Targeting Mitochondrial Dynamics Proteins for the Development of Therapies for Cardiovascular Diseases
Abstract
:1. Introduction
2. The Role of Mitochondria in Cardiac Functioning
2.1. ATP Production
2.2. Calcium Homeostasis
2.3. Lipid Synthesis
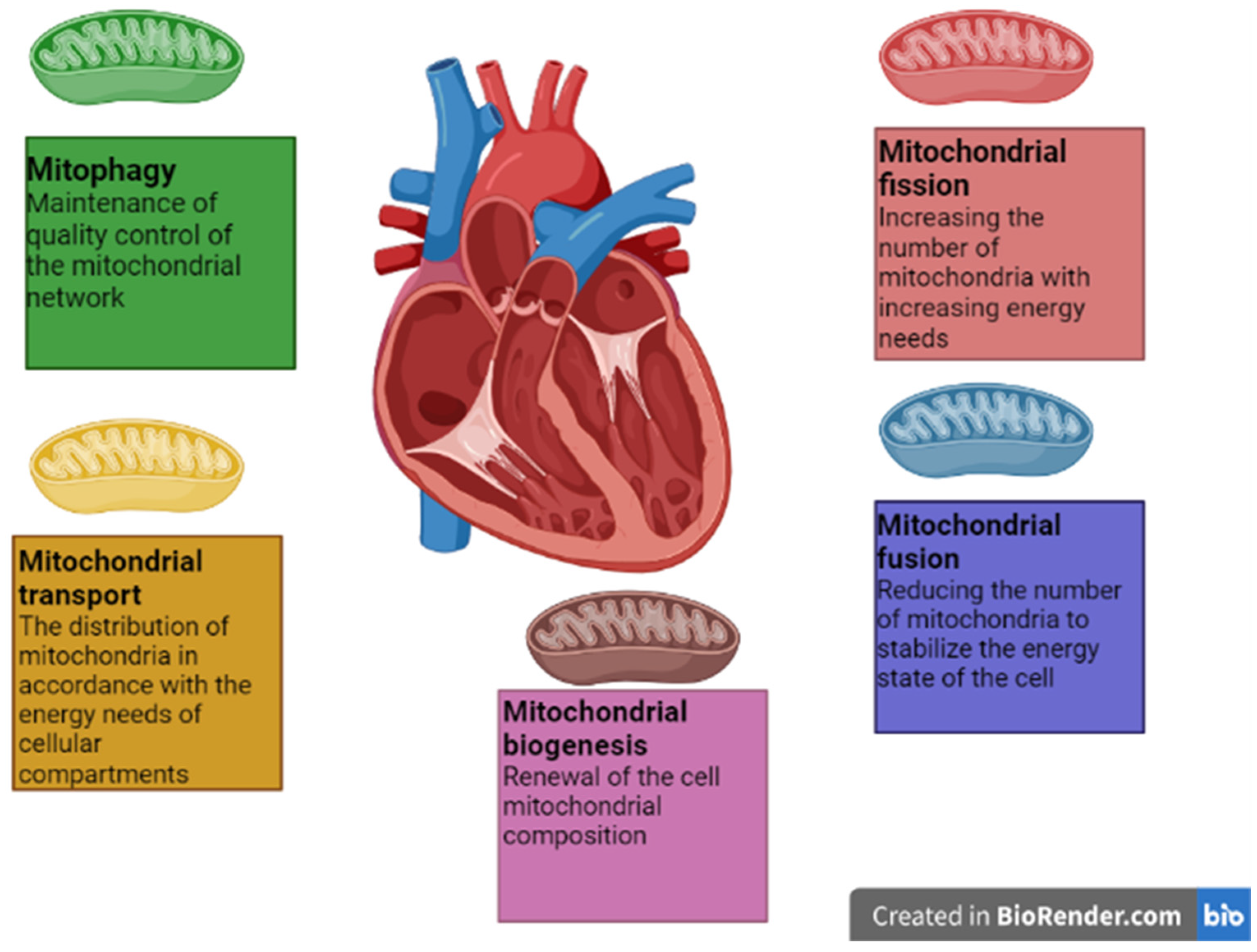
3. Processes of Mitochondrial Dynamics in the Heart Muscle
3.1. Mitochondrial Fusion
3.2. Mitochondrial Fission
3.3. Mitophagy
3.4. Mitochondrial Biogenesis
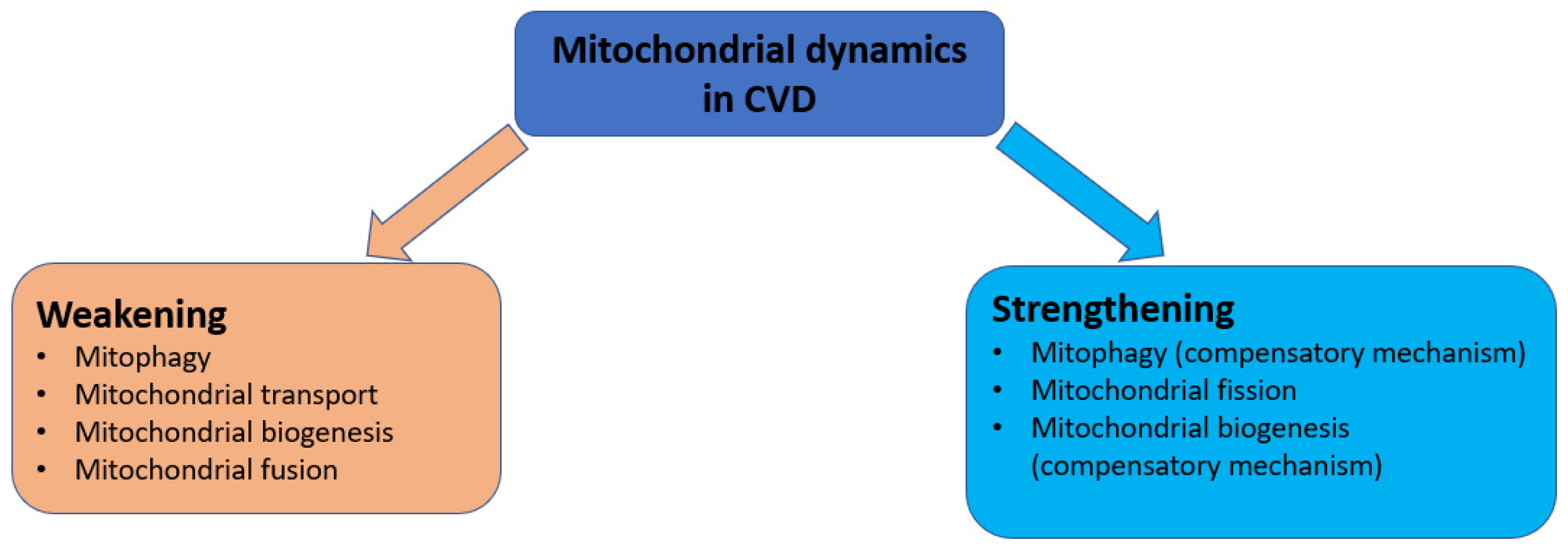
4. Mitochondrial Dynamics Disorders in Cardiovascular Diseases
4.1. Mitophagy Disorders
4.2. Mitochondrial Transport Disorders
4.3. Mitochondrial Biogenesis Disorders
4.4. Mitochondrial Fission Disorders
4.5. Mitochondrial Fusion Disorders
5. Analysis of Mitochondrial Dynamics Proteins as Therapeutic Targets
5.1. Impact on Mitophagy Proteins
5.2. Impact on Mitochondrial Fission Proteins
5.3. Impact on Mitochondrial Fusion Proteins
6. Discussions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stewart, J.; Manmathan, G.; Wilkinson, P. Primary prevention of cardiovascular disease: A review of contemporary guidance and literature. JRSM Cardiovasc. Dis. 2017, 6, 2048004016687211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Sjögren, B.; Bigert, C.; Gustavsson, P. Cardiovascular Disease. Handb. Toxicol. Met. 2015, 2015, 313–331. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [PubMed]
- Felker, G.M.; Ellison, D.H.; Mullens, W.; Cox, Z.L.; Testani, J.M. Diuretic Therapy for Patients with Heart Failure. J. Am. Coll. Cardiol. 2020, 75, 1178–1195. [Google Scholar] [CrossRef] [PubMed]
- Lassiter, G.; Melancon, C.; Rooney, T.; Murat, A.-M.; Kaye, J.; Kaye, A.; Kaye, R.; Cornett, E.; Kaye, A.; Shah, R.; et al. Ozanimod to Treat Relapsing Forms of Multiple Sclerosis: A Comprehensive Review of Disease, Drug Efficacy and Side Effects. Neurol. Int. 2020, 12, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Torregroza, C.; Huhn, R.; Hollmann, M.W.; Preckel, B. Perioperative Cardioprotection: Clinical Implications. Obstet. Anesth. Dig. 2020, 131, 1751–1764. [Google Scholar] [CrossRef]
- Poznyak, A.; Ivanova, E.; Sobenin, I.; Yet, S.-F.; Orekhov, A. The Role of Mitochondria in Cardiovascular Diseases. Biology 2020, 9, 137. [Google Scholar] [CrossRef]
- Murphy, E.; Ardehali, H.; Balaban, R.S.; DiLisa, F.; Dorn, G.W.; Kitsis, R.N.; Otsu, K.; Ping, P.; Rizzuto, R.; Sack, M.N.; et al. Mitochondrial Function, Biology, and Role in Disease. Circ. Res. 2016, 118, 1960–1991. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2019, 176, 4302–4318. [Google Scholar] [CrossRef]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.P.; Hotamisligil, G.S. Calcium Homeostasis and Organelle Function in the Pathogenesis of Obesity and Diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.C.; Liu, J.; Holmström, K.M.; Menazza, S.; Parks, R.J.; Fergusson, M.M.; Yu, Z.