Oleic Acid Facilitates Cd Excretion by Increasing the Abundance of Burkholderia in Cd-Exposed Mice
Abstract
:1. Introduction
2. Results
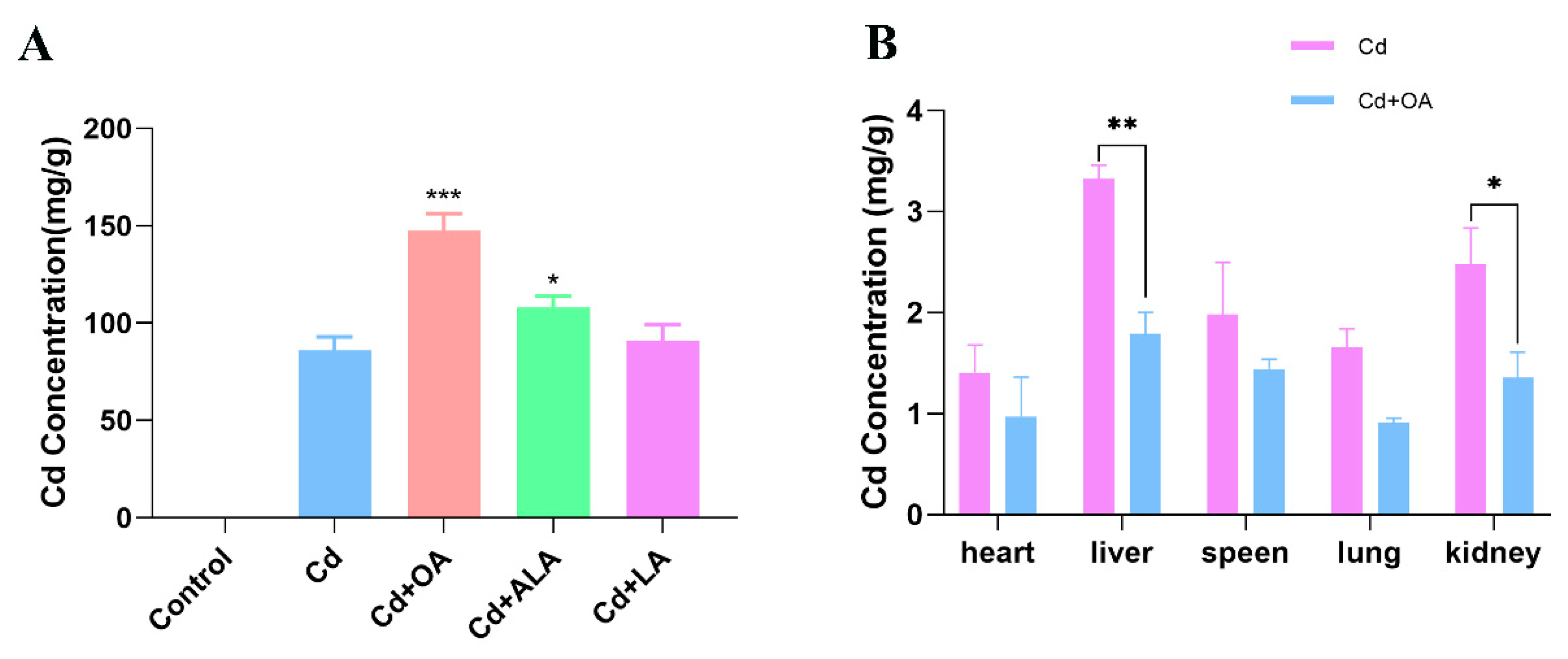
2.1. Effects of Fatty Acids on Cd Excretion in Cd-Exposed Mice
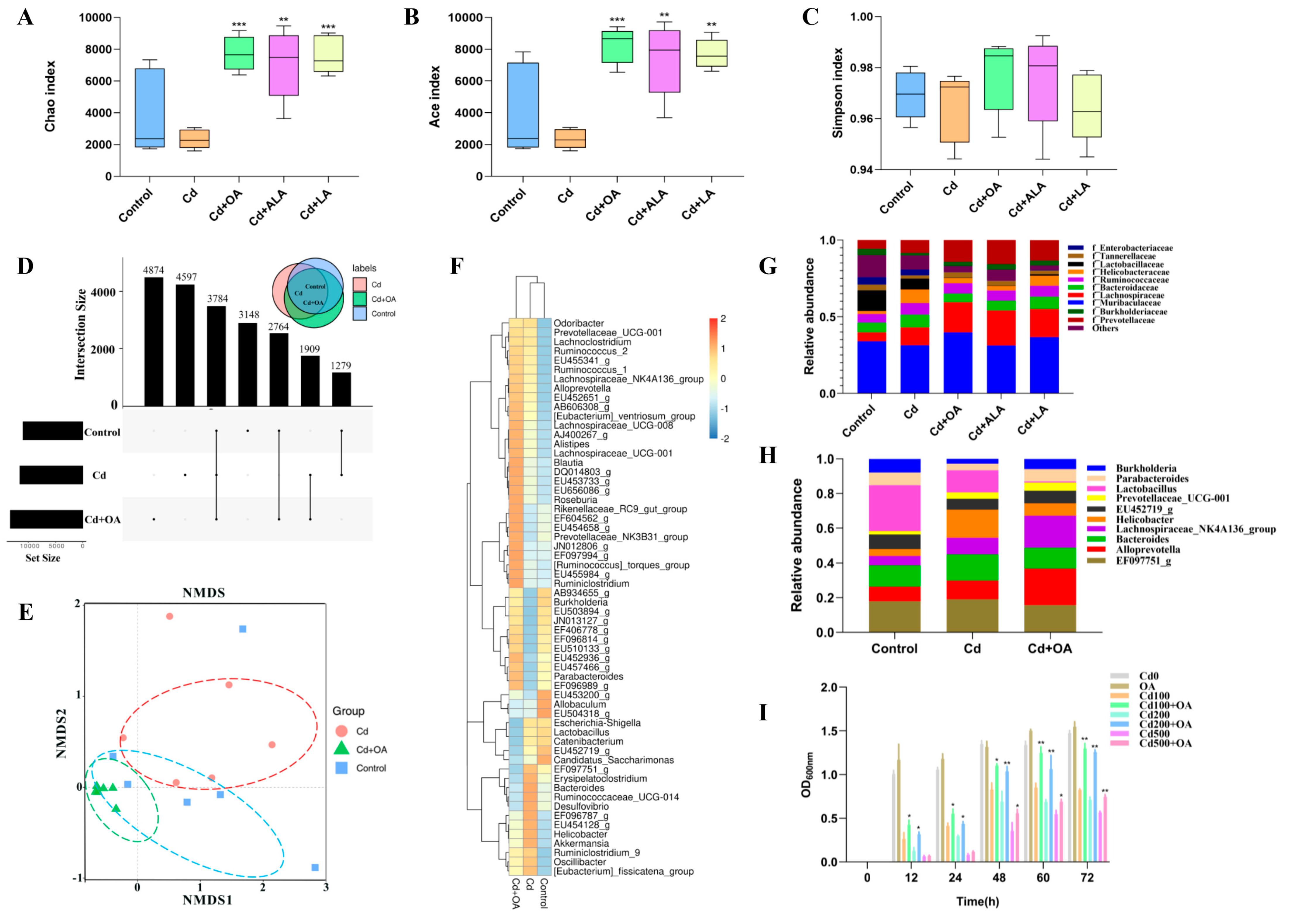
2.2. Effects of Oleic Acid (OA) on Gut Microbiota and B. cepacia against Cd
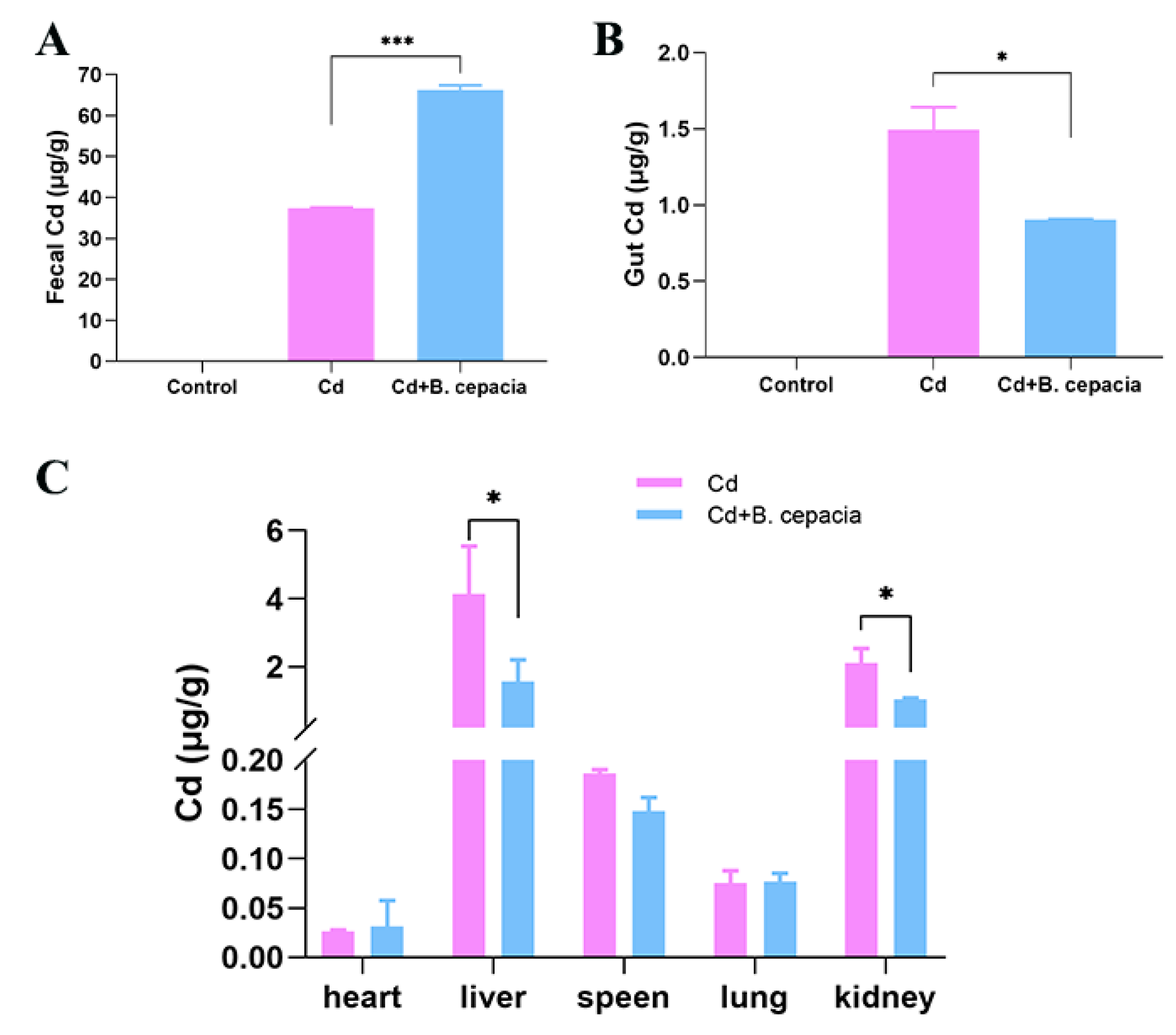
2.3. B. cepacia Facilitates Cd Excretion and Reduces Cd Accumulation in Cd-Exposed Mice
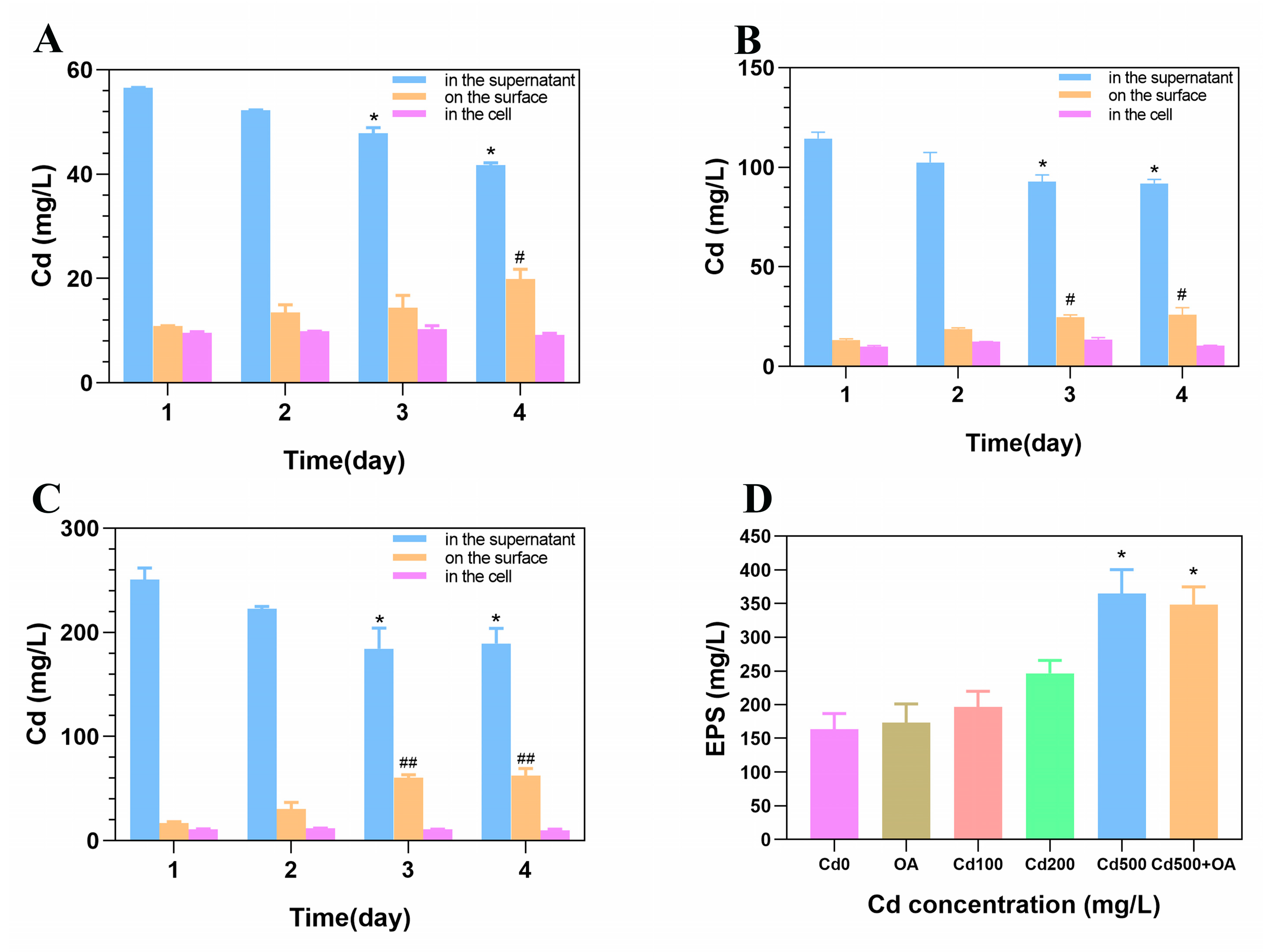
2.4. The Cd Content in B. cepacia and on Its Surface
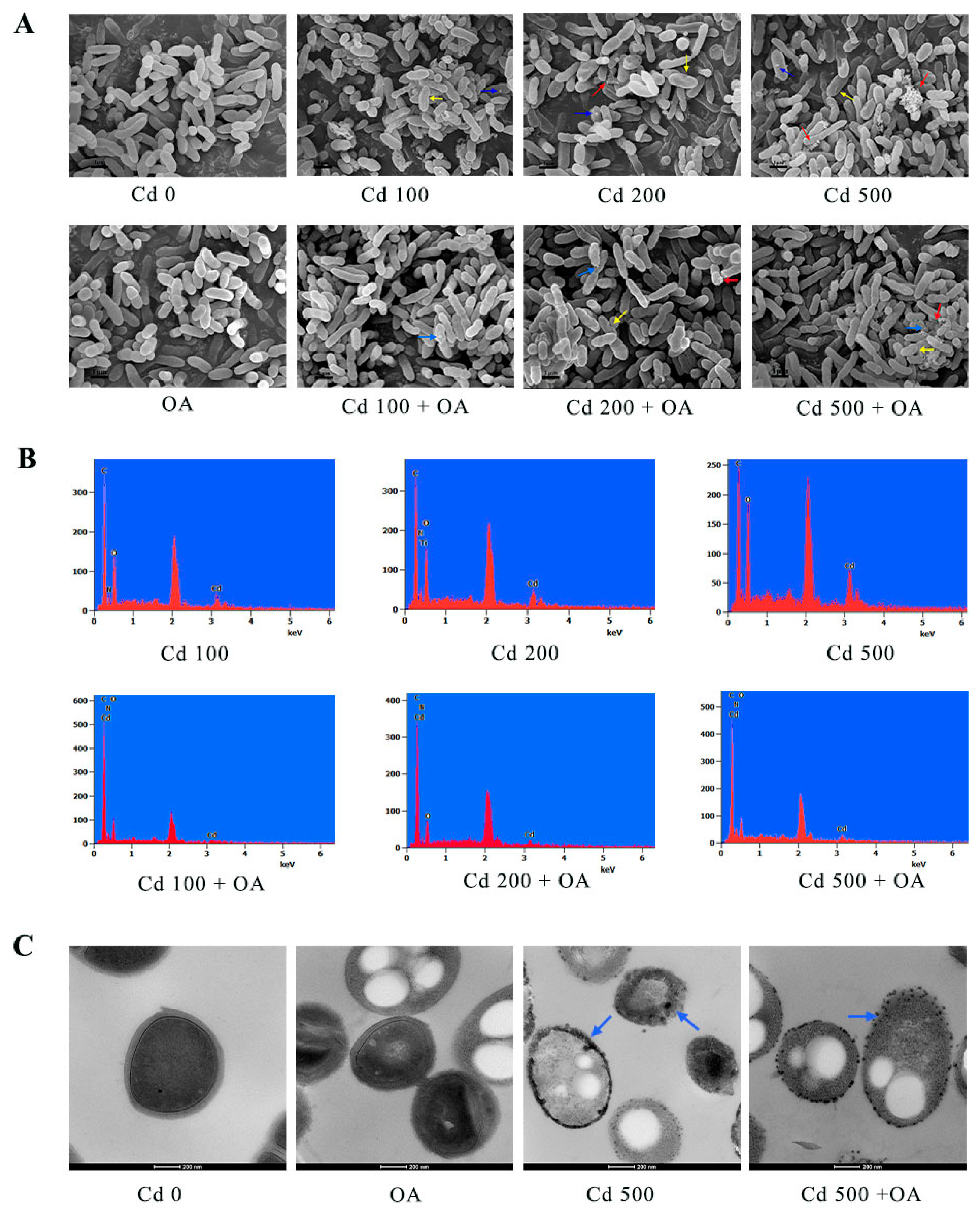
2.5. Analysis of the Cd Distribution in B. cepacia Using Electron Microscopy
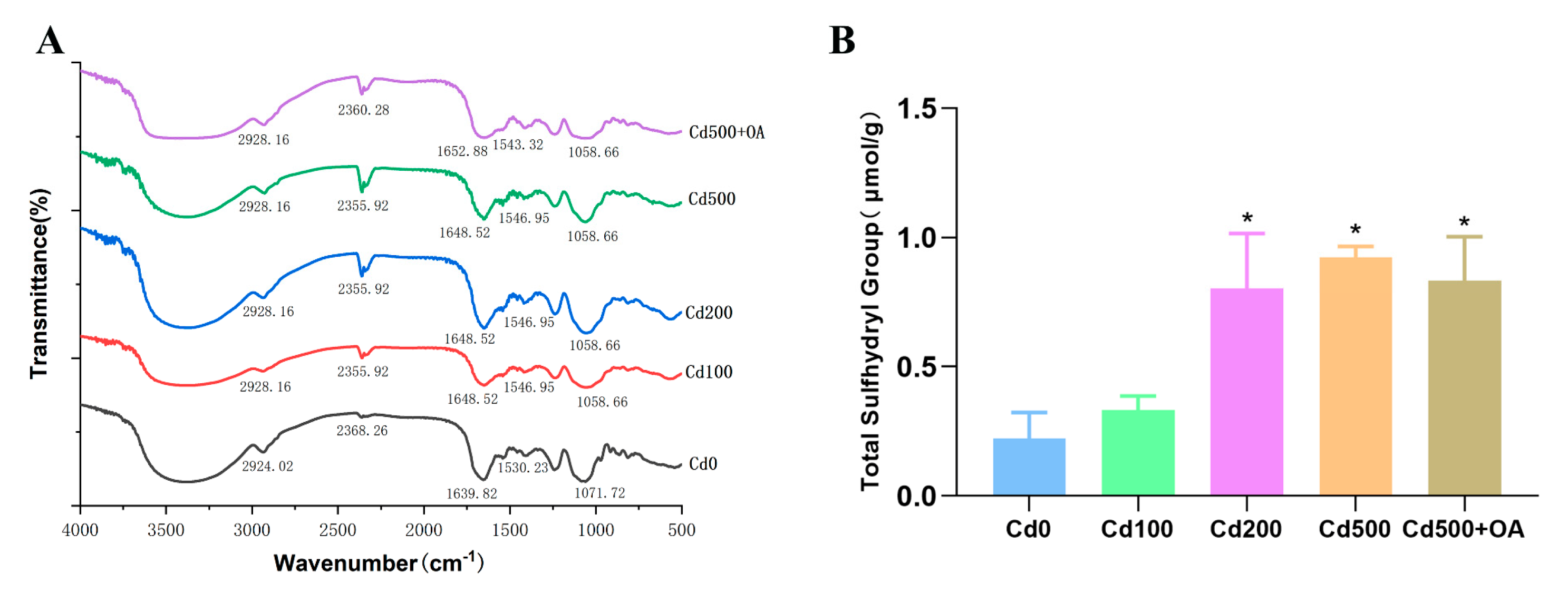
2.6. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of EPS Secreted by B. cepacia under Cd Stress
3. Discussion
3.1. OA Reduced the Cd Content by Facilitating Cd Excretion
3.2. OA Facilitated Cd Excretion by Increasing the Abundance of Gut Burkholderia
3.3. The Cd Absorptive Ability of B. cepacia and Its EPSs
3.4. The Cd Absorption Mechanism of the EPS of B. cepacia and Its Sulfhydryl Group
4. Materials and Methods
4.1. Materials and Reagents
4.2. Animals and Experimental Design
4.3. Determination of Cd Contents in Feces and Tissues
4.4. 16S rRNA Gene Sequencing of the Gut Microbiota
4.5. Cd Sensitivity Assay of B. cepacia by the Growth of B. cepacia Cells
4.6. Determination of Cd Contents Accumulated Intracellularly, Extracellularly, and in the Supernatant of B. cepacia
4.7. EPS Extraction
4.8. Electron Microscopy Analysis
4.9. Fourier Transform Infrared Spectrometer (FTIR) Assay
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bioremediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- RoyChowdhury, A.; Datta, R.; Sarkar, D. Heavy Metal Pollution and Remediation. In Green Chemistry; NUS Biological Sciences: Singapore, 2018; pp. 359–373. [Google Scholar]
- Han, C.; Zhu, Y.; Yang, Z.; Fu, S.; Zhang, W.; Liu, C. Protective effect of Polygonatum sibiricum against cadmium-induced testicular injury in mice through inhibiting oxidative stress and mitochondria-mediated apoptosis. J. Ethnopharmacol. 2020, 261, 113060. [Google Scholar] [CrossRef] [PubMed]
- Yazihan, N.; Kocak, M.K.; Akcil, E.; Erdem, O.; Sayal, A. Role of midkine in cadmium-induced liver, heart and kidney damage. Hum. Exp. Toxicol. 2011, 30, 391–397. [Google Scholar] [CrossRef]
