcalix [4]arene in a Methyl Carbonate Ionic Liquid. Chem. Commun. 2018, 54, 12037–12040. [Google Scholar] [CrossRef]
- Alvarez, S.A. Cartography of the van der Waals Territories. Dalton Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kurur, N.D.; Chawla, H.M.; Varadarajan, R.A. Convenient One Pot One Step Synthesis of p-Nitrocalixarenes via Ipsonitration. Synth. Commun. 2001, 31, 775–779. [Google Scholar] [CrossRef]
- Snayer, T.M.; Bose, S.; Arnott, G. ipso-Bromination of tert-Butylcalix [4]arenes. Arkivoc 2020, v, 108–118. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Bauer, L.J. Calixarenes. 13. The Conformational Properties of Calix [4]arenes, Calix [6]arenes, Calix [8]arenes, and Oxacalixarenes. J. Am. Chem. Soc. 1985, 107, 6052–6059. [Google Scholar] [CrossRef]
- Fatykhova, G.A.; Makarov, E.G.; Mironova, D.A.; Sultanova, E.D.; Burilov, V.A.; Solovieva, S.E.; Antipin, I.S. New Amphiphilic Calix [4]Arene Derivatives with 4,5-Dicarboxytriazolyl Fragments: Synthesis and Use in Micellar Catalysis. Russ. J. Phys. Chem. B 2019, 13, 401–407. [Google Scholar] [CrossRef]
- Piñeiro, L.; Novo, M.; Al-Soufi, W. Fluorescence Emission of Pyrene in Surfactant Solutions. J. Colloid Interface Sci. 2015, 215, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Ding, X.; Guo, X. Assembly Behaviors of Calixarene-based Amphiphile and Supra-amphiphile and the Applications in Drug Delivery and Protein Recognition. Adv. Colloid Interface Sci. 2019, 269, 187–202. [Google Scholar] [CrossRef]
- Cheipesh, T.A.; Mchedlov–Petrossyan, N.O.; Bogdanova, L.N.; Kharchenko, D.V.; Roshal, A.D.; Vodolazkaya, N.A.; Taranets, Y.V.; Shekhovtsov, S.V.; Rodik, R.V.; Kalchenko, V.I. Aggregates of Cationic Calix [4]arenes in Aqueous Solution as Media for Governing Protolytic Equilibrium, Fluorescence, and Kinetics. J. Mol. Liq. 2022, 366, 119940. [Google Scholar] [CrossRef]
- Späth, A.; König, B. Molecular Recognition of Organic Ammonium Ions in Solution Using Synthetic Receptors. Beilstein J. Org. Chem. 2010, 6, 32. [Google Scholar] [CrossRef]
- Shinkai, S.; Araki, K.; Grootenhuis, P.D.J.; Reinhoudt, D.N. pKa Determination of Water-soluble Calix [4]arenes. J. Chem. Soc. Perkin Trans. 2 1991, 12, 1883–1886. [Google Scholar] [CrossRef]
- Slama-Schwok, A.; Lehn, J.M. Interaction of Porphyrin-containing Macrotetracyclic Receptor Molecule with Single-stranded and Double-stranded Polynucleotides. A Photophysical Study. Biochemistry 1990, 29, 7895–7903. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Lu, M.; Marky, L.A.; Kallenbach, N.R. Interaction of the Dye Ethidium Bromide with DNA Containing Guanine repeats. Biochemistry 1992, 31, 2451–2455. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2006; p. 673. [Google Scholar] [CrossRef]
- Akram, M.; Lal, H.; Din, K.-U. Exploring the Binding Mode of Ester-based Cationic Gemini Surfactants with Calf Thymus DNA: A Detailed Physicochemical, Spectroscopic and Theoretical Study. Bioorg.c Chem. 2022, 119, 105555. [Google Scholar] [CrossRef]
- Shahabadi, N.; Zendehcheshm, S. Evaluation of ct-DNA and HSA Binding Propensity of Antibacterial Drug Chloroxine: Multi-spectroscopic Analysis, Atomic Force Microscopy and Docking Simulation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118042. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.; Cheng, R.; Cai, K.; Wang, X.; Xie, C.; Xu, T.; Yuan, C. Interaction of Calf Thymus DNA and Glucose-based Gemini Cationic Surfactants with Different Spacer Length: A Spectroscopy and DLS Study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120606. [Google Scholar] [CrossRef]
- López-López, M.; López-Cornejo, P.; Martín, V.I.; Ostos, F.J.; Checa-Rodríguez, C.; Prados-Carvajal, R.; Prados-Carvajal, R.; Lebron, J.A.; Huertas, P.; Moyá, M.L. Importance of Hydrophobic Interactions in the Single-chained Cationic Surfactant-DNA Complexation. J. Colloid Interface Sci. 2018, 521, 197–205. [Google Scholar] [CrossRef]
