Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae)
Abstract
:1. Introduction
2. Results
2.1. Cloning and Sequence Analysis of CcOctβ1R, CcOctβ2R, and CcOctβ3R
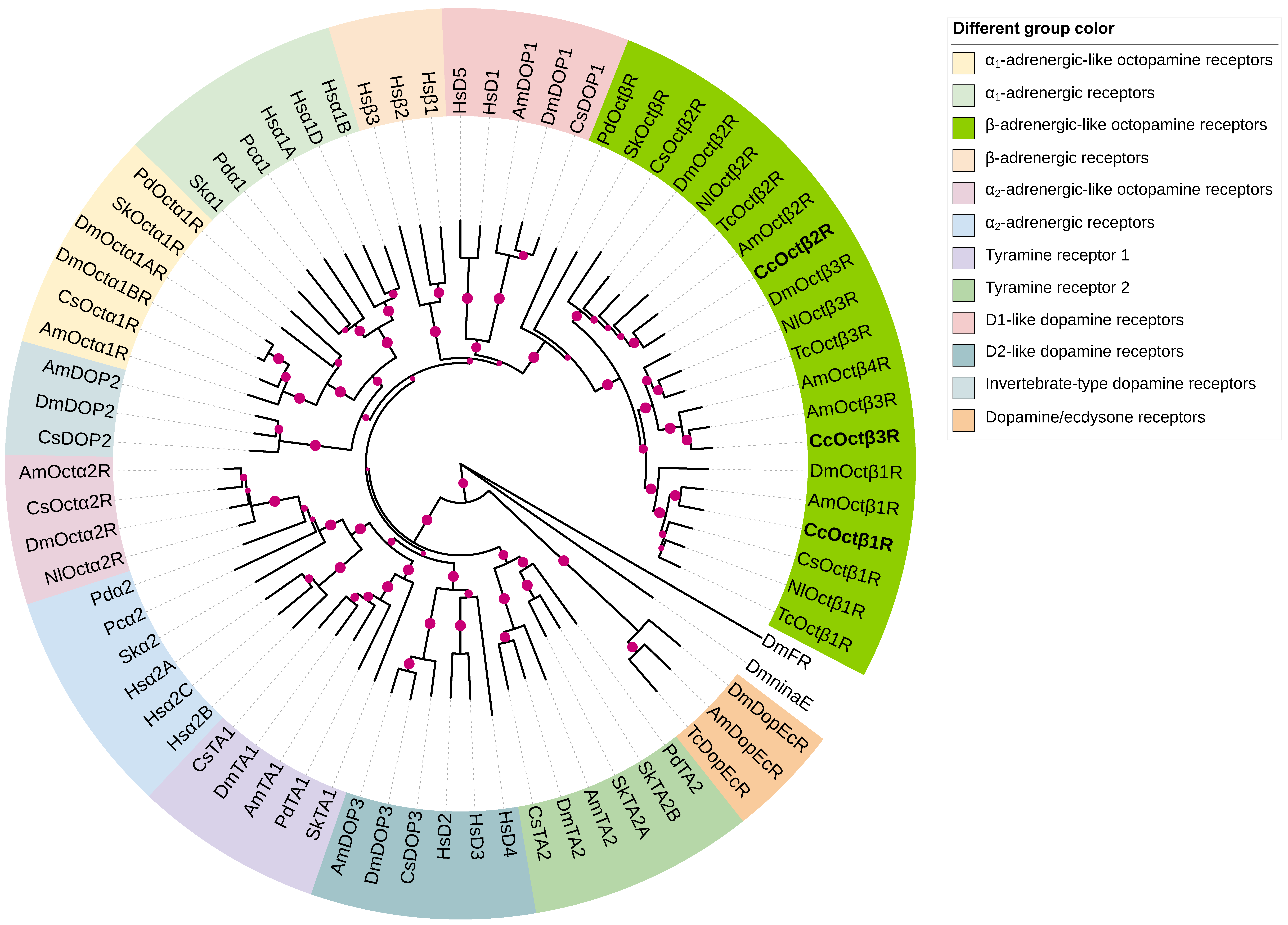
2.2. Phylogenetic Analysis of CcOctβRs
2.3. Expression Profiles of CcOctβRs
2.4. Ligand Specificity of CcOctβRs
2.5. Pharmacological Properties of CcOctβRs
3. Discussion
4. Materials and Methods
4.1. Insects
4.2. Identification and Cloning of CcOctβR Genes
4.3. Sequence Alignment and Phylogenetic Analysis
4.4. Expression Profiles of CcOctβRs
4.5. Construction of Expression Vectors
4.6. Heterologous Expression
4.7. cAMP Assays
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OA | octopamine |
| TA | tyramine |
| DA | dopamine |
| 5-HT | serotonin |
| HA | histamine |
| FK | forskolin |
| OAR | octopamine receptor |
| TAR | tyramine receptor |
| DAR | dopamine receptor |
| Octα1R | α1-adrenergic-like OARs |
| OctβR | β-adrenergic-like OARs |
| Octα2R | α2-adrenergic-like OARs |
| GPCR | G protein-coupled receptor |
| CHO-K1 | Chinese hamster ovary K1 |
| cAMP | cyclic adenosine monophosphate |
| IBMX | 3-isobutyl-1-methylxanthine |
| D-PBS | Dulbecco’s phosphate-buffered saline |
| DMPF | N2-(2,4-dimethylphenyl)-N1-methyformamidine |
| TM | transmembrane |
| RT-PCR | reverse transcription-polymerase chain reaction |
| qRT-PCR | quantitative real-time polymerase chain reaction |
| ANOVA | analysis of variance |
| NCBI | National Center for Biotechnology Information |
References
- White, M.A.; Chen, D.S.; Wolfner, M.F. She’s got nerve: Roles of octopamine in insect female reproduction. J. Neurogenet. 2021, 35, 132–153. [Google Scholar] [CrossRef] [PubMed]
- Roeder, T. The control of metabolic traits by octopamine and tyramine in invertebrates. J. Exp. Biol. 2020, 223, jeb194282. [Google Scholar] [CrossRef] [PubMed]
- Gruteser, N.; Baumann, A. Examination of intracellular GPCR-mediated signaling with high temporal resolution. Int. J. Mol. Sci. 2022, 23, 8516. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, H.; Vleugels, R.; Marchal, E.; Badisco, L.; Pfluger, H.J.; Blenau, W.; Broeck, J.V. The role of octopamine in locusts and other arthropods. J. Insect Physiol. 2010, 56, 854–867. [Google Scholar] [CrossRef]
- Xu, G.; Wu, S.F.; Wu, Y.S.; Gu, G.X.; Fang, Q.; Ye, G.Y. De novo assembly and characterization of central nervous system transcriptome reveals neurotransmitter signaling systems in the rice striped stem borer, Chilo suppressalis. BMC Genom. 2015, 16, 525. [Google Scholar] [CrossRef] [Green Version]
