Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut–Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials
Abstract
:1. Introduction
2. Methods
2.1. Study Protocol
2.2. Data Sources and Search Strategy
2.3. Eligibility Criteria and Screening
2.4. Data Extraction
2.5. Risk of Bias Assessment
3. Results
3.1. Search Results
3.2. Trial Characteristics
3.3. Risk of Bias Assessment
3.4. Effect on Serum Creatinine (Cr)
3.5. Effect on Estimated Glomerular Filtration Rate (eGFR)
3.6. Effect on Urea or Blood Urea Nitrogen (BUN)
3.7. Effect on Urine Albumin/Creatinine Ratio (Alb/Cr)
3.8. Effect on Uric Acid
3.9. Effect on Serum Sodium, Potassium, and Phosphorus
3.10. Effect on Serum Albumin
3.11. Effect on Other Renal Biomarkers
4. Discussion
4.1. Main Findings
4.2. Is there an Optimum Nutraceutical Formulation?
4.3. Findings from Previous Reviews
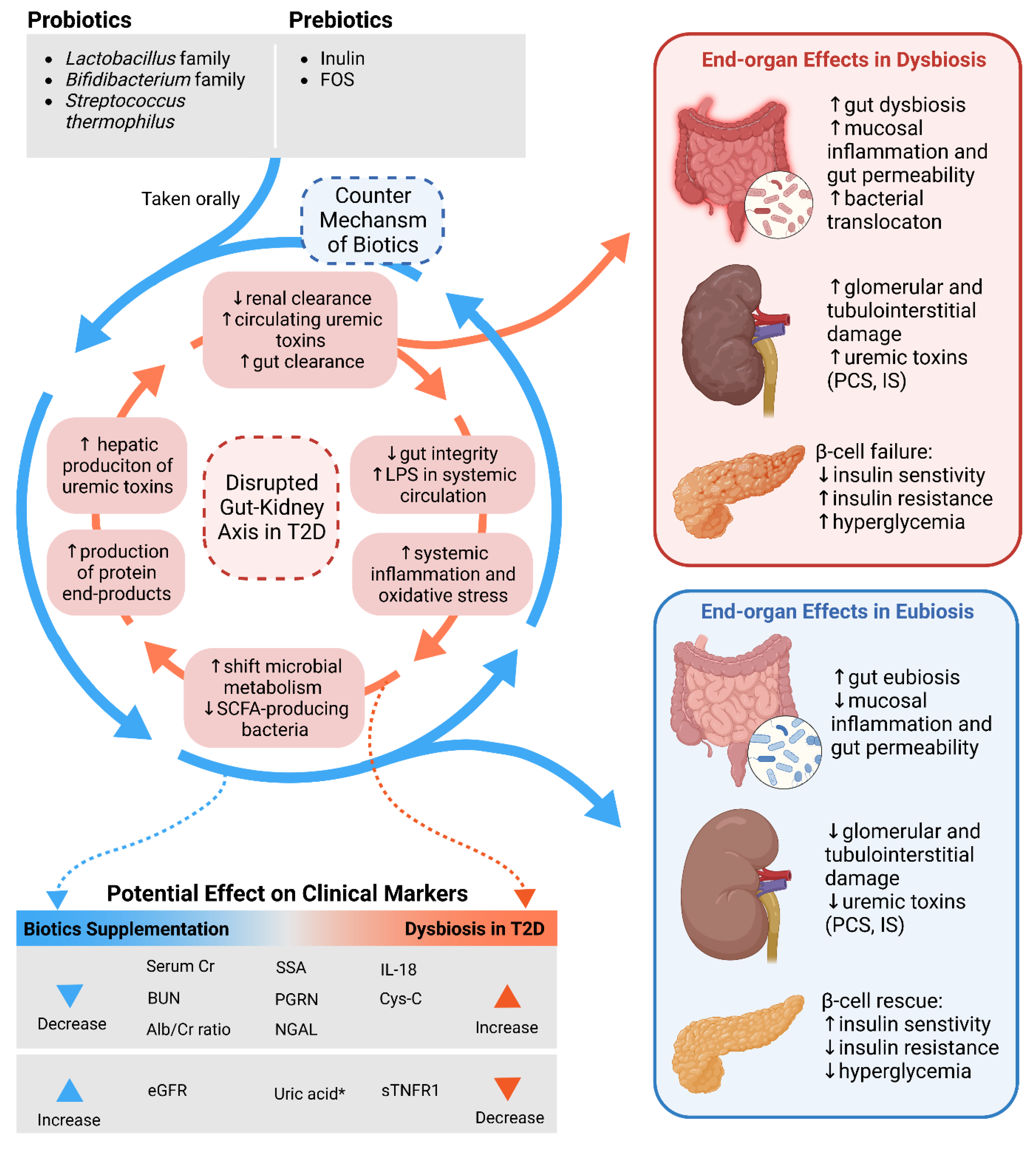
4.4. Gut–Kidney Axis in Diabetes and Diabetic Kidney Disease
4.5. Mechanisms of Action of Microbiome-Modulating Nutraceuticals
4.6. Limitations
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. North Am. 2021, 50, 337–355. [Google Scholar] [CrossRef]
- Kaul, R.; Kaul, R.; Paul, P.; Maksymiuk, V.; Frishman, W.H.; Aronow, W.S. Alcohol and Atrial Fibrillation: A Pathophysiologic Perspective. Cardiol. Rev. 2022. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Abdollahi, S.; Meshkini, F.; Clark, C.C.T.; Heshmati, J.; Soltani, S. The Effect of Probiotics/Synbiotics Supplementation on Renal and Liver Biomarkers in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2022, 128, 625–635. [Google Scholar] [CrossRef]
- Wolf, G. After All Those Fat Years: Renal Consequences of Obesity. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2003, 18, 2471–2474. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative Stress in Diabetic Nephropathy with Early Chronic Kidney Disease. J. Diabetes Res. 2016, 2016, 7047238. [Google Scholar] [CrossRef] [Green Version]
- Gheith, O.; Farouk, N.; Nampoory, N.; Halim, M.A.; Al-Otaibi, T. Diabetic Kidney Disease: World Wide Difference of Prevalence and Risk Factors. J. Nephropharmacol. 2016, 5, 49–56. [Google Scholar] [CrossRef]
- Arnold, S.V.; Tang, F.; Cooper, A.; Chen, H.; Gomes, M.B.; Rathmann, W.; Shimomura, I.; Vora, J.; Watada, H.; Khunti, K.; et al. Global Use of SGLT2 Inhibitors and GLP-1 Receptor Agonists in Type 2 Diabetes. Results from DISCOVER. BMC Endocr. Disord. 2022, 22, 111. [Google Scholar] [CrossRef]
- Global Health & Population Project on Access to Care for Cardiometabolic Diseases (HPACC). Expanding Access to Newer Medicines for People with Type 2 Diabetes in Low-Income and Middle-Income Countries: A Cost-Effectiveness and Price Target Analysis. Lancet Diabetes Endocrinol. 2021, 9, 825–836. [Google Scholar] [CrossRef]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential Beneficial Role of Probiotics on the Outcome of COVID-19 Patients: An Evolving Perspective. Diabetes Metab. Syndr. 2021, 15, 295–301. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Shankar, E.M. SARS-CoV-2-Indigenous Microbiota Nexus: Does Gut Microbiota Contribute to Inflammation and Disease Severity in COVID-19? Front. Cell. Infect. Microbiol. 2021, 11, 590874. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.-D.; Wang, Y.-D. The Relationship Between Gut Microbiota and Inflammatory Diseases: The Role of Macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.D. Human Gut Microbiome: Hopes, Threats and Promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef] [Green Version]
- Frost, F.; Kacprowski, T.; Rühlemann, M.; Pietzner, M.; Bang, C.; Franke, A.; Nauck, M.; Völker, U.; Völzke, H.; Dörr, M.; et al. Long-Term Instability of the Intestinal Microbiome Is Associated with Metabolic Liver Disease, Low Microbiota Diversity, Diabetes Mellitus and Impaired Exocrine Pancreatic Function. Gut 2021, 70, 522–530. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, T.; Chen, Z.; Liu, L.; Luo, T.; Dai, J. Characteristics of the Gut Microbiome in Patients with Prediabetes and Type 2 Diabetes. PeerJ 2021, 9, e10952. [Google Scholar] [CrossRef]
- Vatanen, T.; Franzosa, E.A.; Schwager, R.; Tripathi, S.; Arthur, T.D.; Vehik, K.; Lernmark, Å.; Hagopian, W.A.; Rewers, M.J.; She, J.-X.; et al. The Human Gut Microbiome in Early-Onset Type 1 Diabetes from the TEDDY Study. Nature 2018, 562, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Uusitupa, M.; Khan, T.A.; Viguiliouk, E.; Kahleova, H.; Rivellese, A.A.; Hermansen, K.; Pfeiffer, A.; Thanopoulou, A.; Salas-Salvadó, J.; Schwab, U.; et al. Prevention of Type 2 Diabetes by Lifestyle Changes: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 2611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeshirlarijaney, A.; Gewirtz, A.T. Considering Gut Microbiota in Treatment of Type 2 Diabetes Mellitus. Gut Microbes 2020, 11, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Paul, P.; Kaul, R.; Abdellatif, B.; Arabi, M.; Upadhyay, R.; Saliba, R.; Sebah, M.; Chaari, A. The Promising Role of Microbiome Therapy on Biomarkers of Inflammation and Oxidative Stress in Type 2 Diabetes: A Systematic and Narrative Review. Front. Nutr. 2022, 9, 906243. [Google Scholar] [CrossRef]
- Paul, P.; Kaul, R.; Harfouche, M.; Arabi, M.; Al-Najjar, Y.; Sarkar, A.; Saliba, R.; Chaari, A. The Effect of Microbiome-Modulating Probiotics, Prebiotics and Synbiotics on Glucose Homeostasis in Type 2 Diabetes: A Systematic Review, Meta-Analysis, and Meta-Regression of Clinical Trials. Pharmacol. Res. 2022, 185, 106520. [Google Scholar] [CrossRef] [PubMed]
- Al-Najjar, Y.; Arabi, M.; Paul, P.; Chaari, A. Can Probiotic, Prebiotic, and Synbiotic Supplementation Modulate the Gut-Liver Axis in Type 2 Diabetes? A Narrative and Systematic Review of Clinical Trials. Front. Nutr. 2022. [Google Scholar] [CrossRef]
- Muralitharan, R.R.; Jama, H.A.; Xie, L.; Peh, A.; Snelson, M.; Marques, F.Z. Microbial Peer Pressure: The Role of the Gut Microbiota in Hypertension and Its Complications. Hypertension 2020, 76, 1674–1687. [Google Scholar] [CrossRef]
