Development of the Periventricular Nucleus as a Brain Center, Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis
Abstract
1. Introduction
2. Results
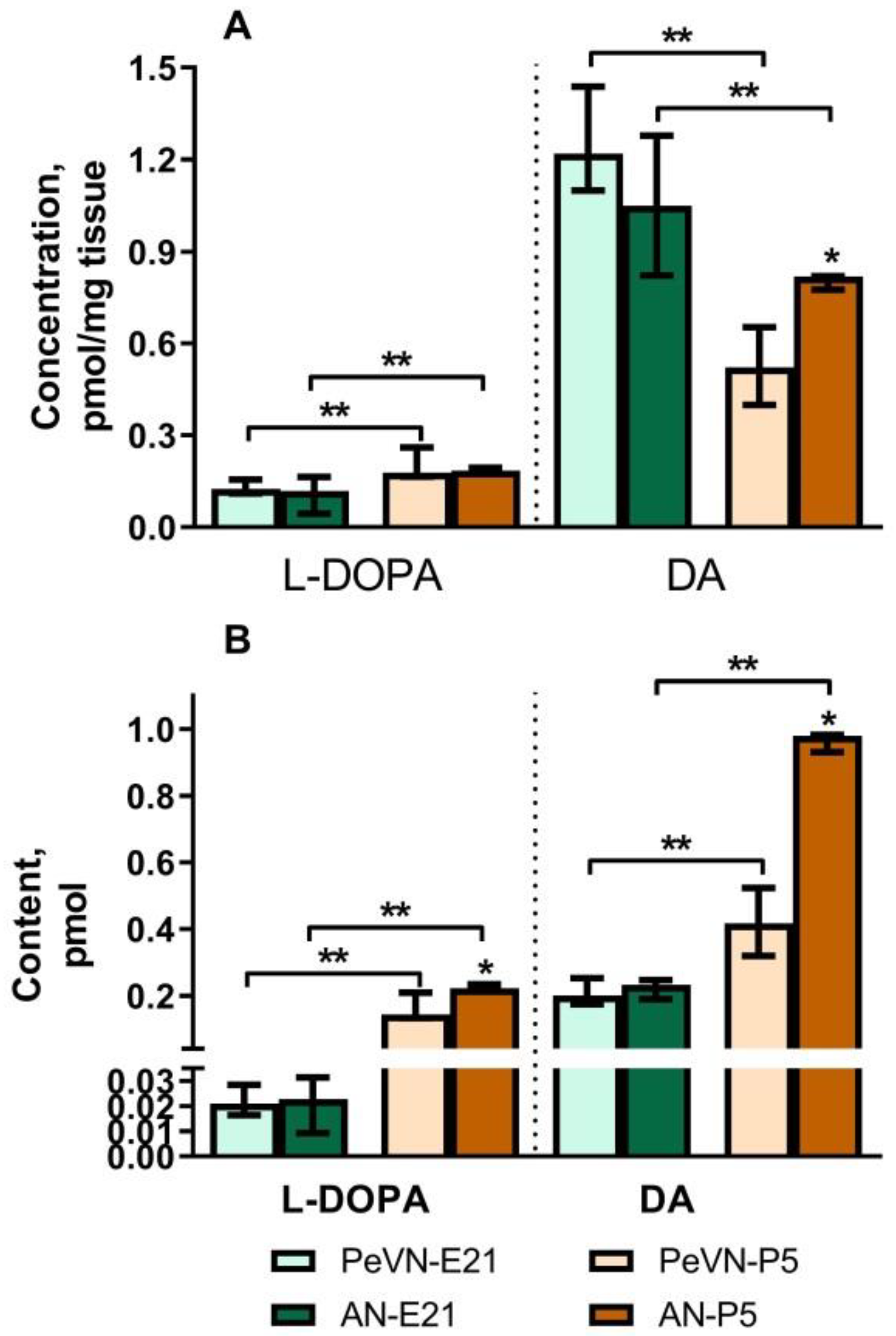
2.1. Dopamine and L-DOPA Levels in the Periventricular and Arcuate Nuclei in Perinatal Rats
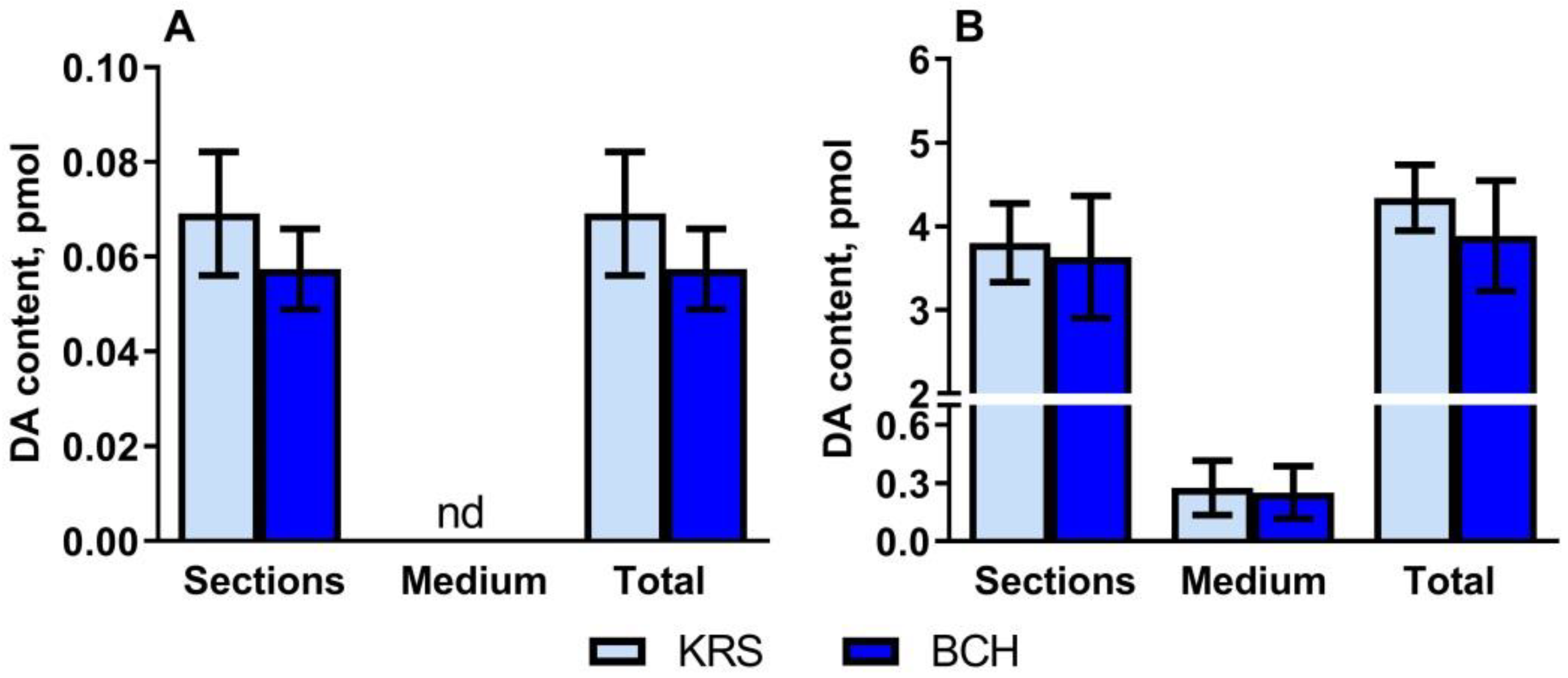
2.2. Dopamine Content in Vibratome Sections of the Periventricular Nucleus and Substantia Nigra in Rats on the 5th Postnatal Day, as Well as in the Incubation Medium after Incubating Sections in the Medium with or without 2-Aminobicyclo[2.2.1]heptane-2-Carboxylic Acid
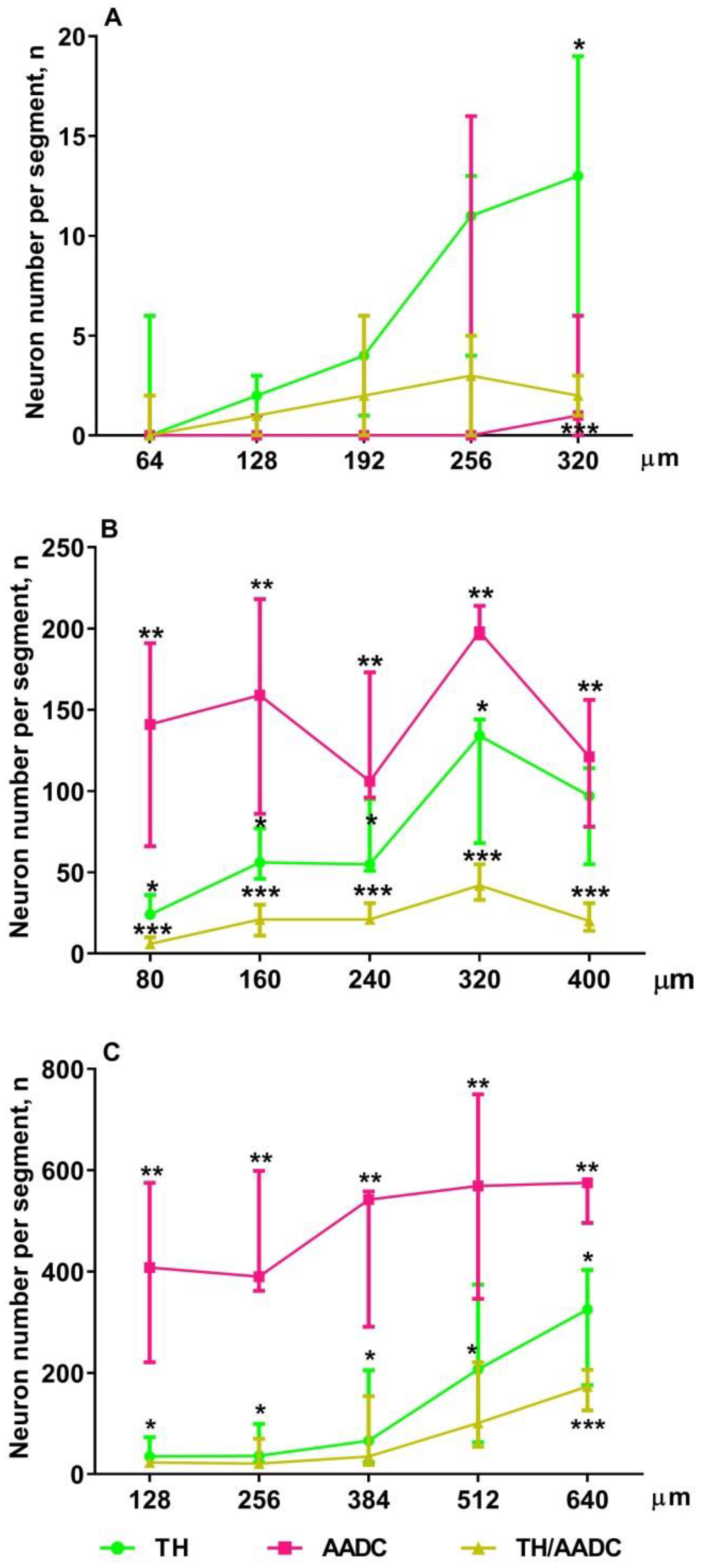
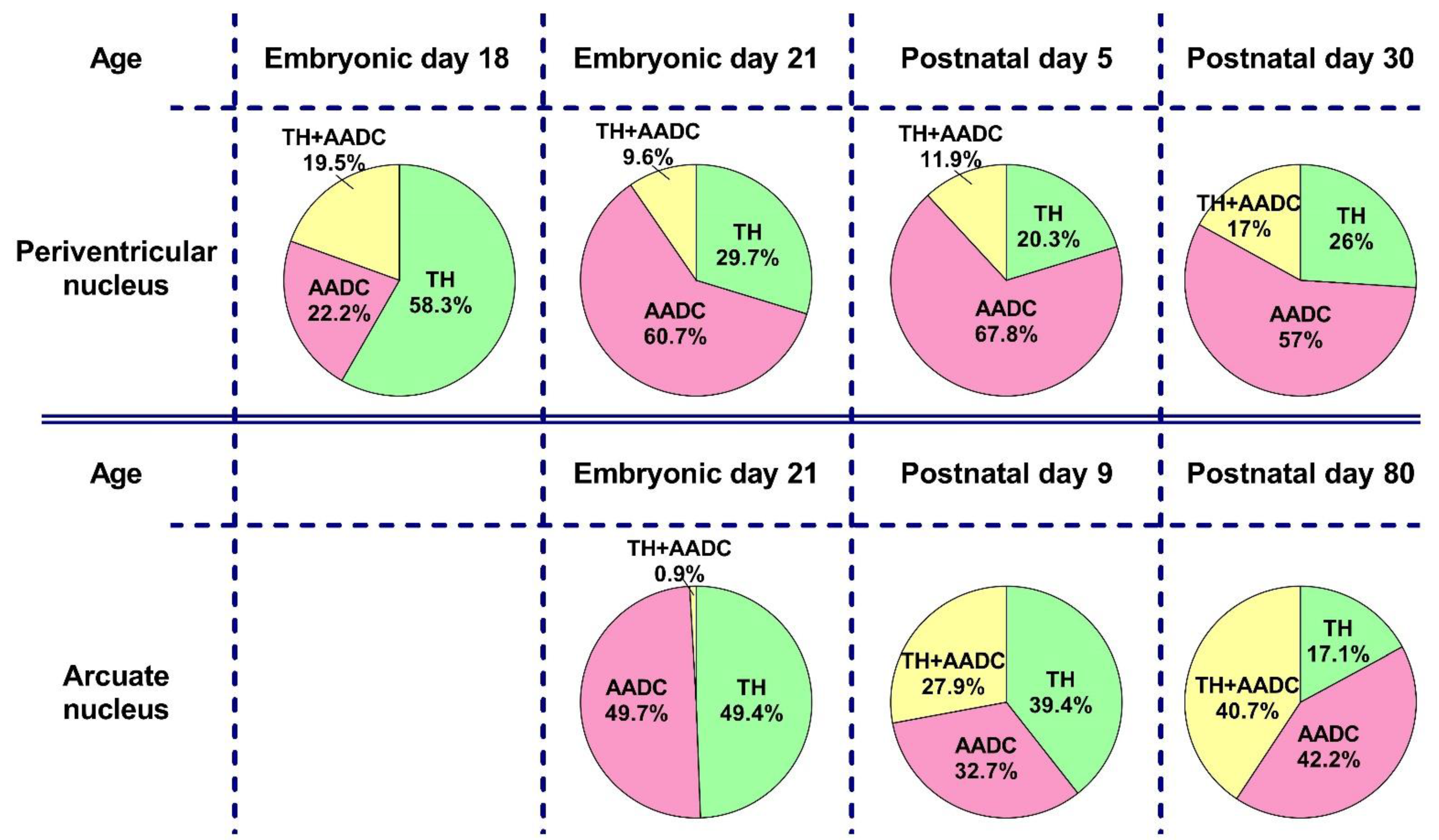
2.3. The Number of Neurons Immunopositive for Tyrosine Hydroxylase, for Aromatic L-amino Acid Decarboxylase, and for Both Enzymes in the Periventricular Nucleus in Rats on the 18th and 21st Embryonic Days and on the 5th Postnatal Day
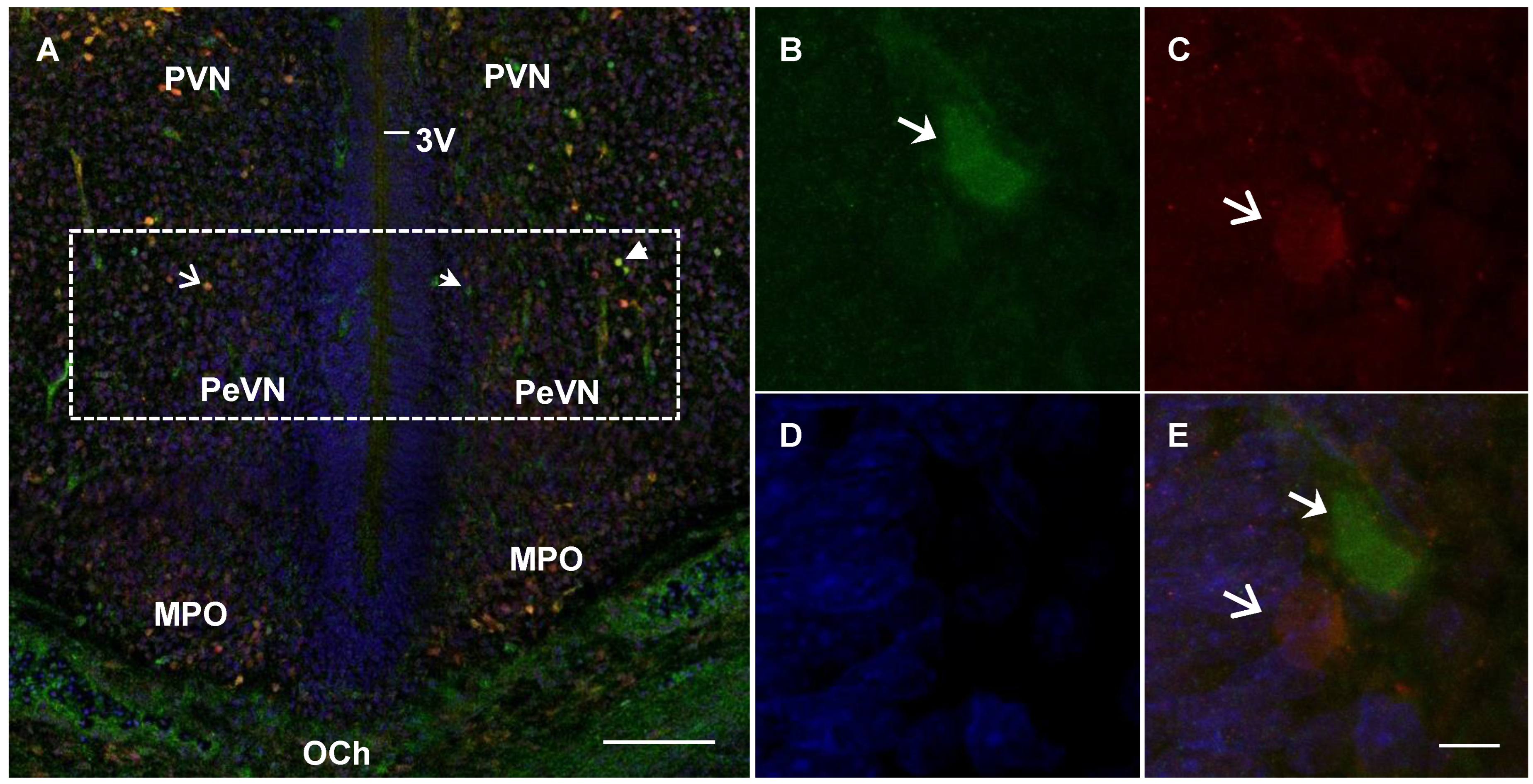
2.4. Confocal Microscopy of Topographic Relationships of Neurons and Nerve Fibers, Immunopositive Only for Tyrosine Hydroxylase, Only for Aromatic L-amino acid Decarboxylase, and for Both Enzymes, with the 3rd Cerebral Ventricle in Rats on Embryonic Days 18 and 21 and on Postnatal Day 5
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experiments and Obtaining Samples for Analysis
4.2.1. Obtaining Periventricular and Arcuate Nuclei Samples for Biochemical Analysis
4.2.2. Incubation of Vibratome Sections of the Periventricular Nucleus and Substantia Nigra of Rats on Postnatal Day 5
4.2.3. Preparing the Brain for Immunohistochemistry
