One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control
Abstract
:1. Introduction
2. Results and Discussion
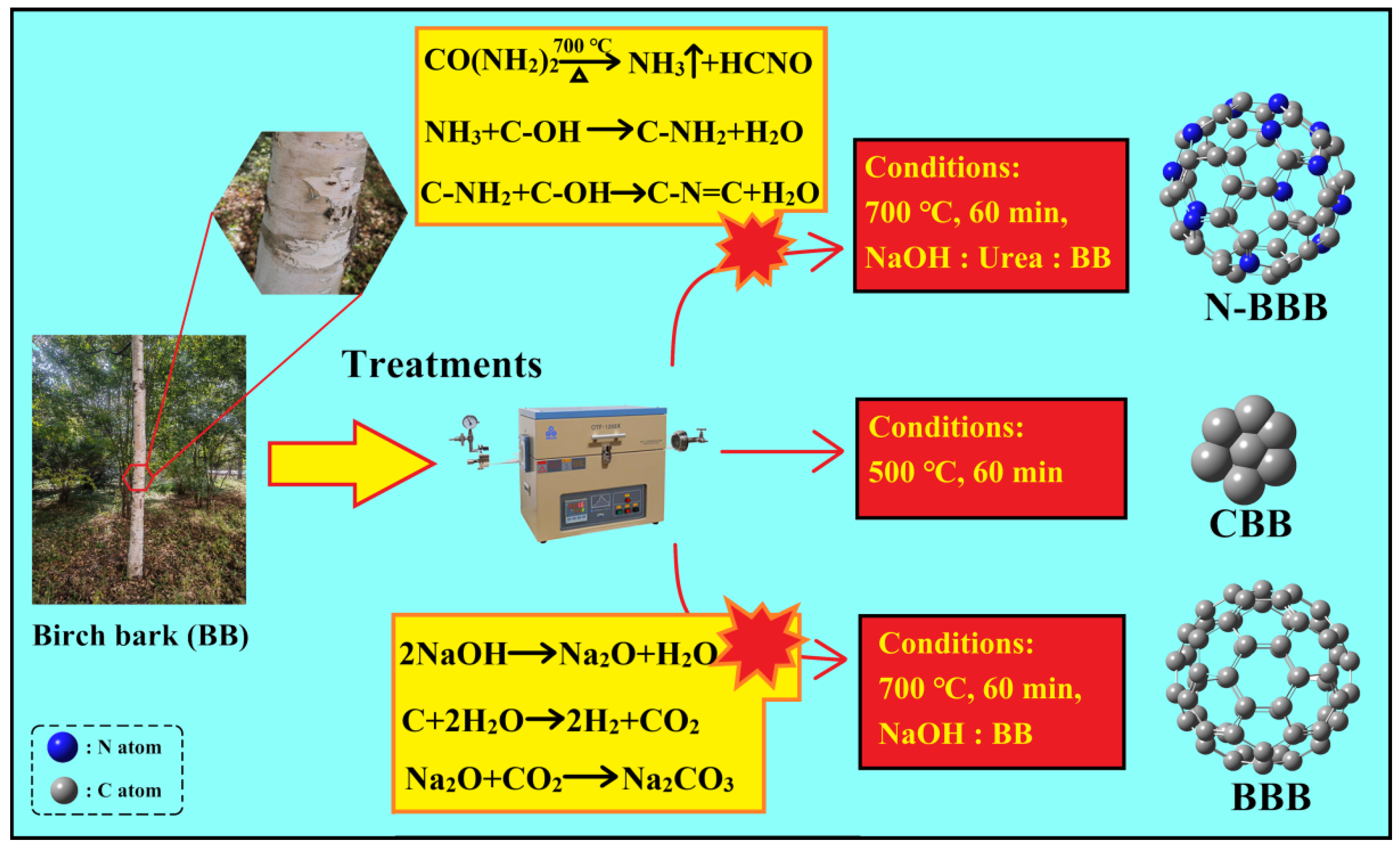
2.1. Preparation of CBB, BBB, and N-BBB
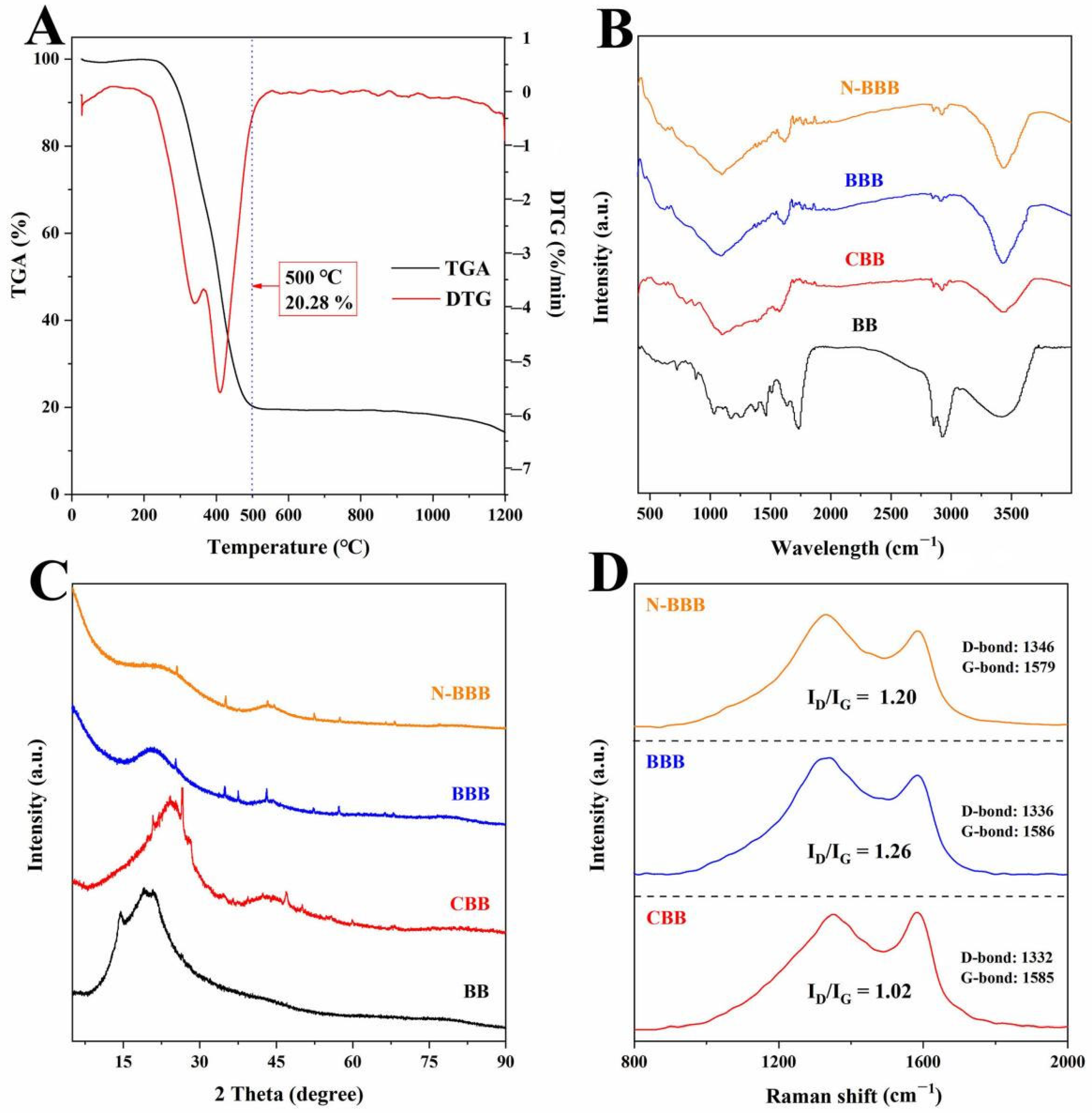
2.2. Results of Characterizations
2.3. Results of Adsorption Performances
2.3.1. Adsorption Kinetics
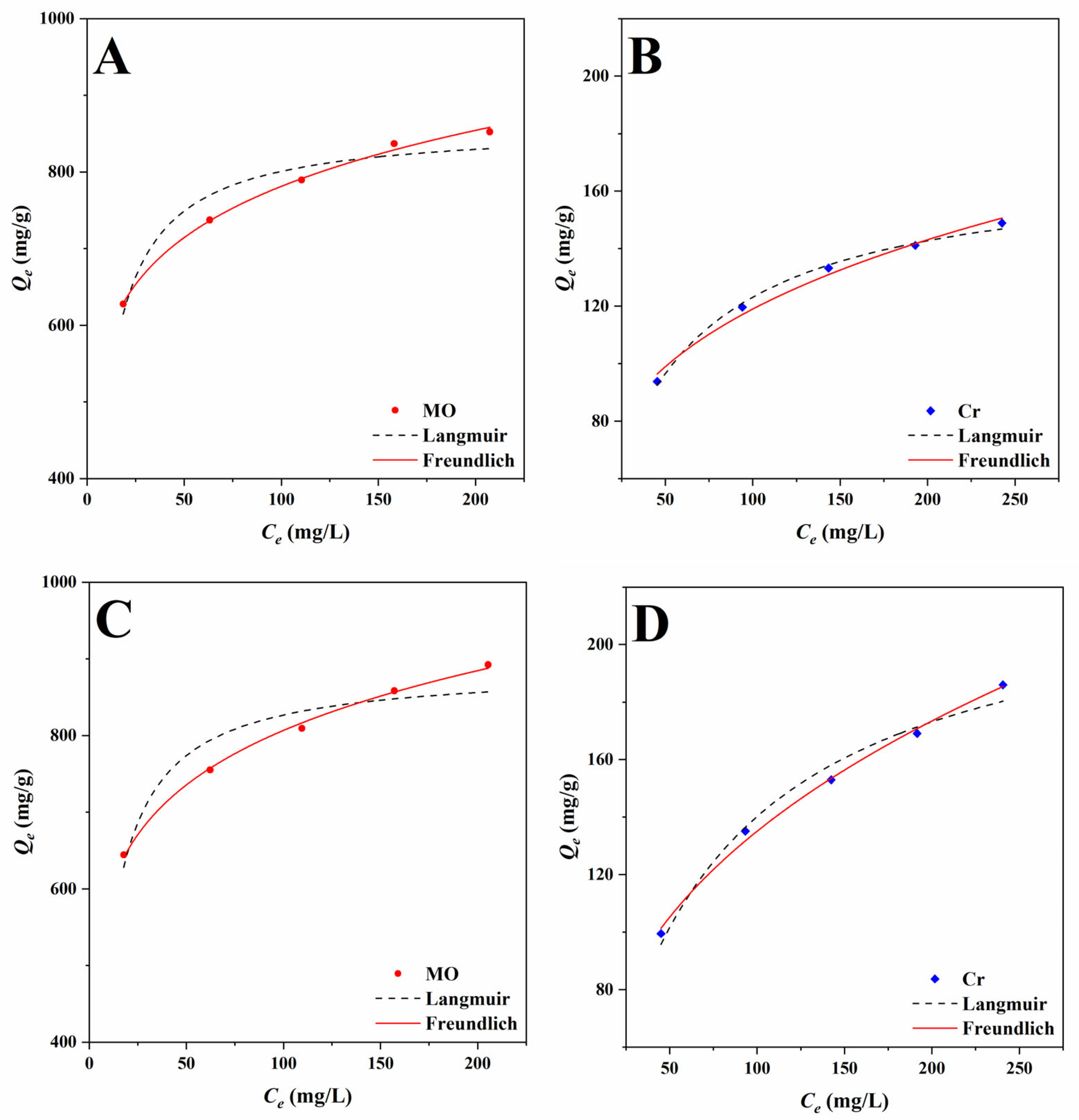
2.3.2. Adsorption Isotherms
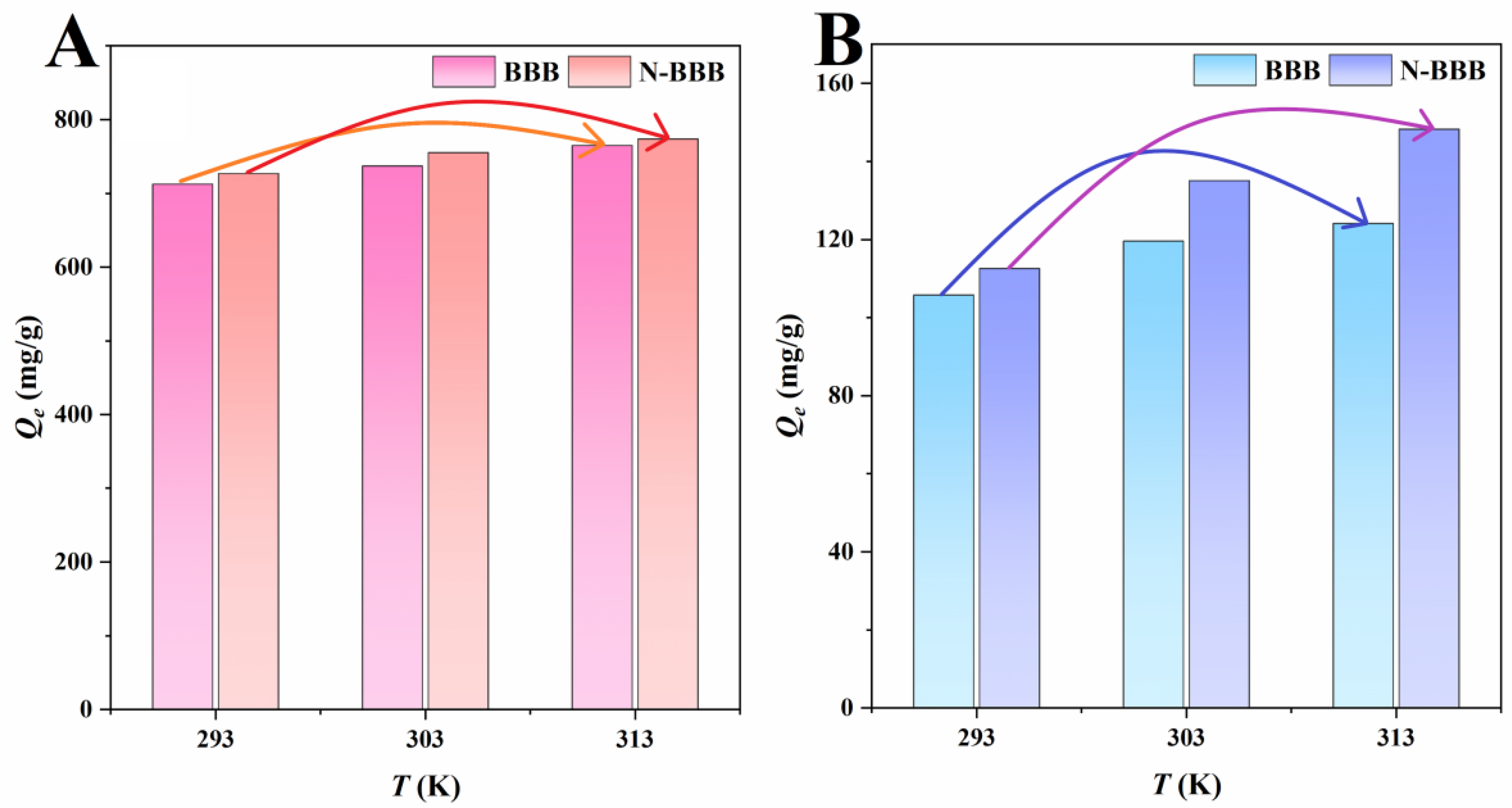
2.3.3. Adsorption Thermodynamics
2.3.4. Effect of pH
2.4. Results of Cycle Tests
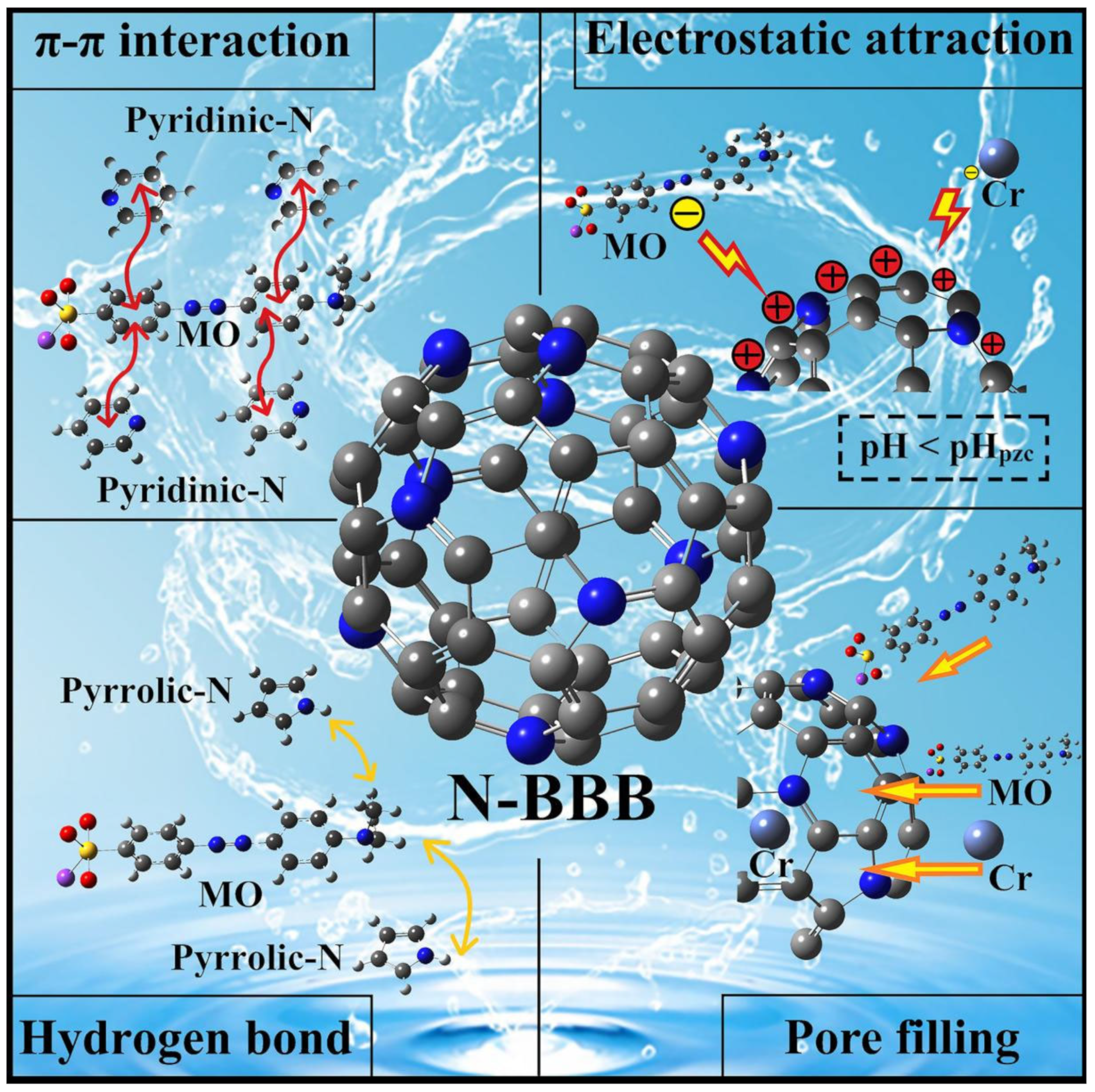
2.5. Probable Mechanism Analysis
2.6. Comparison
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Biochars
3.3. Adsorption Performances
3.4. Cycle Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, L.; Wu, Y.; Zhang, S.; Li, Y.; Gao, Y.; Zhang, W.; Tian, L.; Li, T.; Du, Q.; Sun, S. Recent development in microbial electrochemical technologies: Biofilm formation, regulation, and application in water pollution prevention and control. J. Water Process Eng. 2022, 49, 103135. [Google Scholar] [CrossRef]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal bioremediation of heavy metal pollution in water: Recent advances, challenges, and prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Xu, H.; Fan, Y.; Xia, X.; Liu, Z.; Yang, S. Effect of Ginkgo biloba leaves on the removal efficiency of Cr(VI) in soil and its underlying mechanism. Environ. Res. 2023, 216, 114431. [Google Scholar] [CrossRef]
- Njoya, O.; Zhao, S.; Shen, J.; Kong, X.; Gong, Y.; Wang, B.; Kang, J.; Chen, Z. Acetate improves catalytic performance for rapid removal of Cr(VI) by sodium borohydride in aqueous environments. Sep. Purif. Technol. 2022, 301, 122051. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Q.H.; Ma, K.; Li, H.M.; Guo, Z. Identification and determination of 34 water-soluble synthetic dyes infoodstuff by high performance liquid chromatography–diode arraydetection–ion trap time–of–flight tandem mass spectrometry. Food Chem. 2015, 182, 316–326. [Google Scholar] [CrossRef]
- Moghadas, M.R.S.; Motamedi, E.; Nasiri, J.; Naghavi, M.R.; Sabokdast, M. Proficient dye removal from water using biogenic silver nanoparticles prepared through solid-state synthetic route. Heliyon 2020, 6, e04730. [Google Scholar] [CrossRef]