- Zaky, A.; Glastras, S.J.; Wong, M.Y.W.; Pollock, C.A.; Saad, S. The Role of the Gut Microbiome in Diabetes and Obesity-Related Kidney Disease. Int. J. Mol. Sci. 2021, 22, 9641. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, J.; Wang, C.; Li, S.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Meta-Analysis of Randomized Controlled Trials of the Effects of Probiotics on Type 2 Diabetes in Adults. Clin. Nutr. 2022, 41, 365–373. [Google Scholar] [CrossRef]
- Tang, Q.; Zhong, Y.; Xu, C.; Li, W.; Wang, H.; Hou, Y. Effectiveness of Five Interventions Used for Prevention of Gestational Diabetes: A Network Meta-Analysis. Medicine 2022, 101, e29126. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Wang, J.; Wu, F.; Sui, Y.; Yang, L.; Wang, Z. Lactobacillus Plantarum Strains as Potential Probiotic Cultures with Cholesterol-Lowering Activity. J. Dairy Sci. 2013, 96, 2746–2753. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Quan, J.; Xiong, L.; Luo, Y.; Yi, B. Probiotics Improve Renal Function, Glucose, Lipids, Inflammation and Oxidative Stress in Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail. 2022, 44, 862–880. [Google Scholar] [CrossRef]
- Bohlouli, J.; Namjoo, I.; Borzoo-Isfahani, M.; Hojjati Kermani, M.A.; Balouch Zehi, Z.; Moravejolahkami, A.R. Effect of Probiotics on Oxidative Stress and Inflammatory Status in Diabetic Nephropathy: A Systematic Review and Meta-Analysis of Clinical Trials. Heliyon 2021, 7, e05925. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Glossary of Terms in The Cochrane Collaboration Version 4.2.5 Updated May 2005. Cochrane Handb. Syst. Rev. 2005, 51–79. [Google Scholar]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Abbasi, B.; Mirlohi, M.; Daniali, M.; Ghiasvand, R. Effects of Probiotic Soy Milk on Lipid Panel in Type 2 Diabetic Patients with Nephropathy: A Double-Blind Randomized Clinical Trial. Prog. Nutr. 2018, 20, 70–78. [Google Scholar] [CrossRef]
- Abbasi, B.; Ghiasvand, R.; Mirlohi, M. Kidney Function Improvement by Soy Milk Containing Lactobacillus Plantarum A7 in Type 2 Diabetic Patients With Nephropathy: A Double-Blinded Randomized Controlled Trial. Iran. J. Kidney Dis. 2017, 11, 36–43. [Google Scholar]
- Mosbah, A.G.; Elgharbawy, N.M.; El Bendary, A.S.; Shall, N.D. El Metabolic Effects of Probiotic Supplementation in Diabetic Hemodialysis Patients. J. Adv. Med. Med. Res. 2020, 32, 332–341. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Javid, A.Z.; Dehghan, P. The Effect of Enriched Chicory Inulin on Liver Enzymes, Calcium Homeostasis and Hematological Parameters in Patients with Type 2 Diabetes Mellitus: A Randomized Placebo-Controlled Trial. Prim. Care Diabetes 2016, 10, 265–271. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Dehghan, P.; Namazi, N. Prebiotic Supplementation Modulates Advanced Glycation End-Products (AGEs), Soluble Receptor for AGEs (SRAGE), and Cardiometabolic Risk Factors through Improving Metabolic Endotoxemia: A Randomized-Controlled Clinical Trial. Eur. J. Nutr. 2020, 59, 3009–3021. [Google Scholar] [CrossRef]
- Gonai, M.; Shigehisa, A.; Kigawa, I.; Kurasaki, K.; Chonan, O.; Matsuki, T.; Yoshida, Y.; Aida, M.; Hamano, K.; Terauchi, Y. Galacto-Oligosaccharides Ameliorate Dysbiotic Bifidobacteriaceae Decline in Japanese Patients with Type 2 Diabetes. Benef. Microbes 2017, 8, 705–716. [Google Scholar] [CrossRef]
- Asemi, Z.; Khorrami-Rad, A.; Alizadeh, S.A.; Shakeri, H.; Esmaillzadeh, A. Effects of Synbiotic Food Consumption on Metabolic Status of Diabetic Patients: A Double-Blind Randomized Cross-over Controlled Clinical Trial. Clin. Nutr. 2014, 33, 198–203. [Google Scholar] [CrossRef]
- Ebrahimi, Z.s.; Nasli-Esfahani, E.; Nadjarzade, A.; Mozaffari-khosravi, H. Effect of Symbiotic Supplementation on Glycemic Control, Lipid Profiles and Microalbuminuria in Patients with Non-Obese Type 2 Diabetes: A Randomized, Double-Blind, Clinical Trial. J. Diabetes Metab. Disord. 2017, 16, 23. [Google Scholar] [CrossRef] [Green Version]
- Miraghajani, M.; Zaghian, N.; Dehkohneh, A.; Mirlohi, M.; Ghiasvand, R. Probiotic Soy Milk Consumption and Renal Function Among Type 2 Diabetic Patients with Nephropathy: A Randomized Controlled Clinical Trial. Probiotics Antimicrob. Proteins 2019, 11, 124–132. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Bertéus Forslund, H.; Perkins, R.; Bäckhed, F.; et al. Metabolic Effects of Lactobacillus Reuteri DSM 17938 in People with Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Obes. Metab. 2017, 19, 579–589. [Google Scholar] [CrossRef]
- Mazruei Arani, N.; Emam-Djomeh, Z.; Tavakolipour, H.; Sharafati-Chaleshtori, R.; Soleimani, A.; Asemi, Z. The Effects of Probiotic Honey Consumption on Metabolic Status in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Controlled Trial. Probiotics Antimicrob. Proteins 2019, 11, 1195–1201. [Google Scholar] [CrossRef]
- Asemi, Z.; Zare, Z.; Shakeri, H.; Sabihi, S.S.; Esmaillzadeh, A. Effect of Multispecies Probiotic Supplements on Metabolic Profiles, Hs-CRP, and Oxidative Stress in Patients with Type 2 Diabetes. Ann. Nutr. Metab. 2013, 63, 1–9. [Google Scholar] [CrossRef]
- Firouzi, S.; Mohd-Yusof, B.N.; Majid, H.A.; Ismail, A.; Kamaruddin, N.A. Effect of Microbial Cell Preparation on Renal Profile and Liver Function among Type 2 Diabetics: A Randomized Controlled Trial. BMC Complement. Altern. Med. 2015, 15, 433. [Google Scholar] [CrossRef] [Green Version]
- Jiang, H.; Zhang, Y.; Xu, D.; Wang, Q. Probiotics Ameliorates Glycemic Control of Patients with Diabetic Nephropathy: A Randomized Clinical Study. J. Clin. Lab. Anal. 2021, 35, e23650. [Google Scholar] [CrossRef]
- Mafi, A.; Namazi, G.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Asemi, Z. Metabolic and Genetic Response to Probiotics Supplementation in Patients with Diabetic Nephropathy: A Randomized, Double-Blind, Placebo-Controlled Trial. Food Funct. 2018, 9, 4763–4770. [Google Scholar] [CrossRef]
- Soleimani, A.; Zarrati Mojarrad, M.; Bahmani, F.; Taghizadeh, M.; Ramezani, M.; Tajabadi-Ebrahimi, M.; Jafari, P.; Esmaillzadeh, A.; Asemi, Z. Probiotic Supplementation in Diabetic Hemodialysis Patients Has Beneficial Metabolic Effects. Kidney Int. 2017, 91, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Azadbakht, L.; Esmaillzadeh, A. Soy-Protein Consumption and Kidney-Related Biomarkers among Type 2 Diabetics: A Crossover, Randomized Clinical Trial. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2009, 19, 479–486. [Google Scholar] [CrossRef]
