Novel ROCK Inhibitors, Sovesudil and PHP-0961, Enhance Proliferation, Adhesion and Migration of Corneal Endothelial Cells
Abstract
:1. Introduction
2. Results
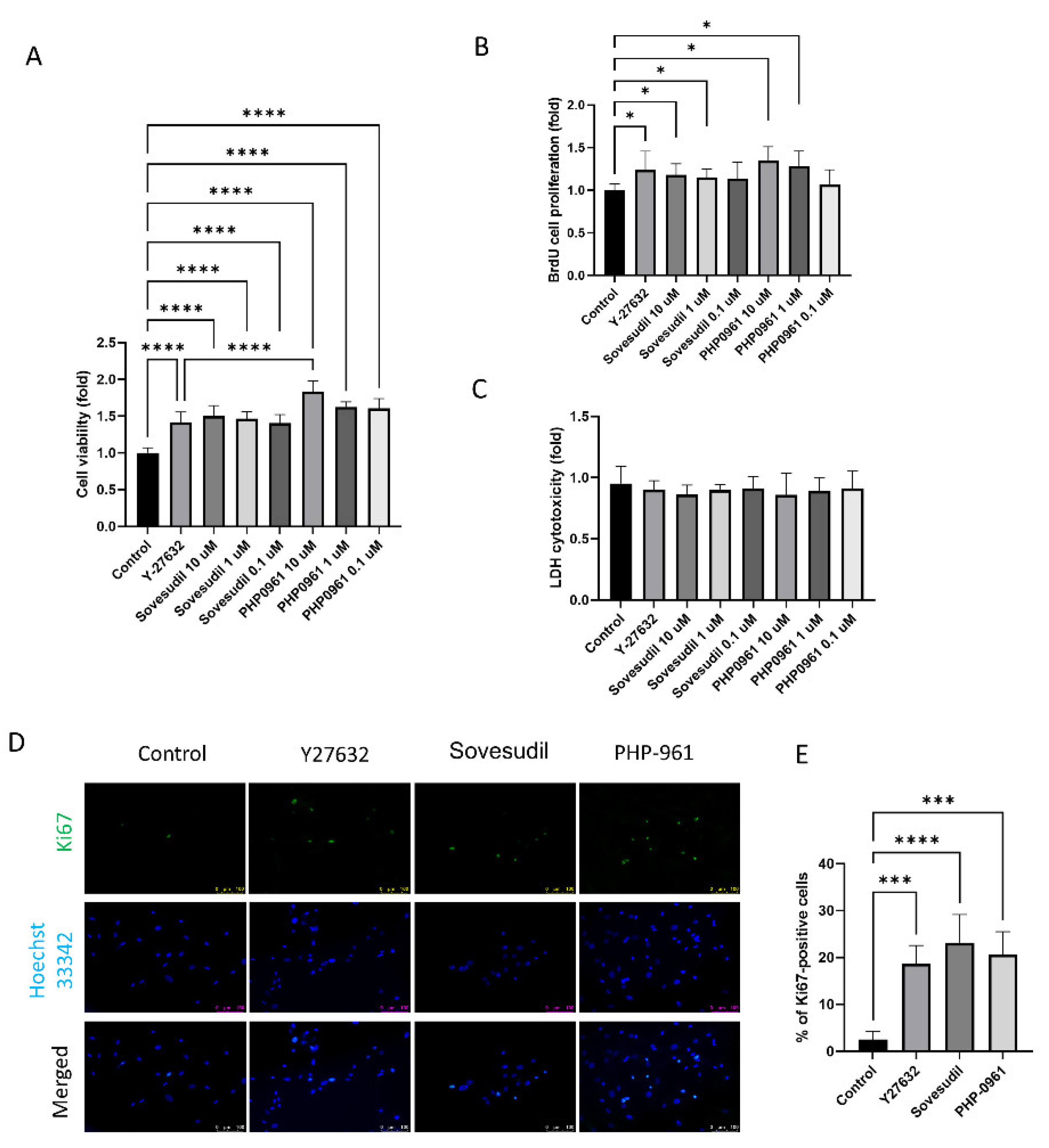
2.1. Effect on Cell Proliferation
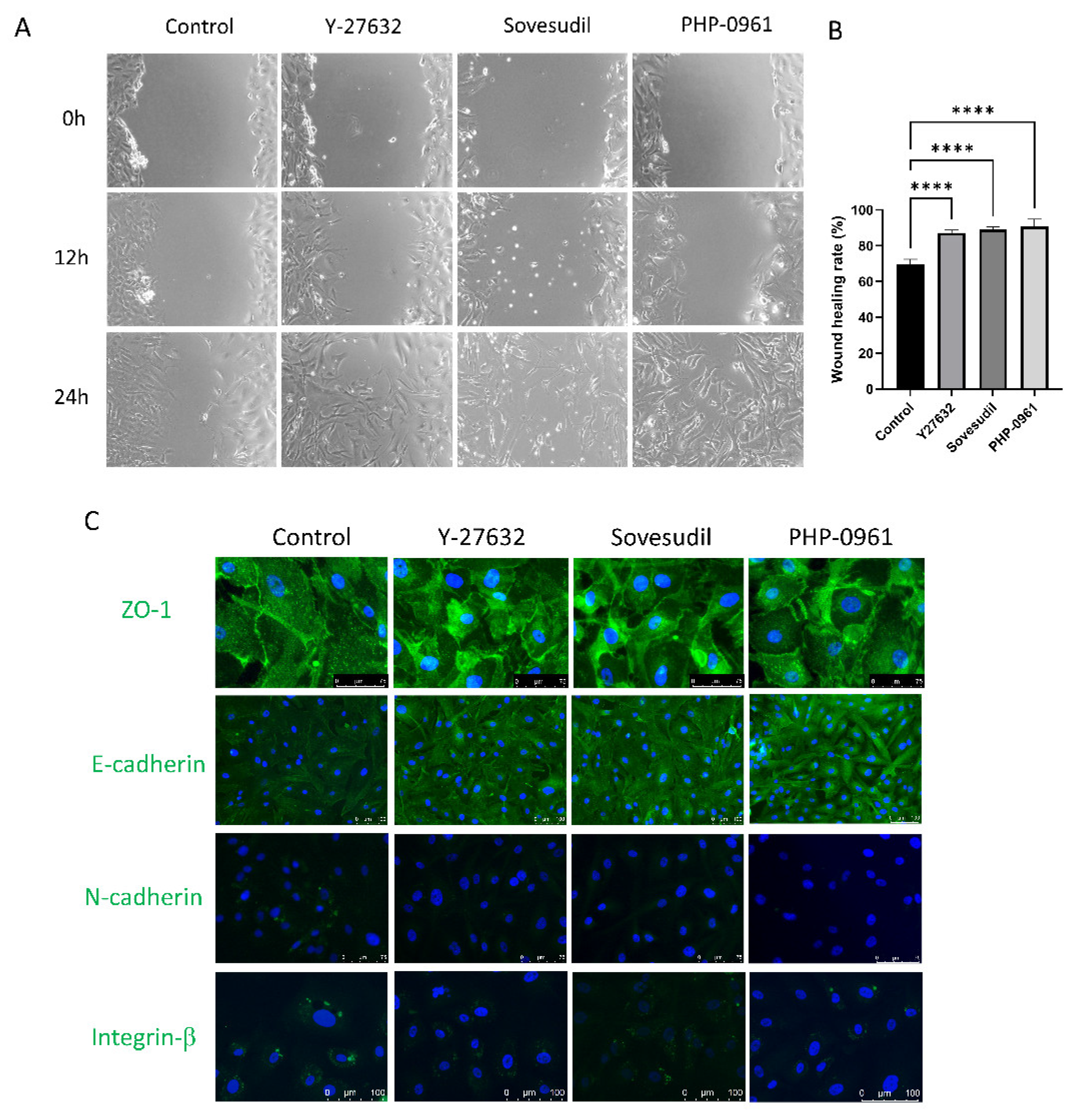
2.2. Cell Migration and Wound Healing
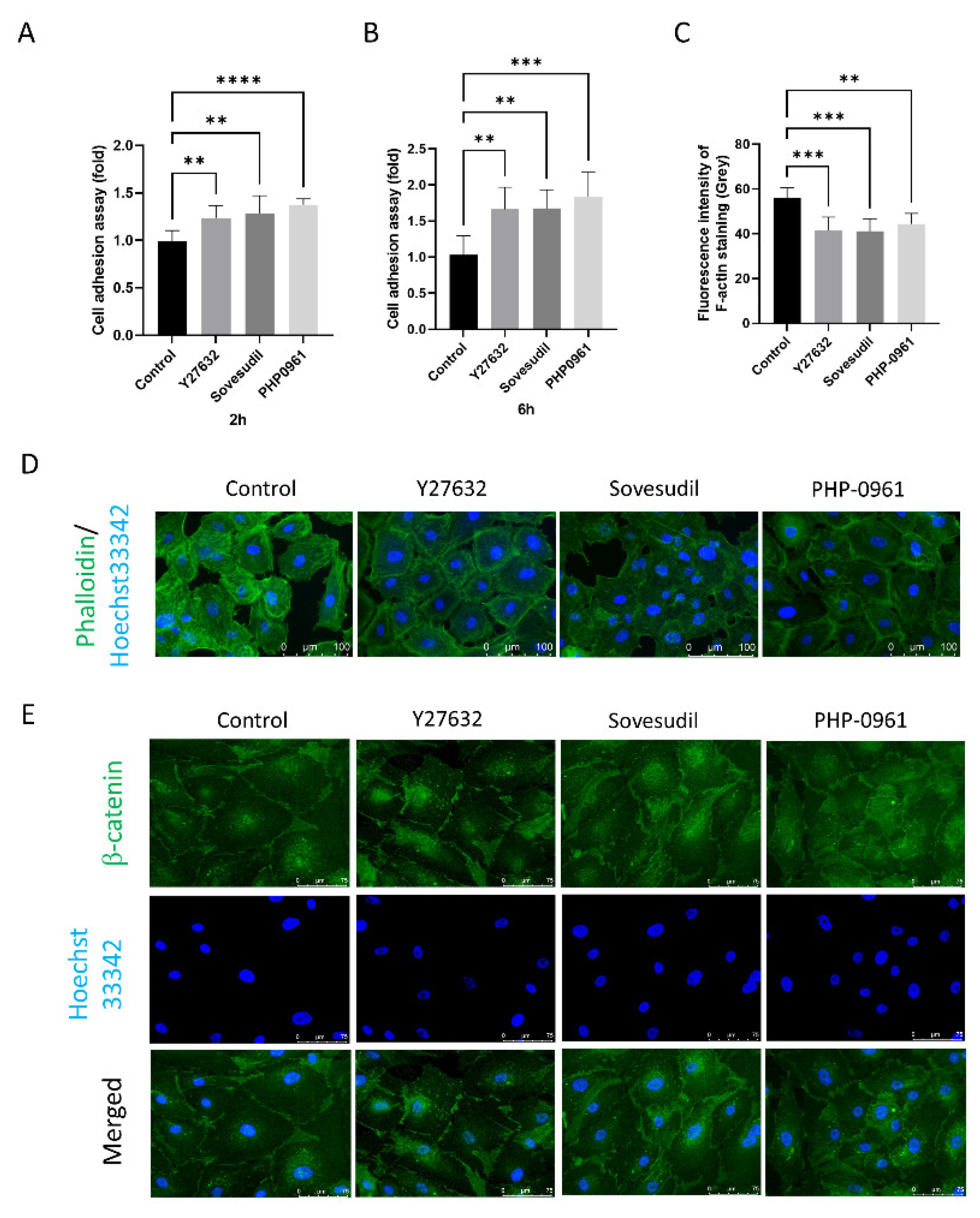
2.3. Effect on the Cell Adhesion Assay
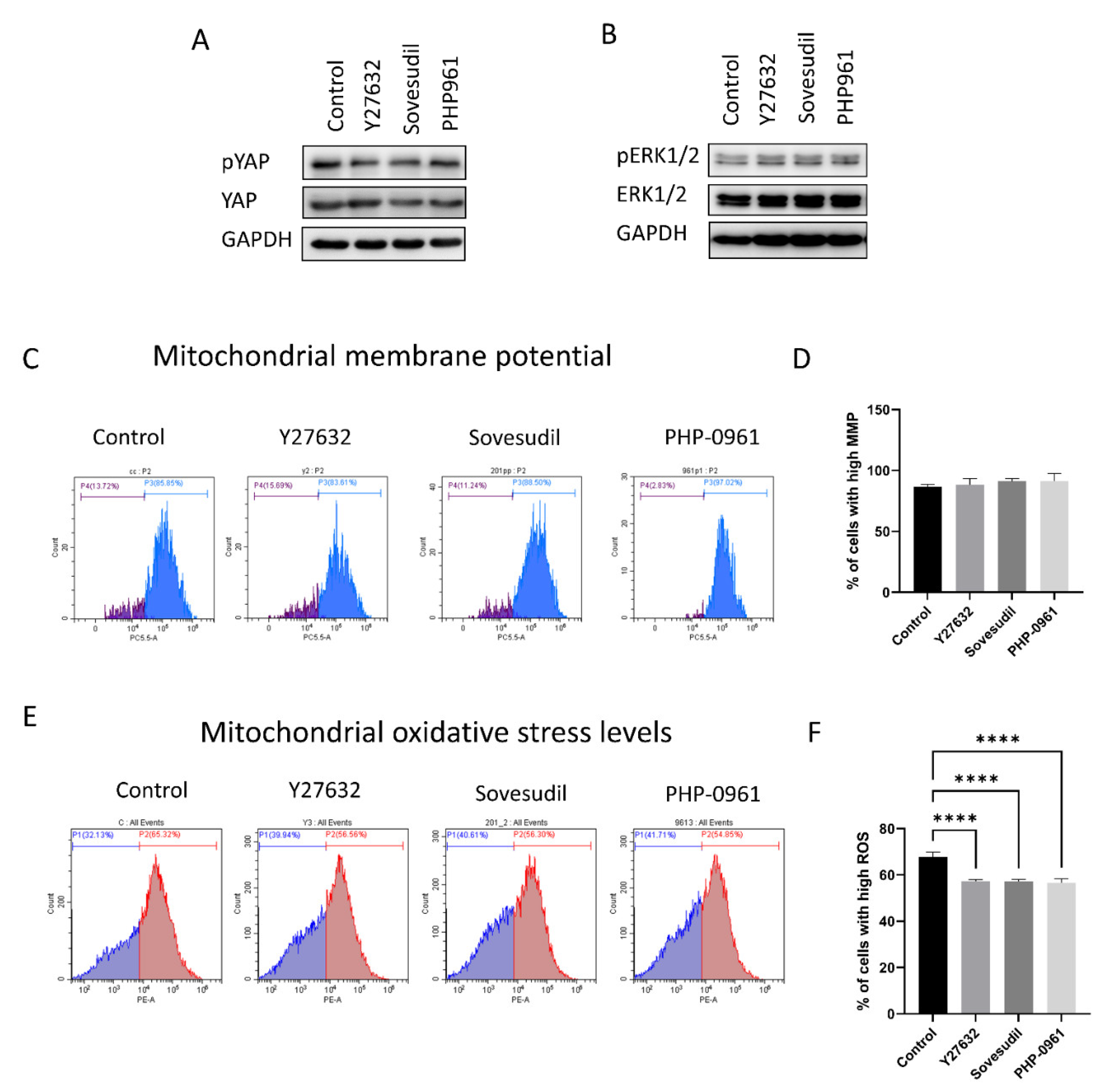
2.4. Cell Signaling
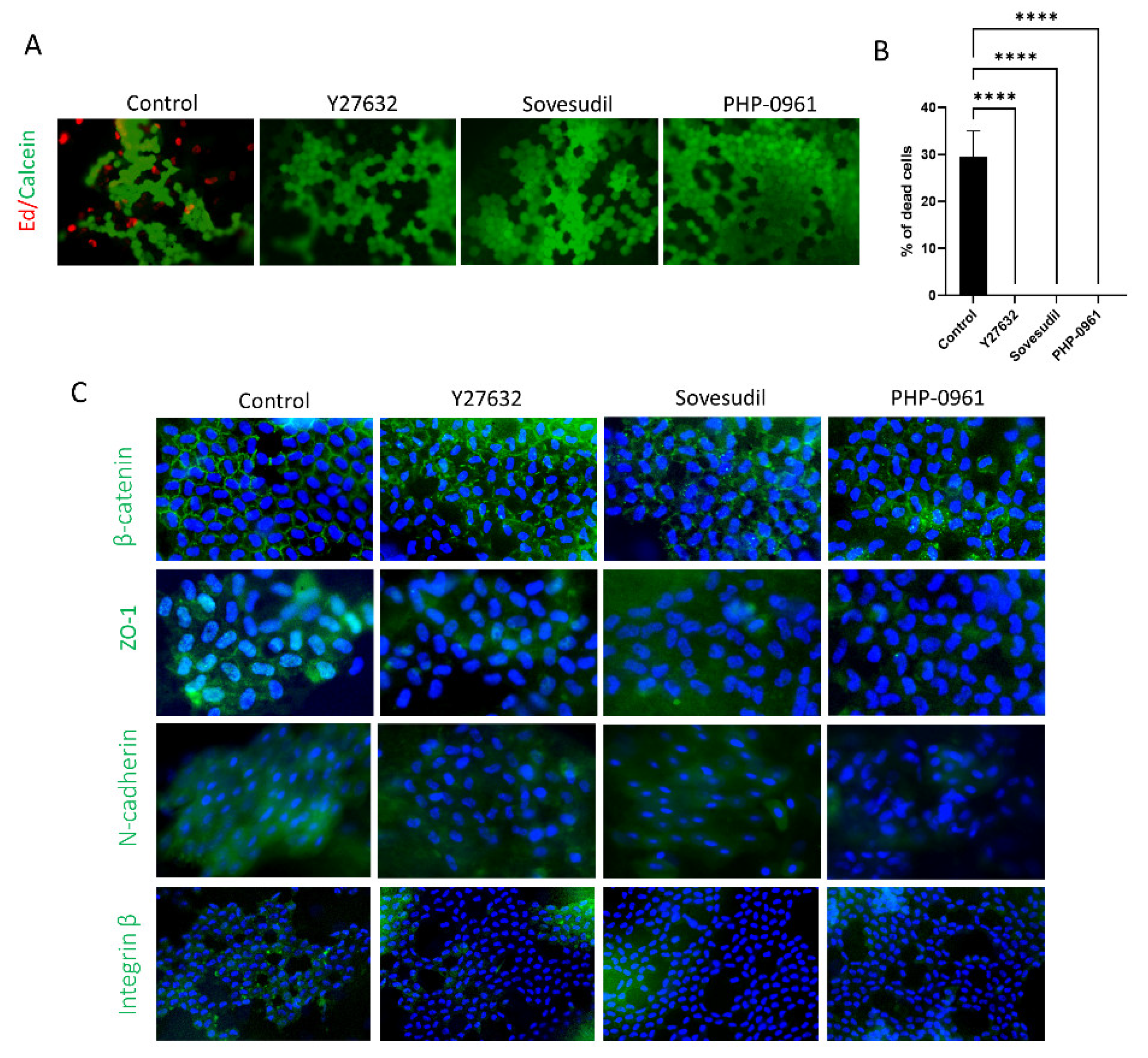
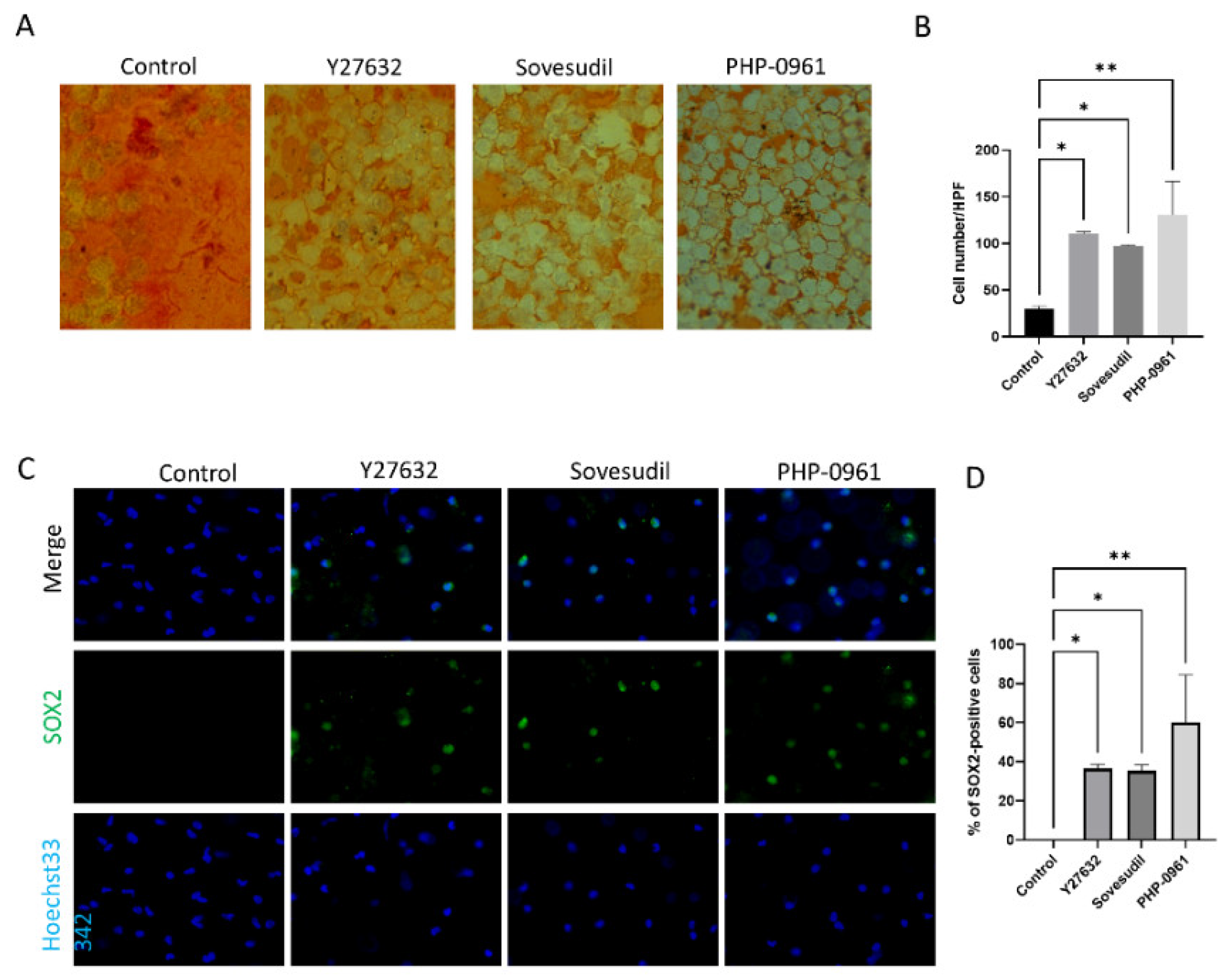
2.5. Porcine Ex Vivo Study
3. Discussion
4. Materials and Methods
4.1. Formatting Cell Culture
4.2. Cell Viability and Toxicity Assay
4.3. Proliferation Assessment
4.4. Immunofluorescence Staining
4.5. Wound Healing Assay
4.6. Cell Adhesion Assay
4.7. Western Blot
4.8. Mitochondrial Assay
4.9. Live/Dead Cell Assay of Porcine Ex Vivo Study
4.10. Immunofluorescence Staining of Porcine Corneas
4.11. Wound Healing Assay of Porcine Corneas
4.12. Alizarin S Red Staining of Porcine Corneas
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joyce, N.C. Proliferative capacity of the corneal endothelium. Prog. Retin. Eye Res. 2003, 22, 359–389. [Google Scholar] [CrossRef] [PubMed]
- Faye, P.A.; Poumeaud, F.; Chazelas, P.; Duchesne, M.; Rassat, M.; Miressi, F.; Lia, A.S.; Sturtz, F.; Robert, P.-Y.; Favreau, F.; et al. Focus on cell therapy to treat corneal endothelial diseases. Exp. Eye Res. 2021, 204, 108462. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Ang, M.; Mehta, J. Evolution of therapies for the corneal endothelium: Past, present and future approaches. Br. J. Ophthalmol. 2020, 105, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Reinprayoon, U.; Srihatrai, P.; Satitpitakul, V.; Puangsricharern, V.; Wungcharoen, T.; Kasetsuwan, N. Survival Outcome and Prognostic Factors of Corneal Transplantation: A 15-Year Retrospective Cohort Study at King Chulalongkorn Memorial Hospital. Clin. Ophthalmol. 