The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy
Abstract
1. Introduction
2. Results
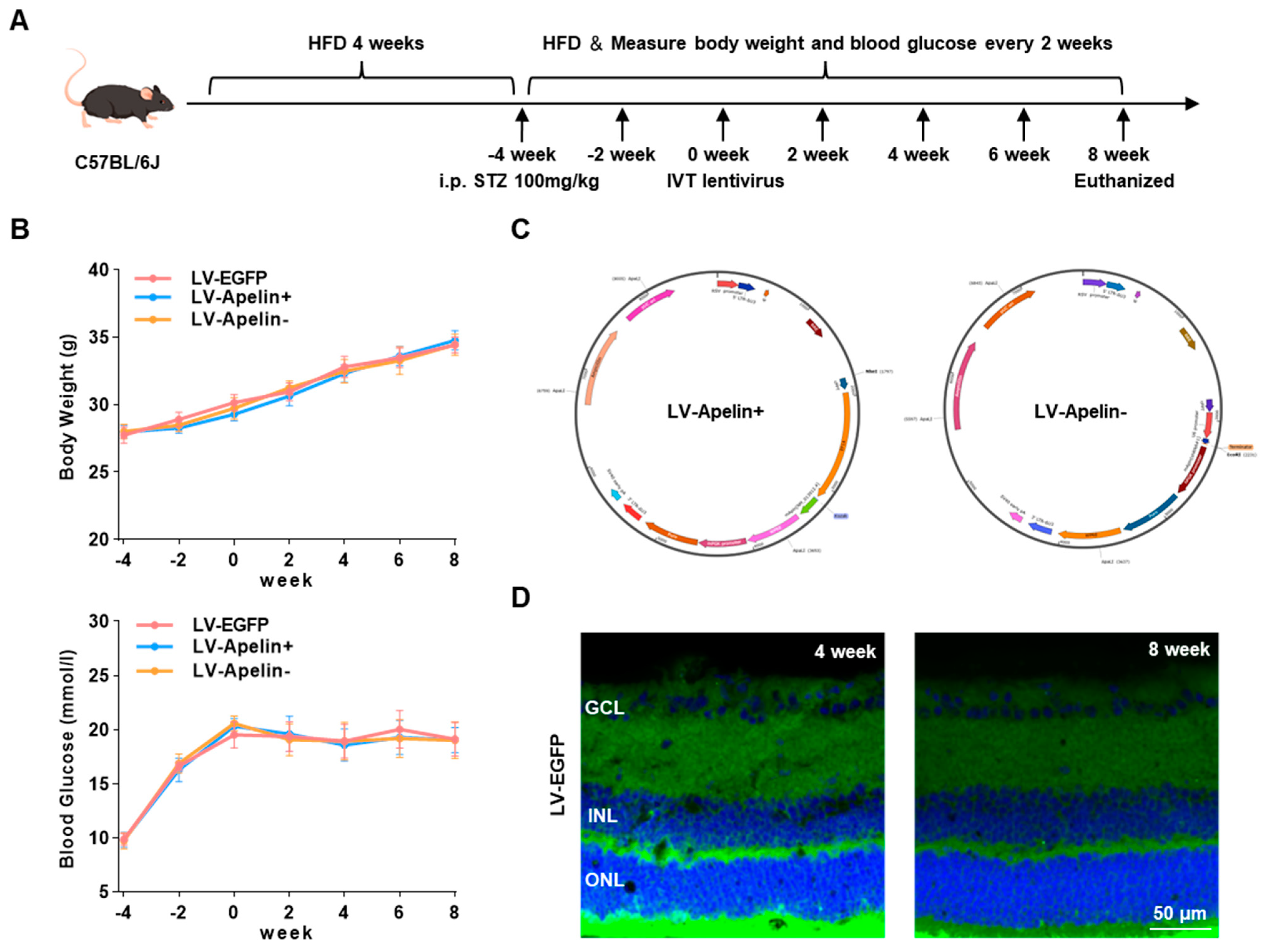
2.1. Construction of an High-Fat Diet/Streptozotocin (HFD/STZ)-Induced Type 2 Diabetic Mouse Model and Various Lentiviral Vectors
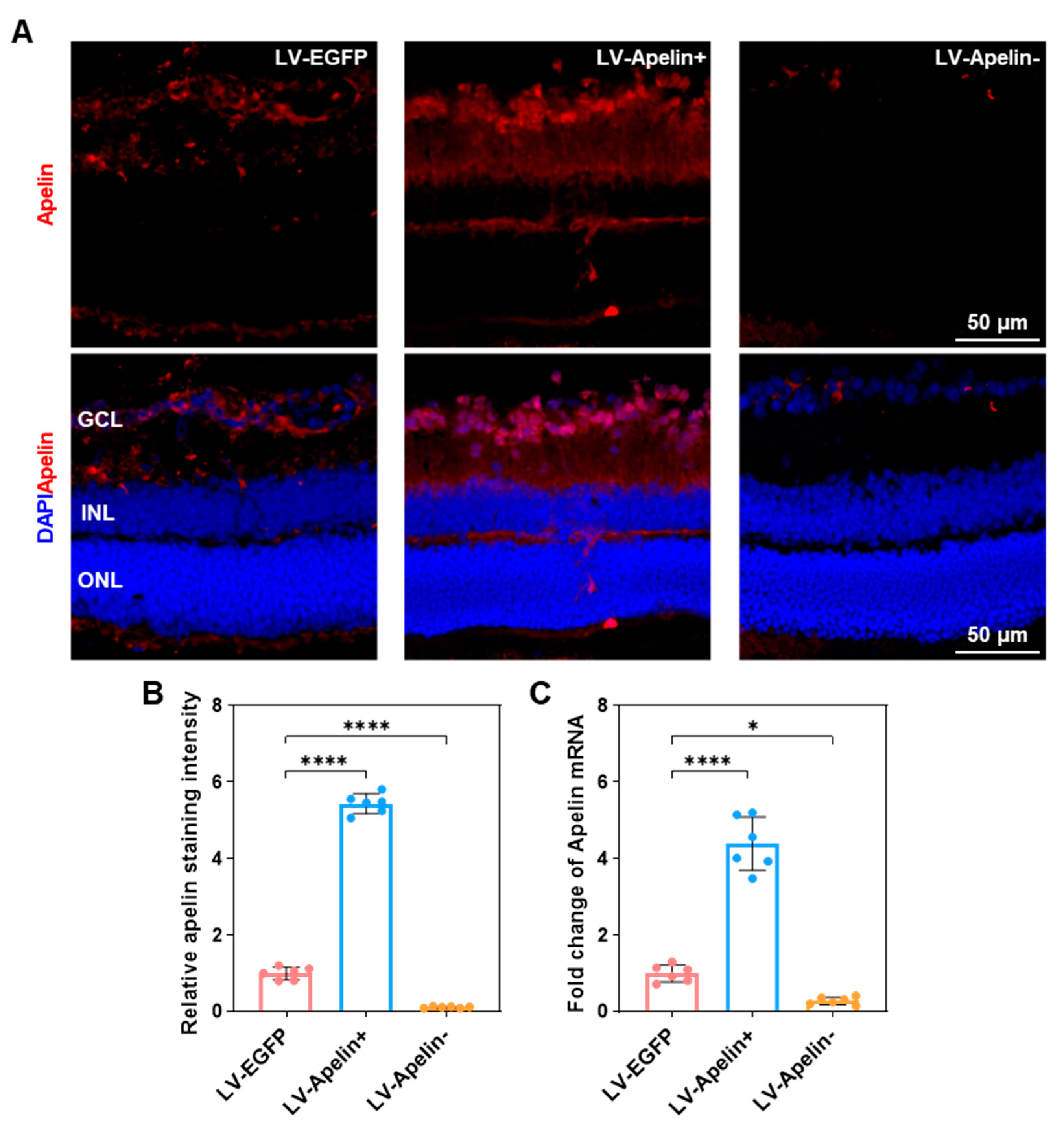
2.2. The Expression of Apelin in DR Mice Treated with LV-EGFP, LV-Apelin+ or LV-Apelin−
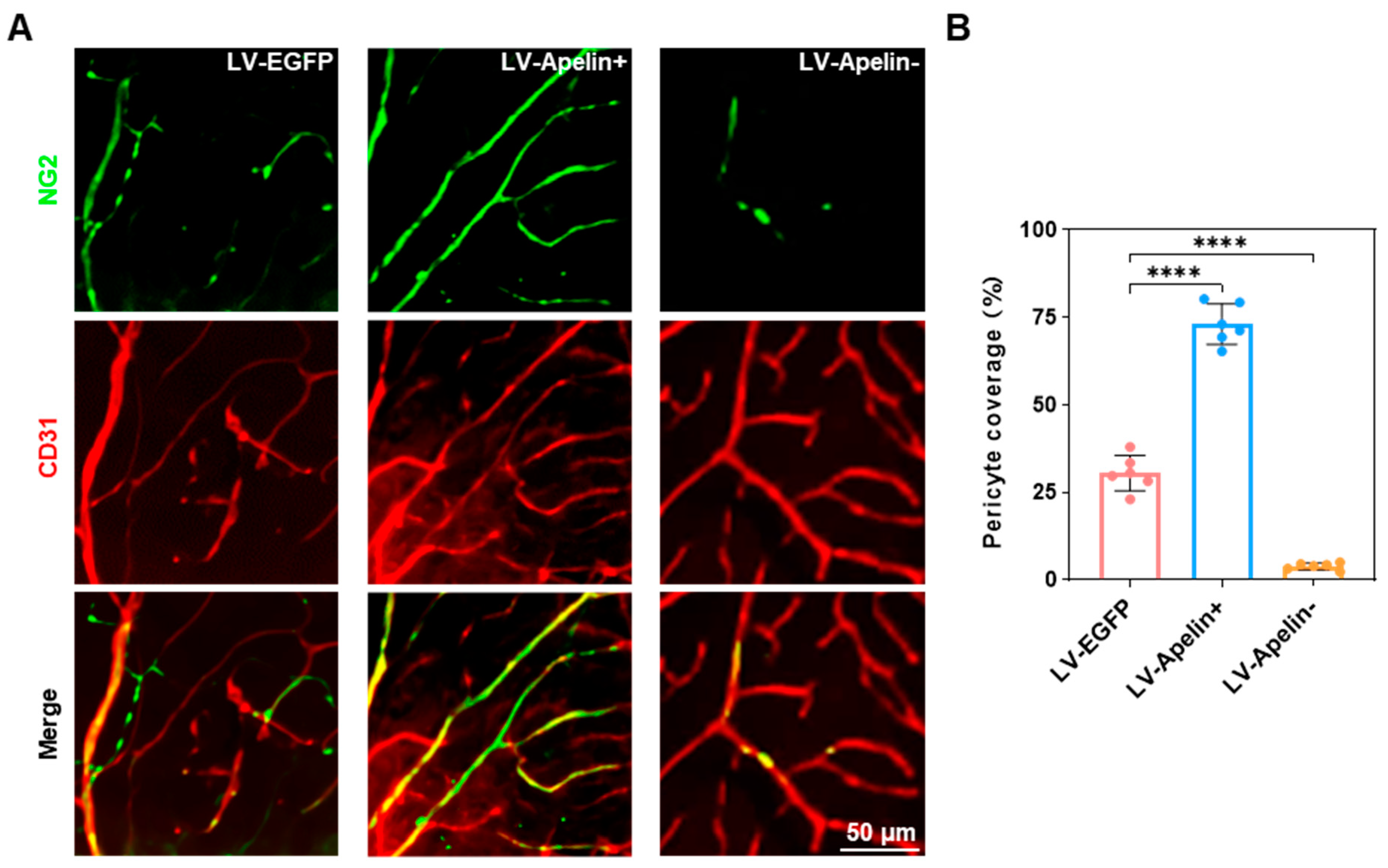
2.3. The Expression of Pericytes in DR Mice Treated with LV-EGFP, LV-Apelin+, or LV-Apelin−
2.4. Retinal Leakage in DR Mice Treated with LV-EGFP, LV-Apelin+, or LV-Apelin−
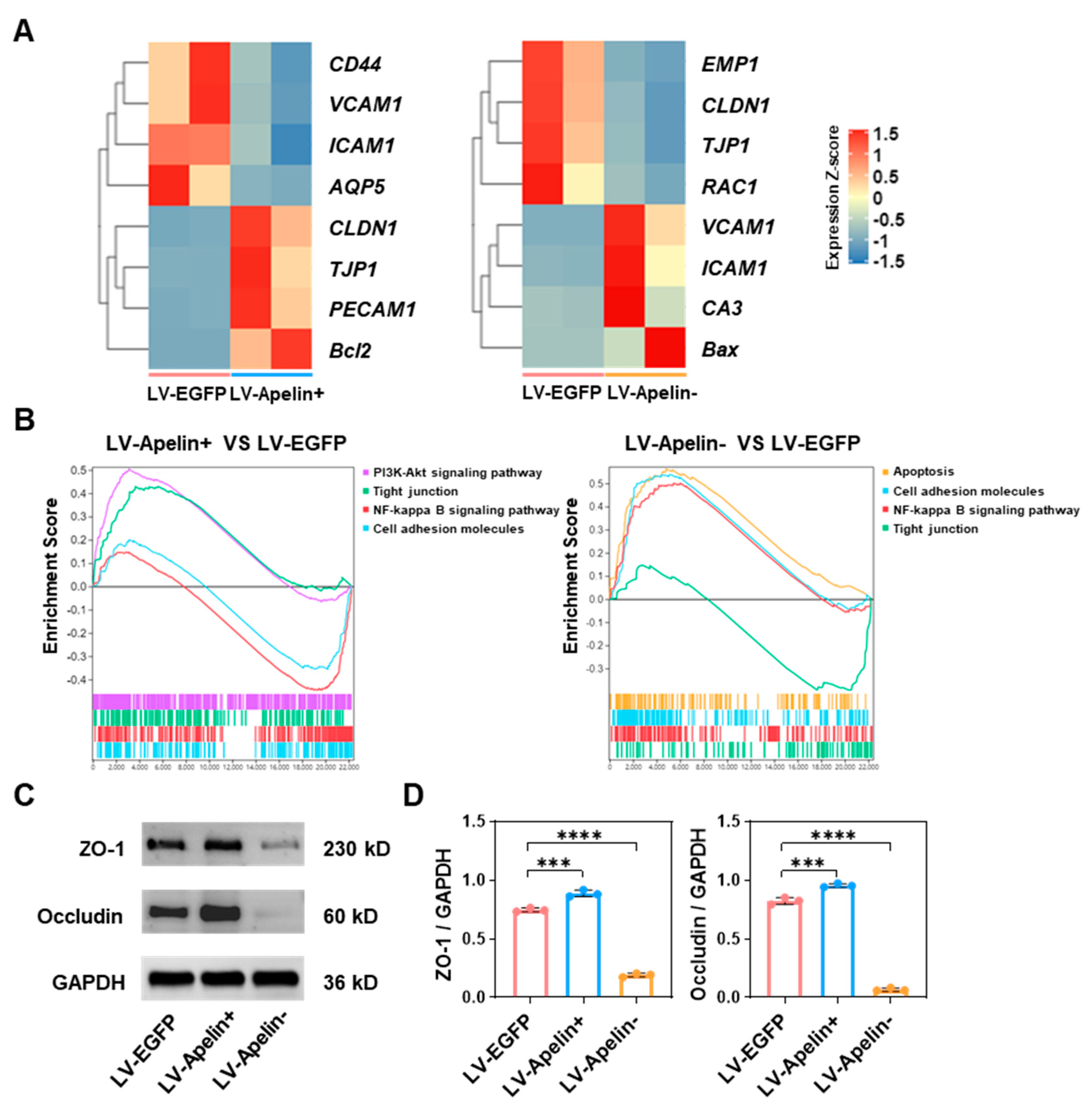
2.5. The Mechanism Underlying the Protective Role of Apelin in the Early Stages of Diabetic Retinopathy
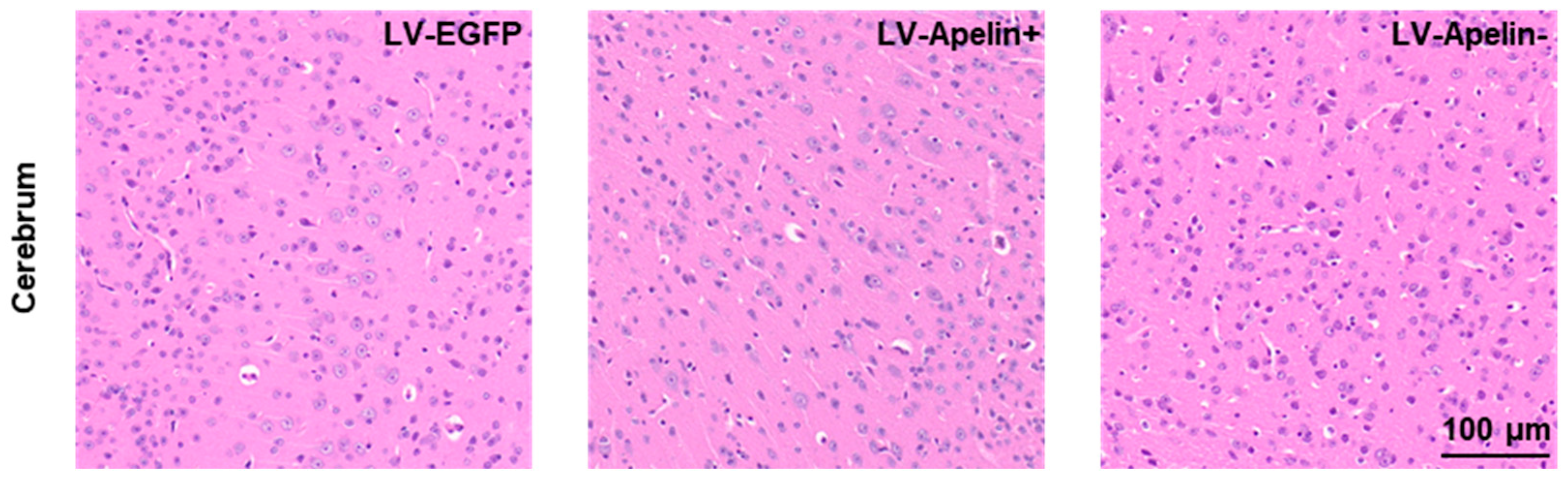
2.6. The Effect on the Cerebrum in DR Mice Treated with LV-EGFP, LV-Apelin+, or LV-Apelin−
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. HFD/STZ-Induced Type 2 Diabetic Mouse Model
4.3. Lentiviral Production
4.4. Immunofluorescence Staining of the Retina
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
4.6. Retinal Leakage Analysis
4.7. Transcriptome Sequencing Analysis
4.8. Western Blot Analysis
4.9. Hematoxylin-Eosin (HE) Staining of the Cerebrum