- Mandal, B.; Ray, S.K. Synthesis of interpenetrating network hydrogel from poly(acrylic acid-co-hydroxyethyl methacrylate) and sodium alginate: Modeling and kinetics study for removal of synthetic dyes from water. Carbohyd. Polym. 2013, 98, 257–269. [Google Scholar] [CrossRef]
- Baig, U.; Kashif Uddin, M.; Gondal, M.A. Removal of hazardous azo dye from water using synthetic nano adsorbent: Facile synthesis, characterization, adsorption, regeneration and design of experiments. Colloids Surf. A 2020, 584, 124031. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Ambika; Kumar, V.; Jamwal, A.; Kumar, V.; Singh, D. Green bioprocess for degradation of synthetic dyes mixture using consortium of laccase-producing bacteria from Himalayan niches. J. Environ. Manag. 2022, 310, 114764. [Google Scholar] [CrossRef] [PubMed]
- Zaafouri, Z.; Batot, G.; Nieto-Draghi, C.; Coasne, B.; Bauer, D. Impact of adsorption kinetics on pollutant dispersion in water flowing in nanopores: A Lattice Boltzmann approach to stationary and transient conditions. Adv. Water Resour. 2022, 162, 104143. [Google Scholar] [CrossRef]
- Tee, G.T.; Gok, X.Y.; Yong, W.F. Adsorption of pollutants in wastewater via biosorbents, nanoparticles and magnetic biosorbents: A review. Environ. Res. 2022, 212, 113248. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal. Sep. Purif. Technol. 2022, 294, 121178. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Zhao, X.; Zhang, L.; Wang, B.; Nie, A.; Mu, C.; Xiang, J.; Zhai, K.; Xue, T.; et al. Lightweight and multifunctional super-hydrophobic aramid nanofiber/multiwalled carbon nanotubes/Fe3O4 aerogel for microwave absorption, thermal insulation and pollutants adsorption. J. Alloys Compd. 2022, 919, 165792. [Google Scholar] [CrossRef]
- Sellaoui, L.; Gomez-Aviles, A.; Dhaouadi, F.; Bedia, J.; Bonilla-Petriciolet, A.; Rtimi, S.; Belver, C. Adsorption of emerging pollutants on lignin-based activated carbon: Analysis of adsorption mechanism via characterization, kinetics and equilibrium studies. Chem. Eng. J. 2023, 452, 139399. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of biochar for the adsorption of organic pollutants from wastewater: Modification strategies, mechanisms and challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Qu, J.; Zhang, Y.; Liu, H. Characterization and mechanism analysis of tylosin biodegradation and simultaneous ammonia nitrogen removal with strain Klebsiella pneumoniae TN-1. Bioresour. Technol. 2021, 336, 125342. [Google Scholar] [CrossRef]