2021, 15, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Sie, N.M.; Yam, G.H.-F.; Soh, Y.Q.; Lovatt, M.; Dhaliwal, D.; Kocaba, V.; Mehta, J.S. Regenerative capacity of the corneal transition zone for endothelial cell therapy. Stem Cell Res. Ther. 2020, 11, 523. [Google Scholar] [CrossRef]
- Peh, G.S.L.; Ang, H.-P.; Lwin, C.N.; Adnan, K.; George, B.L.; Seah, X.-Y.; Lin, S.-J.; Bhogal, M.; Liu, Y.-C.; Tan, D.T.; et al. Regulatory Compliant Tissue-Engineered Human Corneal Endothelial Grafts Restore Corneal Function of Rabbits with Bullous Keratopathy. Sci. Rep. 2017, 7, 14149. [Google Scholar] [CrossRef] [Green Version]
- Ha, A.; Kim, Y.K.; Jeoung, J.W.; Satyal, S.; Kim, J.; Kim, S.; Park, K.H. Sovesudil (locally acting rho kinase inhibitor) for the treatment of normal-tension glaucoma: The randomized phase II study. Acta Ophthalmol. 2021, 100, e470–e477. [Google Scholar] [CrossRef]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK Inhibitor Converts Corneal Endothelial Cells into a Phenotype Capable of Regenerating In Vivo Endothelial Tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef]
- Fujimoto, H.; Setoguchi, Y.; Kiryu, J. The ROCK Inhibitor Ripasudil Shows an Endothelial Protective Effect in Patients with Low Corneal Endothelial Cell Density After Cataract Surgery. Transl. Vis. Sci. Technol. 2021, 10, 18. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Okumura, N.; Ueno, M.; Kinoshita, S. New Therapeutic Modality for Corneal Endothelial Disease Using Rho-Associated Kinase Inhibitor Eye Drops. Cornea 2014, 33, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Bodor, N. Retrometabolic Drug Design-Novel Aspects, Future Directions. ChemInform 2001, 32, S67–S74. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, P.; Bodor, N. Recent advances in the design and development of soft drugs. Pharmazie 2014, 69, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Wu, M.; Feng, J.; Lin, X.; Gu, Z. RhoA/Rho kinase signaling regulates transforming growth factor-β1-induced chondrogenesis and actin organization of synovium-derived mesenchymal stem cells through interaction with the Smad pathway. Int. J. Mol. Med. 2012, 30, 1119–1125. [Google Scholar] [CrossRef] [Green Version]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell Longev. 2015, 2015, 654594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Verschut, V.; Woest, M.E.; Ng-Blichfeldt, J.-P.; Matias, A.; Villetti, G.; Accetta, A.; Facchinetti, F.; Gosens, R.; Kistemaker, L.E.M. Rho-Kinase 1/2 Inhibition Prevents Transforming Growth Factor-β-Induced Effects on Pulmonary Remodeling and Repair. Front. Pharmacol. 2020, 11, 609509. [Google Scholar] [CrossRef]
- Bird, T.G.; Müller, M.; Boulter, L.; Vincent, D.F.; Ridgway, R.A.; Lopez-Guadamillas, E.; Lu, W.-Y.; Jamieson, T.; Govaere, O.; Campbell, A.D.; et al. TGFβ inhibition restores a regenerative response in acute liver injury by suppressing paracrine senescence. Sci. Transl. Med. 2018, 10, eaan1230. [Google Scholar] [CrossRef] [Green Version]
- Feizi, S. Corneal endothelial cell dysfunction: Etiologies and management. Ther. Adv. Ophthalmol. 2018, 10, 2515841418815802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 2014, 6, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Serror, L.; Nir, E.; Dhiraj, D.; Altshuler, A.; Khreish, M.; Tiosano, B.; Hasson, P.; Panman, L.; Luxenburg, C.; et al. SOX2 Regulates P63 and Stem/Progenitor Cell State in the Corneal Epithelium. Stem Cells 2019, 37, 417–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Kaufman, P.D. Ki-67: More than a proliferation marker. Chromosoma 2018, 127, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Schlötzer-Schrehardt, U.; Zenkel, M.; Strunz, M.; Gießl, A.; Schondorf, H.; da Silva, H.; Schmidt, G.A.; Greiner, M.A.; Okumura, N.; Koizumi, N.; et al. Potential Functional Restoration of Corneal Endothelial Cells in Fuchs Endothelial Corneal Dystrophy by ROCK Inhibitor (Ripasudil). Am. J. Ophthalmol. 