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groeneveld, Y.; Tavenier, D.; Blom, J.W.; Polak, B.C.P. Incidence of sight-threatening diabetic retinopathy in people with Type 2 diabetes mellitus and numbers needed to screen: A systematic review. Diabet. Med. 2019, 36, 1199–1208. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, M.; Zhang, L.; Zhang, L.; Kuang, J.; Zhang, G.; Liu, Q.; Guo, H.; Meng, Q. Prevalence and risk factors for diabetic retinopathy in a cross-sectional population-based study from rural southern China: Dongguan Eye Study. BMJ Open 2019, 9, e023586. [Google Scholar] [CrossRef]
- Arboleda-Velasquez, J.F.; Valdez, C.N.; Marko, C.K.; D’Amore, P.A. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr. Diabetes Rep. 2015, 15, 573. [Google Scholar] [CrossRef]
- Caporarello, N.; D’Angeli, F.; Cambria, M.T.; Candido, S.; Giallongo, C.; Salmeri, M.; Lombardo, C.; Longo, A.; Giurdanella, G.; Anfuso, C.D.; et al. Pericytes in Microvessels: From “Mural” Function to Brain and Retina Regeneration. Int. J. Mol. Sci. 2019, 20, 6351. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic roles of pericytes in the blood-retinal barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Ferland-McCollough, D.; Slater, S.; Richard, J.; Reni, C.; Mangialardi, G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol. Ther. 2017, 171, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Uribesalgo, I.; Hoffmann, D.; Zhang, Y.; Kavirayani, A.; Lazovic, J.; Berta, J.; Novatchkova, M.; Pai, T.P.; Wimmer, R.A.; László, V.; et al. Apelin inhibition prevents resistance and metastasis associated with anti-angiogenic therapy. EMBO Mol. Med. 2019, 11, e9266. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, X.L.; Li, L.B.; Huang, L.Y.; Tang, Z.; Luo, J.; Yang, L.; Qin, A.P.; Hu, F. miR-503/Apelin-12 mediates high glucose-induced microvascular endothelial cells injury via JNK and p38MAPK signaling pathway. Regen. Ther. 2020, 14, 111–118. [Google Scholar] [CrossRef]
- Song, B.; Kim, D.; Nguyen, N.H.; Roy, S. Inhibition of Diabetes-Induced Lysyl Oxidase Overexpression Prevents Retinal Vascular Lesions Associated With Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5965–5972. [Google Scholar] [CrossRef]
- Kidoya, H.; Takakura, N. Biology of the apelin-APJ axis in vascular formation. J. Biochem. 2012, 152, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, L.; Jiang, Y.; Tao, Y. The Role of Apelin/APJ in a Mouse Model of Oxygen-induced Retinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 47. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tao, Y.; Feng, J.; Jiang, Y.R. Apelin Protects Primary Rat Retinal Pericytes from Chemical Hypoxia-Induced Apoptosis. J. Ophthalmol. 2015, 2015, 186946. [Google Scholar] [CrossRef] [PubMed]