4.3. Methods
4.3.1. High Performance Liquid Chromatography
4.3.2. Immunohistochemistry
4.3.3. Microscopy and Quantitative Image Analysis
4.3.4. Confocal Microscopy and Image Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AADC | aromatic L-amino acid decarboxylase |
| AN | arcuate nucleus |
| BCH | 2-aminobicyclo[2.2.1]heptane-2-carboxylic acid |
| DA | dopamine |
| E | embryonic day |
| KRS | Krebs-Ringer solution |
| L-DOPA | L-3,4-dihydroxyphenylalanine |
| P | postnatal day |
| PBS | phosphate-buffered saline |
| PeVN | periventricular nucleus |
| SN | substantia nigra |
| TH | tyrosine hydroxylase |
References
- Doummar, D.; Moussa, F.; Nougues, M.-C.; Ravelli, C.; Louha, M.; Whalen, S.; Burglen, L.; Rodriguez, D.; de Villemeur, T.B. Monoamine neurotransmitters and movement disorders in children and adults. Rev. Neurol. 2018, 174, 581–588. [Google Scholar] [CrossRef]
- Kurian, M.A.; Gissen, P.; Smith, M.; Heales, S., Jr.; Clayton, P. The monoamine neurotransmitter disorders: An expanding range of neurological syndromes. Lancet Neurol. 2011, 10, 721–733. [Google Scholar] [CrossRef]
- Ugrumov, M.V. Brain Neurons Partly Expressing Dopaminergic Phenotype: Location, Development, Functional Significance, and Regulation. Adv. Pharmacol. 2013, 68, 37–91. [Google Scholar] [CrossRef]
- Chan-Palay, V.; Záborszky, L.; Köhler, C.; Goldstein, M.; Palay, S.L. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of rats. J. Comp. Neurol. 1984, 227, 467–496. [Google Scholar] [CrossRef]