- Wu, J.; Wang, T.; Liu, Y.; Tang, W.; Geng, S.; Chen, J. Norfloxacin adsorption and subsequent degradation on ball-milling tailored N-doped biochar. Chemosphere 2022, 303, 135264. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, X.; Liu, S.; Li, X.; Wang, S.; Feng, Z.; Wu, Z.; Wang, L.; Jiang, Z.; Zhang, Y. One-step preparation of Fe/N co-doped porous biochar for chromium(VI) and bisphenol a decontamination in water: Insights to co-activation and adsorption mechanisms. Bioresour. Technol. 2022, 361, 127718. [Google Scholar] [CrossRef]
- Li, J.; Lin, Q.; Luo, H.; Fu, H.; Wu, L.; Chen, Y.; Ma, Y. The effect of nanoscale zero-valent iron-loaded N-doped biochar on the generation of free radicals and nonradicals by peroxydisulfate activation. J. Water Process Eng. 2022, 47, 102681. [Google Scholar] [CrossRef]
- Tan, X.; Zhu, S.; Wang, R.; Chen, Y.; Showf, P.; Zhang, F.; Ho, S. Role of biochar surface characteristics in the adsorption of aromatic compounds: Pore structure and functional groups. Chin. Chem. Lett. 2021, 32, 2939–2946. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, D.; Shao, J.; Zhang, X.; Yang, H.; Zhang, S.; Chen, H. Activation-free synthesis of nitrogen-doped biochar for enhanced adsorption of CO2. J. Clean. Prod. 2022, 355, 131642. [Google Scholar] [CrossRef]
- Liew, R.; Azwar, E.; Yek, P.; Lim, X.; Cheng, C.; Ng, J.; Jusoh, A.; Lam, W.; Ibrahim, M.; Ma, N.; et al. Microwave pyrolysis with KOH/NaOH mixture activation: A new approach to produce micro-mesoporous activated carbon for textile dye adsorption. Bioresour. Technol. 2018, 266, 1–10. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Xia, Y.; Chen, H.; Chen, G.; Tang, S. Influence of mixed alkali on the preparation of edible fungus substrate porous carbon material and its application for the removal of dye. Colloids Surf. A 2021, 609, 125675. [Google Scholar] [CrossRef]
- Wei, M.; Marrakchi, F.; Yuan, C.; Cheng, X.; Jiang, D.; Zafar, F.F.; Fu, Y.; Wang, S. Adsorption modeling, thermodynamics, and DFT simulation of tetracycline onto mesoporous and high-surface-area NaOH-activated macroalgae carbon. J. Hazard. Mater. 2022, 425, 127887. [Google Scholar] [CrossRef] [PubMed]