- Hao, R.; Song, X.; Sun-Waterhouse, D.; Tan, X.; Li, F.; Li, D. MiR-34a/Sirt1/p53 signaling pathway contributes to cadmium-induced nephrotoxicity: A preclinical study in mice. Environ. Pollut. 2021, 282, 117029. [Google Scholar] [CrossRef] [PubMed]
- Johri, N.; Jacquillet, G.; Unwin, R. Heavy metal poisoning: The effects of cadmium on the kidney. BioMetals 2010, 23, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Park, J.D.; Cherrington, N.J.; Klaassen, C.D. Intestinal Absorption of Cadmium Is Associated with Divalent Metal Transporter 1 in Rats. Toxicol. Sci. 2002, 68, 288–294. [Google Scholar] [CrossRef] [PubMed]
- McMurray, C.T.; Tainer, J.A. Cancer, cadmium and genome integrity. Nat. Genet. 2003, 34, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Bolm-Audorff, U.; Faldum, A.; Janssen, K.; Reifenrath, M.; Götte, W.; Jung, D.; Mayer-Popken, O.; Fuchs, J.; Gebhard, S.; et al. Occupational exposure to heavy metals: DNA damage induction and DNA repair inhibition prove co-exposures to cadmium, cobalt and lead as more dangerous than hitherto expected. Carcinogenesis 2003, 24, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Purkayastha, D.; Mishra, U.; Biswas, S. A comprehensive review on Cd(II) removal from aqueous solution. J. Water Process Eng. 2014, 2, 105–128. [Google Scholar] [CrossRef]
- Fan, R.; Hu, P.C.; Wang, Y.; Lin, H.Y.; Su, K.; Feng, X.S.; Wei, L.; Yang, F. Betulinic acid protects mice from cadmium chloride-induced toxicity by inhibiting cadmium-induced apoptosis in kidney and liver. Toxicol. Lett. 2018, 299, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yin, H.; Yang, Z.; Tan, M.; Wang, F.; Chen, K.; Zuo, Z.; Shu, G.; Cui, H.; Ouyang, P.; et al. Vitamin E protects against cadmium-induced sub-chronic liver injury associated with the inhibition of oxidative stress and activation of Nrf2 pathway. Ecotoxicol. Environ. Saf. 2021, 208, 111610. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Fang, Z.; Sun, L.; Wang, Y.; Liu, Y.; Xu, D.; Nie, F.; Gooneratne, R. Oleic Acid Alleviates Cadmium-Induced Oxidative Damage in Rat by Its Radicals Scavenging Activity. Biol. Trace Elem. Res. 2019, 190, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Grawe, K.P.; Pickova, J.; Dutta, P.C.; Oskarsson, A. Fatty acid alterations in liver and milk of cadmium exposed rats and in brain of their suckling offspring. Toxicol. Lett. 2004, 148, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Nouairi, I.; Ghnaya, T.; Ben Youssef, N.; Zarrouk, M.; Habib Ghorbel, M. Changes in content and fatty acid profiles of total lipids of two halophytes: Sesuvium portulacastrum and Mesembryanthemum crystallinum under cadmium stress. J. Plant Physiol. 2006, 163, 1198–1202. [Google Scholar] [CrossRef] [PubMed]