- LeDue, E.E.; Mann, K.; Koch, E.; Chu, B.; Dakin, R.; Gordon, M.D. Starvation-induced depotentiation of bitter taste in Drosophila. Curr. Biol. 2016, 26, 2854–2861. [Google Scholar] [CrossRef] [Green Version]
- Selcho, M.; Pauls, D. Linking physiological processes and feeding behaviors by octopamine. Curr. Opin. Insect Sci. 2019, 36, 125–130. [Google Scholar] [CrossRef]
- Tian, Y.J.; Wang, L.M. Octopamine mediates protein-seeking behavior in mated female Drosophila. Cell Discov. 2018, 4, 66. [Google Scholar] [CrossRef]
- Hoyer, S.C.; Eckart, A.; Herrel, A.; Zars, T.; Fischer, S.A.; Hardie, S.L.; Heisenberg, M. Octopamine in male aggression of Drosophila. Curr. Biol. 2008, 18, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Rao, Y.; Rao, Y. A subset of octopaminergic neurons are important for Drosophila aggression. Nat. Neurosci. 2008, 11, 1059–1067. [Google Scholar] [CrossRef]
- Watanabe, K.; Chiu, H.; Pfeiffer, B.D.; Wong, A.M.; Hoopfer, E.D.; Rubin, G.M.; Anderson, D.J. A circuit node that integrates convergent input from neuromodulatory and social behavior-promoting neurons to control aggression in Drosophila. Neuron 2017, 95, 1112–1128. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.C.; Jin, S.; Hu, K.K.; Geng, L.; Han, C.H.; Kang, R.X.; Pang, Y.X.; Ling, E.J.; Tan, E.K.; Pan, Y.F.; et al. Gut microbiome modulates Drosophila aggression through octopamine signaling. Nat. Commun. 2021, 12, 2698. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.R.; Afonso, D.J.; Kenny, A.R.; Oztu Rk-Colak, A.; Moscato, E.H.; Mainwaring, B.; Kayser, M.; Koh, K. Identification of octopaminergic neurons that modulate sleep suppression by male sex drive. eLife 2017, 6, e23130. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Zhao, X.G.; He, T.; Wu, X.Y.; Lv, P.F.; Zhu, A.J.; Du, J. Epigenetic regulator Stuxnet modulates octopamine effect on sleep through a Stuxnet-Polycomb-Octβ2R cascade. EMBO Rep. 2021, 22, e47910. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, H.; Kim, S.M.; Lin, H.W.; Meng, X.L.; Han, K.A.; Chiang, A.S.; Wang, J.W.; Jiao, R.J.; Rao, Y. Molecular genetic analysis of sexual rejection: Roles of octopamine and its receptor OAMB in Drosophila courtship conditioning. J. Neurosci. 2012, 32, 14281–14287. [Google Scholar] [CrossRef] [Green Version]
- Andrews, J.C.; Fernandez, M.P.; Yu, Q.; Leary, G.P.; Leung, A.K.; Kavanaugh, M.P.; Kravitz, E.A.; Certel, S.J. Octopamine neuromodulation regulates Gr32a-linked aggression and courtship pathways in Drosophila males. PLoS Genet. 2014, 10, e1004356. [Google Scholar] [CrossRef] [Green Version]