- Azadbakht, L.; Atabak, S.; Esmaillzadeh, A. Soy Protein Intake, Cardiorenal Indices, and C-Reactive Protein in Type 2 Diabetes with Nephropathy: A Longitudinal Randomized Clinical Trial. Diabetes Care 2008, 31, 648–654. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-M.; Chen, Y.-M.; Ho, S.C. Effects of Soy Intake on Glycemic Control: A Meta-Analysis of Randomized Controlled Trials. Am. J. Clin. Nutr. 2011, 93, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Yeo, S.-K.; Liong, M.-T. Effect of Prebiotics on Viability and Growth Characteristics of Probiotics in Soymilk. J. Sci. Food Agric. 2010, 90, 267–275. [Google Scholar] [CrossRef]
- Vaziri, N.D. CKD Impairs Barrier Function and Alters Microbial Flora of the Intestine: A Major Link to Inflammation and Uremic Toxicity. Curr. Opin. Nephrol. Hypertens. 2012, 21, 587–592. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.-H.; Andersen, G.L. Chronic Kidney Disease Alters Intestinal Microbial Flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Cox, A.J.; West, N.P.; Horn, P.L.; Lehtinen, M.J.; Koerbin, G.; Pyne, D.B.; Lahtinen, S.J.; Fricker, P.A.; Cripps, A.W. Effects of Probiotic Supplementation over 5 Months on Routine Haematology and Clinical Chemistry Measures in Healthy Active Adults. Eur. J. Clin. Nutr. 2014, 68, 1255–1257. [Google Scholar] [CrossRef]
- Snelson, M.; Biruete, A.; McFarlane, C.; Campbell, K. A Renal Clinician’s Guide to the Gut Microbiota. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2020, 30, 384–395. [Google Scholar] [CrossRef]
- Yacoub, R.; Kaji, D.; Patel, S.N.; Simoes, P.K.; Busayavalasa, D.; Nadkarni, G.N.; He, J.C.; Coca, S.G.; Uribarri, J. Association between Probiotic and Yogurt Consumption and Kidney Disease: Insights from NHANES. Nutr. J. 2016, 15, 10. [Google Scholar] [CrossRef]
- Ichii, O.; Otsuka-Kanazawa, S.; Nakamura, T.; Ueno, M.; Kon, Y.; Chen, W.; Rosenberg, A.Z.; Kopp, J.B. Podocyte Injury Caused by Indoxyl Sulfate, a Uremic Toxin and Aryl-Hydrocarbon Receptor Ligand. PLoS ONE 2014, 9, e108448. [Google Scholar]
- Watanabe, H.; Miyamoto, Y.; Honda, D.; Tanaka, H.; Wu, Q.; Endo, M.; Noguchi, T.; Kadowaki, D.; Ishima, Y.; Kotani, S.; et al. P-Cresyl Sulfate Causes Renal Tubular Cell Damage by Inducing Oxidative Stress by Activation of NADPH Oxidase. Kidney Int. 2013, 83, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [Green Version]
- Madani, G.; Mirlohi, M.; Soleimanain-Zad, S.; Hosseini, P.; Babashahi, M. Lactobacillus Plantarum A7, a Potential Probiotic Strain from Infant Fecal Flora. J. Biol. Todays World 2017, 6, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Babashahi, M.; Mirlohi, M.; Ghiasvand, R.; Azadbakht, L.; Mosharaf, L.; Torki-Baghbadorani, S. Effects of Probiotic Soy Milk Fermented by Lactobacillus Plantarum A7 (KC 355240) Added with Cuminum Cyminum Essential Oil on Fasting Blood Glucose Levels, Serum Lipid Profile and Body Weight in Diabetic Wistar Rats. Int. J. Prev. Med. 2020, 11, 8. [Google Scholar] [CrossRef]
- Tarrahi, M.J.; Namjoo, I.; Borzoo-Isfahani, M.; Ebdali, H.; Moravejolahkami, A.R. Can Probiotics Supplementation Improve Glycemic and Renal Status in Diabetic Nephropathy? A Systematic Review and Meta-Analysis of Clinical Trials. Endocr. Metab. Immune Disord.-Drug Targets 2021, 22, 143–158. [Google Scholar] [CrossRef]
- Wei, T.; Na, L.; Yingying, F.; Nutrition, D. of C.; Hospital, Z.C. Effect of Probiotics Supplementation on the Risk of Disease Progression in Elderly with Diabetic Nephropathy. Chin. J. Microecol. 2020, 2020, 570–574. [Google Scholar]
- Wang, H.; Wang, D.; Song, H.; Zou, D.; Feng, X.; Ma, X.; Miao, J.; Yang, W.; Wang, H. The Effects of Probiotic Supplementation on Renal Function, Inflammation, and Oxidative Stress in Diabetic Nephropathy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Mater. Express 2021, 11, 1122–1131. [Google Scholar] [CrossRef]
- Vlachou, E.; Ntikoudi, A.; Govina, O.; Lavdaniti, M.; Kotsalas, N.; Tsartsalis, A.; Dimitriadis, G. Effects of Probiotics on Diabetic Nephropathy: A Systematic Review. Curr. Clin. Pharmacol. 2020, 15, 234–242. [Google Scholar] [CrossRef]
- AbdelQadir, Y.H.; Hamdallah, A.; Sibaey, E.A.; Hussein, A.S.; Abdelaziz, M.; AbdelAzim, A.; Ragab, K.M.; Helmy, S.K.; Nourelden, A.Z. Efficacy of Probiotic Supplementation in Patients with Diabetic Nephropathy: A Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2020, 40, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Haghighatdoost, F. The Effects of Prebiotic, Probiotic, and Synbiotic Supplementation on Blood Parameters of Renal Function: A Systematic Review and Meta-Analysis of Clinical Trials. Nutrition 2018, 51–52, 104–113. [Google Scholar] [CrossRef] [PubMed]
- D’Argenio, V.; Salvatore, F. The Role of the Gut Microbiome in the Healthy Adult Status. Clin. Chim. Acta. 2015, 451, 97–102. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A Metagenome-Wide Association Study of Gut Microbiota in Type 2 Diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut Metagenome in European Women with Normal, Impaired and Diabetic Glucose Control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Chen, Z.; Radjabzadeh, D.; Chen, L.; Kurilshikov, A.; Kavousi, M.; Ahmadizar, F.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Association of Insulin Resistance and Type 2 Diabetes With Gut Microbial Diversity: A Microbiome-Wide Analysis From Population Studies. JAMA Netw. Open 2021, 4, e2118811. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Koppe, L.; Fouque, D.; Soulage, C.O. Metabolic Abnormalities in Diabetes and Kidney Disease: Role of Uremic Toxins. Curr. Diab. Rep. 2018, 18, 97. [Google Scholar] [CrossRef]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular Mechanisms of Diabetic Kidney Disease. J. Clin. Invest. 2014, 124, 2333–2340. [Google Scholar] [CrossRef]
