Assessment of Bone Microstructure by Micro CT in C57BL/6J Mice for Sex-Specific Differentiation
Abstract
:1. Introduction
2. Results
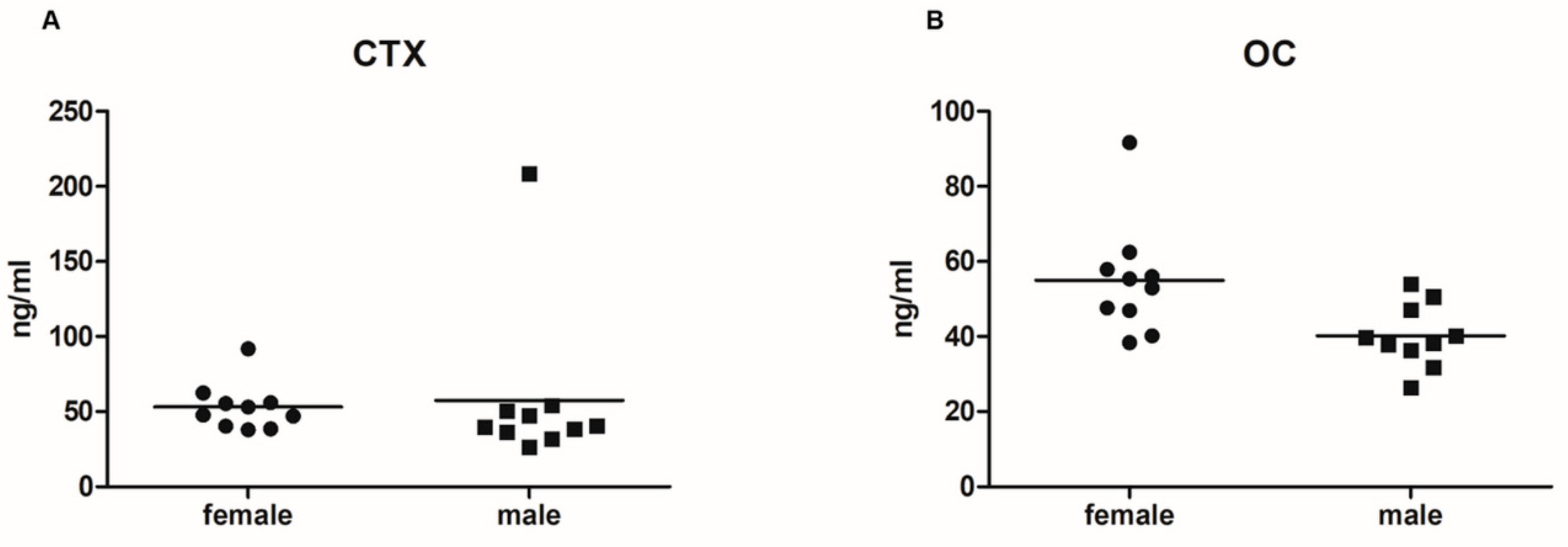
2.1. Serum Levels of Bone Turnover Markers
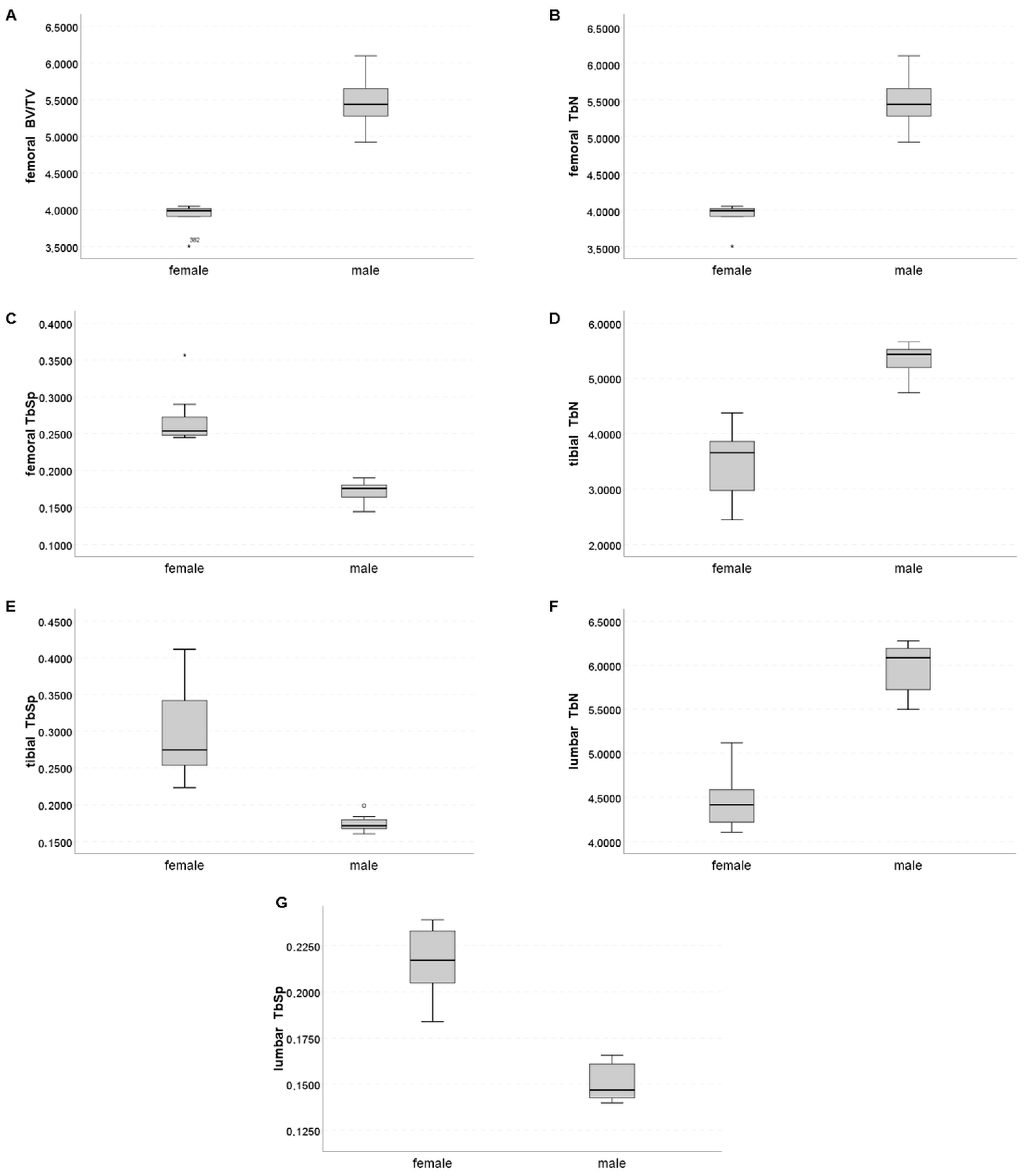
2.2. Trabecular Compartment
2.3. Cortical Compartment
2.4. Sex-Specific Discrimination
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Collection
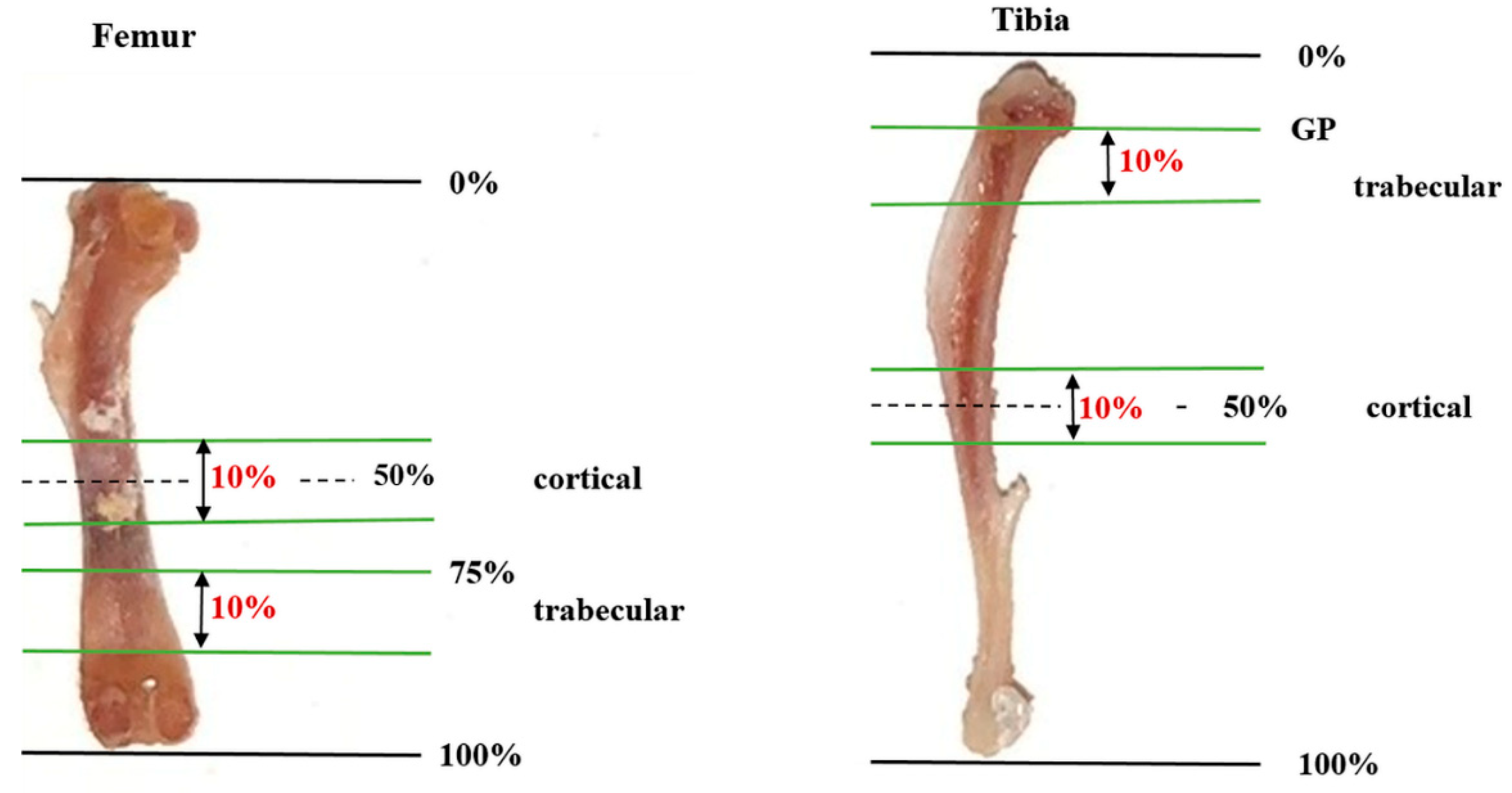
4.3. Analysis of Bone Microstructure
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amling, M.; Herden, S.; Pösl, M.; Hahn, M.; Ritzel, H.; Delling, G. Heterogeneity of the skeleton: Comparison of the trabecular microarchitecture of the spine, the iliac crest, the femur, and the calcaneus. J. Bone Miner. Res. 1996, 11, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Perilli, E.; Bala, Y.; Zebaze, R.; Reynolds, K.J.; Seeman, E. Regional Heterogeneity in the Configuration of the Intracortical Canals of the Femoral Shaft. Calcif. Tissue Int. 2015, 97, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Riggs, B.L.; Atkinson, E.J.; Oberg, A.L.; McDaniel, L.J.; Holets, M.; Peterson, J.M.; Melton, L.J. Effects of Sex and Age on Bone Microstructure at the Ultradistal Radius: A Population-Based Noninvasive In Vivo Assessment. J. Bone Miner. Res. 2005, 21, 124–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalzell, N.; Kaptoge, S.; Morris, N.; Berthier, A.; Koller, B.; Braak, L.; van Rietbergen, B.; Reeve, J. Bone micro-architecture and determinants of strength in the radius and tibia: Age-related changes in a population-based study of normal adults measured with high-resolution pQCT. Osteoporos. Int. 2009, 20, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Burrows, M.; Liu, D.; Moore, S.; McKay, H. Bone microstructure at the distal tibia provides a strength advantage to males in late puberty: An HR-pQCT study. J. Bone Miner. Res. 2010, 25, 1423–1432. [Google Scholar] [CrossRef]