- Kidoya, H.; Naito, H.; Takakura, N. Apelin induces enlarged and nonleaky blood vessels for functional recovery from ischemia. Blood 2010, 115, 3166–3174. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Kong, W. Endothelial tight junctions and their regulatory signaling pathways in vascular homeostasis and disease. Cell Signal 2020, 66, 109485. [Google Scholar] [CrossRef]
- Yun, J.H. Interleukin-1β induces pericyte apoptosis via the NF-κB pathway in diabetic retinopathy. Biochem. Biophys. Res. Commun. 2021, 546, 46–53. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, Y.S.; Roh, G.S.; Choi, W.S.; Cho, G.J. Resveratrol blocks diabetes-induced early vascular lesions and vascular endothelial growth factor induction in mouse retinas. Acta Ophthalmol. 2012, 90, e31–e37. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Mughal, A.; O’Rourke, S.T. Vascular effects of apelin: Mechanisms and therapeutic potential. Pharmacol. Ther. 2018, 190, 139–147. [Google Scholar] [CrossRef]
- Kasai, A.; Ishimaru, Y.; Higashino, K.; Kobayashi, K.; Yamamuro, A.; Yoshioka, Y.; Maeda, S. Inhibition of apelin expression switches endothelial cells from proliferative to mature state in pathological retinal angiogenesis. Angiogenesis 2013, 16, 723–734. [Google Scholar] [CrossRef]
