Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells
Abstract
:1. Introduction
2. Results
2.1. Efficiency of FASN Overexpression and Interference
2.2. Metabolomics Analysis
2.3. Expression Profile of DEGs
2.4. Functional Enrichment Analysis of DEGs
2.5. Network Analysis for DEGs in FASN-Overexpressed phGCs and hGCs
2.6. Combined Metabolome and Transcriptome Analysis
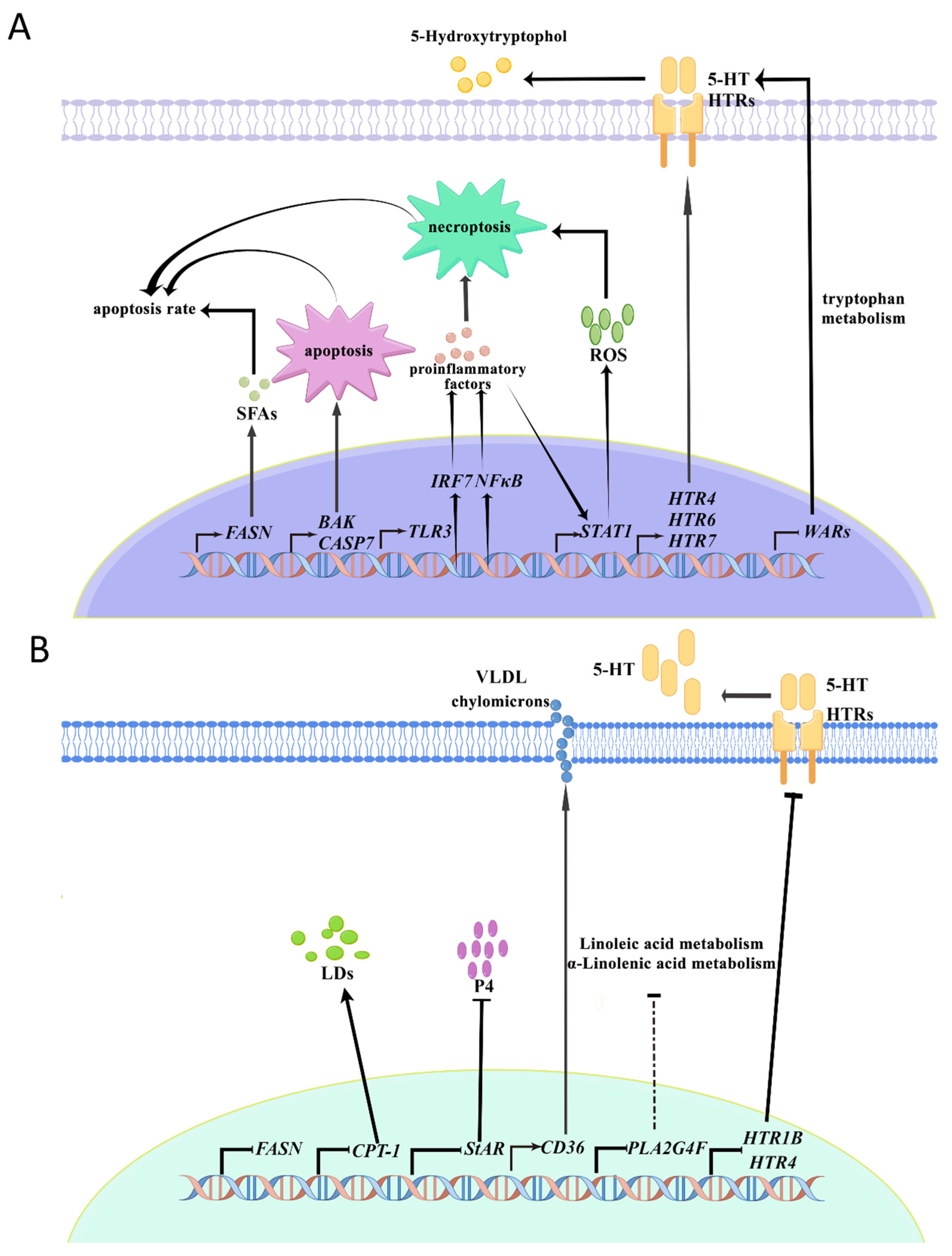
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. GC Culture and Transfection
4.3. Metabolomics Detection
4.4. Metabolites Identification
4.5. Differential Metabolite Analysis
4.6. RNA-Seq and Functional Analysis of the DEGs
4.7. qRT-PCR for Validation of the DEGs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, R.; Gan, X.; Hu, S.; Gao, S.; Deng, Y.; Qiu, J.; Sun, W.; Li, L.; Han, C.; Hu, J.; et al. Evidence for the existence of de novo lipogenesis in goose granulosa cells. Poult. Sci. 2019, 98, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Elis, S.; Desmarchais, A.; Maillard, V.; Uzbekova, S.; Monget, P.; Dupont, J. Cell proliferation and progesterone synthesis depend on lipid metabolism in bovine granulosa cells. Theriogenology 2015, 83, 840–853. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, T.; Leroy, J.; Van Soom, A.; Opsomer, G.; Maes, D.; Coryn, M.; de Kruif, A. Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim. Reprod. Sci. 2005, 87, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Yanase, T.; Nishi, Y.; Tanaka, A.; Saito, M.; Jin, C.H.; Mukasa, C.; Okabe, T.; Nomura, M.; Goto, K.; et al. Saturated FFAs, Palmitic Acid and Stearic Acid, Induce Apoptosis in Human Granulosa Cells. Endocrinology 2001, 142, 3590–3597. [Google Scholar] [CrossRef]
- Chen, X.; Huang, K.; Hu, S.; Lan, G.; Gan, X.; Gao, S.; Deng, Y.; Hu, J.; Li, L.; Hu, B.; et al. FASN-Mediated Lipid Metabolism Regulates Goose Granulosa Cells Apoptosis and Steroidogenesis. Front. Physiol. 2020, 11, 600. [Google Scholar] [CrossRef]
- Griffiths, W.; Wang, Y. Mass spectrometry: From proteomics to metabolomics and lipidomics. Chem. Soc. Rev. 2009, 38, 1882–1896. [Google Scholar] [CrossRef]
- Weatherill, A.; Lee, J.; Zhao, L.; Lemay, D.; Youn, H.; Hwang, D. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. J. Immunol. 2005, 174, 5390–5397. [Google Scholar] [CrossRef] [Green Version]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.; Hoffmann, J. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Frommer, K.; Schäffler, A.; Rehart, S.; Lehr, A.; Müller-Ladner, U.; Neumann, E. Free fatty acids: Potential proinflammatory mediators in rheumatic diseases. Ann. Rheum. Dis. 2015, 74, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Q.; Hu, S.; Wang, G.; Hu, J.; Zhang, J.; Li, L.; Hu, B.; He, H.; Liu, H.; Xia, L.; et