- Sode, M.; Burghardt, A.J.; Kazakia, G.J.; Link, T.M.; Majumdar, S. Regional variations of gender-specific and age-related differences in trabecular bone structure of the distal radius and tibia. Bone 2010, 46, 1652–1660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazakia, G.J.; Nirody, J.A.; Bernstein, G.; Sode, M.; Burghardt, A.J.; Majumdar, S. Age- and gender-related differences in cortical geometry and microstructure: Improved sensitivity by regional analysis. Bone 2012, 52, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Popp, K.L.; Hughes, J.M.; Martinez-Betancourt, A.; Scott, M.; Turkington, V.; Caksa, S.; Guerriere, K.I.; Ackerman, K.E.; Xu, C.; Unnikrishnan, G.; et al. Bone mass, microarchitecture and strength are influenced by race/ethnicity in young adult men and women. Bone 2017, 103, 200–208. [Google Scholar] [CrossRef]
- Maeda, K.; Mochizuki, T.; Kobayashi, K.; Tanifuji, O.; Someya, K.; Hokari, S.; Katsumi, R.; Morise, Y.; Koga, H.; Sakamoto, M.; et al. Cortical thickness of the tibial diaphysis reveals age- and sex-related characteristics between non-obese healthy young and elderly subjects depending on the tibial regions. J. Exp. Orthop. 2020, 7, 78. [Google Scholar] [CrossRef]
- Chakhtoura, M.; Dagher, H.; Sharara, S.; Ajjour, S.; Chamoun, N.; Cauley, J.; Mahfoud, Z.; Boudreau, R.; Fuleihan, G.E.H. Systematic review of major osteoporotic fracture to hip fracture incidence rate ratios worldwide: Implications for Fracture Risk Assessment Tool (FRAX)-derived estimates. J. Bone Miner. Res. 2021, 36, 1942–1956. [Google Scholar] [CrossRef]
- Wronski, T.J.; Smith, J.M.; Jee, W.S.S. Variations in mineral apposition rate of trabecular bone within the beagle skeleton. Calcif. Tissue Int. 1981, 33, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Squire, M.; Donahue, L.-R.; Rubin, C.; Judex, S. Genetic variations that regulate bone morphology in the male mouse skeleton do not define its susceptibility to mechanical unloading. Bone 2004, 35, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-Related Changes in Trabecular Architecture Differ in Female and Male C57BL/6J Mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, M.; Föger-Samwald, U.; Wahl, K.; Kerschan-Schindl, K.; Pietschmann, P. Age- and Strain-Related Differences in Bone Microstructure and Body Composition During Development in Inbred Male Mouse Strains. Calcif. Tissue Int. 2020, 106, 431–443. [Google Scholar] [CrossRef] [Green Version]