- Toth-Manikowski, S.; Atta, M.G. Diabetic Kidney Disease: Pathophysiology and Therapeutic Targets. J. Diabetes Res. 2015, 2015, 697010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- García-García, P.M.; Getino-Melián, M.A.; Domínguez-Pimentel, V.; Navarro-González, J.F. Inflammation in Diabetic Kidney Disease. World J. Diabetes 2014, 5, 431–443. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sun, J.; Liu, C.; Yu, X.; Li, H.; Zhang, W.; Li, Y.; Geng, Y.; Wang, Z. Compositional Alterations of Gut Microbiota in Patients with Diabetic Kidney Disease and Type 2 Diabetes Mellitus. Diabetes. Metab. Syndr. Obes. 2022, 15, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The Impact of Gut Microbiota on Kidney Function and Pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Konstantinidis, T.; Romanidou, G.; Voidarou, C.; Bezirtzoglou, E. Focus on the Gut-Kidney Axis in Health and Disease. Front. Med. 2020, 7, 620102. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [CrossRef]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free Fatty Acid Receptors in Health and Disease. Physiol. Rev. 2019, 100, 171–210. [Google Scholar] [CrossRef] [Green Version]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the Gut-Kidney Axis among Biopsy-Proven Diabetic Nephropathy, Type 2 Diabetes Mellitus and Healthy Controls: An Analysis of the Gut Microbiota Composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef]
- Lili, Z.; Zhisheng, W.; Xiaona, Z.; Lu, Z.; Jinjin, C.; Haibo, L.; Wenchang, S.; Chunjuan, Y.; Hui, W.; Wenqing, D.; et al. Alterations of the Gut Microbiota in Patients with Diabetic Nephropathy. Microbiol. Spectr. 2022, 10, e00324-22. [Google Scholar] [CrossRef]
- Gradisteanu, G.; Stoica, R.; Petcu, L.; Picu, A.; Suceveanu, A.; Salmen, T.; Stefan, D.; Serafinceanu, C.; Chifiriuc, M.; Stoian, A. Microbiota Signatures in Type-2 Diabetic Patients with Chronic Kidney Disease—A Pilot Study. J. Mind Med. Sci. 2019, 6, 130–136. [Google Scholar] [CrossRef]
- Li, Y.J.; Chen, X.; Kwan, T.K.; Loh, Y.W.; Singer, J.; Liu, Y.; Ma, J.; Tan, J.; Macia, L.; Mackay, C.R.; et al. Dietary Fiber Protects against Diabetic Nephropathy through Short-Chain Fatty Acid-Mediated Activation of G Protein-Coupled Receptors GPR43 and GPR109A. J. Am. Soc. Nephrol. 2020, 31, 1267–1281. [Google Scholar] [CrossRef]
- Kikuchi, K.; Saigusa, D.; Kanemitsu, Y.; Matsumoto, Y.; Thanai, P.; Suzuki, N.; Mise, K.; Yamaguchi, H.; Nakamura, T.; Asaji, K.; et al. Gut Microbiome-Derived Phenyl Sulfate Contributes to Albuminuria in Diabetic Kidney Disease. Nat. Commun. 2019, 10, 1835. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Chen, P.P.; Zhang, J.X.; Li, X.Q.; Wang, G.H.; Yuan, B.Y.; Huang, S.J.; Liu, X.Q.; Jiang, T.T.; Wang, M.Y.; et al. GPR43 Deficiency Protects against Podocyte Insulin Resistance in Diabetic Nephropathy through the Restoration of AMPKα Activity. Theranostics 2021, 11, 4728–4742. [Google Scholar] [CrossRef]
- Aronov, P.A.; Luo, F.J.-G.; Plummer, N.S.; Quan, Z.; Holmes, S.; Hostetter, T.H.; Meyer, T.W. Colonic Contribution to Uremic Solutes. J. Am. Soc. Nephrol. 2011, 22, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Gryp, T.; Huys, G.R.B.; Joossens, M.; Van Biesen, W.; Glorieux, G.; Vaneechoutte, M. Isolation and Quantification of Uremic Toxin Precursor-Generating Gut Bacteria in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2020, 21, 1986. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.; Piceno, Y.M.; DeSantis, T.Z.; Pahl, M.; Andersen, G.L.; Vaziri, N.D. Expansion of Urease- and Uricase-Containing, Indole- and p-Cresol-Forming and Contraction of Short-Chain Fatty Acid-Producing Intestinal Microbiota in ESRD. Am. J. Nephrol. 2014, 39, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Mosterd, C.M.; Kanbay, M.; van den Born, B.J.H.; van Raalte, D.H.; Rampanelli, E. Intestinal Microbiota and Diabetic Kidney Diseases: The Role of Microbiota and Derived Metabolites Inmodulation of Renal Inflammation and Disease Progression. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101484. [Google Scholar] [CrossRef]
- Xu, K.-Y.; Xia, G.-H.; Lu, J.-Q.; Chen, M.-X.; Zhen, X.; Wang, S.; You, C.; Nie, J.; Zhou, H.-W.; Yin, J. Impaired Renal Function and Dysbiosis of Gut Microbiota Contribute to Increased Trimethylamine-N-Oxide in Chronic Kidney Disease Patients. Sci. Rep. 2017, 7, 1445. [Google Scholar] [CrossRef] [Green Version]
- Chiavaroli, L.; Mirrahimi, A.; Sievenpiper, J.L.; Jenkins, D.J.A.; Darling, P.B. Dietary Fiber Effects in Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Controlled Feeding Trials. Eur. J. Clin. Nutr. 2015, 69, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Salmean, Y.A.; Segal, M.S.; Langkamp-Henken, B.; Canales, M.T.; Zello, G.A.; Dahl, W.J. Foods with Added Fiber Lower Serum Creatinine Levels in Patients with Chronic Kidney Disease. J. Ren. Nutr. Off. J. Counc. Ren. Nutr. Natl. Kidney Found. 2013, 23, e29–e32. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti Neto, M.P.; de Souza Aquino, J.; de Fátima Romão da Silva, L.; de Oliveira Silva, R.; de Lima Guimarães, K.S.; de Oliveira, Y.; de Souza, E.L.; Magnani, M.; Vidal, H.; de Brito Alves, J.L. Gut Microbiota and Probiotics Intervention: A Potential Therapeutic Target for Management of Cardiometabolic Disorders and Chronic Kidney Disease? Pharmacol. Res. 2018, 130, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Ranganathan, N.; Friedman, E.A.; Tam, P.; Rao, V.; Ranganathan, P.; Dheer, R. Probiotic Dietary Supplementation in Patients with Stage 3 and 4 Chronic Kidney Disease: A 6-Month Pilot Scale Trial in Canada. Curr. Med. Res. Opin. 2009, 25, 1919–1930. [Google Scholar] [CrossRef]
- Dunn, S.R.; Simenhoff, M.L.; Ahmed, K.E.; Gaughan, W.J.; Eltayeb, B.O.; Fitzpatrick, M.-E.D.; Emery, S.M.; Ayres, J.W.; Holt, K.E. Effect of Oral Administration of Freeze-Dried Lactobacillus Acidophilus on Small Bowel Bacterial Overgrowth in Patients with End Stage Kidney Disease: Reducing Uremic Toxins and Improving Nutrition. Int. Dairy J. 1998, 8, 545–553. [Google Scholar] [CrossRef]
- Simenhoff, M.L.; Dunn, S.R.; Zollner, G.P.; Fitzpatrick, M.E.; Emery, S.M.; Sandine, W.E.; Ayres, J.W. Biomodulation of the Toxic and Nutritional Effects of Small Bowel Bacterial Overgrowth in End-Stage Kidney Disease Using Freeze-Dried Lactobacillus Acidophilus. Miner. Electrolyte Metab. 1996, 22, 92–96. [Google Scholar]
