Repeated Episodes of Ischemia/Reperfusion Induce Heme-Oxygenase-1 (HO-1) and Anti-Inflammatory Responses and Protects against Chronic Kidney Disease
Abstract
:1. Introduction
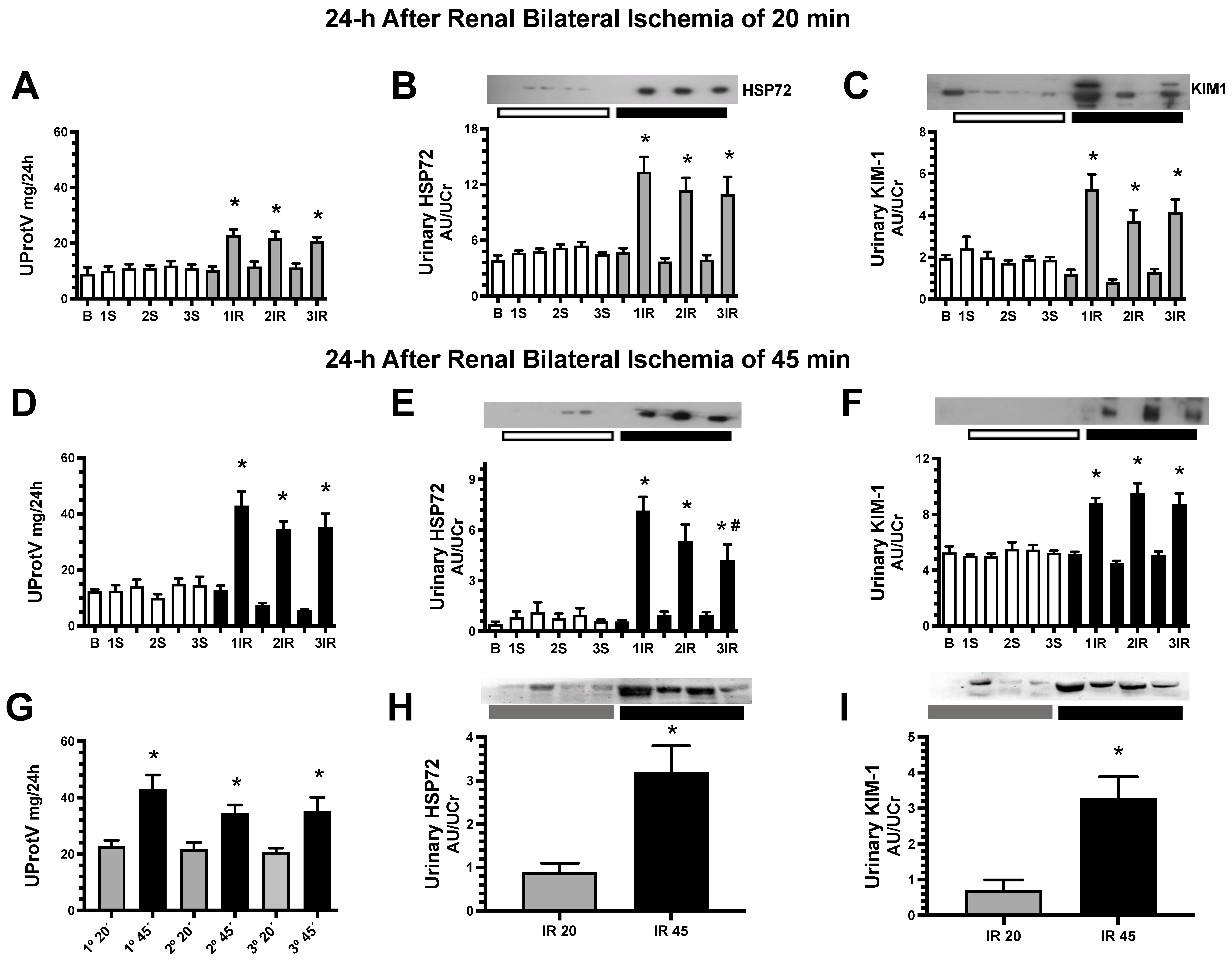
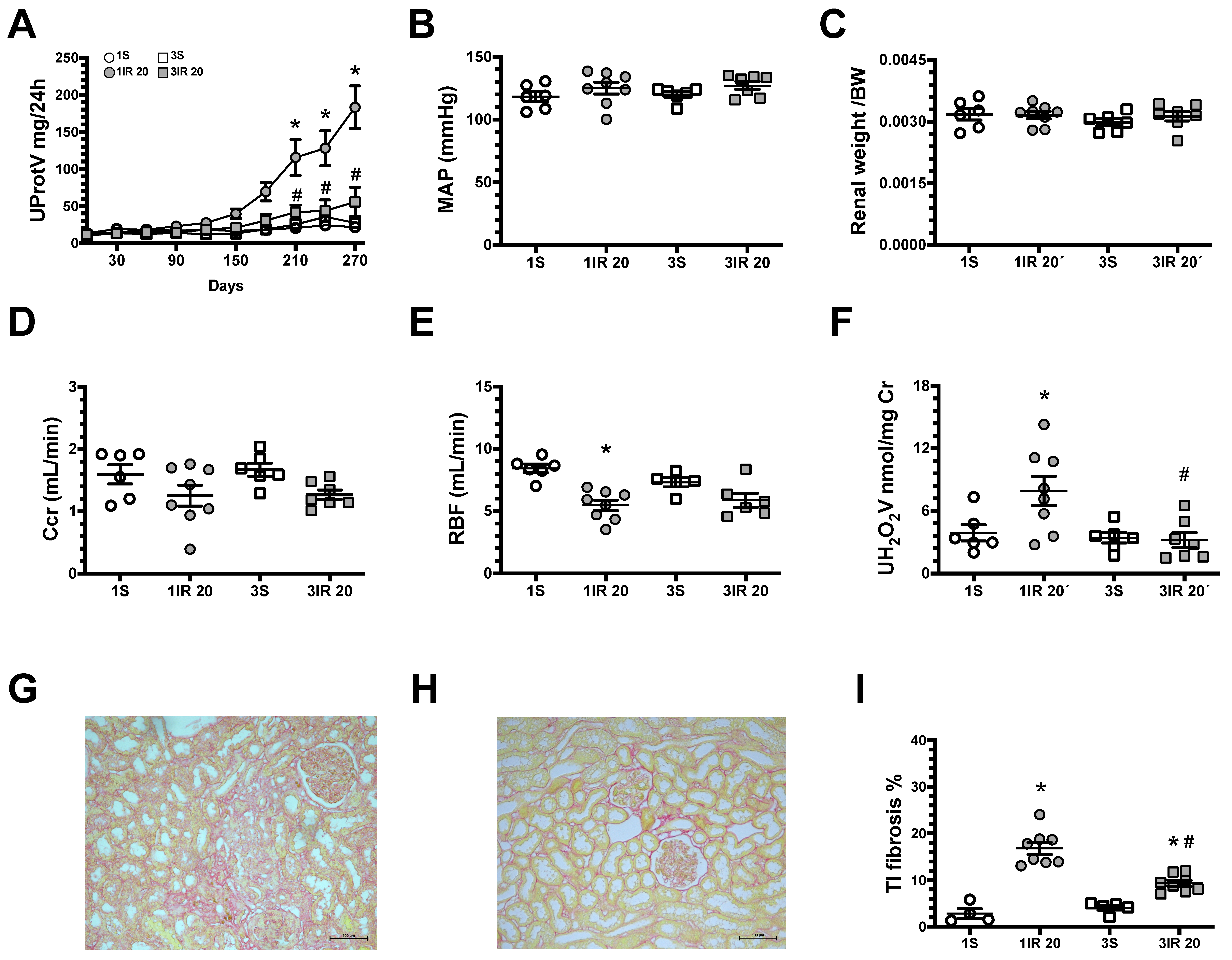
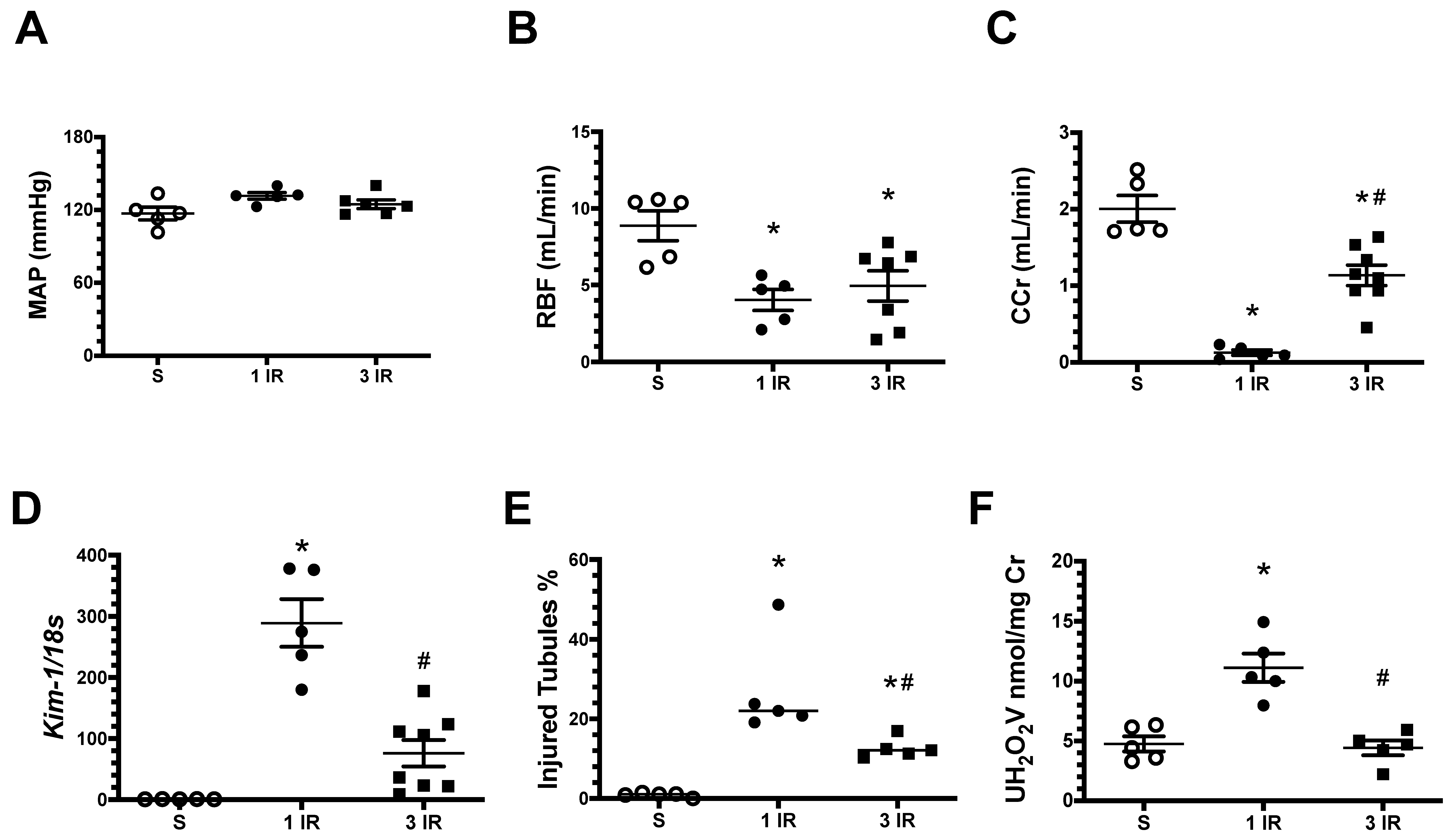
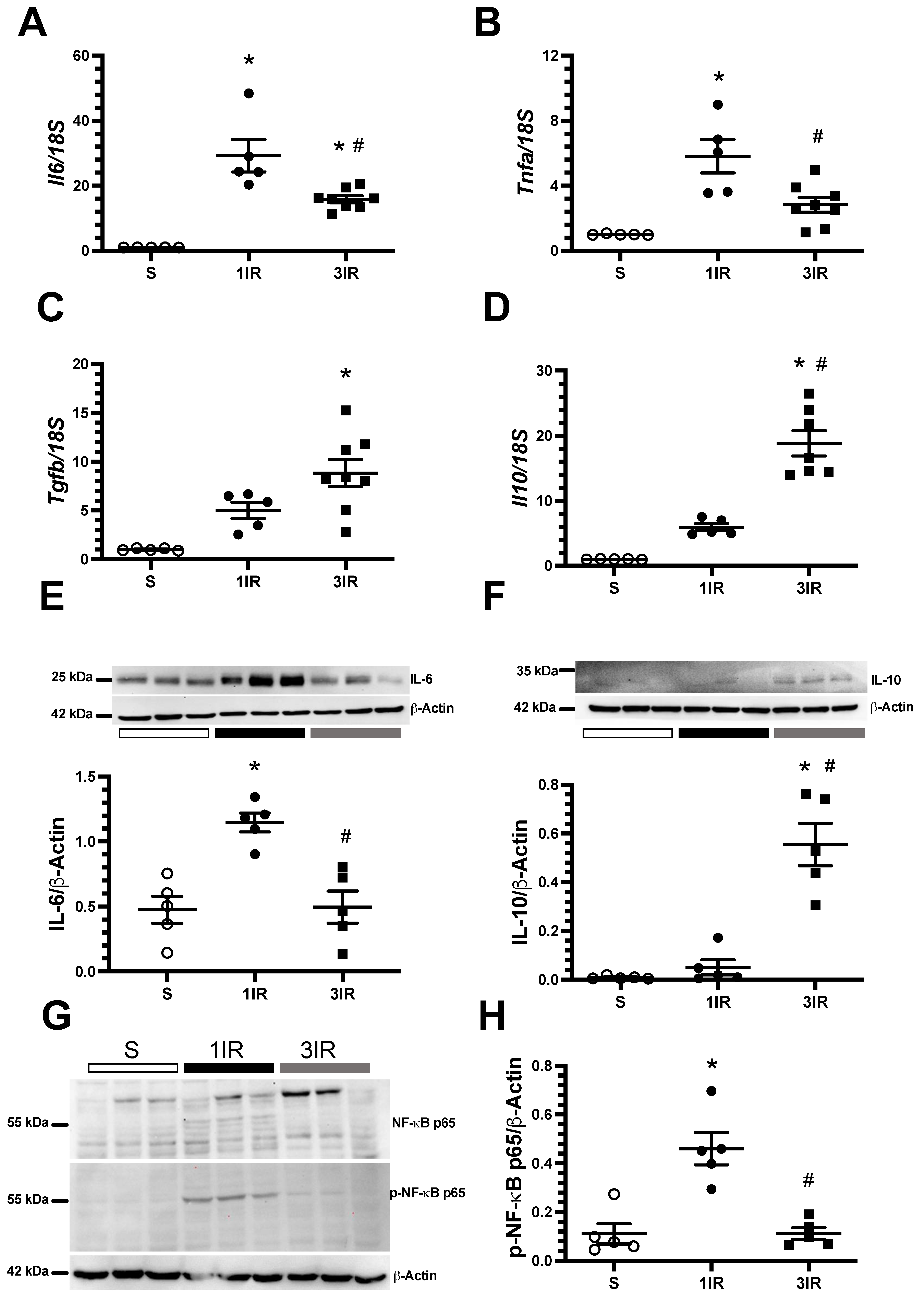
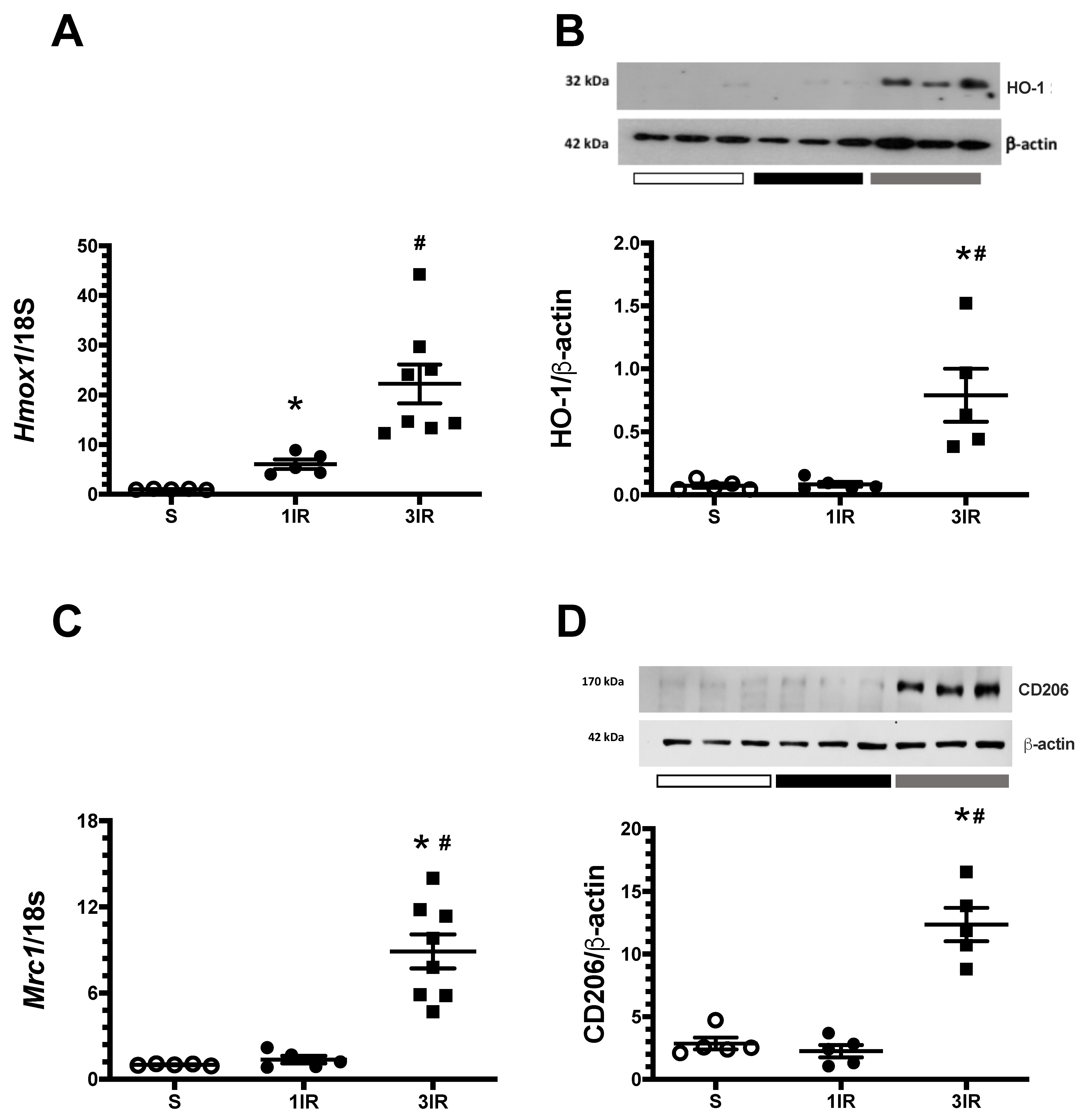
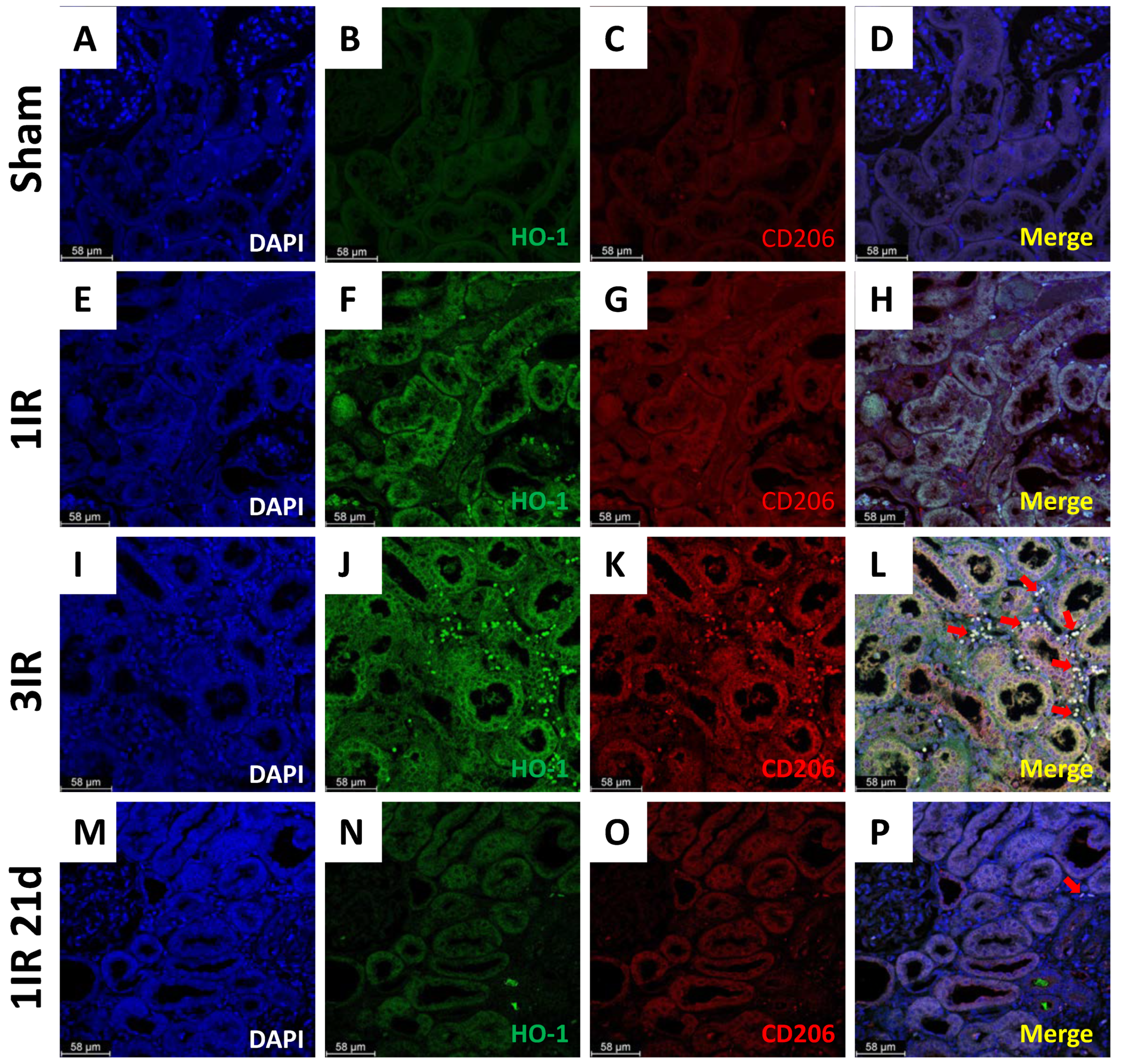
2. Results
3. Discussion
4. Material and Methods
4.1. Protocol 1
4.2. Protocol 2
4.3. Ischemia Reperfusion Model of Acute Kidney Injury
4.4. Biochemical and Renal Hemodynamics
4.5. Histopathological Analysis
4.6. Immunofluorescence
4.7. Semiquantitative RT-PCR
4.8. Western Blot Analysis
5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.L.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic Kidney Disease and Cardiovascular Risk: Epidemiology, Mechanisms, and Prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Barrera-Chimal, J.; Rocha, L.; Amador-Martínez, I.; Pérez-Villalva, R.; González, R.; Cortés-González, C.; Uribe, N.; Ramírez, V.; Berman, N.; Gamba, G.; et al. Delayed Spironolactone Administration Prevents the Transition from Acute Kidney Injury to Chronic Kidney Disease through Improving Renal Inflammation. Nephrol. Dial. Transplant. 2019, 34, 794–801. [Google Scholar] [CrossRef] [PubMed]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-Term Risk of Adverse Outcomes after Acute Kidney Injury: A Systematic Review and Meta-Analysis of Cohort Studies Using Consensus Definitions of Exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Zhu, J.; Liu, Z.; Tang, C.; Cai, J.; Dong, Z. Endoplasmic Reticulum Stress Is Activated in Post-Ischemic Kidneys to Promote Chronic Kidney Disease. EBioMedicine 2018, 37, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Zhang, Q.; Wen, J.; Chen, T.; He, L.; Wang, Y.; Yin, J.; Wu, R.; Xue, R.; Li, S.; et al. Ischemic Duration and Frequency Determines AKI-to-CKD Progression Monitored by Dynamic Changes of Tubular Biomarkers in IRI Mice. Front. Physiol. 