- Eckstein, F.; Lochmüller, E.-M.; Koller, B.; Wehr, U.; Weusten, A.; Rambeck, W.; Hoeflich, A.; Wolf, E. Body composition, bone mass and microstructural analysis in GH-transgenic mice reveals that skeletal changes are specific to bone compartment and gender. Growth Horm. IGF Res. 2002, 12, 116–125. [Google Scholar] [CrossRef]
- Mohan, S.; Hu, Y.; Edderkaoui, B. Identification of gender-specific candidate genes that influence bone microarchitecture in chromosome 1. Calcif. Tissue Int. 2012, 92, 362–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, J.; Choudhary, D.; Khedgikar, V.; Kushwaha, P.; Singh, R.S.; Singh, D.; Tiwari, S.; Trivedi, R. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014, 111, 1811–1821. [Google Scholar] [CrossRef] [Green Version]
- Wejheden, C.; Brunnberg, S.; Larsson, S.; Lind, P.M.; Andersson, G.; Hanberg, A. Transgenic Mice with a Constitutively Active Aryl Hydrocarbon Receptor Display a Gender-Specific Bone Phenotype. Toxicol. Sci. 2009, 114, 48–58. [Google Scholar] [CrossRef]
- Somerville, J.M.; Aspden, R.M.; Armour, K.E.; Reid, D.M. Growth of C57Bl/6 Mice and the Material and Mechanical Properties of Cortical Bone from the Tibia. Calcif. Tissue Int. 2004, 74, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Mun, S.H.; Jastrzebski, S.; Kalinowski, J.; Zeng, S.; Oh, B.; Bae, S.; Eugenia, G.; Khan, N.M.; Drissi, H.; Zhou, P.; et al. Sexual Dimorphism in Differentiating Osteoclast Precursors Demonstrates Enhanced Inflammatory Pathway Activation in Female Cells. J. Bone Miner. Res. 2021, 36, 1104–1116. [Google Scholar] [CrossRef]
- Rauner, M.; Föger-Samwald, U.; Kurz, M.; Brünner-Kubath, C.; Schamall, D.; Kapfenberger, A.; Varga, P.; Kudlacek, S.; Wutzl, A.; Höger, H.; et al. Cathepsin S controls adipocytic and osteoblastic differentiation, bone turnover, and bone microarchitecture. Bone 2014, 64, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Washimi, Y.; Shoumura, S. Three-dimensional microstructure of the bone in a hamster model of senile osteoporosis. Bone 2008, 43, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Jiang, X.; Chen, L.; Josh, P.; Shin, D.G.; Rowe, D. Computer-Automated Static, Dynamic and Cellular Bone Histomorphometry. J. Tissue Sci. Eng. 2014, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Müller, R.; Link, T.M.; Lochmüller, E.