2021, 224, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalczyk, P.; Sulejczak, D.; Kleczkowska, P.; Bukowska-Ośko, I.; Kucia, M.; Popiel, M.; Wietrak, E.; Kramkowski, K.; Wrzosek, K.; Kaczyńska, K. Mitochondrial Oxidative Stress—A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021, 22, 13384. [Google Scholar] [CrossRef] [PubMed]
- Tsogtbaatar, E.; Landin, C.; Minter-Dykhouse, K.; Folmes, C.D.L. Energy Metabolism Regulates Stem Cell Pluripotency. Front. Cell Dev. Biol. 2020, 8, 87. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Zhang, H.; Li, J.; Jiang, X.; Zhang, Y.; Wu, Q.; Shen, L.; Shi, J.; Gao, N. ROCK1 activation-mediated mitochondrial translocation of Drp1 and cofilin are required for arnidiol-induced mitochondrial fission and apoptosis. J. Exp. Clin. Cancer Res. 2020, 39, 37. [Google Scholar] [CrossRef] [Green Version]
- Brand, C.S.; Tan, V.P.; Brown, J.H.; Miyamoto, S. RhoA regulates Drp1 mediated mitochondrial fission through ROCK to protect cardiomyocytes. Cell Signal. 2018, 50, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Zhou, J.; Budhraja, A.; Hu, X.; Chen, Y.; Cheng, Q.; Liu, L.; Zhou, T.; Li, P.; Liu, E.; et al. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget 2015, 6, 1834–1849. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.T.; Park, J.T.; Choi, K.; Choi, H.J.C.; Jung, C.W.; Kim, G.R.; Lee, Y.-S.; Park, S.C. Chemical screening identifies ROCK as a target for recovering mitochondrial function in Hutchinson-Gilford progeria syndrome. Aging Cell 2017, 16, 541–550. [Google Scholar] [CrossRef]
- Morciano, G.; Vezzani, B.; Missiroli, S.; Boncompagni, C.; Pinton, P.; Giorgi, C. An Updated Understanding of the Role of YAP in Driving Oncogenic Responses. Cancers 2021, 13, 3100. [Google Scholar] [CrossRef] [PubMed]