-X.; Springer, D.A.; Halsey, C.; Liu, C.; et al. MICU1 Serves as a Molecular Gatekeeper to Prevent In Vivo Mitochondrial Calcium Overload. Cell Rep. 2016, 16, 1561–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesmin, B. Mitochondrial lipid transport and biosynthesis: A complex balance. J. Cell Biol. 2016, 214, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine Deficiency in Mammalian Mitochondria Impairs Oxidative Phosphorylation and Alters Mitochondrial Morphology. J. Biol. Chem. 2012, 288, 4158–4173. [Google Scholar] [CrossRef] [Green Version]
- Filadi, R.; Pendin, D.; Pizzo, P. Mitofusin 2: From functions to disease. Cell Death Dis. 2018, 9, 330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nottia, M.; Verrigni, D.; Torraco, A.; Rizza, T.; Bertini, E.; Carrozzo, R. Mitochondrial Dynamics: Molecular Mechanisms, Related Primary Mitochondrial Disorders and Therapeutic Approaches. Genes 2021, 12, 247. [Google Scholar] [CrossRef]
- Patten, D.A.; Wong, J.; Khacho, M.; Soubannier, V.; Mailloux, R.; Pilon-Larose, K.; Maclaurin, J.G.; Park, D.; McBride, H.; Trinkle-Mulcahy, L.; et al. OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 2014, 33, 2676–2691. [Google Scholar] [CrossRef] [Green Version]
- Forte, M.; Schirone, L.; Ameri, P.; Basso, C.; Catalucci, D.; Modica, J.; Chimenti, C.; Crotti, L.; Frati, G.; Rubattu, S.; et al. The role of mitochondrial dynamics in cardiovascular diseases. Br. J. Pharmacol. 2020, 178, 2060–2076. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Sadoshima, J. Molecular Mechanisms of Mitochondrial Autophagy/Mitophagy in the Heart. Circ. Res. 2015, 116, 1477–1490. [Google Scholar] [CrossRef] [Green Version]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Michalska, B.; Kwapiszewska, K.; Szczepanowska, J.; Kalwarczyk, T.; Patalas-Krawczyk, P.; Szczepanski, K.; Hołyst, R.; Duszynski, J.; Szymański, J. Insight into the fission mechanism by quantitative characterization of Drp1 protein distribution in the living cell. Sci. Rep. 2018, 8, 8122. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemasters, J.J. Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3). Redox Biol. 2014, 2, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durcan, T.M.; Tang, M.Y.; Pérusse, J.R.; Dashti, E.A.; Aguileta, M.A.; McLelland, G.-L.; Gros, P.; Shaler, T.A.; Faubert, D.; Coulombe, B.; et al. USP 8 regulates mitophagy by removing K 6-linked ubiquitin conjugates from parkin. EMBO J. 2014, 33, 2473–2491. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Maejima, Y.; Zablocki, D.; Sadoshima, J. The Role of Autophagy in the Heart. Annu. Rev. Physiol. 2018, 80, 1–26. [Google Scholar] [CrossRef]
- Hoshino, A.; Mita, Y.; Okawa, Y.; Ariyoshi, M.; Iwai-Kanai, E.; Ueyama, T.; Ikeda, K.; Ogata, T.; Matoba, S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat. Commun. 2013, 4, 2308. [Google Scholar] [CrossRef] [Green Version]