al. Comparative Transcriptome Analysis Suggests Key Roles for 5-Hydroxytryptamlne Receptors in Control of Goose Egg Production. Genes 2020, 11, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmassi, A.; Lü, S.; Hedderich, J.; Oettinghaus, C.; Jonat, W.; Mettler, L. Interaction of interleukin-6 on human granulosa cell steroid secretion. J. Endocrinol. 2001, 170, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, K.; Cheng, L.; Liu, P.; Liu, Z.; Zhao, S.; Zhu, W.; Wang, Q.; Wu, H.; Han, D. Polyinosinic-Polycytidylic Acid Perturbs Ovarian Functions Through Toll-Like Receptor 3-Mediated Tumor Necrosis Factor A Production in Female Mice. Biol. Reprod. 2015, 93, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benco, A.; Sirotkin, A.V.; Vasícek, D.; Pavlová, S.; Zemanová, J.; Kotwica, J.; Darlak, K.; Valenzuela, F. Involvement of the transcription factor STAT1 in the regulation of porcine ovarian granulosa cell functions treated and not treated with ghrelin. Reproduction 2009, 138, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Yu, M.; Hao, C.; Yang, W. Shikonin induces tumor apoptosis in glioma cells via endoplasmic reticulum stress, and Bax/Bak mediated mitochondrial outer membrane permeability. J. Ethnopharmacol. 2020, 263, 113059. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2017, 25, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Dadsena, S.; King, L.E.; García-Sáez, A. Apoptosis regulation at the mitochondria membrane level. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183716. [Google Scholar] [CrossRef] [PubMed]