- Caire, R.; Dalix, E.; Chafchafi, M.; Thomas, M.; Linossier, M.-T.; Normand, M.; Guignandon, A.; Vico, L.; Marotte, H. YAP Transcriptional Activity Dictates Cell Response to TNF In Vitro. Front. Immunol. 2022, 13, 856247. [Google Scholar] [CrossRef] [PubMed]
- Pagliari, S.; Vinarsky, V.; Martino, F.; Perestrelo, A.R.; De La Cruz, J.O.; Caluori, G.; Vrbsky, J.; Mozetic, P.; Pompeiano, A.; Zancla, A.; et al. YAP–TEAD1 control of cytoskeleton dynamics and intracellular tension guides human pluripotent stem cell mesoderm specification. Cell Death Differ. 2021, 28, 1193–1207. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.; Pant, I.; Shen, Y.; Qiao, R.F.; Yang, X.; Bai, Y.; Jin, J.; Poulikakos, P.I.; Aaronson, S.A. ROCK1 mechano-signaling dependency of human malignancies driven by TEAD/YAP activation. Nat. Commun. 2022, 13, 703. [Google Scholar] [CrossRef]
- Jang, J.W.; Kim, M.K.; Bae, S.C. Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway. Small GTPases 2018, 11, 280–288. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, J.; Feng, H.; Peng, S.; Adams, U.; Bai, Y.; Huang, L.; Li, J.; Huang, J.; Meng, S.; et al. YAP/TEAD–Mediated Transcription Controls Cellular Senescence. Cancer Res. 2013, 73, 3615–3624. [Google Scholar] [CrossRef] [Green Version]
- Chung, J.; Huda, M.N.; Shin, Y.; Han, S.; Akter, S.; Kang, I.; Ha, J.; Choe, W.; Choi, T.G.; Kim, S.S. Correlation between Oxidative Stress and Transforming Growth Factor-Beta in Cancers. Int. J. Mol. Sci. 2021, 22, 13181. [Google Scholar] [CrossRef]
- Srinivasan, R.; Zabuawala, T.; Huang, H.; Zhang, J.; Gulati, P.; Fernandez, S.; Karlo, J.C.; Landreth, G.E.; Leone, G.; Ostrowski, M.C. Erk1 and Erk2 Regulate Endothelial Cell Proliferation and Migration during Mouse Embryonic Angiogenesis. PLoS ONE 2009, 4, e8283. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. New York Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Balda, M.S.; Garrett, M.D.; Matter, K. The ZO-1–associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J. Cell Biol. 2003, 160, 423–432. [Google Scholar] [CrossRef] [Green Version]