-M. Sex Differences of Human Trabecular Bone Microstructure in Aging Are Site-Dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Shoumura, S.; Emura, S.; Bunai, Y. Regional variations of vertebral trabecular bone microstructure with age and gender. Osteoporos. Int. 2008, 19, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.W.; Hong, S.-H.; Zhang, C.; Shin, D.-G.; Adams, D.J.; Youngstrom, D.W.; Chen, L.; Wu, Z.; Zhou, Y.; Maye, P. Skeletal screening IMPC/KOMP using μCT and computer automated cryohistology: Application to the Efna4 KO mouse line. Bone 2020, 144, 115688. [Google Scholar] [CrossRef]
- Musy, S.N.; Maquer, G.; Panyasantisuk, J.; Wandel, J.; Zysset, P.K. Not only stiffness, but also yield strength of the trabecular structure determined by non-linear µFE is best predicted by bone volume fraction and fabric tensor. J. Mech. Behav. Biomed. Mater. 2017, 65, 808–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Föger-Samwald, U.; Papageorgiou, M.; Wahl-Figlash, K.; Kerschan-Schindl, K.; Pietschmann, P. Bone and muscle development in three inbred female mouse strains. Osteologie 2021, 30, 173–181. [Google Scholar] [CrossRef]
- MacKenzie, N.C.W.; Zhu, D.; Milne, E.M.; van’t Hof, R.; Martin, A.; Quarles, L.D.; Millán, J.L.; Farquharson, C.; Macrae, V.E. Altered Bone Development and an Increase in FGF-23 Expression in Enpp1−/− Mice. PLoS ONE 2012, 7, e32177. [Google Scholar] [CrossRef]
- Pennisi, P.; Clementi, G.; Prato, A.; Luca, T.; Martinez, G.; Mangiafico, R.A.; Pulvirenti, I.; Muratore, F.; Fiore, C.E. L-arginine supplementation normalizes bone turnover and preserves bone mass in streptozotocin-induced diabetic rats. J. Endocrinol. Investig. 2009, 32, 546–551. [Google Scholar] [CrossRef]