- Alfatah, T.; Mistar, E.M.; Supardan, M.D. Porous structure and adsorptive properties of activated carbon derived from Bambusa vulgaris striata by two-stage KOH/NaOH mixture activation for Hg2+ removal. J. Water Process Eng. 2021, 43, 102294. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S. Tuning ratios of KOH and NaOH on acetic acid-mediated chitosan-based porous carbons for improving their textural features and CO2 uptakes. J. CO2 Util. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Q.; Lai, J.; Cai, Z.; Lv, J.; Chen, X.; Chen, Y.; Zheng, X.; Huang, B.; Lin, G. Nitrogen-doped activated carbons via melamine-assisted NaOH/KOH/urea aqueous system for high performance supercapacitors. Mater. Chem. Phys. 2020, 250, 123201. [Google Scholar] [CrossRef]
- Hou, Z.; Tao, Y.; Bai, T.; Liang, Y.; Huang, S.; Cai, J. Efficient Rhodamine B removal by N-doped hierarchical carbons obtained from KOH activation and urea-oxidation of glucose hydrochar. J. Environ. Chem. Eng. 2021, 9, 105757. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Y.; Gu, Z.; Fan, Q.H. Symmetric supercapacitors using urea-modified lignin derived Ndoped porous carbon as electrode materials in liquid and solid electrolytes. J. Power Sources 2016, 332, 180–186. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Zhang, B.; Chen, G.; Chen, H.; Tang, S. Combining biological and chemical methods to disassemble of cellulose from corn straw for the preparation of porous carbons with enhanced adsorption performance. Int. J. Biol. Macromol. 2022, 209, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xia, Y.; Zhang, B.; Chen, H.; Chen, G.; Tang, S. Disassembly of lignocellulose into cellulose, hemicellulose, and lignin for preparation of porous carbon materials with enhanced performances. J. Hazard. Mater. 2021, 408, 124956. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Porous carbon materials based on Physalis alkekengi L. husk and its application for removal of malachite green. Environ. Technol. Innov. 2021, 21, 101343. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Y.; Qi, J.; Chen, H.; Chen, G.; Tang, S. Preparation of biomass carbon material based on Fomes fomentarius via alkali activation and its application for the removal of brilliant green in wastewater. Environ. Technol. Innov. 2021, 23, 101659. [Google Scholar] [CrossRef]
- Fan, C.; Yan, J.; Huang, Y.; Han, X.; Jiang, X. XRD and TG-FTIR study of the effect of mineral matrix on the pyrolysis and combustion of organic matter in shale char. Fuel 2015, 139, 502–510. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; Han, X.; Liu, J. A TG–FTIR investigation to the catalytic effect of mineral matrix in oil shaleon the pyrolysis and combustion of kerogen. Fuel 2013, 104, 307–317. [Google Scholar] [CrossRef]
