Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water
Abstract
:1. Introduction
2. Results and Discussion
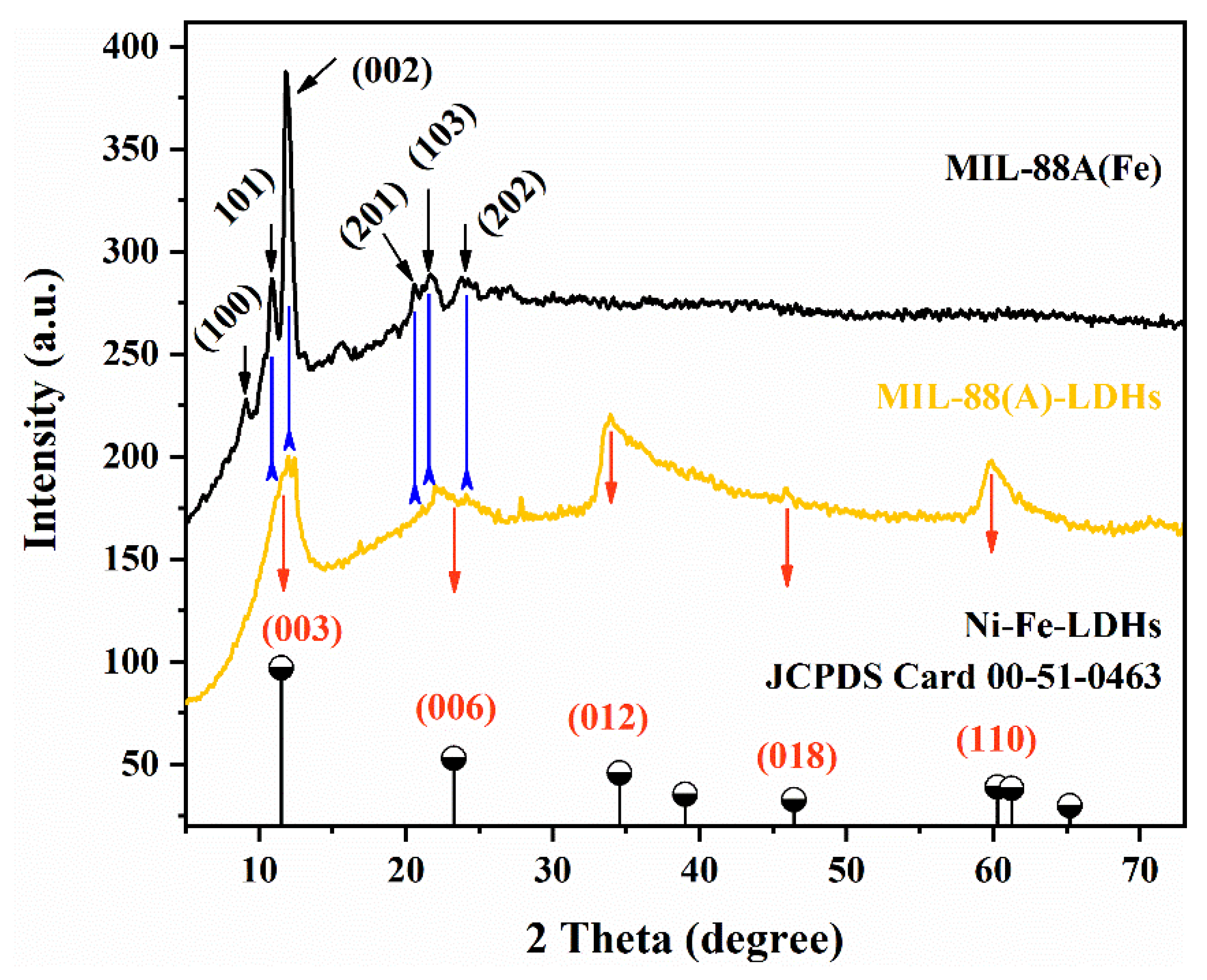
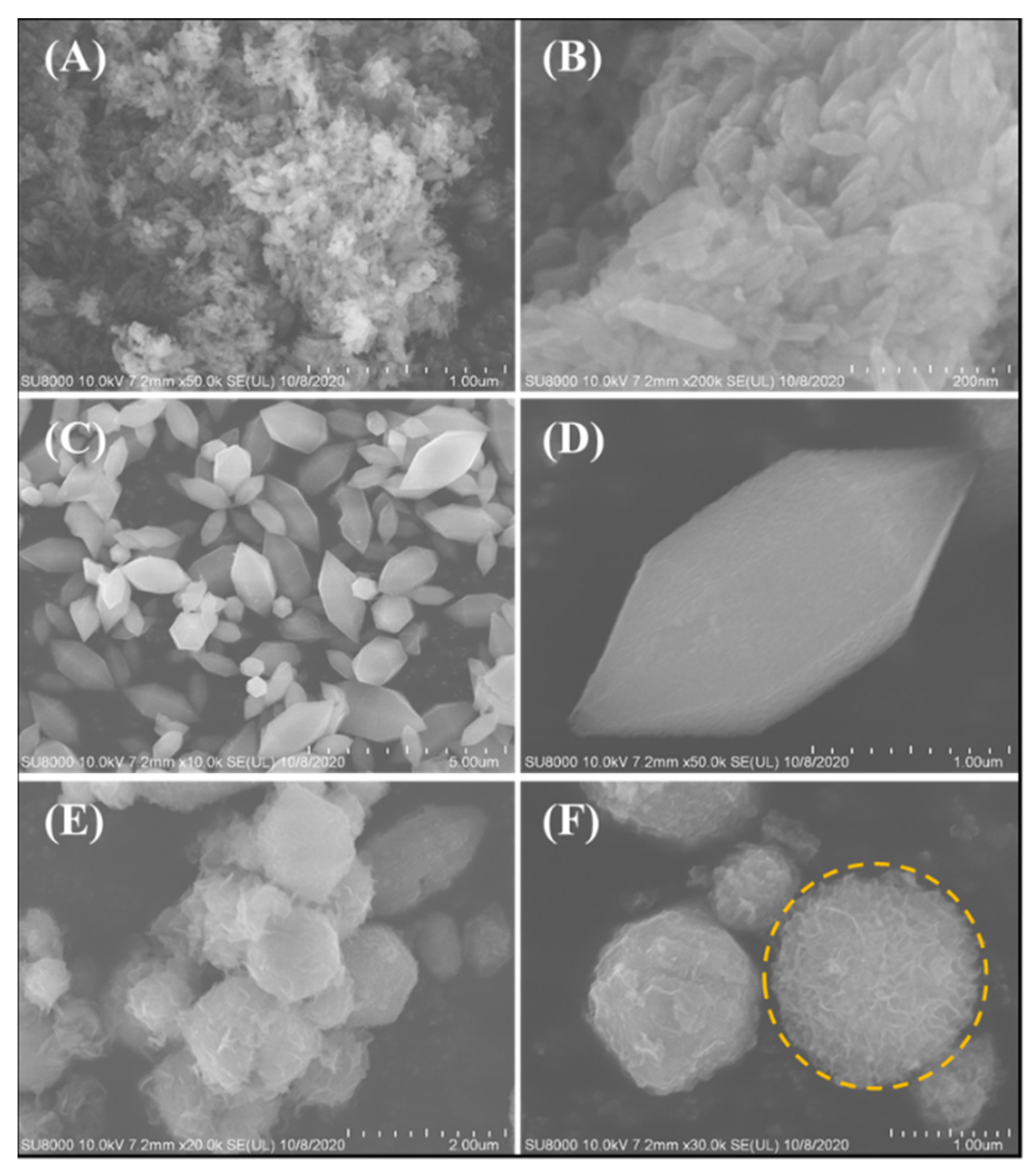
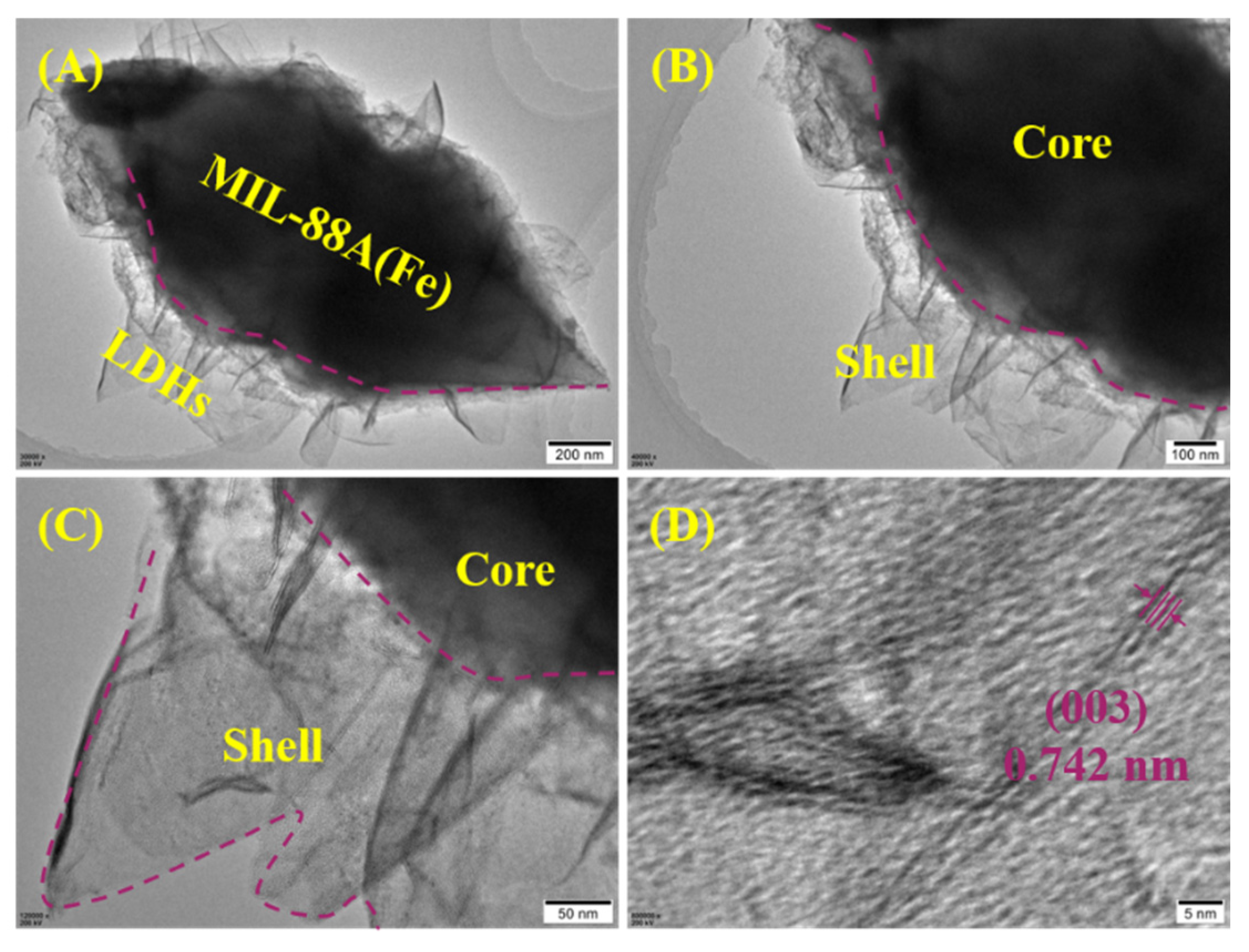
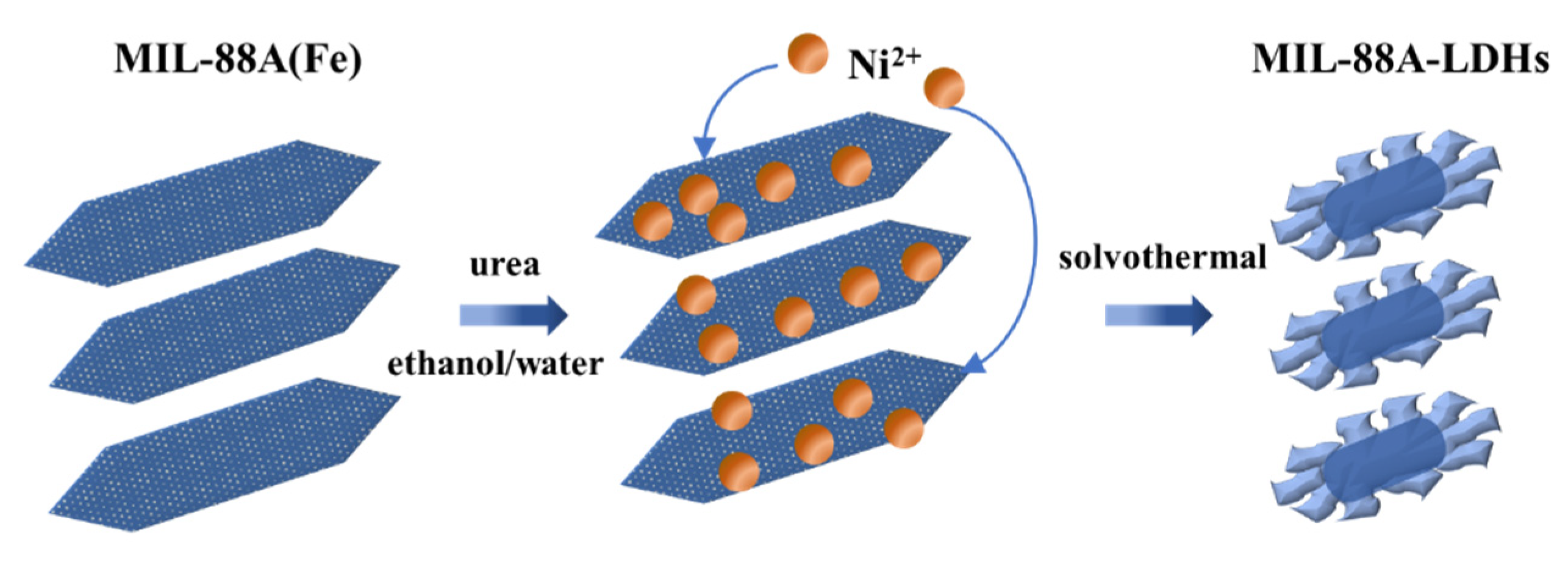
2.1. MIL-88A-LDHs Microstructure
2.2. Adsorption Performance
2.2.1. Adsorption Kinetics
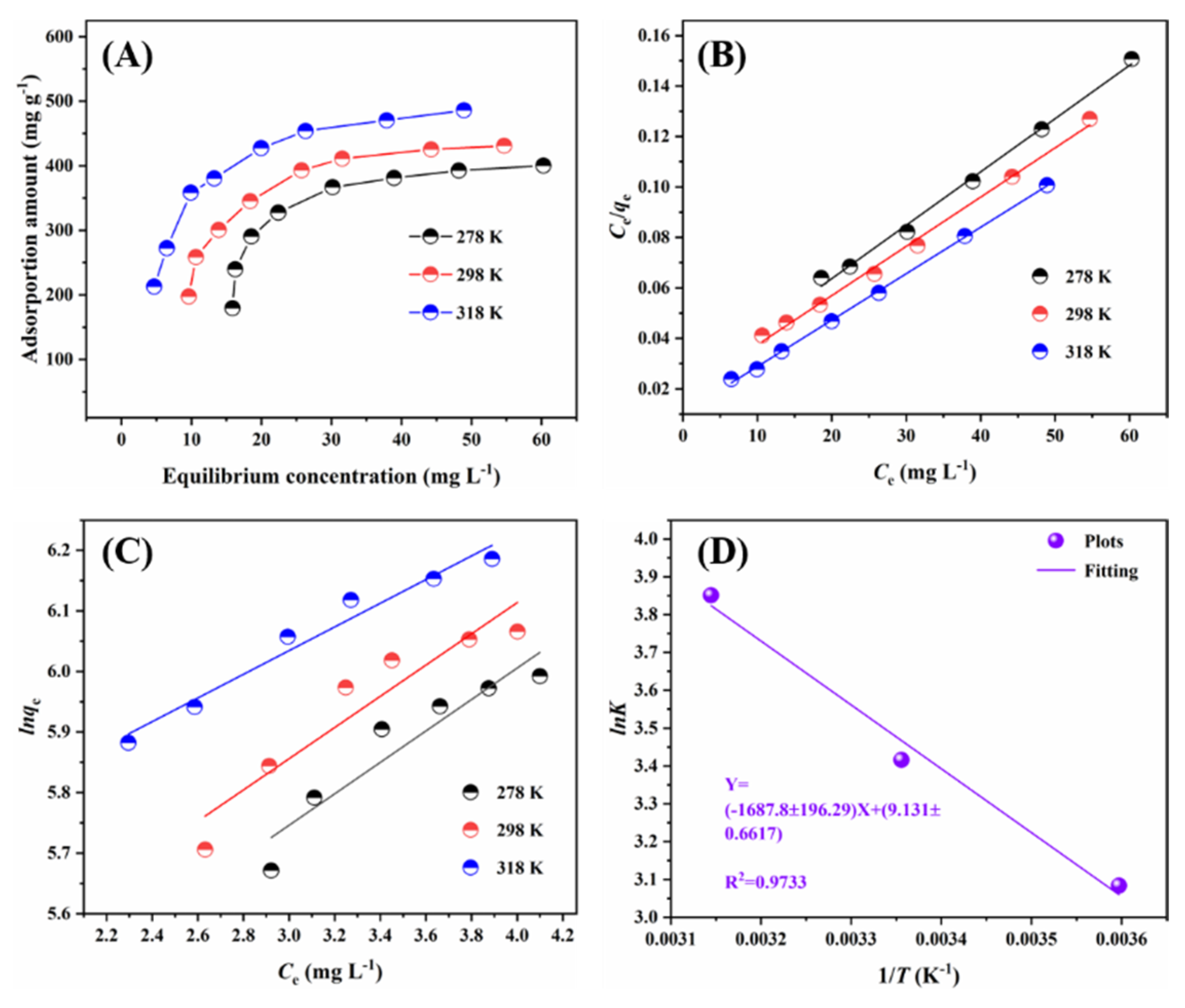
2.2.2. Adsorption Isotherms
2.2.3. Adsorption Thermodynamic
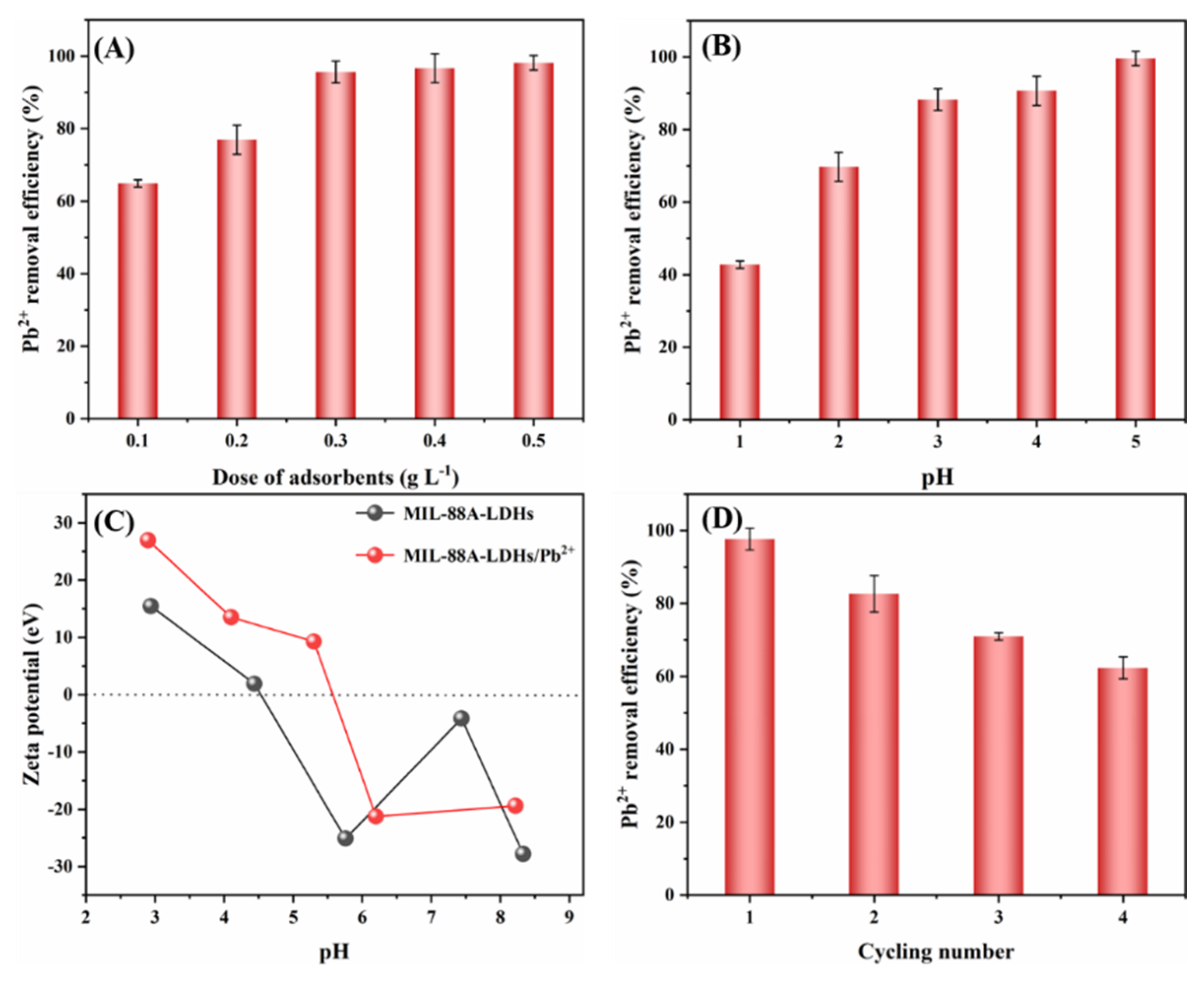
2.2.4. Effects of Adsorbent Dosage and pH
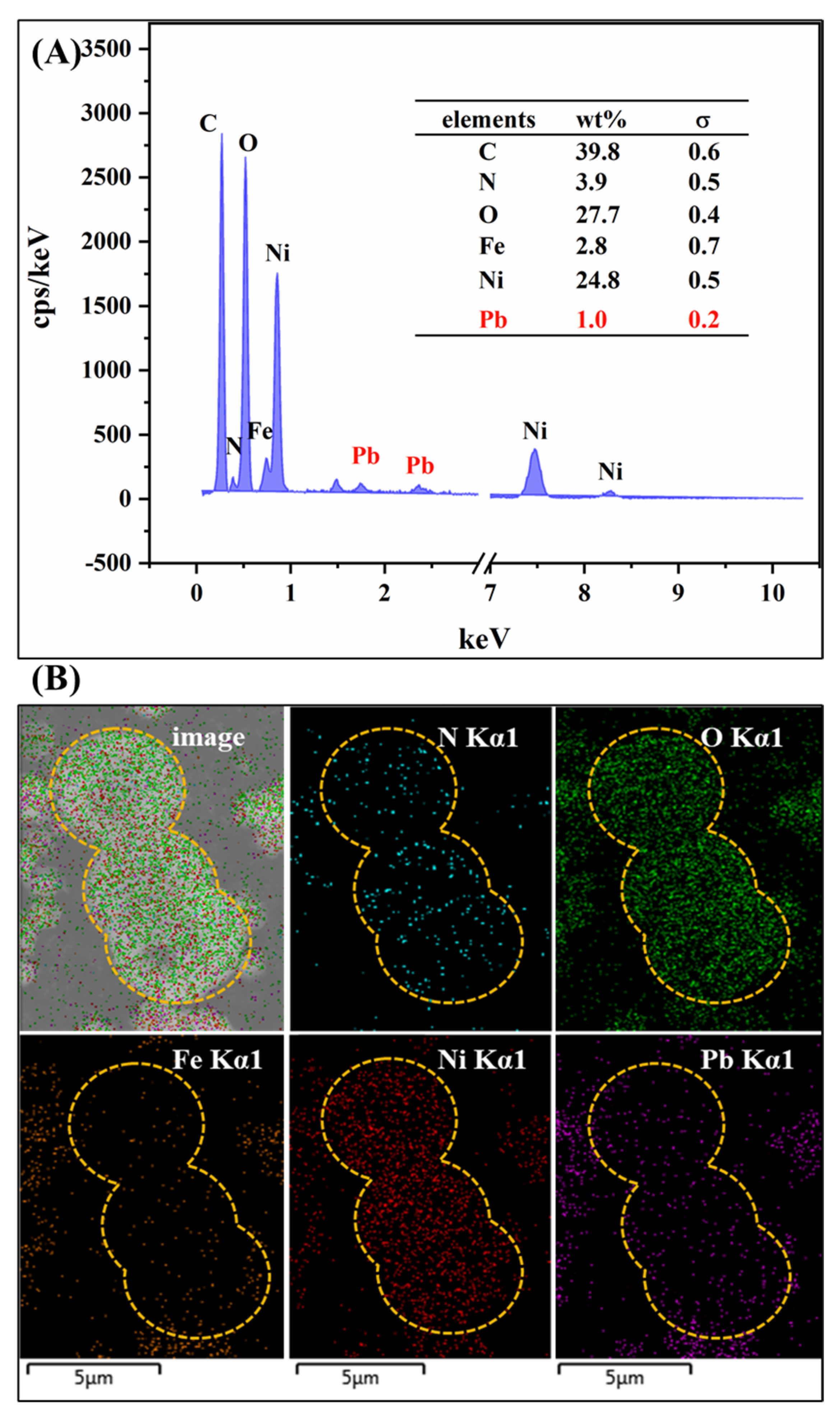
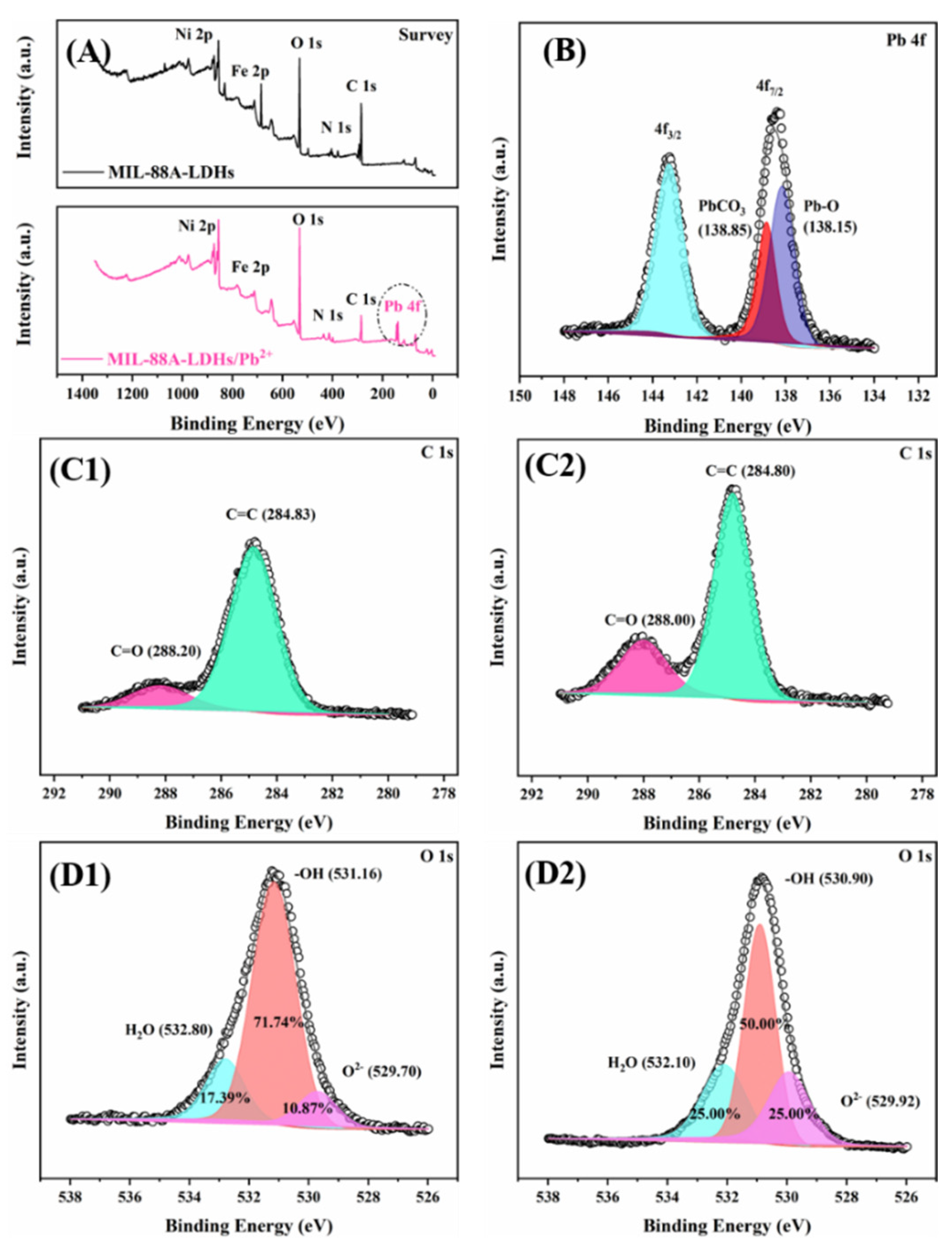
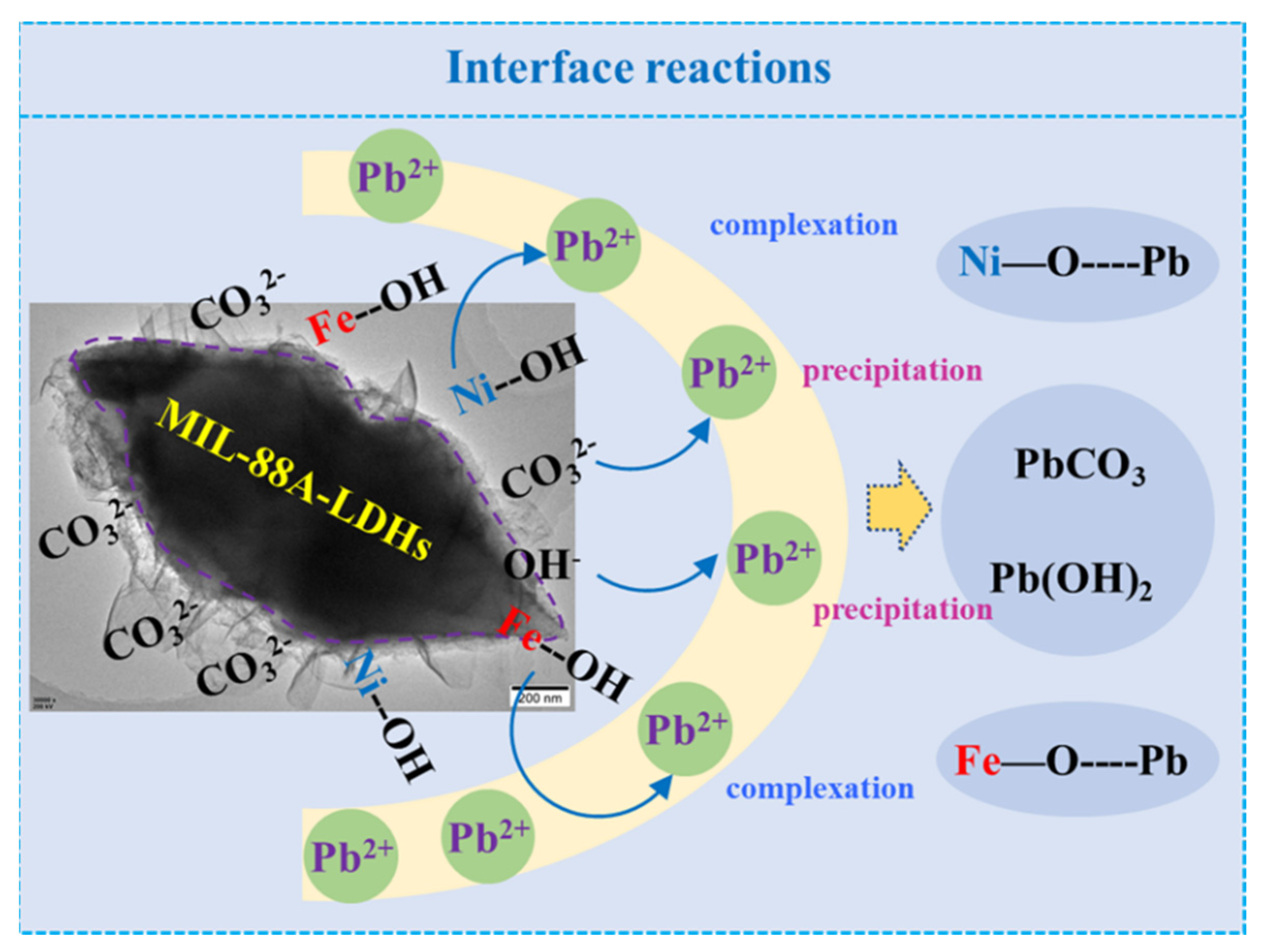
2.2.5. Adsorption Mechanism
2.3. Cycling Performance of MIL-88A-LDHs
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthesis of MIL-88A(Fe), Layered Double Hydroxides (LDHs), and LDHs Derived from MIL-88A(Fe)
3.2.1. Synthesis of MIL-88A(Fe)
3.2.2. Synthesis of Layered Double Hydroxides (LDHs)
3.2.3. Synthesis of LDHs Derived from MIL-88A(Fe)
3.3. Characterization of Materials
3.4. Adsorption Experiments
3.5. Cycling Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zou, Y.; Wang, X.; Khan, A.; Wang, P.; Liu, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Environmental Remediation and Application of Nanoscale Zero-Valent Iron and Its Composites for the Removal of Heavy Metal Ions: A Review. Environ. Sci. Technol. 