- Aboouf, M.A.; Hamdy, N.M.; Amin, A.I.; El-Mesallamy, H.O. Genotype screening of APLN rs3115757 variant in Egyptian women population reveals an association with obesity and insulin resistance. Diabetes Res. Clin. Pract. 2015, 109, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yin, J.; Chang, J.; Zhang, J.; Wang, Y.; Huang, H.; Wang, W.; Zeng, X. Apelin/APJ relieve diabetic cardiomyopathy by reducing microvascular dysfunction. J. Endocrinol. 2021, 249, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Long, H.; Xu, B.; Luo, Y. Apelin attenuates postburn sepsis via a phosphatidylinositol 3-kinase/protein kinase B dependent mechanism: A randomized animal study. Int. J. Surg. 2015, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Yuan, L.Q.; Luo, X.H.; Huang, J.; Cui, R.R.; Guo, L.J.; Zhou, H.D.; Wu, X.P.; Liao, E.Y. Apelin suppresses apoptosis of human osteoblasts. Apoptosis 2007, 12, 247–254. [Google Scholar] [CrossRef]
- Zhong, L.; Simard, M.J.; Huot, J. Endothelial microRNAs regulating the NF-κB pathway and cell adhesion molecules during inflammation. FASEB J. 2018, 32, 4070–4084. [Google Scholar] [CrossRef]
- Neumann, U.H.; Ho, J.S.S.; Chen, S.; Tam, Y.Y.C.; Cullis, P.R.; Kieffer, T.J. Lipid nanoparticle delivery of glucagon receptor siRNA improves glucose homeostasis in mouse models of diabetes. Mol. Metab. 2017, 6, 1161–1172. [Google Scholar] [CrossRef]