- Strohmeier, K.; Hofmann, M.; Jacak, J.; Narzt, M.; Wahlmueller, M.; Mairhofer, M.; Schaedl, B.; Holnthoner, W.; Barsch, M.; Sandhofer, M.; et al. Multi-Level Analysis of Adipose Tissue Reveals the Relevance of Perivascular Subpopulations and an Increased Endothelial Permeability in Early-Stage Lipedema. Biomedicines 2022, 10, 1163. [Google Scholar] [CrossRef] [PubMed]
- Conley, A.; Legacki, E.; Corbin, C.; Stanley, S.; Dahlen, C.; Reynolds, L. Serum and tissue pregnanes and pregnenes after dexamethasone treatment of cows in late gestation. Reproduction 2019, 157, 413–422. [Google Scholar] [CrossRef]
- Das, U. “Cell Membrane Theory of Senescence” and the Role of Bioactive Lipids in Aging, and Aging Associated Diseases and Their Therapeutic Implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, L.; Han, K.; Zhang, X.; Zhang, G.; Dai, G.; Wang, J.; Xie, K. Transcriptome analysis of ovary in relatively greater and lesser egg producing Jinghai Yellow Chicken. Anim. Reprod. Sci. 2019, 208, 106114. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, D.; Zhou, N.; Lin, Y.; Che, L.; Fang, Z.; Wu, D. Reproductive Hormone and Transcriptomic Responses of Pituitary Tissue in Anestrus Gilts Induced by Nutrient Restriction. PLoS ONE 2015, 10, e0143219. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Guo, X.; Wang, Y.; Wang, Y.; Cao, G.; Jiang, Y. Genome-Wide Analysis on the Landscape of Transcriptomes and Their Relationship With DNA Methylomes in the Hypothalamus Reveals Genes Related to Sexual Precocity in Jining Gray Goats. Front. Endocrinol. 2018, 9, 501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berger, M.; Gray, J.; Roth, B. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.W. Effects of free fatty acids on serotonin 2C receptor and calcium homeostasis in pancreatic beta cells. In Proceedings of the Annual Meeting of The Japanese Pharmacological Society, Kyoto, Japan, 4 July 2018; 2018. WCP2018, PO2-7-17. [Google Scholar]
- Deng, Y.; Gan, X.; Chen, D.; Huang, H.; Yuan, J.; Qiu, J.; Hu, S.; Hu, J.; Wang, J. Comparison of growth characteristics of in vitro cultured granulosa cells from geese follicles at different developmental stages. Biosci. Rep. 2018, 38, BSR20171361. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCt Method. Methods 2000, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
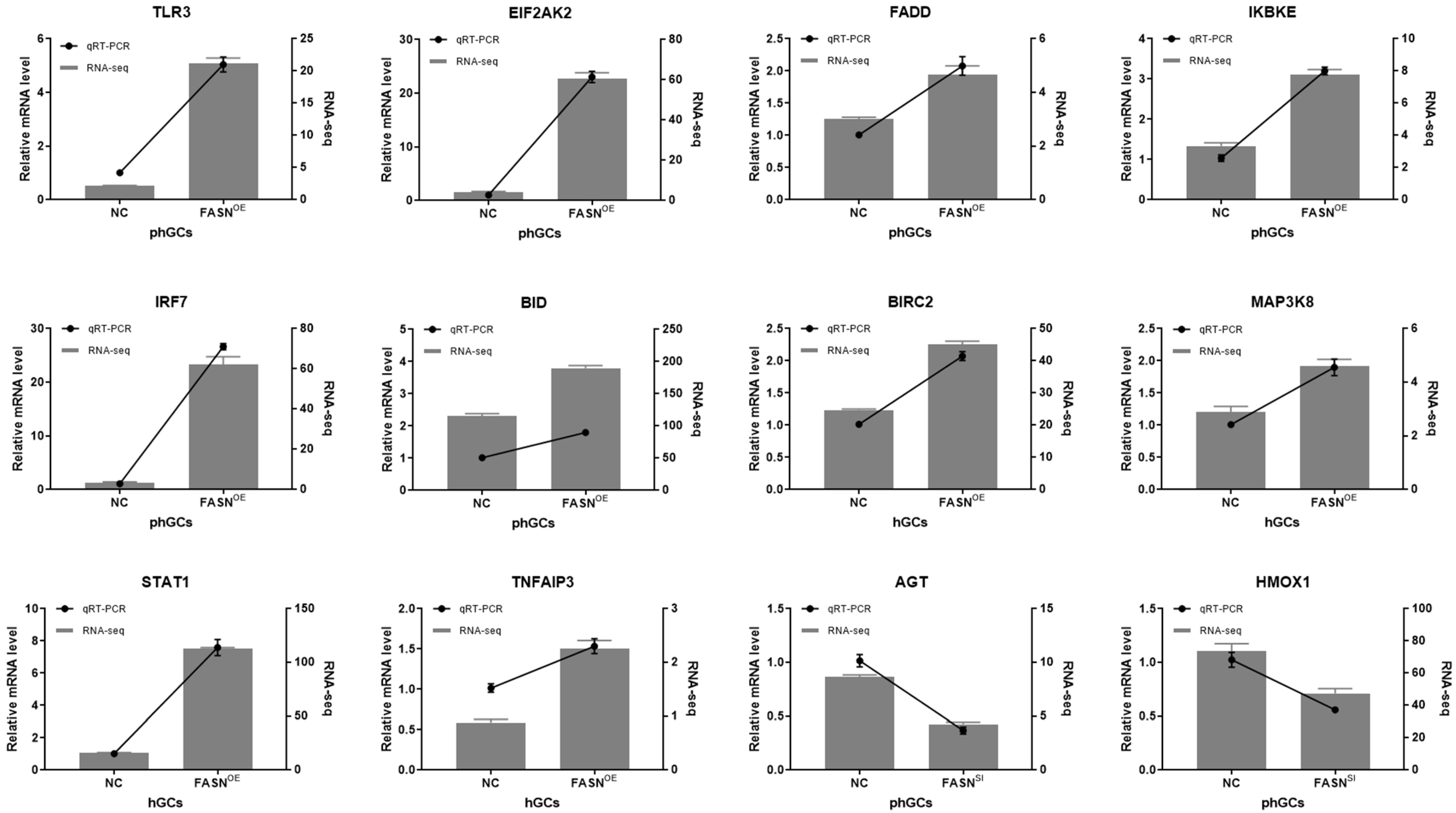
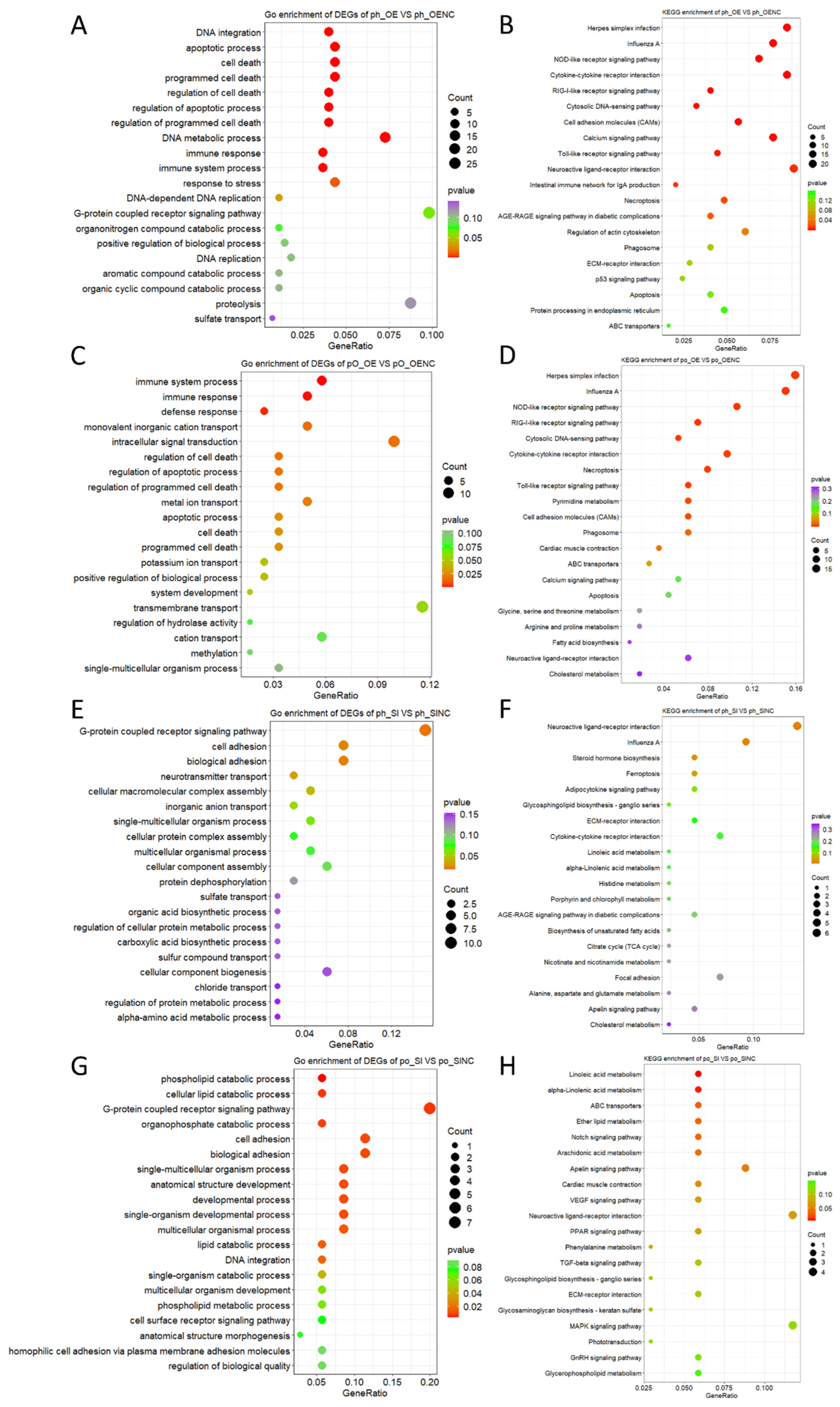
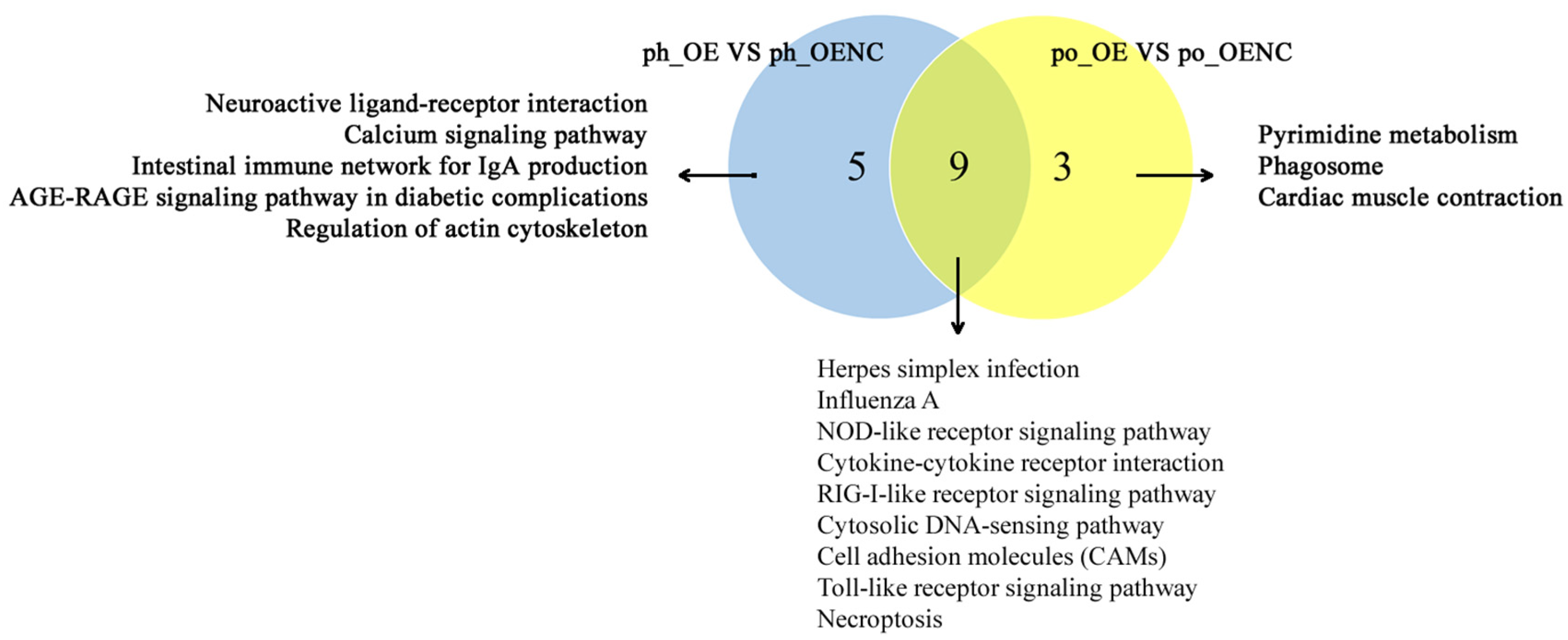
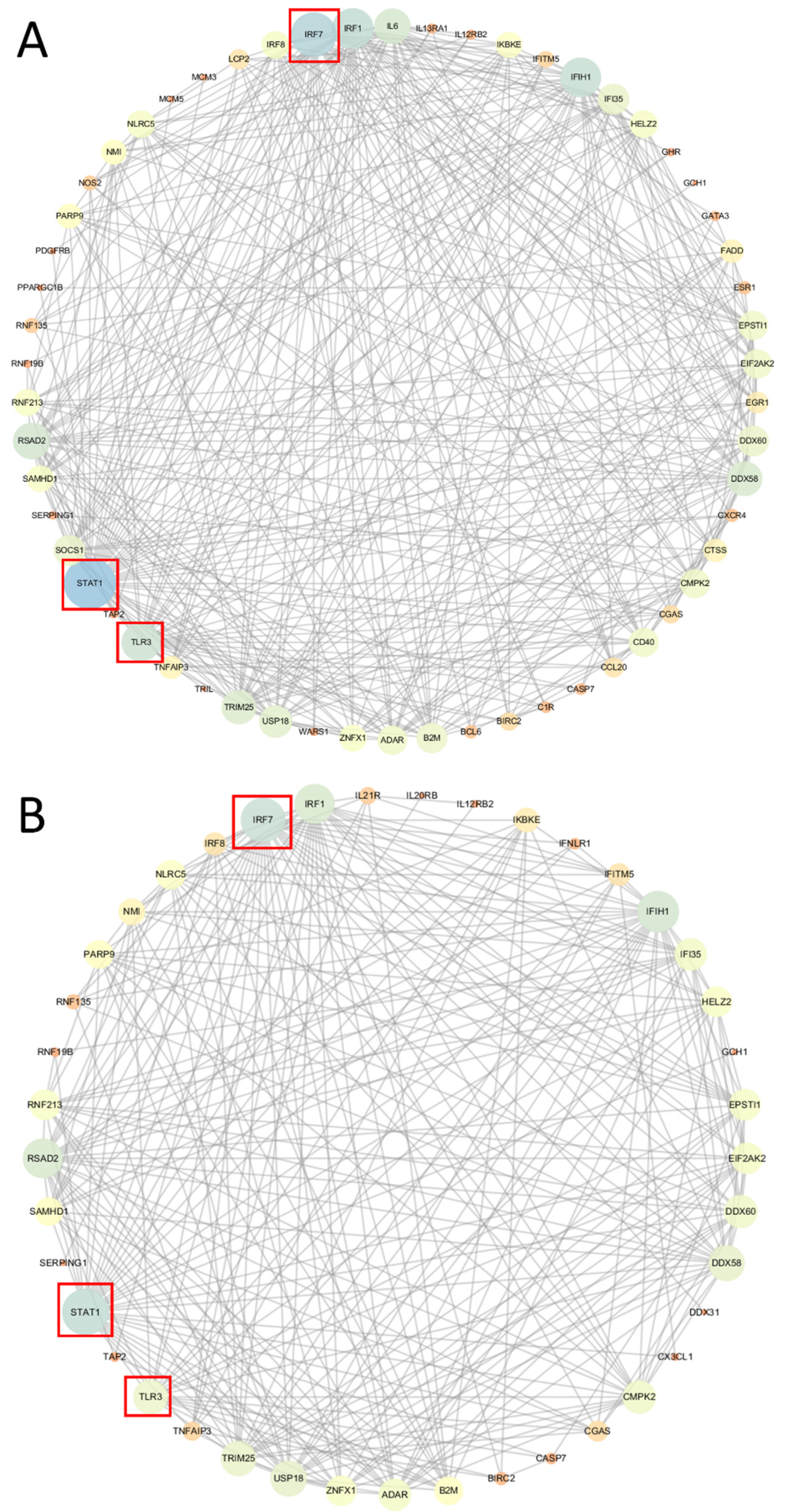
| Compared Samples | Num. of Total Sig. | Num. of Sig. Up | Num. of Sig. Down |