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.-Y. Probiotics and Their Fermented Food Products Are Beneficial for Health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef]
- Tang, S.C.W.; Yiu, W.H. Innate Immunity in Diabetic Kidney Disease. Nat. Rev. Nephrol. 2020, 16, 206–222. [Google Scholar] [CrossRef]
- Al Mamun, A.; Ara Mimi, A.; Wu, Y.; Zaeem, M.; Abdul Aziz, M.; Aktar Suchi, S.; Alyafeai, E.; Munir, F.; Xiao, J. Pyroptosis in Diabetic Nephropathy. Clin. Chim. Acta. 2021, 523, 131–143. [Google Scholar] [CrossRef]
- Maeda, S. Do Inflammatory Cytokine Genes Confer Susceptibility to Diabetic Nephropathy? Kidney Int. 2008, 74, 413–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriwaki, Y.; Yamamoto, T.; Shibutani, Y.; Aoki, E.; Tsutsumi, Z.; Takahashi, S.; Okamura, H.; Koga, M.; Fukuchi, M.; Hada, T. Elevated Levels of Interleukin-18 and Tumor Necrosis Factor-Alpha in Serum of Patients with Type 2 Diabetes Mellitus: Relationship with Diabetic Nephropathy. Metabolism 2003, 52, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Shikata, K.; Hiramatsu, M.; Nakatou, T.; Kitamura, T.; Wada, J.; Itoshima, T.; Makino, H. Serum Interleukin-18 Levels Are Associated with Nephropathy and Atherosclerosis in Japanese Patients with Type 2 Diabetes. Diabetes Care 2005, 28, 2890–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azadbakht, L.; Kimiagar, M.; Mehrabi, Y.; Esmaillzadeh, A.; Hu, F.B.; Willett, W.C. Soy Consumption, Markers of Inflammation, and Endothelial Function: A Cross-over Study in Postmenopausal Women with the Metabolic Syndrome. Diabetes Care 2007, 30, 967–973. [Google Scholar] [CrossRef] [Green Version]
- Wang, I.-K.; Yen, T.-H.; Hsieh, P.-S.; Ho, H.-H.; Kuo, Y.-W.; Huang, Y.-Y.; Kuo, Y.-L.; Li, C.-Y.; Lin, H.-C.; Wang, J.-Y. Effect of a Probiotic Combination in an Experimental Mouse Model and Clinical Patients With Chronic Kidney Disease: A Pilot Study. Front. Nutr. 2021, 8, 661794. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.B.; Bhaktha, G. Relationship between Sialic Acid and Metabolic Variables in Indian Type 2 Diabetic Patients. Lipids Health Dis. 2005, 4, 15. [Google Scholar] [CrossRef]
- Prajna, K.; Kumar, A.; Rai, S.; Shetty, S.K.; Rai, T.; Shrinidhi; Begum, M.; Md, S. Predictive Value of Serum Sialic Acid in Type-2 Diabetes Mellitus and Its Complication (Nephropathy). J. Clin. Diagn. Res. 2013, 7, 2435–2437. [Google Scholar] [CrossRef]
- Shahvali, S.; Shahesmaeili, A.; Sanjari, M.; Karami-Mohajeri, S. The Correlation between Blood Oxidative Stress and Sialic Acid Content in Diabetic Patients with Nephropathy, Hypertension, and Hyperlipidemia. Diabetol. Int. 2020, 11, 19–26. [Google Scholar] [CrossRef]
- Cheeseman, J.; Kuhnle, G.; Stafford, G.; Gardner, R.A.; Spencer, D.I.; Osborn, H.M. Sialic Acid as a Potential Biomarker for Cardiovascular Disease, Diabetes and Cancer. Biomark. Med. 2021, 15, 911–928. [Google Scholar] [CrossRef]
- Varma, V.; Varma, M.; Varma, A.; Kumar, R.; Bharosay, A.; Vyas, S. Serum Total Sialic Acid and Highly Sensitive C-Reactive Protein: Prognostic Markers for the Diabetic Nephropathy. J. Lab. Physicians 2016, 8, 25–29. [Google Scholar] [CrossRef]
- Husain, S.A.; Willey, J.Z.; Park Moon, Y.; Elkind, M.S.V.; Sacco, R.L.; Wolf, M.; Cheung, K.; Wright, C.B.; Mohan, S. Creatinine-versus Cystatin C-Based Renal Function Assessment in the Northern Manhattan Study. PLoS ONE 2018, 13, e0206839. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.F.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.D.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guida, B.; Germanò, R.; Trio, R.; Russo, D.; Memoli, B.; Grumetto, L.; Barbato, F.; Cataldi, M. Effect of Short-Term Synbiotic Treatment on Plasma p-Cresol Levels in Patients with Chronic Renal Failure: A Randomized Clinical Trial. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Sirich, T.L.; Plummer, N.S.; Gardner, C.D.; Hostetter, T.H.; Meyer, T.W. Effect of Increasing Dietary Fiber on Plasma Levels of Colon-Derived Solutes in Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2014, 9, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snelson, M.; de Pasquale, C.; Ekinci, E.I.; Coughlan, M.T. Gut Microbiome, Prebiotics, Intestinal Permeability and Diabetes Complications. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101507. [Google Scholar] [CrossRef] [PubMed]
- Lehto, M.; Groop, P.-H. The Gut-Kidney Axis: Putative Interconnections Between Gastrointestinal and Renal Disorders. Front. Endocrinol. 2018, 9, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pengrattanachot, N.; Thongnak, L.; Lungkaphin, A. The Impact of Prebiotic Fructooligosaccharides on Gut Dysbiosis and Inflammation in Obesity and Diabetes Related Kidney Disease. Food Funct. 2022, 13, 5925–5945. [Google Scholar] [CrossRef]
- Smazal, A.L.; Borcherding, N.C.; Anderegg, A.S.; Schalinske, K.L.; Whitley, E.M.; Rowling, M.J. Dietary Resistant Starch Prevents Urinary Excretion of 25-Hydroxycholecalciferol and Vitamin D-Binding Protein in Type 1 Diabetic Rats. J. Nutr. 2013, 143, 1123–1128. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant Starch Alters Gut Microbiome and Metabolomic Profiles Concurrent with Amelioration of Chronic Kidney Disease in Rats. Am. J. Physiol. Renal Physiol. 2016, 310, F857-71. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Liu, S.-M.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Farzaneh, S.H.; Kieffer, D.A.; Adams, S.H.; Martin, R.J. High Amylose Resistant Starch Diet Ameliorates Oxidative Stress, Inflammation, and Progression of Chronic Kidney Disease. PLoS ONE 2014, 9, e114881. [Google Scholar] [CrossRef] [Green Version]
- Al Theyab, A.; Almutairi, T.; Al-Suwaidi, A.M.; Bendriss, G.; McVeigh, C.; Chaari, A. Epigenetic Effects of Gut Metabolites: Exploring the Path of Dietary Prevention of Type 1 Diabetes. Front. Nutr. 2020, 7, 563605. [Google Scholar] [CrossRef]
- Tzortzis, G.; Vulevic, J. Galacto-Oligosaccharide Prebiotics. In Prebiotics and probiotics science and technology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 207–244. [Google Scholar]