- Wallace, D.C. Mitochondrial genetic medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef]
- Di, W.; Lv, J.; Jiang, S.; Lu, C.; Yang, Z.; Ma, Z.; Hu, W.; Yang, Y.; Xu, B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018, 28, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Islam, H.; Edgett, B.A.; Gurd, B.J. Coordination of mitochondrial biogenesis by PGC-1α in human skeletal muscle: A re-evaluation. Metabolism 2018, 79, 42–51. [Google Scholar] [CrossRef]
- Barshad, G.; Marom, S.; Cohen, T.; Mishmar, D. Mitochondrial DNA Transcription and Its Regulation: An Evolutionary Perspective. Trends Genet. 2018, 34, 682–692. [Google Scholar] [CrossRef]
- Chen, L.; Qin, Y.; Liu, B.; Gao, M.; Li, A.; Li, X.; Gong, G. PGC-1α-Mediated Mitochondrial Quality Control: Molecular Mechanisms and Implications for Heart Failure. Front. Cell Dev. Biol. 2022, 10, 871357. [Google Scholar] [CrossRef] [PubMed]
- Sygitowicz, G.; Sitkiewicz, D. Mitochondrial Quality Control: The Role in Cardiac Injury. Front. Biosci. 2022, 27, 96. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.-B.; Xiu, J.; Wei, J.-X.; Pan, P.-P.; Sun, B.-H.; Liu, L. Shen Qi Li Xin formula improves chronic heart failure through balancing mitochondrial fission and fusion via upregulation of PGC-1α. J. Physiol. Sci. 2021, 71, 32. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Sadoshima, J. Mitochondrial autophagy in cardiomyopathy. Curr. Opin. Genet. Dev. 2016, 38, 8. [Google Scholar] [CrossRef] [Green Version]
- Sergin, I.; Bhattacharya, S.; Emanuel, R.; Esen, E.; Stokes, C.J.; Evans, T.D.; Arif, B.; Curci, J.A.; Razani, B. p62-enriched inclusion bodies in macrophages protect against atherosclerosis. Sci. Signal. 2016, 9, ra2. [Google Scholar]
- Poznyak, A.V.; Nikiforov, N.G.; Wu, W.K.; Kirichenko, T.V.; Orekhov, A.N. Autophagy and Mitophagy as Essential Components of Atherosclerosis. Cells 2021, 10, 443. [Google Scholar] [CrossRef]
- Morciano, G.; Patergnani, S.; Bonora, M.; Pedriali, G.; Tarocco, A.; Bouhamida, E.; Marchi, S.; Ancora, G.; Anania, G.; Wieckowski, M.R.; et al. Mitophagy in Cardiovascular Diseases. J. Clin. Med. 2020, 9, 892. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Calvani, R.; Bernabei, R.; Leeuwenburgh, C.; Marzetti, E. Contribution of Impaired Mitochondrial Autophagy to Cardiac Aging. Circ. Res. 2012, 110, 1125–1138. [Google Scholar] [CrossRef] [Green Version]
- Luan, Y.; Luan, Y.; Feng, Q.; Chen, X.; Ren, K.D.; Yang, Y. Emerging Role of Mitophagy in the Heart: Therapeutic Potentials to Modulate Mitophagy in Cardiac Diseases. Oxid. Med. Cell. Longev. 2021, 2021, 3259963. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; Gaude, E.; Aksentijević, D.; Sundier, S.Y.; Robb, E.L.; Logan, A.; Nadtochiy, S.M.; Ord, E.N.J.; Smith, A.C.; et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 2014, 515, 431. [Google Scholar] [CrossRef] [PubMed]