2019, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- García-Ortuño, L.E.; Barrera-Chimal, J.; Pérez-Villalva, R.; Ortega-Trejo, J.A.; Luna-Bolaños, E.; Lima-Posada, I.; Sánchez-Navarro, A.; Reyes-Castro, L.; Gamba, G.; Zambrano, E.; et al. Resilience to Acute Kidney Injury in Offspring of Maternal Protein Restriction. Am. J. Physiol. Ren. Physiol. 2019, 317, F1637–F1648. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Horino, T.; Nagata, K.; Matsumoto, T.; Terada, Y.; Okuhara, Y. Transition from Acute Kidney Injury to Chronic Kidney Disease: A Single-Centre Cohort Study. Clin. Exp. Nephrol. 2018, 22, 1281–1293. [Google Scholar] [CrossRef]
- Kapitsinou, P.P.; Jaffe, J.; Michael, M.; Swan, C.E.; Duffy, K.J.; Erickson-Miller, C.L.; Haase, V.H. Preischemic Targeting of HIF Prolyl Hydroxylation Inhibits Fibrosis Associated with Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2012, 302, 1172–1179. [Google Scholar] [CrossRef] [Green Version]
- Noble, R.A.; Lucas, B.J.; Selby, N.M. Long-Term Outcomes in Patients with Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2020, 15, 423–429. [Google Scholar] [CrossRef]
- Rodríguez-Romo, R.; Benítez, K.; Barrera-Chimal, J.; Pérez-Villalva, R.; Gómez, A.; Aguilar-León, D.; Rangel-Santiago, J.F.; Huerta, S.; Gamba, G.; Uribe, N.; et al. AT1 Receptor Antagonism before Ischemia Prevents the Transition of Acute Kidney Injury to Chronic Kidney Disease. Kidney Int. 2016, 89, 363–373. [Google Scholar] [CrossRef]
- Hoste, E.A.J.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of Acute Kidney Injury in Critically Ill Patients: The Multinational AKI-EPI Study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.A.; Schnellmann, R.G. Persistent Disruption of Mitochondrial Homeostasis after Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2012, 302, 853–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linkermann, A.; Bräsen, J.H.; Darding, M.; Jin, M.K.; Sanz, A.B.; Heller, J.O.; De