- Grote, H.; Amling, M.; Vogel, M.; Hahn, M.; Pösl, M.; Delling, G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone 1995, 16, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Kubo, K.-Y. Segmental variations in trabecular bone density and microstructure of the spine in senescence-accelerated mouse (SAMP6): A murine model for senile osteoporosis. Exp. Gerontol. 2012, 47, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
| Femur | Tibia | Th12 | L5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p Value | Female | Male | p Value | Female | Male | p Value | Female | Male | p Value | |
| BV/TV (%) | 0.162 [0.104; 0.199] | 0.366 [0.285; 0.397] | 0.0009 | 0.141 [0.113; 0.141] | 0.282 [0.223; 0.306] | 0.0040 | 0.362 [0.352; 0.380] | 0.438 [0.423; 0.452] | 0.0060 | 0.327 [0.313; 0.362] | 0.413 [0.388; 0.461] | 0.0060 |
| TbN (mm−1) | 3.99 [3.91; 4.03] | 5.44 [5.25; 5.66] | 0.0021 | 3.65 [2.89; 4.01] | 5.43 [5.19; 5.54] | 0.0004 | 5.15 [4.90; 5.34] | 6.41 [6.27; 6.70] | 0.0009 | 4.42 [4.20; 4.62] | 6.08 [5.69; 6.19] | 0.0009 |
| TbTh (mm) | 0.073 [0.060; 0.079] | 0.083 [0.073; 0.088] | n.s. | 0.067 [0.063; 0.070] | 0.068 [0.061; 0.069] | n.s. | 0.071 [0.067; 0.072] | 0.067 [0.066; 0.070] | n.s. | 0.070 [0.070; 0.071] | 0.071 [0.064; 0.075] | n.s. |
| TbSp (mm) | 0.254 [0.247; 0.281] | 0.176 [0.164; 0.180] | 0.0009 | 0.274 [0.246; 0.358] | 0.171 [0.168; 0.181] | 0.0004 | 0.178 [0.167; 0.187] | 0.132 [0.126; 0.132] | 0.0080 | 0.217 [0.202; 0.233] | 0.147 [0.142; 0.161] | 0.0009 |
| TbBMD (mgHA/cm3) | 651.0 [627.4; 686.2] | 686.6 [658.4; 704.5] | n.s. | 728.0 [706.9; 759.7] | 716.1 [703.3; 730.4] | n.s. | 676.2 [670.0; 703.9] | 668.7 [646.0; 680.6] | n.s. | 704.0 [684.6; 710.2] | 698.3 [664.5; 717.3] | n.s. |
| Femur | Tibia | Skull | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p-Value | Female | Male | p-Value | Female | Male | p-Value | |
| CtAr/TtAr (%) | 96.85 [96.28; 97.21] | 96.40 [96.66; 96.96] | n.s. | 95.47 [94.32; 96.43] | 96.56 [96.32; 96.84] | n.s. | 82.64 [79.90; 83.84] | 82.27 [80.21; 82.98] | n.s. |
| CtTh (mm) | 0.21 [0.21; 0.22] | 0.20 [0.20; 0.20] | 0.0348 | 0.21 [0.20; 0.21] | 0.21 [0.20; 0.22] | n.s. | 0.11 [0.11; 0.12] | 0.12 [0.12; 0.12] | n.s. |