2016, 50, 7290–7304. [Google Scholar] [CrossRef] [PubMed]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y. One-pot synthesis of magnetic graphene oxide composites as an efficient and recoverable adsorbent for Cd(II) and Pb(II) removal from aqueous solution. J. Hazard. Mater. 2020, 381, 120914. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-supported nanoscale zero-valent iron: New findings on simultaneous adsorption of Cd(II), Pb(II), and As(III) in aqueous solution and soil. J. Hazard. Mater. 2018, 344, 1–11. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Adsorption of divalent heavy metal ions from water using carbon nanotube sheets. J. Hazard. Mater. 2011, 185, 140–147. [Google Scholar] [CrossRef] [PubMed]
- El Rouby, W.M.A.; El-Dek, S.I.; Goher, M.E.; Noaemy, S.G. Efficient water decontamination using layered double hydroxide beads nanocomposites. Environ. Sci. Pollut. Res. 2020, 27, 18985–19003. [Google Scholar] [CrossRef]
- Gu, P.; Zhang, S.; Li, X.; Wang, X.; Wen, T.; Jehan, R.; Alsaedi, A.; Hayat, T.; Wang, X. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution. Environ. Pollut. 2018, 240, 493–505. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Kumar, P.; Pournara, A.; Kim, K.-H.; Bansal, V.; Rapti, S.; Manos, M.J. Metal-organic frameworks: Challenges and opportunities for ion-exchange/sorption applications. Prog. Mater. Sci. 2017, 86, 25–74. [Google Scholar] [CrossRef]
- Pang, H.; Wu, Y.; Wang, X.; Hu, B.; Wang, X. Recent Advances in Composites of Graphene and Layered Double Hydroxides for Water Remediation: A Review. Chem. Asian J. 2019, 14, 2542–2552. [Google Scholar] [CrossRef]
- Yu, S.; Liu, Y.; Ai, Y.; Wang, X.; Zhang, R.; Chen, Z.; Chen, Z.; Zhao, G.; Wang, X. Rational design of carbonaceous nanofiber/Ni-Al layered double hydroxide nanocomposites for high-efficiency removal of heavy metals from aqueous solutions. Environ. Pollut. 2018, 242, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liao, F.; Ye, L.; Yeh, L. Controlled pyrolysis of MIL-88A to prepare iron/carbon composites for synergistic persulfate oxidation of phenol: Catalytic performance and mechanism. J. Hazard. Mater. 2020, 398, 122938. [Google Scholar] [CrossRef]
- Dang, S.; Zhu, Q.-L.; Xu, Q. Nanomaterials derived from metal–organic frameworks. Nat. Rev. Mater. 2017, 3, 17075. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Guan, B.Y.; Song, S.; Lou, X.W.D. Metal-Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution. Adv. Mater. 