- Hökfelt, T.; Johansson, O.; Fuxe, K.; Goldstein, M.; Park, D. Immunohistochemical Studies on the Localization and Distribution of Monoamine Neuron Systems in the Rat Brain. I. Tyrosine Hydroxylase in the Mes- and Diencephalon. Med. Biol. 1976, 54, 427–453. [Google Scholar]
- Kitahama, K.; Luppi, P.-H.; Bérod, A.; Goldstein, M.; Jouvet, M. Localization of tyrosine hydroxylase immunoreactive neurons in the cat hypothalamus, with special reference to fluorescence histochemistry. J. Comp. Neurol. 1987, 262, 578–593. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Friedmann, D.; Xiong, J.; Liu, C.D.; Ferguson, B.R.; Weerakkody, T.; DeLoach, K.E.; Ran, C.; Pun, A.; Sun, Y.; et al. Anatomically Defined and Functionally Distinct Dorsal Raphe Serotonin Sub-systems. Cell 2018, 175, 472–487.e20. [Google Scholar] [CrossRef] [PubMed]
- Steinbusch, H.W.M.; Nieuwenhuys, R. Localization of Serotonin-Like Immunoreactivity in the Central Nervous System and Pituitary of the Rat, with Special References to the Innervation of the Hypothalamus. Adv. Exp. Med. Biol. 1981, 133, 7–35. [Google Scholar] [CrossRef]
- Godefroy, D.; Boukhzar, L.; Dubessy, C.; Montero-Hadjadje, M.; Yon, L.; Eiden, L.E.; Anouar, Y. Three-dimensional mapping of tyrosine hydroxylase in the transparent brain and adrenal of prenatal and pre-weaning mice: Comprehensive methodological flowchart and quantitative aspects of 3D mapping. J. Neurosci. Methods 2020, 335, 108596. [Google Scholar] [CrossRef] [PubMed]
- Weihe, E.; Depboylu, C.; Schütz, B.; Schäfer, M.K.-H.; Eiden, L.E. Three Types of Tyrosine Hydroxylase-Positive CNS Neurons Distinguished by Dopa Decarboxylase and VMAT2 Co-Expression. Cell. Mol. Neurobiol. 2006, 26, 657–676. [Google Scholar] [CrossRef] [PubMed]