- Fransson, A.; Ruusala, A.; Aspenstrém, P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J. Biol. Chem. 2003, 278, 6495–6502. [Google Scholar] [CrossRef] [Green Version]
- Caporizzo, M.A.; Chen, C.Y.; Prosser, B.L. Cardiac microtubules in health and heart disease. Exp. Biol. Med. 2019, 244, 1255–1272. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, M.; Giordano, C.; Nediani, C.; Travaglini, C.; Borchi, E.; Zani, M.; Feccia, M.; Mancini, M.; Petrozza, V.; Cossarizza, A.; et al. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J. Am. Coll. Cardiol. 2007, 50, 1362–1369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishio, M.L.; Ornatsky, O.I.; Craig, E.E.; Hood, D.A. Mitochondrial biogenesis during pressure overload induced cardiac hypertrophy in adult rats. Can. J. Physiol. Pharmacol. 1995, 73, 630–637. [Google Scholar] [CrossRef]
- Rosca, M.G.; Tandler, B.; Hoppel, C.L. Mitochondria in cardiac hypertrophy and heart failure. J. Mol. Cell. Cardiol. 2013, 55, 31. [Google Scholar] [CrossRef] [Green Version]
- Pisano, A.; Cerbelli, B.; Perli, E.; Pelullo, M.; Bargelli, V.; Preziuso, C.; Mancini, M.; He, L.; Bates, M.G.; Lucena, J.R.; et al. Impaired mitochondrial biogenesis is a common feature to myocardial hypertrophy and end-stage ischemic heart failure. Cardiovasc. Pathol. 2016, 25, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Quiles, J.M.; Gustafsson, A.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Rijzewijk, L.J.; van der Meer, R.W.; Smit, J.W.; Diamant, M.; Bax, J.J.; Hammer, S.; Romijn, J.A.; de Roos, A.; Lamb, H.J. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J. Am. Coll. Cardiol. 2008, 52, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.-R.; Zheng, D.-L.; Liu, P.-M.; Yang, H.; Li, L.-A.; Kuang, S.-J.; Lai, Y.-Y.; Rao, F.; Xue, Y.-M.; Lin, J.-J.; et al. High glucose induces Drp1-mediated mitochondrial fission via the Orail calcium channel to participate in diabetic cardiomyocyte hypertrophy. Cell Death Dis. 2021, 12, 216. [Google Scholar] [CrossRef]
- Jin, J.-y.; Wei, X.-x.; Zhi, X.-l.; Wang, X.-h.; Meng, D. Drp1-dependent mitochondrial fission in cardiovascular disease. Acta Pharmacol. Sin. 2020, 42, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, X.; Yan, Y.; Liu, B. Role of GTPase-Dependent Mitochondrial Dynamics in Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 720085. [Google Scholar] [CrossRef] [PubMed]
- Vizziello, M.; Borellini, L.; Franco, G.; Ardolino, G. Disruption of Mitochondrial Homeostasis: The Role of PINK1 in Parkinson’s Disease. Cells 2021, 10, 3022. [Google Scholar] [CrossRef]