- Inaba, T.; Kobayashi, E.; Suwazono, Y.; Uetani, M.; Oishi, M.; Nakagawa, H.; Nogawa, K. Estimation of cumulative cadmium intake causing Itai–itai disease. Toxicol. Lett. 2005, 159, 192–201. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Liu, K.; Shen, J. Exposing to cadmium stress cause profound toxic effect on microbiota of the mice intestinal tract. PLoS ONE 2014, 9, e85323. [Google Scholar] [CrossRef]
- Duan, H.; Yu, L.; Tian, F.; Zhai, Q.; Fan, L.; Chen, W. Gut microbiota: A target for heavy metal toxicity and a probiotic protective strategy. Sci. Total Environ. 2020, 742, 140429. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.; Hassanzadeh, P.; Alaei, S. Cadmium chloride exhibits a profound toxic effect on bacterial microflora of the mice gastrointestinal tract. Hum. Exp. Toxicol. 2011, 30, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, Y.; Zeng, Z.; Liu, Z.; Fu, Z. Subchronic Exposure of Mice to Cadmium Perturbs Their Hepatic Energy Metabolism and Gut Microbiome. Chem. Res. Toxicol. 2015, 28, 2000–2009. [Google Scholar] [CrossRef]
- Tinkov, A.A.; Gritsenko, V.A.; Skalnaya, M.G.; Cherkasov, S.V.; Aaseth, J.; Skalny, A.V. Gut as a target for cadmium toxicity. Environ. Pollut. 2018, 235, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, W.; Sun, Y.; Liu, J.; Zhang, W. Effects of cadmium on organ function, gut microbiota and its metabolomics profile in adolescent rats. Ecotoxicol. Environ. Saf. 2021, 222, 112501. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Breton, J.; Daniel, C.; Dewulf, J.; Pothion, S.; Froux, N.; Sauty, M.; Thomas, P.; Pot, B.; Foligne, B. Gut microbiota limits heavy metals burden caused by chronic oral exposure. Toxicol. Lett. 2013, 222, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, Y.; Huang, Y.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Influence of oral administration of Akkermansia muciniphila on the tissue distribution and gut microbiota composition of acute and chronic cadmium exposure mice. FEMS Microbiol. Lett. 2019, 366, fnz160. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Hou, M.; Liu, C.; Li, J.; Ba, Q.; Wang, H. Cadmium accelerates bacterial oleic acid production to promote fat accumulation in Caenorhabditis elegans. J. Hazard. Mater. 2022, 421, 126723. [Google Scholar] [CrossRef]