- Georgiadis, A.; Tschernutter, M.; Bainbridge, J.W.; Balaggan, K.S.; Mowat, F.; West, E.L.; Munro, P.M.; Thrasher, A.J.; Matter, K.; Balda, M.S.; et al. The Tight Junction Associated Signalling Proteins ZO-1 and ZONAB Regulate Retinal Pigment Epithelium Homeostasis in Mice. PLoS ONE 2010, 5, e15730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, S.V.; Hopkins, A.M.; Chen, J.; Narumiya, S.; Parkos, C.A.; Nusrat, A. Rho kinase regulates tight junction function and is necessary for tight junction assembly in polarized intestinal epithelia. Gastroenterology 2001, 121, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.; Luo, H.; Huang, W.; Zhang, J.; Wu, D.; Wang, J.; Pi, J.; Liu, C.; Qu, X.; Liu, H.; et al. Modulation of the EMT/MET Process by E-Cadherin in Airway Epithelia Stress Injury. Biomolecules 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- van Roy, F.; Berx, G. The cell-cell adhesion molecule E-cadherin. Cell Mol. Life Sci. 2008, 65, 3756–3788. [Google Scholar] [CrossRef]
- Das, S.; Becker, B.N.; Hoffmann, F.M.; Mertz, J.E. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009, 10, 94. [Google Scholar] [CrossRef] [Green Version]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; VanDyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef] [Green Version]
- Bianchi, A.; Gervasi, M.E.; Bakin, A. Role of β5-integrin in epithelial-mesenchymal transition in response to TGF-β. Cell Cycle 2010, 9, 1647–1659. [Google Scholar] [CrossRef] [Green Version]
- Bhowmick, N.A.; Zent, R.; Ghiassi, M.; McDonnell, M.; Moses, H.L. Integrin β1 Signaling Is Necessary for Transforming Growth Factor-β Activation of p38MAPK and Epithelial Plasticity. J. Biol. Chem. 2001, 276, 46707–46713. [Google Scholar] [CrossRef]
- Pipparelli, A.; Arsenijevic, Y.; Thuret, G.; Gain, P.; Nicolas, M.; Majo, F. ROCK Inhibitor Enhances Adhesion and Wound Healing of Human Corneal Endothelial Cells. PLoS ONE 2013, 8, e62095. [Google Scholar] [CrossRef]