- Rezaval, C.; Nojima, T.; Neville, M.C.; Lin, A.C.; Goodwin, S.F. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Curr. Biol. 2014, 24, 725–730. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; Chen, Y.; Zhang, J.Y.; Stanley, D.; Song, Q.S.; Ge, L.Q. Octopamine signaling is involved in the female postmating state in Nilaparvata lugens Stål (Hemiptera: Delphacidae). Arch. Insect Biochem. Physiol. 2021, 107, e21825. [Google Scholar] [CrossRef]
- Li, F.; Li, K.; Wu, L.J.; Fan, Y.L.; Liu, T.X. Role of biogenic amines in oviposition by the diamondback moth, Plutella xylostella L. Front. Physiol. 2020, 11, 475. [Google Scholar] [CrossRef]
- Liu, D.D.; Zhang, X.X.; Fang, C.Q.; Nyamwasa, I.; Cao, Y.Z.; Yin, J.; Zhang, S.; Feng, H.L.; Li, K.B. Octopamine modulates insect mating and oviposition. J. Chem. Ecol. 2022, 48, 628–640. [Google Scholar] [CrossRef]
- Pang, L.; Liu, Z.G.; Chen, J.N.; Dong, Z.; Zhou, S.C.; Zhang, Q.C.; Lu, Y.Q.; Sheng, Y.F.; Chen, X.X.; Huang, J.H. Search performance and octopamine neuronal signaling mediate parasitoid induced changes in Drosophila oviposition behavior. Nat. Commun. 2022, 13, 4476. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Lee, H.G.; Lim, J.; Han, K.A. Appetitive learning requires the alpha1-like octopamine receptor OAMB in the Drosophila mushroom body neurons. J. Neurosci. 2013, 33, 1672–1677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabandal, J.M.; Sabandal, P.R.; Kim, Y.C.; Han, K.A. Concerted actions of octopamine and dopamine receptors drive olfactory learning. J. Neurosci. 2020, 40, 4240–4250. [Google Scholar] [CrossRef]
- Burke, C.J.; Huetteroth, W.; Owald, D.; Perisse, E.; Krashes, M.J.; Das, G.; Gohl, D.; Silies, M.; Certel, S.; Waddell, S. Layered reward signalling through octopamine and dopamine in Drosophila. Nat. 2012, 492, 433–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Wu, S.F.; Li, X.H.; Adamo, S.A.; Ye, G.Y. The characterization of a concentration-sensitive α-adrenergic-like octopamine receptor found on insect immune cells and its possible role in mediating stress hormone effects on immune function. Brain Behav. Immun. 2012, 26, 942–950. [Google Scholar] [CrossRef]
- Kong, H.L.; Dong, C.L.; Tian, Z.; Mao, N.; Wang, C.; Cheng, Y.X.; Zhang, L.; Jiang, X.F.; Luo, L.Z. Altered immunity in crowded Mythimna separata is mediated by octopamine and dopamine. Sci. Rep. 2018, 8, 3215. [Google Scholar] [CrossRef] [Green Version]
- Sujkowski, A.; Ramesh, D.; Brockmann, A.; Wessells, R. Octopamine drives endurance exercise adaptations in Drosophila. Cell Rep. 2017, 21, 1809–1823. [Google Scholar] [CrossRef] [Green Version]