- Ugrumov, M.V.; Pavlova, E.N.; Kolacheva, A.A.; Dil’Mukhametova, L.K.; Bogdanov, V.V.; Blokhin, V.; Pronina, T.S. The Periventricular Nucleus as a Brain Center Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis. Int. J. Mol. Sci. 2022, 23, 6739. [Google Scholar] [CrossRef]
- Asmus, S.E.; Cocanougher, B.T.; Allen, D.L.; Boone, J.B.; Brooks, E.A.; Hawkins, S.M.; Hench, L.A.; Ijaz, T.; Mayfield, M.N. Increasing proportions of tyrosine hydroxylase-immunoreactive interneurons colocalize with choline acetyltransferase or vasoactive intestinal peptide in the developing rat cerebral cortex. Brain Res. 2011, 1383, 108–119. [Google Scholar] [CrossRef]
- Ikemoto, K.; Kitahama, K.; Nishimura, A.; Jouvet, A.; Nishi, K.; Arai, R.; Jouvet, M.; Nagatsu, I. Tyrosine hydroxylase and aromatic l-amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci. Lett. 1999, 269, 37–40. [Google Scholar] [CrossRef]
- Karasawa, N.; Hayashi, M.; Yamada, K.; Nagatsu, I.; Iwasa, M.; Takeuchi, T.; Uematsu, M.; Watanabe, K.; Onozuka, M. Tyrosine Hydroxylase (TH)- and Aromatic-L-Amino Acid Decarboxylase (AADC)-Immunoreactive Neurons of the Common Marmoset (Callithrix jacchus) Brain: An Immunohistochemical Analysis. Acta Histochem. Cytochem. 2007, 40, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Kitahama, K.; Ikemoto, K.; Jouvet, A.; Nagatsu, I.; Sakamoto, N.; Pearson, J. Aromatic l-amino acid decarboxylase- and tyrosine hydroxylase-immunohistochemistry in the adult human hypothalamus. J. Chem. Neuroanat. 