Zen, F.; Weinlich, R.; Ortiz, A.; Walczak, H.; et al. Two Independent Pathways of Regulated Necrosis Mediate Ischemia-Reperfusion Injury. Proc. Natl. Acad. Sci. USA 2013, 110, 12024–12029. [Google Scholar] [CrossRef] [Green Version]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation Determines Fibroblast Activation and Fibrogenesis in the Kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial Cell Cycle Arrest in G2/M Mediates Kidney Fibrosis after Injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef] [Green Version]
- Sykes, L.; Asar, O.; Ritchie, J.; Raman, M.; Vassallo, D.; Alderson, H.V.; O’Donoghue, D.J.; Green, D.; Diggle, P.J.; Kalra, P.A. The Influence of Multiple Episodes of Acute Kidney Injury on Survival and Progression to End Stage Kidney Disease in Patients with Chronic Kidney Disease. PLoS ONE 2019, 14, e0219828. [Google Scholar] [CrossRef] [Green Version]
- Thakar, C.V.; Christianson, A.; Himmelfarb, J.; Leonard, A.C. Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin. J. Am. Soc. Nephrol. 2011, 6, 2567–2572. [Google Scholar] [CrossRef] [Green Version]
- Siew, E.D.; Parr, S.K.; Abdel-Kader, K.; Eden, S.K.; Peterson, J.F.; Bansal, N.; Hung, A.M.; Fly, J.; Speroff, T.; Ikizler, T.A.; et al. Predictors of Recurrent AKI. J. Am. Soc. Nephrol. 2016, 27, 1190–1200. [Google Scholar] [CrossRef] [Green Version]
- Sawhney, S.; Marks, A.; Fluck, N.; Levin, A.; Prescott, G.; Black, C. Intermediate and Long-Term Outcomes of Survivors of Acute Kidney Injury Episodes: A Large Population-Based Cohort Study. Am. J. Kidney Dis. 2017, 69, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, E.; Arias-Cabrales, C.; Bermejo, S.; Sierra, A.; Burballa, C.; Soler, M.J.; Barrios, C.; Pascual, J. Impact of Recurrent Acute Kidney Injury on Patient Outcomes. Kidney Blood Press. Res. 2018, 43, 34–44. [Google Scholar] [CrossRef]