- Agans, R.; Gordon, A.; Kramer, D.L.; Perez-Burillo, S.; Rufián-Henares, J.A.; Paliy, O. Dietary Fatty Acids Sustain the Growth of the Human Gut Microbiota. Appl. Environ. Microbiol. 2018, 84, e01525-18. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Chang, S.; Liu, S.; Peng, L.; Xie, J.; Dong, W.; Tian, Y.; Sheng, J. Correlations between α-Linolenic Acid-Improved Multitissue Homeostasis and Gut Microbiota in Mice Fed a High-Fat Diet. mSystems 2020, 5, e00391-20. [Google Scholar] [CrossRef]
- Shrestha, N.; Sleep, S.L.; Cuffe, J.S.M.; Holland, O.J.; McAinch, A.J.; Nitert, M.D.; Hryciw, D.H. Pregnancy and diet-related changes in the maternal gut microbiota following exposure to an elevated linoleic acid diet. Am. J. Physiol-Endoc. Metab. 2020, 318, E276–E285. [Google Scholar] [CrossRef]
- Serrato, R.V.; Sassaki, G.L.; Gorin, P.A.; Cruz, L.M.; Pedrosa, F.O.; Choudhury, B.; Carlson, R.W.; Iacomini, M. Structural characterization of an acidic exoheteropolysaccharide produced by the nitrogen-fixing bacterium Burkholderia tropica. Carbohydr. Polym. 2008, 73, 564–572. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Zeng, Y.; Zhang, J.; Lu, G.; Dang, Z.; Guo, C. Bioaccumulation and distribution of cadmium by Burkholderia cepacia GYP1 under oligotrophic condition and mechanism analysis at proteome level. Ecotoxicol. Environ. Saf. 2019, 176, 162–169. [Google Scholar] [CrossRef]
- Gan, L.; Zhou, F.; Owens, G.; Chen, Z. Burkholderia cepacia immobilized on eucalyptus leaves used to simultaneously remove malachite green (MG) and Cr(VI). Colloids Surf. B. Biointerfaces 2018, 172, 526–531. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; El-Khatib, R.M.; Abdel-Fatah, S.M. Some new nano-sized Fe(II), Cd(II) and Zn(II) Schiff base complexes as precursor for metal oxides: Sonochemical synthesis, characterization, DNA interaction, in vitro antimicrobial and anticancer activities. Bioorg. Chem. 2016, 69, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, L.H.; Ismail, N.M.; Ismael, M.; Abu-Dief, A.M.; Ahmed, E.A.-H. Synthesis, characterization, DFT calculations and biological studies of Mn(II), Fe(II), Co(II) and Cd(II) complexes based on a tetradentate ONNO donor Schiff base ligand. J. Mol. Struct. 2017, 1134, 851–862. [Google Scholar] [CrossRef]