- Kypr, J.; Kejnovska, I.; Renciuk, D.; Vorlickova, M. Circular Dichroism and Conformational Polymorphism of DNA. Nucleic Acids Res. 2009, 37, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Yu, J.S.; Liang, Y.; Fan, K.; Lai, L. Chlorobenzylidine–calf Thymus DNA Interaction II: Circular Dichroism and Nuclear Magnetic Resonance Studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 2985–2992. [Google Scholar] [CrossRef]
- Xu, L.; Feng, L.; Hao, J.; Dong, S. Compaction and Decompaction of DNA Dominated by the Competition between Counterions and DNA Associating with Cationic Aggregates. Colloids Surf. B Biointerfaces 2015, 134, 105–112. [Google Scholar] [CrossRef]
- Watts, C.; Marsh, M. Endocytosis: What Goes in and How? J. Cell. Sci. 1992, 103, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, H.; He, C.; Qiao, F.; Wang, S.; Wang, Y. DNA Condensation Induced by a Star-Shaped Hexameric Cationic Surfactant. ACS Appl. Mater. Interfaces 2017, 9, 23333–23341. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ardoy, A.; Gomez-Garcia, M.; Ortiz, M.C.; Sevillano, N.; Giron, M.D.; Salto, R.; Santoyo-Gonzalez, F.; Garcia Fernández, J.M. Preorganized Macromolecular Gene Delivery Systems: Amphiphilic β-Cyclodextrin “Click Clusters”. Org. Biomol. Chem. 2009, 7, 2681–2684. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Iqbal, M. p-tert-Butylcalix [4]arene. Org. Synth. 1993, 8, 75. [Google Scholar] [CrossRef]
- Zhang, T.-X.; Zhang, Z.-Z.; Yue, Y.-X.; Hu, X.-Y.; Huang, F.; Shi, L.; Liu, Y.; Guo, D.S. A General Hypoxia-Responsive Molecular Container for Tumor-Targeted Therapy. Adv. Mater. 2020, 32, 1908435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrating Space Group Determination and Structure Solution. Acta Crystallogr. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2007, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]


| System | CAC, μM | D Average, nm | d1, nm | d2, nm | PDI | ζ, mV |
|---|---|---|---|---|---|---|
| 5 | - | 221 ± 8 | - | - | 0.256 ± 0.017 | +42 ± 6 |
| 5 2 | - | 167 ± 32 | - | - | 0.331 ± 0.042 | +46 ± 2 |
| 5 3 | - | 1679 ± 83 | 2548 ± 79 (78%) | 443 ± 42 (22%) | 0.486 ± 0.040 | −6 ± 1 |
| 6 | 5.0 | 145 ± 60 | 146 ± 34 (73%) | 10 ± 1 (27%) | 0.339 ± 0.053 | +43 ± 6 |
| 7 | 3.4 | 19 ± 4 | 16 ± 2 (74%) | 201 ± 72 (26%) | 0.346 ± 0.122 | +48 ± 8 |
| Macrocycle | Ksv × 105, M−1 | R2 | Kq × 1013, M−1 | Ka × 105, M−1 | R2 | n |
|---|---|---|---|---|---|---|
| 5 | 0.85 | 0.98 | 0.85 | 0.11 | 0.99 | 0.74 |
| 6 | 1.29 | 0.99 | 1.29 | 0.17 | 0.99 | 0.83 |
| 7 | 2.29 | 0.99 | 2.29 | 5.59 | 0.99 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burilov, V.; Makarov, E.; Mironova, D.; Sultanova, E.; Bilyukova, I.; Akyol, K.; Evtugyn, V.; Islamov, D.; Usachev, K.; Mukhametzyanov, T.; et al. Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding. Int. J. Mol. Sci. 2022, 23, 14889. https://doi.org/10.3390/ijms232314889
Burilov V, Makarov E, Mironova D, Sultanova E, Bilyukova I, Akyol K, Evtugyn V, Islamov D, Usachev K, Mukhametzyanov T, et al. Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding. International Journal of Molecular Sciences. 2022; 23(23):14889. https://doi.org/10.3390/ijms232314889
Chicago/Turabian StyleBurilov, Vladimir, Egor Makarov, Diana Mironova, Elza Sultanova, Islamiya Bilyukova, Kevser Akyol, Vladimir Evtugyn, Daut Islamov, Konstantin Usachev, Timur Mukhametzyanov, and et al. 2022. "Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding" International Journal of Molecular Sciences 23, no. 23: 14889. https://doi.org/10.3390/ijms232314889
APA StyleBurilov, V., Makarov, E., Mironova, D., Sultanova, E., Bilyukova, I., Akyol, K., Evtugyn, V., Islamov, D., Usachev, K., Mukhametzyanov, T., Solovieva, S., & Antipin, I. (2022). Calix[4]arene Polyamine Triazoles: Synthesis, Aggregation and DNA Binding. International Journal of Molecular Sciences, 23(23), 14889. https://doi.org/10.3390/ijms232314889