- Hinz, S.W.A.; van den Brock, L.A.M.; Beldman, G.; Vincken, J.-P.; Voragen, A.G.J. Beta-Galactosidase from Bifidobacterium Adolescentis DSM20083 Prefers Beta(1,4)-Galactosides over Lactose. Appl. Microbiol. Biotechnol. 2004, 66, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Depeint, F.; Tzortzis, G.; Vulevic, J.; I’anson, K.; Gibson, G.R. Prebiotic Evaluation of a Novel Galactooligosaccharide Mixture Produced by the Enzymatic Activity of Bifidobacterium Bifidum NCIMB 41171, in Healthy Humans: A Randomized, Double-Blind, Crossover, Placebo-Controlled Intervention Study. Am. J. Clin. Nutr. 2008, 87, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulevic, J.; Rastall, R.A.; Gibson, G.R. Developing a Quantitative Approach for Determining the in Vitro Prebiotic Potential of Dietary Oligosaccharides. FEMS Microbiol. Lett. 2004, 236, 153–159. [Google Scholar] [CrossRef]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic Effects: Metabolic and Health Benefits. Br. J. Nutr. 2010, 104 (Suppl. 2), S1–S63. [Google Scholar] [CrossRef] [Green Version]
- Nagase, N.; Ikeda, Y.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Efficacy of Probiotics on the Modulation of Gut Microbiota in the Treatment of Diabetic Nephropathy. World J. Diabetes 2022, 13, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Man, Y.; Gao, C.; Zhou, L.; Gu, J.; Xu, H.; Wan, Q.; Long, Y.; Chai, L.; Xu, Y.; et al. Short-Chain Fatty Acids Ameliorate Diabetic Nephropathy via GPR43-Mediated Inhibition of Oxidative Stress and NF-ΚB Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 4074832. [Google Scholar] [CrossRef] [PubMed]
- Favero, C.; Giordano, L.; Mihaila, S.M.; Masereeuw, R.; Ortiz, A.; Sanchez-Niño, M.D. Postbiotics and Kidney Disease. Toxins 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Abdelazez, A.; Alshehry, G.; Algarni, E.; Al Jumayi, H.; Abdel-Motaal, H.; Meng, X.-C. Postbiotic Gamma-Aminobutyric Acid and Camel Milk Intervention as Innovative Trends Against Hyperglycemia and Hyperlipidemia in Streptozotocin-Induced C(57)BL/6J Diabetic Mice. Front. Microbiol. 2022, 13, 943930. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Precup, G.; Pocol, C.B.; Teleky, B.-E.; Vodnar, D.C. Awareness, Knowledge, and Interest about Prebiotics-A Study among Romanian Consumers. Int. J. Environ. Res. Public Health 2022, 19, 1208. [Google Scholar] [CrossRef]
- Kühbacher, T.; Ott, S.J.; Helwig, U.; Mimura, T.; Rizzello, F.; Kleessen, B.; Gionchetti, P.; Blaut, M.; Campieri, M.; Fölsch, U.R.; et al. Bacterial and Fungal Microbiota in Relation to Probiotic Therapy (VSL#3) in Pouchitis. Gut 2006, 55, 833–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Hu, S.; Wu, C.; Gu, F.; Yang, Y. Probiotics: Potential Novel Therapeutics Against Fungal Infections. Front. Cell. Infect. Microbiol. 2021, 11, 793419. [Google Scholar] [CrossRef] [PubMed]

| Type of Nutraceutical | Study Design, Country | Participant Demographics * Sample Size and Sex (n, F/M) Age (Mean ± SD; Years) BMI (Mean ± SD; kg/m2) | Control/Placebo Substance | Intervention Nutraceutical | Dose × Frequency | Trial Duration | Marker and Effect (If Significant) | Control/Placebo Change Φ | Intervention Change Φ | Overall Effect and Statistical Significance Φ | Author, Year | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control/Placebo | Intervention | |||||||||||
| Probiotic (SS) | PG, DB, RCT (Iran) | T2D-DN n = 20 (10M/10F) 53.6 ± 7.19 26.58 ± 3.27 | T2D-DN n = 20 (9M/11F) 56.9 ± 8.1 26.68 ± 3.19 | Conventional soy milk | Probiotic soy milk containing Lactobacillus plantarum A7 (2 × 107 CFU/mL) | 200 mL/d | 8 weeks | ↓ Serum Cr (mg/dL) (§) | Cb: 1.03 ± 0.16 Ce: 1.00 ± 0.14 CΔ: −0.03 ± 0.08 | Ib: 1.01 ± 0.11 Ie: 0.83 ± 0.16 IΔ: −0.17 ± 0.11 (Ib vs. Ie p < 0.05) (§) | CΔ vs. IΔ (adjusted) p < 0.0001 (§) | [38] χ |
| ↑ eGFR (mL·min−1 (1.73 m2)−1) (§) | Cb: 72.1 ± 9.1 Ce: 75.4 ± 11.13 CΔ: 3.2 ± 8.4 | Ib: 71.5 ± 9.5 Ie: 87.5 ± 14.2 IΔ: 15.9 ± 10.8 (Ib vs. Ie p < 0.05) (§) | CΔ vs. IΔ (adjusted) p < 0.0001 (§) | |||||||||
| Serum Phosphorous (mg/dL) | Cb: 4.38 ± 0.67 Ce: 4.44 ± 0.59 CΔ: 0.05 ± 0.5 | Ib: 4.48 ± 0.47 Ie: 4.33 ± 0.44 IΔ: −0.14 ± 0.10 | CΔ vs. IΔ (adjusted) p = 0.106 (No significant effect) | |||||||||
| Probiotic (SS) | PG, DB, RCT (Iran) | T2D-DN n = 20 (10M/10F) 53.6 ± 7.19 26.58 ± 3.27 | T2D-DN n = 20 (9M/11F) 56.9 ± 8.1 26.68 ± 3.19 | Conventional soy milk | Probiotic soy milk containing Lactobacillus plantarum A7 (2 × 107 CFU/mL) | 200 mL/d | 8 weeks | ↓ Serum IL-18 (pg/mL) (§) | Cb: 335.14 ± 266.65 Ce: 326.1 ± 260.34 CΔ: −9.03 ± 18.65 | Ib: 286.14 ± 207.8 Ie: 236.96 ± 181.87 IΔ: −49.18 ± 48.22 | CΔ vs. IΔ (adjusted) p = 0.002 (§) | [39] χ |
| ↓ Urine Alb/Cr (mg/g) | Cb: 147.0 ± 38.6 Ce: 141.36 ± 37.9 CΔ: −5.7 ± 15.04 | Ib: 145.8 ± 29.1 Ie: 129.36 ± 31.9 IΔ: −16.5 ± 12.2 | CΔ vs. IΔ (adjusted) p = 0.03 (§) | |||||||||
| ↓ Serum sialic acid (mg/dL) (§) | Cb: 232.33 ± 40.79 Ce: 227.95 ± 40.5 CΔ: 4.37 ± 9.91 | Ib: 223.6 ± 44.72 Ie: 206.2 ± 43.24 IΔ: −17.4 ± 11.43 | CΔ vs. IΔ (adjusted) p = 0.001 (§) | |||||||||
| Probiotic (SS) | PG, DB, RCT (Iran) | T2D-DN n = 20 (10M/10F) 53.60 ± 1.60 26.58 ± 0.73 | T2D-DN n = 20 (9M/11F) 56.90 ± 1.81 26.68 ± 0.71 | Conventional soy milk | Probiotic soy milk containing Lactobacillus plantarum A7 (2 × 107 CFU/mL) | 200 mL/d | 8 weeks | ↓ NGAL (ng/mL) (§) | Cb: 1667.41 ± 420.66 Ce: 2417.61 ± 392.47 (Cb vs. Ce p = 0.75) | Ib: 1808.73 ± 510.20 Ie: 1164.68 ± 379.64 (Ib vs. Ie p = 0.07) | CΔ vs. IΔ (adjusted) p = 0.05 (§) | [46] χ |
| ↑ sTNFR1 (ng/mL) (§) | Cb: 424.80 ± 47.04 Ce: 348.79 ± 80.89 (Cb vs. Ce p = 0.04) | Ib: 292.53 ± 40.87 Ie: 353.33 ± 88.02 (Ib vs. Ie p = 0.95) | CΔ vs. IΔ (adjusted) p = 0.03 (§) | |||||||||
| ↓ Cys-C (ng/mL) (§) | Cb: 50.40 ± 3.84 Ce: 58.86 ± 5.44 (Cb vs. Ce p = 0.09) | Ib: 47.85 ± 2.76 Ie: 26.82 ± 6.70 (Ib vs. Ie p = 0.12) | CΔ vs. IΔ (adjusted) p = 0.01 (§) | |||||||||
| ↓ PGRN (ng/mL) (§) | Cb: 328.85 ± 76.18 Ce: 399.56 ± 105.20 (Cb vs. Ce p = 0.60) | Ib: 339.66 ± 109.61 Ie: 180.90 ± 69.25 (Ib vs. Ie p = 0.83) | CΔ vs. IΔ (adjusted) p = 0.01 (§) | |||||||||