1998, 16, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Mura, A.; Linder, J.; Young, S.; Groves, P. Striatal cells containing aromatic l-amino acid decarboxylase: An immunohistochemical comparison with other classes of striatal neurons. Neuroscience 2000, 98, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Novak, C.M.; Nunez, A.A. Tyrosine hydroxylase- and/or aromatic l-amino acid decarboxylase-containing cells in the suprachiasmatic nucleus of the Syrian hamster (Mesocricetus auratus). J. Chem. Neuroanat. 1998, 14, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Kitahama, K.; Ikemoto, K.; Jouvet, A.; Araneda, S.; Nagatsu, I.; Raynaud, B.; Nishimura, A.; Nishi, K.; Niwa, S.-I. Aromatic l-amino acid decarboxylase-immunoreactive structures in human midbrain, pons, and medulla. J. Chem. Neuroanat. 2009, 38, 130–140. [Google Scholar] [CrossRef]
- Meister, B.; Hökfelt, T.; Steinbusch, H.W.; Skagerberg, G.; Lindvall, O.; Geffard, M.; Joh, T.H.; Cuello, A.C.; Goldstein, M. Do Tyrosine Hydroxylase-Immunoreactive Neurons in the Ventrolateral Arcuate Nucleus Produce Dopamine or Only L-Dopa? J. Chem. Neuroanat. 1988, 1, 59–64. [Google Scholar] [PubMed]
- Ugrumov, M.; Taxi, J.; Pronina, T.; Kurina, A.; Sorokin, A.; Sapronova, A.; Calas, A. Neurons expressing individual enzymes of dopamine synthesis in the mediobasal hypothalamus of adult rats: Functional significance and topographic interrelations. Neuroscience 2014, 277, 45–54. [Google Scholar] [CrossRef]
- Ershov, P.; Ugrumov, M.; Calas, A.; Krieger, M.; Thibault, J. Degeneration of dopaminergic neurons triggers an expression of individual enzymes of dopamine synthesis in non-dopaminergic neurons of the arcuate nucleus in adult rats. J. Chem. Neuroanat. 2005, 30, 27–33. [Google Scholar] [CrossRef]