- Zhang, B.; Jin, Y.; Huang, X.; Tang, S.; Chen, H.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Biological self-assembled hyphae/starch porous carbon composites for removal of organic pollutants from water. Chem. Eng. J. 2022, 450, 138264. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, B.; Guo, Z.; Tang, S.; Su, Y.; Yu, X.; Chen, S.; Chen, G. Fungal mycelium modified hierarchical porous carbon with enhanced performance and its application for removal of organic pollutants. J. Environ. Chem. Eng. 2022, 10, 108699. [Google Scholar] [CrossRef]
- Chen, X.; Yu, G.; Chen, Y.; Tang, S.; Su, Y. Cow dung-based biochar materials prepared via mixed base and its application in the removal of organic pollutants. Int. J. Mol. Sci. 2022, 23, 10094. [Google Scholar] [CrossRef]
- Chauhdary, Y.; Hanif, M.A.; Rashid, U.; Bhatti, I.A.; Anwar, H.; Jamil, Y.; Alharthi, F.A.; Kazerooni, E.A. Effective removal of reactive and direct dyes from colored wastewater using low-cost novel bentonite nanocomposites. Water 2022, 14, 3604. [Google Scholar] [CrossRef]
- Lin, L.; Li, L.; Xiao, L.; Zhang, C.; Li, X.; Pervez, M.N.; Zhang, Y.; Nuruzzaman, M.; Mondal, M.I.H.; Cai, Y.; et al. Adsorption behaviour of reactive blue 194 on raw Ramie Yarn in palm oil and water media. Materials 2022, 15, 7818. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, N.S.; Salama, A.; Guarino, V. Coupling of 3-Aminopropyl sulfonic acid to cellulose nanofifibers for effificient removal of cationic dyes. Materials 2022, 15, 6964. [Google Scholar] [CrossRef] [PubMed]
- Shirendev, N.; Bat-Amgalan, M.; Kano, N.; Kim, H.-J.; Gunchin, B.; Ganbat, B.; Yunden, G. A natural zeolite developed with 3-Aminopropyltriethoxylane and adsorption of Cu(II) from aqueous media. Appl. Sci. 2022, 12, 11344. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, L.; Sun, H.; Deng, Y.; Zhang, T.; Liu, G. Synthesis of benzxazine-based nitrogen-doped mesoporous carbon spheres for methyl orange dye adsorption. J. Porous Mater. 2017, 24, 1565–1574. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Y.; Cha, L. Removal of methyl orange dye using activated biochar derived from pomelo peel wastes: Performance, isotherm, and kinetic studies. J. Disper. Sci. Technol. 2018, 41, 1561298. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.; Zhang, Z. A versatile EDTA and chitosan bi-functionalized magnetic bamboo biochar for simultaneous removal of methyl orange and heavy metals from complex wastewater. Environ. Pollut. 2022, 293, 118517. [Google Scholar] [CrossRef]