- Qiao, H.; Ren, H.; Du, H.; Zhang, M.; Xiong, X.; Lv, R. Liraglutide repairs the infarcted heart: The role of the SIRT 1/Parkin/mitophagy pathway. Mol. Med. Rep. 2018, 17, 3722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Chen, J.; Feng, J.; Zhang, R.; Fan, M.; Han, D.; Li, X.; Li, C.; Ren, J.; Wang, Y.; et al. Melatonin Ameliorates the Progression of Atherosclerosis via Mitophagy Activation and NLRP3 Inflammasome Inhibition. Oxid. Med. Cell. Longev. 2018, 2018, 9286458. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Z.; Feng, X.; Cheng, Z.; Xiong, Z.; Wang, T.; Lin, J.; Zhang, M.; Hu, J.; Fan, Y.; et al. Melatonin activates Parkin translocation and rescues the impaired mitophagy activity of diabetic cardiomyopathy through Mst1 inhibition. J. Cell. Mol. Med. 2018, 22, 5132–5144. [Google Scholar] [CrossRef]
- Ong, S.B.; Subrayan, S.; Lim, S.Y.; Yellon, D.M.; Davidson, S.M.; Hausenloy, D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation 2010, 121, 20122022. [Google Scholar] [CrossRef] [Green Version]
- Nan, J.; Zhu, W.; Rahman, M.S.; Liu, M.; Li, D.; Su, S.; Zhang, N.; Hu, X.; Yu, H.; Gupta, M.P.; et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim. Biophys. Acta Mol. Cell. Res. 2017, 1864, 1260–1273. [Google Scholar] [CrossRef]
- Branchetti, E.; Bavaria, J.E.; Grau, J.B.; Shaw, R.E.; Poggio, P.; Lai, E.K.; Desai, N.D.; Gorman, J.H.; Gorman, R.C.; Ferrari, G. Circulating Soluble Receptor for Advanced Glycation End Product Identifies Patients with Bicuspid Aortic Valve and Associated Aortopathies. Arter. Thromb. Vasc. Biol. 2014, 34, 2349–2357. [Google Scholar] [CrossRef] [Green Version]
- Poggio, P.; Branchetti, E.; Grau, J.B.; Lai, E.K.; Gorman, R.C.; Gorman, J.H., III; Sacks, M.S.; Bavaria, J.E.; Ferrari, G. Osteopontin–CD44v6 Interaction Mediates Calcium Deposition via Phospho-Akt in Valve Interstitial Cells from Patients with Noncalcified Aortic Valve Sclerosis. Arter. Thromb. Vasc. Biol. 2014, 34, 2086–2094. [Google Scholar] [CrossRef] [Green Version]
- Haileselassie, B.; Mukherjee, R.; Joshi, A.U.; Napier, B.A.; Massis, L.M.; Ostberg, N.P.; Queliconi, B.B.; Monack, D.; Bernstein, D.; Mochly-Rosen, D. Drp1/Fis1 Interaction Mediates Mitochondrial Dysfunction in Septic Cardiomyopathy. J. Mol. Cell. Cardiol. 2019, 130, 160. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.H.H.; Jumbo, J.C.C.; Wang, Y.; Li, P. Therapeutic potential and recent advances on targeting mitochondrial dynamics in cardiac hypertrophy: A concise review. Mol. Ther. Nucleic Acids. 2021, 25, 416–443. [Google Scholar] [CrossRef] [PubMed]
- Zanobini, M.; Saccocci, M.; Tamborini, G.; Veglia, F.; Di Minno, A.; Poggio, P.; Pepi, M.; Alamanni, F.; Loardi, C. Postoperative Echocardiographic Reduction of Right Ventricular Function: Is Pericardial Opening Modality the Main Culprit? BioMed Res. Int. 2017, 2017, 4808757. [Google Scholar] [CrossRef] [PubMed]