- Kozina, E.A.; Kim, A.; Kurina, A.Y.; Ugrumov, M.V. Cooperative synthesis of dopamine by non-dopaminergic neurons as a compensatory mechanism in the striatum of mice with MPTP-induced Parkinsonism. Neurobiol. Dis. 2017, 98, 108–121. [Google Scholar] [CrossRef]
- Ben-Jonathan, N.; Hnasko, R. Dopamine as a Prolactin (PRL) Inhibitor. Endocr. Rev. 2001, 22, 724–763. [Google Scholar] [CrossRef]
- Lopez, V.M.; Decatur, C.L.; Stamer, W.D.; Lynch, R.M.; McKay, B.S. L-DOPA Is an Endogenous Ligand for OA1. PLoS Biol. 2008, 6, e236. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Ershov, P.V.; Ugrumov, M.V.; Krieger, M.; Thibault, J. Differentiation of tyrosine hydroxylase-synthesizing and/or aromaticL-amino acid decarboxylase-synthesizing neurons in the rat mediobasal hypothalamus: Quantitative double-immunofluorescence study. J. Comp. Neurol. 2002, 446, 114–122. [Google Scholar] [CrossRef]
- Ugrumov, M.; Melnikova, V.; Lavrentyeva, A.; Kudrin, V.; Rayevsky, K. Dopamine synthesis by non-dopaminergic neurons expressing individual complementary enzymes of the dopamine synthetic pathway in the arcuate nucleus of fetal rats. Neuroscience 2004, 124, 629–635. [Google Scholar] [CrossRef]
- Ugrumov, M. Non-dopaminergic neurons partly expressing dopaminergic phenotype: Distribution in the brain, development and functional significance. J. Chem. Neuroanat. 2009, 38, 241–256. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Shmukler, Y.B.; Lauder, J.M. From oocyte to neuron: Do neurotransmitters function in the same way throughout development? Cell. Mol. Neurobiol. 1996, 16, 533–559. [Google Scholar] [CrossRef]