- Khalaf, M.M.; Abd El-Lateef, H.M.; Gouda, M.; Sayed, F.N.; Mohamed, G.G.; Abu-Dief, A.M. Design, Structural Inspection and Bio-Medicinal Applications of Some Novel Imine Metal Complexes Based on Acetylferrocene. Materials 2022, 15, 4842. [Google Scholar] [CrossRef] [PubMed]
- Fein, J.B.; Yu, Q.; Nam, J.; Yee, N. Bacterial cell envelope and extracellular sulfhydryl binding sites: Their roles in metal binding and bioavailability. Chem. Geol. 2019, 521, 28–38. [Google Scholar] [CrossRef]
- Nouairi, I.; Ammar, W.B.; Youssef, N.B.; Daoud, D.B.M.; Ghorbal, M.H.; Zarrouk, M. Comparative study of cadmium effects on membrane lipid composition of Brassica juncea and Brassica napus leaves. Plant Sci. 2006, 170, 511–519. [Google Scholar] [CrossRef]
- Omotoso, O.; Owolabi, J.; Akpan, H.; Dare, B.; Ashamu, E.; Adelakun, S. Positive Anti-Cadmium Poisoning Anti-Oxidant Effects of Moringa Seed Oil and Cashew Nut Oil in Juvenile Wistar Rats Hippocampus. J. Adv. Med. Pharm. Sci. 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Shin, S.; Lim, Y.; Chung, J.; Park, S.; Han, S.N. Effect of Korean pine nut oil on hepatic iron, copper, and zinc status and expression of genes and proteins related to iron absorption in diet-induced obese mice. J. Nutr. Health 2021, 54, 435–447. [Google Scholar] [CrossRef]
- Shafaei, N.; Barkhordar, S.M.A.; Rahmani, F.; Nabi, S.; Idliki, R.B.; Alimirzaei, M.; Karimi, E.; Oskoueian, E. Protective Effects of Anethum graveolens Seed’s Oil Nanoemulsion Against Cadmium-Induced Oxidative Stress in Mice. Biol. Trace Elem. Res. 2020, 198, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liang, X.; Lei, C.; Huang, Q.; Song, W.; Fang, R.; Li, C.; Li, X.; Mo, H.; Sun, N.; et al. High-Fat Diet Affects Heavy Metal Accumulation and Toxicity to Mice Liver and Kidney Probably via Gut Microbiota. Front. Microbiol. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Pal, P.K.; Chattopadhyay, A.; Bandyopadhyay, D. Oleic acid protects against cadmium induced cardiac and hepatic tissue injury in male Wistar rats: A mechanistic study. Life Sci. 2020, 244, 117324. [Google Scholar] [CrossRef]