| Probiotic (SS) | DB, R, PG, PC (Sweden) | T2D-Abdominal obesity n = 15 (11M/4F) 65 ± 5 30.7 ± 4.0 | T2D-Abdominal obesity n = 15 (12M/3F) 66 ± 6 30.6 ± 4.5 (low-dose group) | Capsule with mildly sweet tasting powder in an aluminum laminate stick pack | Capsule containing low-dose Lactobacillus reuteri DSM 17938 (108 CFU/capsule) | 1 capsule/d | 12 weeks | Urine Alb/Cr | Cb: 2.0 ± 2.9 Ce: 2.2 ± 2.3 | Ib: 2.2 ± 5.9 Ie: 3.1 ± 8.3 | No significant effect | [47] φ |
| T2D-Abdominal obesity n = 14 (11M/3F) 64 ± 6 32.3 ± 3.4 (high-dose group) | Capsule with mildly sweet tasting powder in an aluminum laminate stick pack | Capsule containing high-dose Lactobacillus reuteri DSM 17938 (1010 CFU/capsule) | 1 capsule/d | 12 weeks | Urine Alb/Cr | Cb: 2.0 ± 2.9 Ce: 2.2 ± 2.3 | Ib: 6.7 ± 15.9 Ie: 6.5 ± 13.4 | No significant effect | ||||
| Probiotic (SS) | R, DB, CT (Iran) | DN n = 30 (Sex NS) 60.3 ± 8.5 31.1 ± 4.6 | DN n = 30 (Sex NS) 62.7 ± 9.1 30.3 ± 5.6 | Control honey | Probiotic honey containing viable and heat-resistant Bacillus coagulans T4 (108 CFU/g) | 25 g/d | 12 weeks | BUN (mg/dL) | Cb: 19.6 ± 6.2 Ce: 19.9 ± 7.3 CΔ: 0.3 ± 4.3 | Ib: 19.6 ± 7.1 Ie: 19.3 ± 6.8 IΔ: 0.3 ± 2.1 | CΔ vs. IΔ p = 0.54 (No significant effect) | [48] |
| Serum Cr (mg/dL) | Cb: 1.3 ± 0.5 Ce: 1.5 ± 0.8 CΔ: 0.2 ± 0.7 | Ib: 1.6 ± 0.6 Ie: 1.5 ± 0.5 IΔ: −0.1 ± 0.5 | CΔ vs. IΔ p = 0.09 (No significant effect) | |||||||||
| Probiotic (MS) | R, DB, PC, CT (Iran) | n = 27 (Sex NS) 52.59 ± 7.14 30.17 ± 4.23 | n = 27 (Sex NS) 50.51 ± 9.82 31.61 ± 6.36 | 100 mg fructo-oligosaccharide with lactose/capsule | Freeze-dried Lactobacillus acidophilus (2 × 109 CFU), L. casei (7 × 109 CFU), L. rhamnosus (1.5 × 109 CFU), L. bulgaricus (2 × 108 CFU), Bifidobacterium breve (2 × 1010 CFU), B. longum (7 × 109 CFU), Streptococcus thermophilus (1.5 × 109 CFU), and 100 mg FOS with lactose carrier per capsule | 1 capsule/d | 8 weeks | Uric Acid (mg/dL) | Cb: 4.73 ± 0.27 Ce: 4.88 ± 0.24 CΔ: 0.15 ± 0.21 (Cb vs. Ce p = 0.47) | Ib: 4.71 ± 0.27 Ie: 5.51 ± 0.28 IΔ: 0.8 ± 0.27 (Ib vs. Ie p = 0.008) (§) | CΔ vs. IΔ p = 0.07 (No significant effect) | [49] |
| Probiotic (MS) | DB, R, PG, PC (Malaysia) | n = 68 (34M/34F) 54.2 ± 8.3 29.3 ± 5.3 | n = 68 (31M/37F) 52.9 ± 9.2 29.2 ± 5.6 | Organoleptically similar sachets without probiotic | Sachets containing viable microbial cell preparation of Lactobacillus acidophilus, L. casei, L. lactis, Bifidobacterium bifidum, B. longum and B. infantis (6.0 × 1010 CFU/d total) mixed in water | 2 sachets/d | 12 weeks | ↓ Urea (mmol/L) (§) | Cb: 4.03 ± 0.89 C1/2: 4.07 ± 1.10 Ce: 4.24 ± 1.14 (Cb vs. Ce p = 0.081) | Ib: 4.26 ± 1.29 I1/2: 4.03 ± 1.00 Ie: 4.04 ± 1.04 (Ib vs. Ie p = 0.086) | CΔ vs. IΔ (ITT) p < 0.05 (§) | [50] |
| Serum Cr (µmol/L) | Cb: 72.10 ± 18.84 C1/2: 71.95 ± 18.60 Ce: 75.17 ± 18.93 (Cb vs. Ce p < 0.05) (§) | Ib: 69.20 ± 17.36 I1/2: 70.87 ± 18.70 Ie: 72.26 ± 19.73 (Ib vs. Ie p < 0.05) (§) | CΔ vs. IΔ (ITT) p = 0.329 (No significant effect) | |||||||||
| eGFR (mL/min) | Cb: 73.66 ± 13.38 C1/2: 73.91 ± 13.58 Ce: 68.89 ± 13.55 (Cb vs. Ce p < 0.05) (§) | Ib: 74.45 ± 18.5 I1/2: 74.14 ± 16.94 Ie: 73.07 ± 17.13 (Ib vs. Ie p = 0.710) | CΔ vs. IΔ (ITT) p = 0.147 (No significant effect) | |||||||||
| Serum Sodium (mmol/L) | Cb: 137.9 ± 2.5 C1/2: 138.8 ± 2.9 Ce: 138.5 ± 3.1 (Cb vs. Ce p = 0.167) | Ib: 138.5 ± 2.2 I1/2: 138.9 ± 2.7 Ie: 138.1 ± 3.5 (Ib vs. Ie p = 0.147) | CΔ vs. IΔ (ITT) p = 0.235 (No significant effect) | |||||||||
| Serum Potassium (mmol/L) | Cb: 4.40 ± 0.40 C1/2: 4.34 ± 0.36 Ce: 4.37 ± 0.43 (Cb vs. Ce p = 0.360) | Ib: 4.42 ± 0.30 I1/2: 4.42 ± 0.31 Ie: 4.35 ± 0.31 (Ib vs. Ie p = 0.060) | CΔ vs. IΔ (ITT) p = 0.164 (No significant effect) | |||||||||
| Probiotic (MS) | RCT (China) | T2D-DN n = 34 (12M/22F) 56.12 ± 8.23 26.44 ± 2.78 | T2D-DN n = 42 (15M/27F) 55.96 ± 8.45 27.51 ± 3.22 | Starch | Probiotic supplements containing Bifidobacterium bifidum, Lactobacillus acidophilus, Streptococcus thermophilus (3.2 × 109 CFU/d in total) | 1 capsule/d | 12 weeks | ↓ Urine Alb/Cr (mg/g) (§) | Cb: 99. 66 ± 25.24 Ce: 87.71 ± 23.01 | Ib: 101.60 ± 22.17 Ie: 67.53 ± 20.11 | Ce vs. Ie p < 0.05 (§) | [51] |
| eGFR (ml·min−1 (1.73 m2) −1) | Cb: 83.12 ± 7.2 Ce: 84.28 ± 7.13 (Cb vs. Ce p = 0.77) | Ib: 82.8 ± 8.72 Ie: 84.34 ± 6.97 (Ib vs. Ie p = 0.45) | Ce vs. Ie p = 0.08 (No significant effect) | |||||||||
| Probiotic (MS) | R, DB, PC (Iran) | DN n = 30 (28/30 = T2D) (2/30 = T1D) Sex NS 60.9 ± 4.4 26.3 ± 3.2 | DN n = 30 (28/30 = T2D) (2/30 = T1D) Sex NS 58.9 ± 8.8 25.3 ± 2.3 | Starch | Probiotic supplements with Lactobacillus acidophilus ZT-L1, Bifidobacterium bifidum ZT-B1, L. reuteri ZT-Lre, and L. fermentum ZT-L3 (8 × 109 CFU/d in total) | 1 capsule/d | 12 weeks | ↓ BUN (mg/dL) (§) | Cb: 22.2 ± 9.9 Ce: 22.6 ± 12.1 CΔ: 0.4 ± 7.7 | Ib: 23.5 ± 10.6 Ie: 20.0 ± 8.3 IΔ: −3.5 ± 5.8 | CΔ vs. IΔ p = 0.03 (§) | [52] |
| ↓ Serum Cr (mg/dL) (§) | Cb: 1.3 ± 0.5 Ce: 1.4 ± 0.5 CΔ: 0.1 ± 0.2 | Ib: 1.5 ± 0.5 Ie: 1.3 ± 0.5 IΔ: −0.2 ± 0.3 | CΔ vs. IΔ p = 0.001 (§) | |||||||||
| ↑ eGFR (mL/min) (§) | Cb: 68.4 ± 25.1 Ce: 65.1 ± 24.1 CΔ: −3.2 ± 6.4 | Ib: 58.4 ± 22.8 Ie: 66.7 ± 25.8 IΔ: 8.3 ± 17.3 | CΔ vs. IΔ p = 0.001 (§) | |||||||||
| Urine Protein (mg/day) | Cb: 1330.0 ± 637 Ce: 1331.3 ± 640 CΔ: 0.13 ± 33.5 | Ib: 1261.7 ± 698.3 Ie: 1247.3 ± 713.4 IΔ: −14.3 ± 40.1 | CΔ vs. IΔ p = 0.10 (No significant effect) | |||||||||
| Probiotic (MS) | R, DB, PC, CT (Iran) | Diabetic hemodialysis n = 30 (20M/10F) (27/30 = T2D) (3/30 = T1D) 59.4 ± 16.0 27.0 ± 6.4 | Diabetic hemodialysis n = 30 (20M/10F) (27/30 = T2D) (3/30 = T1D) 54.0 ± 16.0 25.5 ± 5.6 | ‘Placebo’ | Probiotic capsule containing Lactobacillus acidophilus, L. casei, and Bifidobacterium bifidum (2 × 109 CFU/g each) | 1 capsule/d | 12 weeks | eGFR (ml·min−1 (1.73 m2)−1) | Cb: 2.22 ± 0.86 Ce: 2.25 ± 0.93 CΔ: 0.02 ± 0.20 (Cb vs. Ce p = 0.46) | Ib: 2.49 ± 1.15 Ie: 2.54 ± 1.16 IΔ: 0.04 ± 0.18 (Ib vs. Ie p = 0.23) | CΔ vs. IΔ p = 0.77; adjusted p = 0.74 (No significant effect) | [53] |
| Serum Cr (mg/dL) | Cb: 7.8 ± 3.0 Ce: 7.7 ± 2.9 CΔ: −0.1 ± 0.8 (Cb vs. Ce p = 0.48) | Ib: 7.4 ± 3.1 Ie: 7.2 ± 2.6 IΔ: −0.2 ± 1.2 (Ib vs. Ie p = 0.39) | CΔ vs. IΔ p = 0.73; adjusted p = 0.33 (No significant effect) | |||||||||