- Torres, G.; Morales, P.E.; García-Miguel, M.; Norambuena-Soto, I.; Cartes-Saavedra, B.; Vidal-Peña, G.; Moncada-Ruff, D.; Sanhueza-Olivares, F.; San Martín, A.; Chiong, M. Glucagon-like peptide-1 inhibits vascular smooth muscle cell dedifferentiation through mitochondrial dynamics regulation. Biochem. Pharmacol. 2016, 104, 52–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.J.Y.; Diamant, M.; de Boer, K.; Harms, H.J.; Robbers, L.F.H.J.; van Rossum, A.C.; Kramer, M.H.H.; Lammertsma, A.A.; Knaapen, P. Effects of exenatide on cardiac function, perfusion, and energetics in type 2 diabetic patients with cardiomyopathy: A randomized controlled trial against insulin glargine. Cardiovasc. Diabetol. 2017, 16, 67. [Google Scholar] [CrossRef] [Green Version]
- Magyar, K.; Halmosi, R.; Palfi, A.; Feher, G.; Czopf, L.; Fulop, A.; Battyany, I.; Sumegi, B.; Toth, K.; Szabados, E. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin. Hemorheol. Microcirc. 2012, 50, 179–187. [Google Scholar] [CrossRef]
- Sainger, R.; Grau, J.B.; Poggio, P.; Branchetti, E.; Bavaria, J.E.; Gorman, J.H.; Gorman, R.C.; Ferrari, G. Dephosphorylation of circulating human osteopontin correlates with severe valvular calcification in patients with calcific aortic valve disease. Biomarkers 2012, 17, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Hull, T.D.; Boddu, R.; Guo, L.; Tisher, C.C.; Traylor, A.M.; Patel, B.; Joseph, R.; Prabhu, S.D.; Suliman, H.B.; Piantadosi, C.A.; et al. Heme oxygenase-1 regulates mitochondrial quality control in the heart. JCI Insight 2016, 1, 85817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, H.; Du, J.; Song, X.; He, L.; Zhang, Y.; Li, X.; Qiu, C.; Zhang, Y.; Hou, J.; Feng, J.; et al. Melatonin prevents adverse myocardial infarction remodeling via Notch1/Mfn2 pathway. Free Radic. Biol. Med. 2016, 97, 408–417. [Google Scholar] [CrossRef]
- Franco, A.; Kitsis, R.N.; Fleischer, J.A.; Gavathiotis, E.; Kornfeld, O.S.; Gong, G.; Biris, N.; Benz, A.; Qvit, N.; Donnelly, S.K.; et al. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature 2016, 540, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Tong, M.; Zablocki, D.; Sadoshima, J. The role of Drp1 in mitophagy and cell death in the heart. J. Mol. Cell. Cardiol. 2020, 142, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Ashley, N.; Diot, A.; Morten, K.; Phadwal, K.; Williams, A.; Fearnley, I.; Rosser, L.; Lowndes, J.; Fratter, C.; et al. Dysregulated mitophagy and mitochondrial organization in optic atrophy due to OPA1 mutations. Neurology 2017, 88, 131–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twig, G.; Shirihai, O.S. The Interplay between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed]

| Disease | Model | Disruption of Mitochondrial Dynamics |
|---|---|---|
| Atherosclerosis | Mice, human cardiomyocyte culture | Decreased mitophagy |
| Heart failure | Mice, human cardiomyocyte culture | Decreased mitophagy |
| Ischemic heart disease | Mice | Increased mitophagy at the beginning of the IRI and decreased at the end of the IRI |
| Heart failure | Mice | Decreased mitochondrial transport |
| Mitochondrial cardiomyopathy | Human cardiomyocyte culture | Increased mitochondrial biogenesis |