- Sujkowski, A.; Gretzinger, A.; Soave, N.; Todi, S.V.; Wessells, R. Alpha- and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations. PLoS Genet. 2020, 16, e1008778. [Google Scholar] [CrossRef] [PubMed]
- Kaya-Zeeb, S.; Engelmayer, L.; Straßburger, M.; Bayer, J.; Bähre, H.; Seifert, R.; Scherf-Clavel, O.; Thamm, M. Octopamine drives honeybee thermogenesis. eLife 2022, 11, e74334. [Google Scholar] [CrossRef]
- Deshpande, S.A.; Rohrbach, E.W.; Asuncion, J.D.; Harrigan, J.; Eamani, A.; Schlingmann, E.H.; Suto, D.J.; Lee, P.T.; Schweizer, F.E.; Bellen, H.J.; et al. Regulation of Drosophila oviduct muscle contractility by octopamine. iScience 2022, 25, 104697. [Google Scholar] [CrossRef]
- Hill, C.A.; Sharan, S.; Watts, V.J. Genomics, GPCRs and new targets for the control of insect pests and vectors. Curr. Opin. Insect Sci. 2018, 30, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Xu, G.; Qi, Y.X.; Xia, R.Y.; Huang, J.; Ye, G.Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.X.; Xu, G.; Gu, G.X.; Mao, F.; Ye, G.Y.; Liu, W.; Huang, J. A new Drosophila octopamine receptor responds to serotonin. Insect Biochem. Mol. Biol. 2017, 90, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Hana, S.; Lange, A.B. Cloning and functional characterization of Octβ2-receptor and Tyr1-receptor in the Chagas disease vector, Rhodnius prolixus. Front. Physiol. 2017, 8, 744. [Google Scholar] [CrossRef] [Green Version]
- Kita, T.; Hayashi, T.; Ohtani, T.; Takao, H.; Takasu, H.; Liu, G.Y.; Ohta, H.; Ozoe, F.; Ozoe, Y. Amitraz and its metabolite differentially activate α- and β-adrenergic-like octopamine receptors. Pest Manag. Sci. 2017, 73, 984–990. [Google Scholar] [CrossRef]
- Takata, M.; Misato, S.; Ozoe, F.; Ozoe, Y. A point mutation in the β-adrenergic-like octopamine receptor: Possible association with amitraz resistance. Pest Manag. Sci. 2020, 76, 3720–3728. [Google Scholar] [CrossRef]
- Lou, Y.G.; Zhang, G.R.; Zhang, W.Q.; Hu, Y.; Zhang, J. Biological control of rice insect pests in China. Biol. Control 2013, 67, 8–20. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xu, G.; Gan, S.Y.; Chen, X.; Fang, Q.; Ye, G.Y. Effects of the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae) parasitism, venom, and calyx fluid on cellular and humoral immunity of its host Chilo suppressalis (Lepidoptera: Crambidae) larvae. J. Insect Physiol. 2016, 85, 46–56. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.H.; Xiong, S.J.; Teng, Z.W.; Yang, Y.; Wang, J.L.; Yu, K.L.; Wu, H.Z.; Mei, Y.; Xue, C.; Yan, Z.C.; et al. Genome of the parasitoid wasp Cotesia chilonis sheds light on amino acid resource exploitation. BMC Biol. 2022, 20, 118. [Google Scholar] [CrossRef]