- Holmes, J.; Geen, J.; Williams, J.D.; Phillips, A.O. Recurrent Acute Kidney Injury: Predictors and Impact in a Large Population-Based Cohort. Nephrol. Dial. Transplant. 2020, 35, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Wever, K.E.; Menting, T.P.; Rovers, M.; Van Der Vliet, J.A.; Rongen, G.A.; Masereeuw, R.; Ritskes-Hoitinga, M.; Hooijmans, C.R.; Warlé, M. Ischemic Preconditioning in the Animal Kidney, a Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e32296. [Google Scholar] [CrossRef] [Green Version]
- Cho, W.Y.; Choi, H.M.; Lee, S.Y.; Kim, M.G.; Kim, H.-K.; Jo, S.-K. The Role of Tregs and CD11c+ Macrophages/Dendritic Cells in Ischemic Preconditioning of the Kidney. Kidney Int. 2010, 78, 981–992. [Google Scholar] [CrossRef] [Green Version]
- Zager, R.A.; Baltes, L.A.; Sharma, H.M.; Jurkowitz, M.S. Responses of the Ischemic Acute Renal Failure Kidney to Additional Ischemic Events. Kidney Int. 1984, 26, 689–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.M.; Byun, J.Y.; Kramers, C.; Kim, J.I.; Huang, P.L.; Bonventre, J.V. Inducible Nitric-Oxide Synthase Is an Important Contributor to Prolonged Protective Effects of Ischemic Preconditioning in the Mouse Kidney. J. Biol. Chem. 2003, 278, 27256–27266. [Google Scholar] [CrossRef] [Green Version]
- Park, K.M.; Chen, A.; Bonventre, J.V. Prevention of Kidney Ischemia/Reperfusion-Induced Functional Injury and JNK, P38, and MAPK Kinase Activation by Remote Ischemic Pretreatment. J. Biol. Chem. 2001, 276, 11870–11876. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.S.; Kim, J.; Park, Y.K.; Park, K.M. Infiltrated Macrophages Contribute to Recovery after Ischemic Injury but Not to Ischemic Preconditioning in Kidneys. Transplantation 2008, 85, 447–455. [Google Scholar] [CrossRef]
- Barrera-chimal, J.; Pérez-villalva, R.; Ortega, J.A.; Sánchez, A.; Durand, M.; Jaisser, F.; Bobadilla, N.A. Mild Ischemic Injury Leads to Long-Term Alterations in the Kidney: Amelioration by Spironolactone Admin-Istration. Int. J. Biol. Sci. 2015, 11, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Barrera-Chimal, J.; Pérez-Villalva, R.; Rodríguez-Romo, R.; Reyna, J.; Uribe, N.; Gamba, G.; Bobadilla, N.A. Spironolactone Prevents Chronic Kidney Disease Caused by Ischemic Acute Kidney Injury. Kidney Int. 2013, 83, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buss, H.; Handschick, K.; Jurrmann, N.; Pekkonen, P.; Beuerlein, K.; Müller, H.; Wait, R.; Saklatvala, J.; Ojala, P.M.; Schmitz, M.L.; et al. Cyclin-Dependent Kinase 6 Phosphorylates NF-ΚB P65 at Serine 536 and Contributes to the Regulation of Inflammatory Gene Expression. PLoS ONE 2012, 7, e51847. [Google Scholar] [CrossRef]
- Barakat, M.; Gabr, M.M.; Zhran, F.; El-Adl, M.; Hussein, A.M.; Barakat, N.; Eldemerdash, R. Upregulation of Heme Oxygenase 1 (HO-1) Attenuates Kidney Damage, Oxidative Stress and Inflammatory Reaction during Renal Ischemia/Reperfusion Injury. Gen. Physiol. Biophys. 2018, 37, 193–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferenbach, D.A.; Ramdas, V.; Spencer, N.; Marson, L.; Anegon, I.; Hughes, J.; Kluth, D.C. Macrophages Expressing Heme Oxygenase-1 Improve Renal Function in Ischemia/Reperfusion Injury. Mol. Ther. 2010, 18, 1706–1713. [Google Scholar] [CrossRef]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased Risk of Death and de Novo Chronic Kidney Disease Following Reversible Acute Kidney Injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lafrance, J.-P.; Miller, D.R. Acute Kidney Injury Associates with Increased Long-Term Mortality. J. Am. Soc. Nephrol. 2010, 21, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with Ischemia: A Delay of Lethal Cell Injury in Ischemic Myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [Green Version]
- Ozbilgin, S.; Ozkardesler, S.; Akan, M.; Boztas, N.; Ozbilgin, M.; Ergur, B.U.; Derici, S.; Guneli, M.E.; Meseri, R. Renal Ischemia/Reperfusion Injury in Diabetic Rats: The Role of Local Ischemic Preconditioning. Biomed Res. Int. 2016, 2016, 8580475. [Google Scholar] [CrossRef] [Green Version]