- He, X.; Qi, Z.; Hou, H.; Qian, L.; Gao, J.; Zhang, X.X. Structural and functional alterations of gut microbiome in mice induced by chronic cadmium exposure. Chemosphere 2020, 246, 125747. [Google Scholar] [CrossRef]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Chen, Z.; Wang, S.; Shi, P.; Shen, Y.; Zhang, Y.; Xiao, J.; Huang, Z. Overexpression of OLE1 enhances cytoplasmic membrane stability and confers resistance to cadmium in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017, 83, e02319-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Yu, Y.; Fang, Z.; Deng, Y.; Shen, Y.; Shi, P. OLE1 reduces cadmium-induced oxidative damage in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 2018, 365, fny193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Huang, Y.; Yang, X.; Xue, W.; Zhang, X.; Zhang, Y.; Pang, J.; Liu, Y.; Liu, Z. Burkholderia sp. Y4 inhibits cadmium accumulation in rice by increasing essential nutrient uptake and preferentially absorbing cadmium. Chemosphere 2020, 252, 126603. [Google Scholar] [CrossRef]
- You, L.-X.; Zhang, R.-R.; Dai, J.-X.; Lin, Z.-T.; Li, Y.-P.; Herzberg, M.; Zhang, J.-L.; Al-Wathnani, H.; Zhang, C.-K.; Feng, R.-W.; et al. Potential of cadmium resistant Burkholderia contaminans strain ZCC in promoting growth of soy beans in the presence of cadmium. Ecotoxicol. Environ. Saf. 2021, 211, 111914. [Google Scholar] [CrossRef]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice indicate routes of protection besides intestinal sequestration. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef] [Green Version]
- Beveridge, T.J. Role of cellular design in bacterial metal accumulation and mineralization. Annu. Rev. Microbiol. 1989, 43, 147–171. [Google Scholar] [CrossRef]
- Chakravarty, R.; Banerjee, P.C. Mechanism of cadmium binding on the cell wall of an acidophilic bacterium. Bioresour. Technol. 2012, 108, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Luo, X.; Liu, S.; Wan, W.; Huang, Q.; Chen, W. Synergistic effect of biofilm growth and cadmium adsorption via compositional changes of extracellular matrix in montmorillonite system. Bioresour. Technol. 2020, 315, 123742. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Wei, J.; Ma, K.; Zhang, J.; Yang, Y.; Yu, S. Extracellular polymeric substances, microbial activity and microbial community of biofilm and suspended sludge at different divalent cadmium concentrations. Bioresour. Technol. 2016, 205, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Pan, X.; Mostofa, K.M.G.; Chen, X.; Mu, G.; Wu, F.; Liu, J.; Song, W.; Yang, J.; Liu, Y.; et al. Complexation between Hg(II) and biofilm extracellular polymeric substances: An application of fluorescence spectroscopy. J. Hazard. Mater. 