|---|---|---|---|
| ph_OE vs. ph_OENC | 63 | 59 | 4 |
| po_OE vs. po_OENC | 54 | 33 | 21 |
| ph_SI vs. ph_SINC | 36 | 19 | 17 |
| po_SI vs. po_SINC | 35 | 17 | 18 |
| Group | Map ID | Map Title | p-Value |
|---|---|---|---|
| ph_OE vs. ph_OENC | map00240 | Pyrimidine metabolism | 0.019931862 |
| map00520 | Amino sugar and nucleotide sugar metabolism | 0.042016807 | |
| map00480 | Glutathione metabolism | 0.031746032 | |
| ph_SI vs. ph_SINC | map00780 | Biotin metabolism | 0.015873016 |
| po_SI vs. po_SINC | map04726 | Serotonergic synapse | 0.001424299 |
| map04080 | Neuroactive ligand-receptor interaction | 0.001914757 | |
| map04540 | Gap junction | 0.004199855 | |
| map04742 | Taste transduction | 0.004199855 | |
| map04750 | Inflammatory mediator regulation of TRP channels | 0.004199855 | |
| map04024 | cAMP signaling pathway | 0.008255516 | |
| map04721 | Synaptic vesicle cycle | 0.008255516 | |
| map03320 | PPAR signaling pathway | 0.042016807 | |
| map04261 | Adrenergic signaling in cardiomyocytes | 0.042016807 | |
| map04270 | Vascular smooth muscle contraction | 0.042016807 | |
| map04924 | Renin secretion | 0.042016807 | |
| map04970 | Salivary secretion | 0.042016807 |
| Group | Num. of DEG | Num. of Sig. Up | Num. of Sig. Down |
|---|---|---|---|
| ph_OE vs. ph_OENC | 1099 | 724 | 375 |
| po_OE vs. po_OENC | 591 | 373 | 128 |