- Shu, B.; Feng, Y.; Gui, Y.; Lu, Q.; Wei, W.; Xue, X.; Sun, X.; He, W.; Yang, J.; Dai, C. Blockade of CD38 Diminishes Lipopolysaccharide-Induced Macrophage Classical Activation and Acute Kidney Injury Involving NF-ΚB Signaling Suppression. Cell Signal. 2018, 42, 249–258. [Google Scholar] [CrossRef]
- Guo, Q.; Du, X.; Zhao, Y.; Zhang, D.; Yue, L.; Wang, Z. Ischemic Postconditioning Prevents Renal Ischemia Reperfusion Injury through the Induction of Heat Shock Proteins in Rats. Mol. Med. Rep. 2014, 10, 2875–2881. [Google Scholar] [CrossRef] [Green Version]
- Li, J.R.; Ou, Y.C.; Wu, C.C.; Der Wang, J.; Lin, S.Y.; Wang, Y.Y.; Chen, W.Y.; Chen, C.J. Ischemic Preconditioning Improved Renal Ischemia/Reperfusion Injury and Hyperglycemia. IUBMB Life 2019, 71, 321–329. [Google Scholar] [CrossRef]
- Pan, T.; Jia, P.; Chen, N.; Fang, Y.; Liang, Y.; Guo, M.; Ding, X. Delayed Remote Ischemic Preconditioning Confers Renoprotection against Septic Acute Kidney Injury via Exosomal MiR-21. Theranostics 2019, 9, 405–423. [Google Scholar] [CrossRef]
- Tracz, M.J.; Juncos, J.P.; Croatt, A.J.; Ackerman, A.W.; Grande, J.P.; Knutson, K.L.; Kane, G.C.; Terzic, A.; Griffin, M.D.; Nath, K.A. Deficiency of Heme Oxygenase-1 Impairs Renal Hemodynamics and Exaggerates Systemic Inflammatory Responses to Renal Ischemia. Kidney Int. 2007, 72, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, M.; Delbauve, S.; Wespes, E.; Roumeguère, T.; Leo, O.; Flamand, V.; Le Moine, A.; Hougardy, J.M. Dual Effect of Hemin on Renal Ischemia-Reperfusion Injury. Biochem. Biophys. Res. Commun. 2018, 503, 2820–2825. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. How Many Transcription Factors Does It Take to Turn on the Heme Oxygenase-1 Gene? Am. J. Respir. Cell Mol. Biol. 2007, 36, 166–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potteti, H.R.; Tamatam, C.R.; Marreddy, R.; Reddy, N.M.; Noel, S.; Rabb, H.; Reddy, S.P. Nrf2-AKT Interactions Regulate Heme Oxygenase 1 Expression in Kidney Epithelia during Hypoxia and Hypoxia-Reoxygenation. Am. J. Physiol. Ren. Physiol. 