- Lauder, J.M. Neurotransmitters as growth regulatory signals: Role of receptors and second messengers. Trends Neurosci. 1993, 16, 233–240. [Google Scholar] [CrossRef]
- Ugrumov, M.V. Developing Brain as an Endocrine Organ: A Paradoxical Reality. Neurochem. Res. 2010, 35, 837–850. [Google Scholar] [CrossRef]
- Melnikova, V.; Orosco, M.; Rouch, C.; Calas, A.; Nicolaidis, S.; Proshlyakova, E.; Sapronova, A.; Ugrumov, M. Prolactin secretion and its dopamine inhibitory control in rat fetuses. Eur. J. Endocrinol. 1998, 139, 337–342. [Google Scholar] [CrossRef]
- Lehman, M.N.; Hileman, S.M.; Goodman, R.L. Neuroanatomy of the Kisspeptin Signaling System in Mammals: Comparative and Developmental Aspects. Adv. Exp. Med. Biol. 2013, 784, 27–62. [Google Scholar] [CrossRef]
- Stephens, S.B.Z.; Rouse, M.L.; Tolson, K.P.; Liaw, R.B.; Parra, R.A.; Chahal, N.; Kauffman, A.S. Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 2017, 4, 0150–17. [Google Scholar] [CrossRef]
- Murtazina, A.R.; Bondarenko, N.S.; Pronina, T.S.; Chandran, K.I.; Bogdanov, V.V.; Dilmukhametova, L.K.; Ugrumov, M.V. A Comparative Analysis of CSF and the Blood Levels of Monoamines as Neurohormones in Rats during Ontogenesis. Acta Naturae 2021, 13, 89–97. [Google Scholar] [CrossRef]