| ph_SI vs. ph_SINC | 224 | 102 | 122 |
| po_SI vs. po_SINC | 161 | 70 | 91 |
| Group | Experimental Group | Control Group |
|---|---|---|
| ph_OE vs. ph_OENC | phGCs transfected with pEGFP-N1-FASN | phGCs transfected with pEGFP-N1 |
| po_OE vs. po_OENC | hGCs transfected with pEGFP-N1-FASN | hGCs transfected with pEGFP-N1 |
| ph_SI vs. ph_SINC | phGCs transfected with siRNA-FASN | phGCs transfected with siRNA-NC |
| po_SI vs. po_SINC | hGCs transfected with siRNA-FASN | hGCs transfected with siRNA-NC |
| Genes | AccessionNumber | Primer (5′-3′) | Tm (°C) | Size (bp) |
|---|---|---|---|---|
| TLR3 | KC292270.1 | F:CCCCCAGACTGAAGGAGGTAR:TGACATTGCTTTCCGACCGA | 63 | 129 |
| EIF2AK2 | XM_013170695.1 | F:GACCTCACCATGTGGACCAGR:CTTCTTTTGCAGCGGCTTGT | 63 | 227 |
| FADD | XM_013194898.1 | F:ACCATGGACCCGTTTCTGACR:AGGAAGTTGAAGAGCTCCGC | 63 | 149 |
| IKBKE | XM_013189616.1 | F:GGAGCATCGAGTGGAGCTAC R:TCGAACCCCCAGCATTTCTC | 65 | 124 |
| IRF7 | XM_013174398.1 | F:CAGCAGCGACATCGAGATCTR:TCGCTGTTCTTGGAGTGGTC | 63 | 156 |
| BID | XM_013172977.1 | F:CGAGAGAAGGCCATGTTGGTR:GCTGGAGATCTCTCACTCGC | 63 | 162 |
| BIRC2 | XM_013178135.1 | F:CTCTGGTACCTGGAGCTCCT R:AGAATGGACTCACTGCTGGC | 63 | 179 |
| MAP3K8 | XM_013191750.1 | F:ATTCCTCGGGGAGCTTTTGGR:GATGGATCGTCTCGTCCCAC | 63 | 184 |
| STAT1 | XM_013187488.1 | F:GGATCTCAAAACGAGCCCCAR:ATGTAGCCGTTTCCCTTGGG | 63 | 245 |