2016, 311, F1025–F1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.J.; Jiang, B.H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A.M.K. Hypoxia-Inducible Factor-1 Mediates Transcriptional Activation of the Heme Oxygenase-1 Gene in Response to Hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, J.L.; Zand, B.A.; Yang, L.M.; Sabaawy, H.E.; Lianos, E.; Abraham, N.G. Heme Oxygenase Isoform-Specific Expression and Distribution in the Rat Kidney. Kidney Int. 2001, 59, 1448–1457. [Google Scholar] [CrossRef] [Green Version]
- Lee, T.S.; Chau, L.Y. Heme Oxygenase-1 Mediates the Anti-Inflammatory Effect of Interleukin-10 in Mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Vanella, L.; Barbagallo, I.; Tibullo, D.; Forte, S.; Zappalà, A.; Li Volti, G. The Non-Canonical Functions of the Heme Oxygenases. Oncotarget 2016, 7, 69075–69086. [Google Scholar] [CrossRef] [Green Version]
- Yayeh, T.; Im, E.J.; Kwon, T.H.; Roh, S.S.; Kim, S.; Kim, J.H.; Hong, S.B.; Cho, J.Y.; Park, N.H.; Rhee, M.H. Hemeoxygenase 1 Partly Mediates the Anti-Inflammatory Effect of Dieckol in Lipopolysaccharide Stimulated Murine Macrophages. Int. Immunopharmacol. 2014, 22, 51–58. [Google Scholar] [CrossRef]
- Luo, D.; Guo, Y.; Cheng, Y.; Zhao, J.; Wang, Y.; Rong, J. Natural Product Celastrol Suppressed Macrophage M1 Polarization against Inflammation in Diet-Induced Obese Mice via Regulating Nrf2/HO-1, MAP Kinase and NF-ΚB Pathways. Aging 2017, 9, 2068–2081. [Google Scholar] [CrossRef]
- Ferenbach, D.A.; Nkejabega, N.C.J.; McKay, J.; Choudhary, A.K.; Vernon, M.A.; Beesley, M.F.; Clay, S.; Conway, B.C.; Marson, L.P.; Kluth, D.C.; et al. The Induction of Macrophage Hemeoxygenase-1 Is Protective during Acute Kidney Injury in Aging Mice. Kidney Int. 2011, 79, 966–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Huen, S.; Nishio, H.; Nishio, S.; Lee, H.K.; Choi, B.S.; Ruhrberg, C.; Cantley, L.G. Distinct Macrophage Phenotypes Contribute to Kidney Injury and Repair. J. Am. Soc. Nephrol. 