- Cheng, Y.; Yu, X.; Zhang, J.; Chang, Y.; Xue, M.; Li, X.; Lu, Y.; Li, T.; Meng, Z.; Su, L.; et al. Pancreatic kallikrein protects against diabetic retinopathy in KK Cg-A(y)/J and high-fat diet/streptozotocin-induced mouse models of type 2 diabetes. Diabetologia 2019, 62, 1074–1086. [Google Scholar] [CrossRef]
- Zhuang, P.; Muraleedharan, C.K.; Xu, S. Intraocular Delivery of miR-146 Inhibits Diabetes-Induced Retinal Functional Defects in Diabetic Rat Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1646–1655. [Google Scholar] [CrossRef]
- Laliberté, A.M.; MacPherson, T.C.; Micks, T.; Yan, A.; Hill, K.A. Vision deficits precede structural losses in a mouse model of mitochondrial dysfunction and progressive retinal degeneration. Exp. Eye Res. 2011, 93, 833–841. [Google Scholar] [CrossRef]
- Qin, Y.J.; Xiao, K.; Zhong, Z.; Zhao, Y.; Yu, T.; Sun, X.F. LECT2 Ameliorates Blood-Retinal Barrier Impairment Secondary to Diabetes Via Activation of the Tie2/Akt/mTOR Signaling Pathway. Investig. Ophthalmol. Vis. Sci. 2022, 63, 7. [Google Scholar] [CrossRef]
- Lee, K.; Choi, J.O.; Hwang, A.; Bae, H.W.; Kim, C.Y. Ciliary Neurotrophic Factor Derived From Astrocytes Protects Retinal Ganglion Cells Through PI3K/AKT, JAK/STAT, and MAPK/ERK Pathways. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, F.; Ye, T.; Wang, J.; Lyu, C.; Qing, S.; Ding, Z.; Gao, X.; Jia, R.; Yu, D.; et al. Macrophage-tumor chimeric exosomes accumulate in lymph node and tumor to activate the immune response and the tumor microenvironment. Sci. Transl. Med. 2021, 13, eabb6981. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Yang, W.; Luan, F.; Ma, F.; Wang, Y.; Zhang, Y.; Liu, X.; Chen, L.; Hu, X.; Tao, Y. The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy. Int. J. Mol. Sci. 2022, 23, 14680. https://doi.org/10.3390/ijms232314680
Feng J, Yang W, Luan F, Ma F, Wang Y, Zhang Y, Liu X, Chen L, Hu X, Tao Y. The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy. International Journal of Molecular Sciences. 2022; 23(23):14680. https://doi.org/10.3390/ijms232314680
Chicago/Turabian StyleFeng, Jing, Weiqiang Yang, Fuxiao Luan, Fang Ma, Yingjie Wang, Yiquan Zhang, Xuhui Liu, Li Chen, Xiaofeng Hu, and Yong Tao. 2022. "The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy" International Journal of Molecular Sciences 23, no. 23: 14680. https://doi.org/10.3390/ijms232314680
APA StyleFeng, J., Yang, W., Luan, F., Ma, F., Wang, Y., Zhang, Y., Liu, X., Chen, L., Hu, X., & Tao, Y. (2022). The Protective Role of Apelin in the Early Stages of Diabetic Retinopathy. International Journal of Molecular Sciences, 23(23), 14680. https://doi.org/10.3390/ijms232314680