| BUN (mg/dL) | Cb: 53.6 ± 19.5 Ce: 52.3 ± 12.7 CΔ: −1.3 ± 16.1 (Cb vs. Ce p = 0.65) | Ib: 64.9 ± 29.5 Ie: 63.9 ± 26.0 IΔ: −1.0 ± 32.6 (Ib vs. Ie p = 0.85) | CΔ vs. IΔ p = 0.96; adjusted p = 0.17 (No significant effect) | |||||||||
| Serum Albumin (g/dL) | Cb: 4.0 ± 0.4 Ce: 4.1 ± 0.4 CΔ: 0.1 ± 0.4 (Cb vs. Ce p = 0.38) | Ib: 4.2 ± 0.4 Ie: 4.3 ± 0.4 IΔ: 0.1 ± 0.3 (Ib vs. Ie p = 0.45) | CΔ vs. IΔ p = 0.84; adjusted p = 0.48 (No significant effect) | |||||||||
| Serum Sodium (mmol/L) | Cb: 137.1 ± 4.2 Ce: 138.1 ± 2.9 CΔ: 1.0 ± 3.8 (Cb vs. Ce p = 0.10) | Ib: 135.9 ± 3.3 Ie: 136.2 ± 3.1 IΔ: 0.3 ± 3.8 (Ib vs. Ie p = 0.63) | CΔ vs. IΔ p = 0.51; adjusted p = 0.07 (No significant effect) | |||||||||
| Serum Potassium (mmol/L) | Cb: 4.6 ± 0.7 Ce: 4.4 ± 0.4 CΔ: −0.1 ± 0.6 (Cb vs. Ce p = 0.10) | Ib: 4.8 ± 0.6 Ie: 4.7 ± 0.7 IΔ: −0.1 ± 0.6 (Ib vs. Ie p = 0.19) | CΔ vs. IΔ p = 0.87; adjusted p = 0.18 (No significant effect) | |||||||||
| Probiotic (MS) | SB, PC, CT (Egypt) | Diabetic ESRD hemodialysis n = 30 (18M/12F) 50.9 ± 16.9 BMI NS | Diabetic ESRD hemodialysis n = 30 (12M/18F) 57.7 ± 11.4 BMI NS | Placebo capsules, ESA, and anti-diabetic agents | Capsules containing study agent (5 × 106 of Lactobacillus delbrueckii and L. fermentum), ESA, and antidiabetic agents | 1 capsule/d | 12 weeks | Serum Albumin (g/dL) | Cb: 3.5 (IQR: 3.1−4.0) Ce: 3.5 (IQR: 3.0–3.6) (Cb vs. Ce p = 0.116) | Ib: 3.4 (IQR: 3.2–3.5) Ie: 3.5 (IQR: 3.1–3.8) (Ib vs. Ie p = 0.039) (§) | Effect NS | [40] |
| Prebiotic | DB, PC (Iran) | T2D-Overwight n = 22 (22F) 48.61 ± 9.16 29.98 ± 4.01 | T2D-Overwight n = 27 (27F) 48.07 ± 8.70 31.43 ± 3.50 | Maltodextrin | Oligofructose-enriched chicory inulin | 5 × 2 g/d | 2 months | Serum Cr (mg/dL) | Cb: 0.78 ± 0.09 Ce: 0.82 ± 0.14 (Cb vs. Ce p = 0.16) | Ib: 0.77 ± 0.11 Ie: 0.79 ± 0.10 (Ib vs. Ie p = 0.47) | Ce vs. Ie p = 0.44 (No significant effect) | [41] |
| Serum Phosphorous (mg/dL) | Cb: 4.23 ± 0.45 Ce: 4.00 ± 0.45 (Cb vs. Ce p = 0.013) | Ib: 3.96 ± 0.48 Ie: 3.96 ± 0.56 (Ib vs. Ie p = 0.97) | Ce vs. Ie p = 0.80 (No significant effect) | |||||||||
| eGFR (ml·min−1 (1.73 m2)−1) | Cb: 85.30 ± 13.45 Ce: 82.05 ± 16.06 (Cb vs. Ce p = 0.28) | Ib: 86.34 ± 13.96 Ie: 84.30 ± 13.57 (Ib vs. Ie p = 0.44) | Ce vs. Ie p = 0.65 (No significant effect) | |||||||||
| Prebiotic | R, PC, TB, CT (Iran) | T2D-Overwight n = 33 (33F) 48.6 ± 7.9 32.0 ± 3.9 | T2D-Overwight n = 32 (32F) 49.5 ± 8.0 31.5 ± 4.5 | Maltodextrin | Resistant dextrin supplement (NUTRIOSE®06) | 5 × 2 g/d | 8 weeks | Uric Acid (mg/dL) | Cb: 5.40 ± 0.61 Ce: 5.50 ± 0.33 CΔ MD: 0.10 (95% CI: −0.80; 1.12) (Cb vs. Ce p = 0.28) | Ib: 4.80 ± 0.40 Ie: 5.60 ± 0.20 IΔ MD: 1.85 (95% CI: 0.91; 1.24) (Ib vs. Ie p < 0.05) (§) | CΔ vs. IΔ MD: 0.10 (95% CI: −1.55; 0.75) (No significant effect) | [42] |
| Prebiotic | R, DB, PC (Japan) | n = 25 (17M/8F) 54 ± 12 27.2 ± 4.6 | n = 27 (21M/6F) 55 ± 11 27.9 ± 3.6 | Maltodextrin syrup | GOS syrup (Oligomate55N) | 10 g/d | 4 weeks | BUN (mg/dL) | Cb: 14.0 ± 4.0 Ce: 13.0 ± 3.0 (Cb vs. Ce p > 0.05) | Ib: 15.0 ± 5.0 Ie: 15.0 ± 5.0 (Ib vs. Ie p > 0.05) | No significant effect | [43] |
| Serum Cr (mg/dL) | Cb: 0.7 ± 0.2 Ce: 0.8 ± 0.2 (Cb vs. Ce p < 0.05) (§) | Ib: 0.9 ± 0.5 Ie: 0.9 ± 0.4 (Ib vs. Ie p > 0.05) | No significant effect | |||||||||
| eGFR (mL/min) | Cb: 85.1 ± 21.3 Ce: 79.9 ± 18.7 (Cb vs. Ce p < 0.05) (§) | Ib: 75.1 ± 24.4 Ie: 71.2 ±2 1.6 (Ib vs. Ie p < 0.05) (§) | Effect NS | |||||||||
| Synbiotic (SS) | R, DB, CC, CT (Iran) | n = 62 (19M/43F) 53.1 ± 8.7 29.90 ± 5.18 | n = 62 (19M/43F) 53.1 ± 8.7 29.60 ± 4.53 | 0.38 g isomalt, 0.36 g sorbitol and 0.05 g stevia per 1g | Heat-resistant Lactobacillus sporogenes (1 × 107 CFU), 0.04 g inulin, 0.38 g isomalt, 0.36 g sorbitol and 0.05 g stevia per gram | 9 × 3 g/d | 6 × 2 weeks | ↑ Uric acid (mg/dL) (§) | Cb: 5.5 ± 0.3 Ce: 5.4 ± 0.2 CΔ: −0.1 ± 0.3 | Ib: 4.9 ± 0.2 Ie: 5.6 ± 0.2 IΔ: 0.7 ± 0.2 | CΔ vs. IΔ p = 0.03 (§) | [44] ψ |
| Synbiotic (MS) | SC, R, DB, PC (Iran) | n = 35 (19M/16F) 58.63 ± 8.06 27.30 ± 3.81 | n = 35 (23M/12F) 58.71 ± 8.20 28.13 ± 3.78 | Capsules containing row starch, B group vitamins (1 mg), lactose (0.5 mg), malt-dextrin, magnesium saturate and talc | Capsules containing Lactobacillus family, Bifidobacterium family, Streptococcus thermophilus, FOS, B group vitamins (1 mg), lactose (0.5 mg), maltodextrin, magnesium saturate and talc | 1 × 500 mg capsule/d | 9 weeks | Urea (mg/dL) | Cb: 36.80 ± 14.79 Ce: 37.94 ± 14.57 CΔ: −1.14 ± 7.30 (Cb vs. Ce p = 0.36) | Ib: 31.20 ± 7.67 Ie: 33.25 ± 7.61 IΔ: −2.05 ± 7.31 (Ib vs. Ie p = 0.10) | Ce vs. Ie p = 0.09 CΔ vs. IΔ p = 0.60 (No significant effect) | [45] |
| Serum Cr (mg/dL) | Cb: 1.05 ± 0.22 Ce: 1.03 ± 0.24 CΔ: 0.02 ± 0.11 (Cb vs. Ce p = 0.22) | Ib: 1.04 ± 0.26 Ie: 1.05 ± 0.26 IΔ: −0.00 ± 0.09 (Ib vs. Ie p = 0.82) | Ce vs. Ie p = 0.73 CΔ vs. IΔ p = 0.73 (No significant effect) | |||||||||
| ↓ Urine Alb/Cr (mg/g) (§) | Cb: 62.77 ± 59.6 Ce: 81.09 ± 81.58 CΔ: 18.31 ± 46.78 (Cb vs. Ce p = 0.027) (§) | Ib: 45.39 ± 38.85 Ie: 34.94 ± 13.1 IΔ: −10.44 ± 35.26 (Ib vs. Ie p = 0.089) | Ce vs. Ie p = 0.00 (§) CΔ vs. IΔ p < 0.0001 (§) | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, P.; Kaul, R.; Chaari, A. Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut–Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials. Int. J. Mol. Sci. 2022, 23, 14838. https://doi.org/10.3390/ijms232314838
Paul P, Kaul R, Chaari A. Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut–Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials. International Journal of Molecular Sciences. 2022; 23(23):14838. https://doi.org/10.3390/ijms232314838
Chicago/Turabian StylePaul, Pradipta, Ridhima Kaul, and Ali Chaari. 2022. "Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut–Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials" International Journal of Molecular Sciences 23, no. 23: 14838. https://doi.org/10.3390/ijms232314838
APA StylePaul, P., Kaul, R., & Chaari, A. (2022). Renal Health Improvement in Diabetes through Microbiome Modulation of the Gut–Kidney Axis with Biotics: A Systematic and Narrative Review of Randomized Controlled Trials. International Journal of Molecular Sciences, 23(23), 14838. https://doi.org/10.3390/ijms232314838