2010, 175, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.W.; Miller, C.D.; Dimkpa, C.O.; Anderson, A.J.; McLean, J.E. Defining the surface adsorption and internalization of copper and cadmium in a soil bacterium, Pseudomonas putida. Chemosphere 2010, 81, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Liu, X.; Huang, Z.; Niu, S.; Xu, T.; Zeng, J.; Li, H.; Wang, T.; Gao, Y.; et al. Physiological, biochemical and proteomic insight into integrated strategies of an endophytic bacterium Burkholderia cenocepacia strain YG-3 response to cadmium stress. Metallomics 2019, 11, 1252–1264. [Google Scholar] [CrossRef]
- Hansda, A.; Kumar, V. Anshumali A comparative review towards potential of microbial cells for heavy metal removal with emphasis on biosorption and bioaccumulation. World J. Microbiol. Biotechnol. 2016, 32, 170. [Google Scholar] [CrossRef]
- Fein, J.B.; Daughney, C.J.; Yee, N.; Davis, T.A. A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim. Cosmochim. Acta 1997, 61, 3319–3328. [Google Scholar] [CrossRef]
- Holmes, J.D.; Smith, P.R.; Evans-Gowing, R.; Richardson, D.J.; Russell, D.A.; Sodeau, J.R. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch. Microbiol. 1995, 163, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Guo, C.-L.; Lu, G.-N.; Yi, X.-Y.; Zhu, L.-D.; Dang, Z. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere 2014, 109, 134–142. [Google Scholar] [CrossRef]
- Guibaud, G.; Comte, S.; Bordas, F.; Dupuy, S.; Baudu, M. Comparison of the complexation potential of extracellular polymeric substances (EPS), extracted from activated sludges and produced by pure bacteria strains, for cadmium, lead and nickel. Chemosphere 2005, 59, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Kumar, R.; Yadav, P.; Singh, A.; Yadav, A.; Chowdhary, P.; Raj, A. Biofilm formation and extracellular polymeric substance (EPS) production by Bacillus haynesii and influence of hexavalent chromium. Bioresour. Technol. 2022, 352, 127109. [Google Scholar] [CrossRef] [PubMed]
- Raj, R.; Dalei, K.; Chakraborty, J.; Das, S. Extracellular polymeric substances of a marine bacterium mediated synthesis of CdS nanoparticles for removal of cadmium from aqueous solution. J. Colloid Interface Sci. 2016, 462, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, J.; Das, S. Characterization and cadmium-resistant gene expression of biofilm-forming marine bacterium Pseudomonas aeruginosa JP-11. Environ. Sci. Pollut. Res. 2014, 21, 14188–14201. [Google Scholar] [CrossRef] [PubMed]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and Redox Modulation of Cysteine Protein Function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalups, R.K.; Ahmad, S. Molecular handling of cadmium in transporting epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188. [Google Scholar] [CrossRef]