- Ouedrhiri, A.; Himi, M.A.; Youbi, B.; Lghazi, Y.; Bahar, J.; Haimer, C.E.; Aynaou, A.; Bimaghra, I. Biochar material derived from natural waste with superior dye adsorption performance. Mater. Today. Proc. 2022, 66, 259–267. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, Y.; Hu, H.; Tao, Y.; Zhou, J.; Cai, J. Facile preparation of multi-porous biochar from lotus biomass for methyl orange removal: Kinetics, isotherms, and regeneration studies. Bioresour. Technol. 2021, 329, 124877. [Google Scholar] [CrossRef]
- Aichour, A.; Zaghouane-Boudiaf, H.; Khodja, H.D. Highly removal of anionic dye from aqueous medium using a promising biochar derived from date palm petioles: Characterization, adsorption properties and reuse studies. Arab. J. Chem. 2022, 15, 103542. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Liu, L.; Liu, P.; Zhou, Z.; Huhetaoli; Wu, Y.; Lei, T. Characteristics and adsorption of Cr(VI) of biochar pyrolyzed from landfill leachate sludge. J. Anal. Appl. Pyrolysis 2022, 162, 105449. [Google Scholar] [CrossRef]
- Zhen, Z.; Duan, X.; Tie, J. One-pot synthesis of a magnetic Zn/iron-based sludge/biochar composite for aqueous Cr(VI) adsorption. Environ. Technol. Innov. 2022, 28, 102661. [Google Scholar] [CrossRef]
- Xu, D.; Sun, T.; Jia, H.; Sun, Y.; Zhu, X. The performance and mechanism of Cr(VI) adsorption by biochar derived from Potamogeton crispus at different pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2022, 167, 105662. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, X.; Zhang, Y.; Ma, J.; Ning, P. Adsorption properties and mechanism of Cr(VI) by Fe2(SO4)3 modified biochar derived from Egeria najas. Colloids Surf. A 2022, 645, 128938. [Google Scholar] [CrossRef]
- Kuang, Q.; Liu, K.; Wang, Q.; Chang, Q. Three-dimensional hierarchical pore biochar prepared from soybean protein and its excellent Cr(VI) adsorption. Sep. Purif. Technol. 2023, 304, 122295. [Google Scholar] [CrossRef]






| Samples | SBET (m2/g) | Vmicro (cm3/g) | Vtotal (cm3/g) |
|---|---|---|---|
| CBB | 49.5 | 0.0187 | 0.0222 |
| BBB | 2502.3 | 1.1118 | 1.1389 |