2018, 30, 1801211. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Zeng, Z.; Zeng, G.; Yang, Z.; Xiao, R.; Li, X.; Cao, J.; Zhou, C.; Chen, H.; Jia, M.; et al. Metal-organic frameworks derived magnetic carbon-αFe/Fe3C composites as a highly effective adsorbent for tetracycline removal from aqueous solution. Chem. Eng. J. 2019, 374, 91–99. [Google Scholar] [CrossRef]
- Palomino Cabello, C.; Picó, M.F.F.; Maya, F.; del Rio, M.; Turnes Palomino, G. UiO-66 derived etched carbon/polymer membranes: High-performance supports for the extraction of organic pollutants from water. Chem. Eng. J. 2018, 346, 85–93. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, L.; Chen, Y.; Lu, X.F.; Gao, S.; Lou, X.W.D. Designed Formation of Double-Shelled Ni-Fe Layered-Double-Hydroxide Nanocages for Efficient Oxygen Evolution Reaction. Adv. Mater. 2020, 32, 1906432. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, W.; Wu, H.; Li, Y.; Ren, X.; Li, M.; Liu, J.; Sun, J.; Yue, T.; Wang, J. In Situ Cascade Derivation toward a Hierarchical Layered Double Hydroxide Magnetic Absorbent for High-Performance Protein Separation. ACS Sustain. Chem. Eng. 2020, 8, 4966–4974. [Google Scholar] [CrossRef]