- Pérez-Rama, M.; Abalde Alonso, J.; Herrero López, C.; Torres Vaamonde, E. Cadmium removal by living cells of the marine microalga Tetraselmis suecica. Bioresour. Technol. 2002, 84, 265–270. [Google Scholar] [CrossRef]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral Administration of Probiotics Inhibits Absorption of the Heavy Metal Cadmium by Protecting the Intestinal Barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Deng, Y.; Deng, Q.; Sun, D.; Fang, Z.; Sun, L.; Wang, Y.; Gooneratne, R. Vibrio parahaemolyticus Infection in Mice Reduces Protective Gut Microbiota, Augmenting Disease Pathways. Front. Microbiol. 2020, 11, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, L.; Devi, P.; Divya Shridhar, M.P.; Naik, C.G. Use of Fourier Transform Infrared (FTIR) Spectroscopy to Study Cadmium-Induced Changes in Padina Tetrastromatica (Hauck). Anal. Chem. Insights 2008, 3, 135–143. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.; Chen, Y.; Li, Y.; Sun, L.; Deng, Q.; Wang, J.; Gooneratne, R. Oleic Acid Facilitates Cd Excretion by Increasing the Abundance of Burkholderia in Cd-Exposed Mice. Int. J. Mol. Sci. 2022, 23, 14718. https://doi.org/10.3390/ijms232314718
Fang Z, Chen Y, Li Y, Sun L, Deng Q, Wang J, Gooneratne R. Oleic Acid Facilitates Cd Excretion by Increasing the Abundance of Burkholderia in Cd-Exposed Mice. International Journal of Molecular Sciences. 2022; 23(23):14718. https://doi.org/10.3390/ijms232314718
Chicago/Turabian StyleFang, Zhijia, Yinyan Chen, Yongbin Li, Lijun Sun, Qi Deng, Jingwen Wang, and Ravi Gooneratne. 2022. "Oleic Acid Facilitates Cd Excretion by Increasing the Abundance of Burkholderia in Cd-Exposed Mice" International Journal of Molecular Sciences 23, no. 23: 14718. https://doi.org/10.3390/ijms232314718
APA StyleFang, Z., Chen, Y., Li, Y., Sun, L., Deng, Q., Wang, J., & Gooneratne, R. (2022). Oleic Acid Facilitates Cd Excretion by Increasing the Abundance of Burkholderia in Cd-Exposed Mice. International Journal of Molecular Sciences, 23(23), 14718. https://doi.org/10.3390/ijms232314718