| N-BBB | 2292.7 | 1.0170 | 1.0356 |
| Adsorbates | Adsorbents | Models | Parameters | C0 (mg L−1) | ||
|---|---|---|---|---|---|---|
| 50 | 100 | 200 | ||||
| MO | BBB | Qe (mg/g) | 627.9 | 737.2 | 836.9 | |
| PFK | k1 (min−1) | 0.0028 | 0.0074 | 0.0058 | ||
| Qe.cat (mg/g) | 611.8 | 720.4 | 800.3 | |||
| R2 | 0.9880 | 0.9944 | 0.9780 | |||
| PSK | k2 (g mg−1 min−1) | 0.0003 | 0.0003 | 0.0005 | ||
| Qe.cat (mg/g) | 664.5 | 769.0 | 844.2 | |||
| R2 | 0.9848 | 0.9896 | 0.9997 | |||
| IPD | k3 (mg g−1 min−0.5) | 27.12 | 23.62 | 19.90 | ||
| C | 350.1 | 497.9 | 628.2 | |||
| R2 | 0.6464 | 0.5822 | 0.7517 | |||
| N-BBB | Qe (mg/g) | 644.4 | 755.1 | 858.3 | ||
| PFK | k1 (min−1) | 0.0044 | 0.0054 | 0.0051 | ||
| Qe.cat (mg/g) | 631.3 | 732.9 | 817.1 | |||
| R2 | 0.9952 | 0.9885 | 0.9721 | |||
| PSK | k2 (g mg−1 min−1) | 0.0004 | 0.0004 | 0.0005 | ||
| Qe.cat (mg/g) | 672.1 | 777.9 | 862.8 | |||
| R2 | 0.9873 | 0.9980 | 0.9989 | |||
| IPD | k3 (mg g−1 min−0.5) | 19.4 | 22.0 | 20.9 | ||
| C | 448.7 | 530.3 | 637.3 | |||
| R2 | 0.5471 | 0.6549 | 0.7830 | |||
| Cr6+ | BBB | Qe (mg/g) | 93.8 | 119.6 | 141.1 | |
| PFK | k1 (min−1) | 0.0014 | 0.0017 | 0.0020 | ||
| Qe.cat (mg/g) | 85.3 | 109.2 | 130.4 | |||
| R2 | 0.9741 | 0.9614 | 0.9550 | |||
| PSK | k2 (g mg−1 min−1) | 0.0007 | 0.0008 | 0.0008 | ||
| Qe.cat (mg/g) | 98.4 | 122.3 | 143.6 | |||
| R2 | 0.9923 | 0.9919 | 0.9913 | |||
| IPD | k3 (mg g−1 min−0.5) | 6.2 | 6.9 | 7.3 | ||
| C | 22.7 | 41.4 | 59.1 | |||
| R2 | 0.9138 | 0.9280 | 0.9279 | |||
| N-BBB | Qe (mg/g) | 99.5 | 135.0 | 169.1 | ||
| PFK | k1 (min−1) | 0.0015 | 0.0017 | 0.0017 | ||
| Qe.cat (mg/g) | 89.7 | 122.4 | 153.0 | |||
| R2 | 0.9500 | 0.9506 | 0.9318 | |||
| PSK | k2 (g mg−1 min−1) | 0.0007 | 0.0007 | 0.0006 | ||
| Qe.cat (mg/g) | 102.2 | 135.9 | 170.6 | |||
| R2 | 0.9843 | 0.9869 | 0.9765 | |||
| IPD | k3 (mg g−1 min−0.5) | 6.2 | 7.3 | 9.6 | ||
| C | 27.7 | 50.5 | 58.4 | |||
| R2 | 0.9537 | 0.9432 | 0.9746 | |||
| Adsorbates | Adsorbents | Types | Parameters | |
|---|---|---|---|---|
| MO | BBB | Langmuir | Qm (mg/g) | 860.3 |
| KL (L/mg) | 0.1345 | |||
| R2 | 0.9327 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 431.5 | ||
| nF | 7.75 | |||
| R2 | 0.9967 | |||
| N-BBB | Langmuir | Qm (mg/g) | 887.4 | |
| KL (L/mg) | 0.1364 | |||
| R2 | 0.9041 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 437.1 | ||
| nF | 7.51 | |||