| Hypertrophic cardiomyopathy | Human cardiomyocyte culture | Decreased mitochondrial biogenesis |
| Heart failure | Mice | Increased mitochondrial fission and decreased fusion |
| Metabolic cardiomyopathy | Rats | Increased mitochondrial fission and decreased fusion |
| Therapy Compound | Action Mechanism | Study Type | Results |
|---|---|---|---|
| Liraglutide | Mitophagy strengthening through SIRT1 expression increasing | Animal model | Restoring myocardial function after a heart attack |
| Melatonin (1) | Activation of mitophagy | Animal models | Improving clinical symptoms of atherosclerosis and diabetic cardiomyopathy |
| Berberine | Activation of mitophagy | Animal model | Restoration of cardiac function in heart failure |
| mitoTEMPOL | Inhibition of mitophagy | Animal model | |
| mdivi-1 | Suppression of the GTPase activity of DRP1 | Animal model | Protection from ischemia/reperfusion and reduction in the possibility of myocardial infarction |
| P110 peptides | Inhibition of the DRP1 binding with Fis1 | Animal model and cell model | Protection from septic cardiomyopathy development |
| Exenatide | Inhibition of the mitochondrial localization of Drp1 | Cell model and clinical trial | Heart regeneration in cell model but no significant improvement in cardiac function in clinical trial |
| Resveratrol | Inhibition of the Drp1expression | Animal models and clinical trial | Improvement of clinical symptoms from patients with coronary heart disease |
| HO1 | Increase in Mfn1/2 expression | Animal model | |
| Melatonin (2) | Increase in Mfn2 expression | Animal model | Attenuation of post-infarction injury |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blagov, A.V.; Kozlov, S.; Blokhina, T.; Sukhorukov, V.N.; Orekhov, A.N. Targeting Mitochondrial Dynamics Proteins for the Development of Therapies for Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 14741. https://doi.org/10.3390/ijms232314741
Blagov AV, Kozlov S, Blokhina T, Sukhorukov VN, Orekhov AN. Targeting Mitochondrial Dynamics Proteins for the Development of Therapies for Cardiovascular Diseases. International Journal of Molecular Sciences. 2022; 23(23):14741. https://doi.org/10.3390/ijms232314741
Chicago/Turabian StyleBlagov, Alexander V., Sergey Kozlov, Tatiana Blokhina, Vasily N. Sukhorukov, and Alexander N. Orekhov. 2022. "Targeting Mitochondrial Dynamics Proteins for the Development of Therapies for Cardiovascular Diseases" International Journal of Molecular Sciences 23, no. 23: 14741. https://doi.org/10.3390/ijms232314741
APA StyleBlagov, A. V., Kozlov, S., Blokhina, T., Sukhorukov, V. N., & Orekhov, A. N. (2022). Targeting Mitochondrial Dynamics Proteins for the Development of Therapies for Cardiovascular Diseases. International Journal of Molecular Sciences, 23(23), 14741. https://doi.org/10.3390/ijms232314741