2011, 22, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Navarro, A.; Pérez-Villalva, R.; Murillo-de-Ozores, A.R.; Martínez-Rojas, M.Á.; Rodríguez-Aguilera, J.R.; González, N.; Castañeda-Bueno, M.; Gamba, G.; Recillas-Targa, F.; Bobadilla, N.A. Vegfa Promoter Gene Hypermethylation at HIF1α Binding Site Is an Early Contributor to CKD Progression after Renal Ischemia. Sci. Rep. 2021, 11, 8769. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Morales-Buenrostro, L.E.; Ortega-Trejo, J.A.; Pérez-Villalva, R.; Marino, L.A.; González-Bobadilla, Y.; Juárez, H.; Zamora-Mejía, F.M.; González, N.; Espinoza, R.; Barrera-Chimal, J.; et al. Spironolactone Reduces Oxidative Stress in Living Donor Kidney Transplantation: A Randomized Controlled Trial. Am. J. Physiol. Ren. Physiol. 2019, 317, F519–F528. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Trejo, J.A.; Pérez-Villalva, R.; Sánchez-Navarro, A.; Marquina, B.; Rodríguez-Iturbe, B.; Bobadilla, N.A. Repeated Episodes of Ischemia/Reperfusion Induce Heme-Oxygenase-1 (HO-1) and Anti-Inflammatory Responses and Protects against Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 14573. https://doi.org/10.3390/ijms232314573
Ortega-Trejo JA, Pérez-Villalva R, Sánchez-Navarro A, Marquina B, Rodríguez-Iturbe B, Bobadilla NA. Repeated Episodes of Ischemia/Reperfusion Induce Heme-Oxygenase-1 (HO-1) and Anti-Inflammatory Responses and Protects against Chronic Kidney Disease. International Journal of Molecular Sciences. 2022; 23(23):14573. https://doi.org/10.3390/ijms232314573
Chicago/Turabian StyleOrtega-Trejo, Juan Antonio, Rosalba Pérez-Villalva, Andrea Sánchez-Navarro, Brenda Marquina, Bernardo Rodríguez-Iturbe, and Norma A. Bobadilla. 2022. "Repeated Episodes of Ischemia/Reperfusion Induce Heme-Oxygenase-1 (HO-1) and Anti-Inflammatory Responses and Protects against Chronic Kidney Disease" International Journal of Molecular Sciences 23, no. 23: 14573. https://doi.org/10.3390/ijms232314573