| R2 | 0.9883 | |||
| Cr6+ | BBB | Langmuir | Qm (mg/g) | 169.8 |
| KL (L/mg) | 0.0264 | |||
| R2 | 0.9949 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 34.9 | ||
| nF | 3.76 | |||
| R2 | 0.9976 | |||
| N-BBB | Langmuir | Qm (mg/g) | 226.2 | |
| KL (L/mg) | 0.0163 | |||
| R2 | 0.9822 | |||
| Freundlich | KF (mg g−1(L mg−1)1/n) | 25.7 | ||
| nF | 2.77 | |||
| R2 | 0.9959 | |||
| Adsorbents | Adsorbates | T (K) | ∆G (kJ/mol) | ∆H (kJ/mol) | ∆S (J mol−1 K−1) |
|---|---|---|---|---|---|
| BBB | MO | 293 | −5.86 | 4.29 | 34.61 |
| 303 | −6.19 | ||||
| 313 | −6.55 | ||||
| Cr6+ | 293 | −0.27 | 6.47 | 23.01 | |
| 303 | −0.61 | ||||
| 313 | −0.73 | ||||
| N-BBB | MO | 293 | −5.93 | 3.82 | 33.27 |
| 303 | −6.29 | ||||
| 313 | −6.60 | ||||
| Cr6+ | 293 | −0.43 | 11.23 | 39.78 | |
| 303 | −0.93 | ||||
| 313 | −123 |
| Adsorbents | Qe for MO (mg/g) | Qe for Cr6+ (mg/g) | References |
|---|---|---|---|
| BBB | 836.9 | 141.1 | This work |
| N-BBB | 858.3 | 169.1 | This work |
| Pomelo peel biochar | 147.9 | - | [46] |
| Magnetic bamboo biochar | 305.4 | - | [47] |
| Date seeds biochar | 334.0 | - | [48] |
| Lotus root biochar | 449.0 | - | [49] |
| Date palm petioles biochar | 461.0 | - | [50] |
| Landfill leachate sludge biochar | - | 17.5 | [51] |
| Zn/iron-based sludge/biochar | - | 27.0 | [52] |
| Potamogeton crispus biochar | - | 34.4 | [53] |
| Egeria najas biochar | - | 138.8 | [54] |
| Soybean protein biochar | - | 489.7 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.; Shi, Y.; Jiang, M.; Chen, S. One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control. Int. J. Mol. Sci. 2022, 23, 14618. https://doi.org/10.3390/ijms232314618
Su Y, Shi Y, Jiang M, Chen S. One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control. International Journal of Molecular Sciences. 2022; 23(23):14618. https://doi.org/10.3390/ijms232314618
Chicago/Turabian StyleSu, Yingjie, Yuqing Shi, Meiyi Jiang, and Siji Chen. 2022. "One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control" International Journal of Molecular Sciences 23, no. 23: 14618. https://doi.org/10.3390/ijms232314618
APA StyleSu, Y., Shi, Y., Jiang, M., & Chen, S. (2022). One-Step Synthesis of Nitrogen-Doped Porous Biochar Based on N-Doping Co-Activation Method and Its Application in Water Pollutants Control. International Journal of Molecular Sciences, 23(23), 14618. https://doi.org/10.3390/ijms232314618