| CtPo (%) | 3.2 [2.8; 3.7] | 3.6 [3.0; 4.3] | n.s. | 4.5 [3.6; 5.7] | 3.4 [3.2; 3.7] | n.s. | 17.4 [16.2; 20.1] | 17.7 [17.0; 19.8] | n.s. |
| CtBMD (mgHA/cm3) | 992.5 [974.6; 999.1] | 971.2 [949.4; 983.9] | n.s. | 932.7 [904.4; 966.3] | 960.5 [952.5; 967.3] | n.s. | 717.5 [708.6; 725.0] | 721.3 [709.4; 734.1] | n.s. |
| AUC—Femur | AUC—Tibia | AUC—Th12 | AUC—L5 | AUC—Skull | |
|---|---|---|---|---|---|
| Trabecular compartment | |||||
| BV/TV (%) | 1.000 | 0.937 | 0.956 | 0.962 | / |
| TbN (mm−1) | 1.000 | 1.000 | 0.989 | 1.000 | / |
| TbTh (mm) | 0.800 | 0.522 | 0.689 | 0.656 | / |
| TbSp (mm) | 1.000 | 1.000 | 0989 | 1.000 | / |
| TbBMD (mgHA/cm3) | 0.743 | 0.633 | 0.717 | 0.625 | / |
| Cortical compartment | |||||
| CtAr/TtAr (%) | 0.729 | 0.839 | / | / | 0.570 |
| CtTh (mm) | 0.914 | 0.622 | / | / | 0.675 |
| CtPo (%) | 0.729 | 0.839 | / | / | 0.570 |
| CtBMD (mgHA/cm3) | 0.771 | 0.711 | / | / | 0.560 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerschan-Schindl, K.; Papageorgiou, M.; Föger-Samwald, U.; Butylina, M.; Weber, M.; Pietschmann, P. Assessment of Bone Microstructure by Micro CT in C57BL/6J Mice for Sex-Specific Differentiation. Int. J. Mol. Sci. 2022, 23, 14585. https://doi.org/10.3390/ijms232314585
Kerschan-Schindl K, Papageorgiou M, Föger-Samwald U, Butylina M, Weber M, Pietschmann P. Assessment of Bone Microstructure by Micro CT in C57BL/6J Mice for Sex-Specific Differentiation. International Journal of Molecular Sciences. 2022; 23(23):14585. https://doi.org/10.3390/ijms232314585
Chicago/Turabian StyleKerschan-Schindl, Katharina, Maria Papageorgiou, Ursula Föger-Samwald, Maria Butylina, Michael Weber, and Peter Pietschmann. 2022. "Assessment of Bone Microstructure by Micro CT in C57BL/6J Mice for Sex-Specific Differentiation" International Journal of Molecular Sciences 23, no. 23: 14585. https://doi.org/10.3390/ijms232314585
APA StyleKerschan-Schindl, K., Papageorgiou, M., Föger-Samwald, U., Butylina, M., Weber, M., & Pietschmann, P. (2022). Assessment of Bone Microstructure by Micro CT in C57BL/6J Mice for Sex-Specific Differentiation. International Journal of Molecular Sciences, 23(23), 14585. https://doi.org/10.3390/ijms232314585