- Cheng, R.; Kang, M.; Zhuang, S.; Shi, L.; Zheng, X.; Wang, J. Adsorption of Sr(II) from water by mercerized bacterial cellulose membrane modified with EDTA. J. Hazard. Mater. 2019, 364, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, J. A general kinetic model for adsorption: Theoretical analysis and modeling. J. Mol. Liq. 2019, 288, 111100. [Google Scholar] [CrossRef]
- Li, J.; Zan, Y.; Zhang, Z.; Dou, M.; Wang, F. Prussian blue nanocubes decorated on nitrogen-doped hierarchically porous carbon network for efficient sorption of radioactive cesium. J. Hazard. Mater. 2020, 385, 121568. [Google Scholar] [CrossRef]
- Xing, M.; Zhuang, S.; Wang, J. Efficient removal of Cs(I) from aqueous solution using graphene oxide. Prog. Nucl. Energy 2020, 119, 103167. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Z.; Xiao, B.; Gu, P.; Yao, W.; Xing, J.; Asiri, A.M.; Almaty, K.A.; Wang, X.; Wang, S. Enhanced removal of lead ions from aqueous solution by iron oxide nanomaterials with cobalt and nickel doping. J. Clean. Prod. 2019, 211, 1250–1258. [Google Scholar] [CrossRef]
- Goyal, P.; Tiwary, C.S.; Misra, S.K. Ion exchange based approach for rapid and selective Pb(II) removal using iron oxide decorated metal organic framework hybrid. J. Environ. Manag. 2021, 277, 111469. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.M.; Choi, J.H.; Kim, D.H.; Han, G.S.; Jung, H.S. Synthesis and adsorption properties of gelatin-conjugated hematite (alpha-Fe2O3) nanoparticles for lead removal from wastewater. J. Hazard. Mater. 2021, 416, 125696. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chen, Z. A facile one-step synthesized epsilon-MnO2 nanoflowers for effective removal of lead ions from wastewater. Chemosphere 2020, 250, 126329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, Z.; Chen, Z.; Sun, J.; Gao, Y.; Wu, E. Resource utilization of swine sludge to prepare modified biochar adsorbent for the efficient removal of Pb(II) from water. J. Clean. Prod. 2020, 257, 120322. [Google Scholar] [CrossRef]
- Luo, J.; Fu, K.; Sun, M.; Yin, K.; Wang, D.; Liu, X.; Crittenden, J.C. Phase-Mediated Heavy Metal Adsorption from Aqueous Solutions Using Two-Dimensional Layered MoS2. ACS Appl. Mater. Interfaces 2019, 11, 38789–38797. [Google Scholar] [CrossRef]
- Tang, N.; Niu, C.G.; Li, X.T.; Liang, C.; Guo, H.; Lin, L.S.; Zheng, C.W.; Zeng, G.M. Efficient removal of Cd(2+) and Pb(2+) from aqueous solution with amino- and thiol-functionalized activated carbon: Isotherm and kinetics modeling. Sci. Total Environ. 2018, 635, 1331–1344. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, J.; Zhang, Y.; Cheng, G.; Huang, S.; Chen, J.; Xu, R.; Ming, Y.A.; Wang, Y.; Chen, R. Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with excellent performance for arsenic removal from water. J. Hazard. Mater. 2020, 383, 121172. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ren, G.; Ma, Y.; Wang, C.; Li, M.; Pang, X.; Pan, Q.; Li, J. Adsorption and reutilization of Pb(II) based on acid-resistant metal-organic gel. Sep. Purif. Technol. 2022, 295, 121253. [Google Scholar] [CrossRef]
- Ma, Y.; You, D.; Fang, Y.; Luo, J.; Pan, Q.; Liu, Y.; Wang, F.; Yang, W. Confined growth of MOF in chitosan matrix for removal of trace Pb(Ⅱ) from reclaimed water. Sep. Purif. Technol. 2022, 294, 121223. [Google Scholar] [CrossRef]


| PFO | PSO | Intra-Particle Diffusion | |||
|---|---|---|---|---|---|
| R2 | 0.9443 | R2 | 0.9736 | R2 | 0.9512 |
| k1 | 0.2971 | k2 | 0.0020 | ki1 | 51.29 |
| qe | 108.5 | qe | 157.2 | qe | 17.30 |
| R2 | 0.3366 | ||||
| ki2 | 3.13 | ||||
| qe | 117.95 | ||||
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| KL | R2 | qm | KF | R2 | n | |
| 278 K | 0.0983 | 0.9945 | 473.9 | 143.6 | 0.8665 | 3.853 |
| 298 K | 0.1087 | 0.9946 | 512.8 | 161.2 | 0.8682 | 3.881 |
| 318 K | 0.1767 | 0.9989 | 649.4 | 232.3 | 0.9591 | 5.117 |
| No. | Adsorbent | pH | Maximum Capacity (mg g−1) | Ref |