| TNFAIP3 | XM_013175520.1 | F:GACAGCCCGAAAGAACAGGAR:CACAGCTCCTCACTTCTGCA | 63 | 107 |
| AGT | XM_048045900.1 | F:AAACAGGGATGAGGAGCTGCR:TGGCCTCCACAAAGGTGTTT | 63 | 182 |
| HMOX1 | XM_013181078.2 | F:GGAGGAGGCCAAGAAAGCATR:GCCTTTGACTGCACAAGGTG | 63 | 105 |
| β-actin * | M26111.1 | F:CAACGAGCGGTTCAGGTGTR:TGGAGTTGAAGGTGGTCTCG | 60 | 170 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Huang, K.; Hu, S.; Lan, G.; Gan, X.; Gao, S.; Deng, Y.; Hu, J.; Li, L.; Hu, B.; et al. Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 14717. https://doi.org/10.3390/ijms232314717
Chen X, Huang K, Hu S, Lan G, Gan X, Gao S, Deng Y, Hu J, Li L, Hu B, et al. Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells. International Journal of Molecular Sciences. 2022; 23(23):14717. https://doi.org/10.3390/ijms232314717
Chicago/Turabian StyleChen, Xi, Kailiang Huang, Shenqiang Hu, Gang Lan, Xiang Gan, Shanyan Gao, Yan Deng, Jiwei Hu, Liang Li, Bo Hu, and et al. 2022. "Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells" International Journal of Molecular Sciences 23, no. 23: 14717. https://doi.org/10.3390/ijms232314717
APA StyleChen, X., Huang, K., Hu, S., Lan, G., Gan, X., Gao, S., Deng, Y., Hu, J., Li, L., Hu, B., He, H., Liu, H., Xia, L., & Wang, J. (2022). Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells. International Journal of Molecular Sciences, 23(23), 14717. https://doi.org/10.3390/ijms232314717