- Schaefer, T.; Lengerke, C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: The PI3K/AKT/SOX2 axis and beyond. Oncogene 2019, 39, 278–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodor, N.; Buchwald, P. Soft drug design: General principles and recent applications. Med. Res. Rev. 2000, 20, 58–101. [Google Scholar] [CrossRef]
- Van De Velde, S.; Van Bergen, T.; Sijnave, D.; Hollanders, K.; Castermans, K.; Defert, O.; Leysen, D.; Vandewalle, E.; Moons, L.; Stalmans, I. AMA0076, a Novel, Locally Acting Rho Kinase Inhibitor, Potently Lowers Intraocular Pressure in New Zealand White Rabbits with Minimal Hyperemia. Investig. Opthalmol. Vis. Sci. 2014, 55, 1006–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Yeo, M.K.; Kim, S.-H.; Kim, J.M.; Huang, S.-M.; Kim, M.-R.; Song, K.S.; Kim, K.-H. Correlation of expression of phosphorylated and non-phosphorylated Yes-associated protein with clinicopathological parameters in esophageal squamous cell carcinoma in a Korean population. Anticancer Res. 2012, 32, 3835–3840. [Google Scholar]
- Dasgupta, I.; McCollum, D. Control of cellular responses to mechanical cues through YAP/TAZ regulation. J. Biol. Chem. 2019, 294, 17693–17706. [Google Scholar] [CrossRef] [Green Version]
- Nardone, G.; Oliver-De La Cruz, J.; Vrbsky, J.; Martini, C.; Pribyl, J.; Skládal, P.; Pešl, M.; Caluori, G.; Pagliari, S.; Martino, F.; et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 2017, 8, 15321. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Estarás, C.; Moresco, J.J.; Yates, J.R., 3rd; Jones, K.A. α-Catenin interacts with APC to regulate β-catenin proteolysis and transcriptional repression of Wnt target genes. Genes Dev. 2013, 27, 2473–2488. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Qu, R.; Fan, T.; Yang, Y.; Jiang, X.; Khan, A.U.; Zhou, Z.; Zhang, J.; Wei, K.; Ouyang, J.; et al. Actin polymerization state regulates osteogenic differentiation in human adipose-derived stem cells. Cell Mol. Biol. Lett. 2021, 26, 15. [Google Scholar] [CrossRef]