|---|---|---|---|---|
| 1 | Co-Fe2O3/Ni-Fe2O3 | 5.0 | 136.0/97.5 | [24] |
| 2 | Fe3O4-Cu-MOF | 4.0 | 610.0 | [25] |
| 3 | HT NPs | - | 169.5 | [26] |
| 4 | Epsilon-MnO2 | - | 239.7 | [27] |
| 5 | Modified biochar | 5.0 | 145.0 | [28] |
| 6 | MIL-88A-LDHs | 3.0 | 512.8 | This study |
| Temperature (K) | ΔG0 (kJ mol−1) | ΔH0 (kJ mol−1) | ΔS0 (J mol−1 K−1) |
|---|---|---|---|
| 278 | −7.128 | 14.03 | 75.91 |
| 298 | −8.463 | ||
| 318 | −10.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, J.-B.; Yu, G. Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water. Int. J. Mol. Sci. 2022, 23, 14556. https://doi.org/10.3390/ijms232314556
Huo J-B, Yu G. Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water. International Journal of Molecular Sciences. 2022; 23(23):14556. https://doi.org/10.3390/ijms232314556
Chicago/Turabian StyleHuo, Jiang-Bo, and Guoce Yu. 2022. "Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water" International Journal of Molecular Sciences 23, no. 23: 14556. https://doi.org/10.3390/ijms232314556
APA StyleHuo, J.-B., & Yu, G. (2022). Layered Double Hydroxides Derived from MIL-88A(Fe) as an Efficient Adsorbent for Enhanced Removal of Lead (II) from Water. International Journal of Molecular Sciences, 23(23), 14556. https://doi.org/10.3390/ijms232314556