- Ashwell, K.W.S.; Paxinos, G. Atlas of the Developing Rat Nervous System, COMPACT, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Yanagida, O.; Kanai, Y.; Chairoungdua, A.; Kim, D.K.; Segawa, H.; Nii, T.; Cha, S.H.; Matsuo, H.; Fukushima, J.-I.; Fukasawa, Y.; et al. Human L-type amino acid transporter 1 (LAT1): Characterization of function and expression in tumor cell lines. Biochim. Biophys. Acta Biomembr. 2001, 1514, 291–302. [Google Scholar] [CrossRef]
- Abercrombie, M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946, 94, 239–247. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pronina, T.; Pavlova, E.; Dil’mukhametova, L.; Ugrumov, M. Development of the Periventricular Nucleus as a Brain Center, Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis. Int. J. Mol. Sci. 2022, 23, 14682. https://doi.org/10.3390/ijms232314682
Pronina T, Pavlova E, Dil’mukhametova L, Ugrumov M. Development of the Periventricular Nucleus as a Brain Center, Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis. International Journal of Molecular Sciences. 2022; 23(23):14682. https://doi.org/10.3390/ijms232314682
Chicago/Turabian StylePronina, Tatiana, Ekaterina Pavlova, Liliya Dil’mukhametova, and Michael Ugrumov. 2022. "Development of the Periventricular Nucleus as a Brain Center, Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis" International Journal of Molecular Sciences 23, no. 23: 14682. https://doi.org/10.3390/ijms232314682
APA StylePronina, T., Pavlova, E., Dil’mukhametova, L., & Ugrumov, M. (2022). Development of the Periventricular Nucleus as a Brain Center, Containing Dopaminergic Neurons and Neurons Expressing Individual Enzymes of Dopamine Synthesis. International Journal of Molecular Sciences, 23(23), 14682. https://doi.org/10.3390/ijms232314682