- Heuberger, J.; Birchmeier, W. Interplay of Cadherin-Mediated Cell Adhesion and Canonical Wnt Signaling. Cold Spring Harb. Perspect. Biol. 2009, 2, a002915. [Google Scholar] [CrossRef]
- He, J.; Shi, J.; Zhang, K.; Xue, J.; Li, J.; Yang, J.; Chen, J.; Wei, J.; Ren, H.; Liu, X. Sox2 inhibits Wnt-β-catenin signaling and metastatic potency of cisplatin-resistant lung adenocarcinoma cells. Mol. Med. Rep. 2017, 15, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Somani, A.N.; Vaidyanathan, U.; Patel, B.C. Fuchs Endothelial Dystrophy. In StatPearls; StatPearls: Treasure Island, FL, USA; Cassell & Company Ltd.: London, UK, 2022. [Google Scholar]
- Koizumi, N.; Kinoshita, S.; Okumura, N. The Role of Rho Kinase Inhibitors in Corneal Endothelial Dysfunction. Curr. Pharm. Des. 2017, 23, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.E.; He, P.C.; Veldman, P.B. Management of endothelial disease without keratoplasty. Curr. Opin. Ophthalmol. 2022, 33, 332–337. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.W.; Shin, Y.J.; Lee, S.C.S. Novel ROCK Inhibitors, Sovesudil and PHP-0961, Enhance Proliferation, Adhesion and Migration of Corneal Endothelial Cells. Int. J. Mol. Sci. 2022, 23, 14690. https://doi.org/10.3390/ijms232314690
Kim KW, Shin YJ, Lee SCS. Novel ROCK Inhibitors, Sovesudil and PHP-0961, Enhance Proliferation, Adhesion and Migration of Corneal Endothelial Cells. International Journal of Molecular Sciences. 2022; 23(23):14690. https://doi.org/10.3390/ijms232314690
Chicago/Turabian StyleKim, Kyung Wook, Young Joo Shin, and Sammy Chi Sam Lee. 2022. "Novel ROCK Inhibitors, Sovesudil and PHP-0961, Enhance Proliferation, Adhesion and Migration of Corneal Endothelial Cells" International Journal of Molecular Sciences 23, no. 23: 14690. https://doi.org/10.3390/ijms232314690