- Maqueira, B.; Chatwin, H.; Evans, P.D. Identification and characterization of a novel family of Drosophila β-adrenergic-like octopamine G-protein coupled receptors. J. Neurochem. 2005, 94, 547–560. [Google Scholar] [CrossRef]
- Balfanz, S.; Jordan, N.; Langenstuck, T.; Breuer, J.; Bergmeier, V.; Baumann, A. Molecular, pharmacological, and signaling properties of octopamine receptors from honeybee (Apis mellifera) brain. J. Neurochem. 2014, 129, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Jv, X.M.; Huang, J.M.; Gao, C.F. Molecular features and expression profiles of octopamine receptors in the brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2019, 75, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.S.; Zhan, X.; Yu, Y.; Wang, S.Z.; Lu, C.; Lin, G.F.; Zhu, X.Y.; He, W.Y.; You, M.S.; You, S.J. Molecular and pharmacological characterization of biogenic amine receptors from the diamondback moth, Plutella Xylostella. Pest Manag. Sci. 2021, 77, 4462–4475. [Google Scholar] [CrossRef] [PubMed]
- Li, H.M.; Jiang, H.B.; Gui, S.H.; Liu, X.Q.; Liu, H.; Lu, X.P.; Smagghe, G.; Wang, J.J. Characterization of a β-adrenergic-like octopamine receptor in the oriental fruit fly, Bactrocera dorsalis (Hendel). Int. J. Mol. Sci. 2016, 17, 1577. [Google Scholar] [CrossRef] [Green Version]
- Ohhara, Y.; Shimada-Niwa, Y.; Niwa, R.; Kayashima, Y.; Hayashi, Y.; Akagi, K.; Ueda, H.; Yamakawa-Kobayashi, K.; Kobayashi, S. Autocrine regulation of ecdysone synthesis by β3-octopamine receptor in the prothoracic gland is essential for Drosophila metamorphosis. Proc. Natl. Acad. Sci. USA 2015, 112, 1452–1457. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Liu, Z.M.; Ma, H.H.; Zheng, W.; Liu, J.; Zhou, Y.; Man, Y.L.; Zhou, X.A.; Zeng, A.P. Pharmacological properties and function of the PxOctβ3 octopamine receptor in Plutella xylostella (L.). Insects 2022, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Sabandal, P.R.; Fernandez, A.; Sabandal, J.M.; Lee, H.G.; Evans, P.; Han, K.A. The octopamine receptor Octβ2R regulates ovulation in Drosoph. Melanogaster. PLoS ONE 2014, 9, e104441. [Google Scholar]
- Li, Y.; Fink, C.; El-Kholy, S.; Roeder, T. The octopamine receptor octβ2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster. Arch. Insect Biochem. Physiol. 2015, 88, 168–178. [Google Scholar] [CrossRef]
- Wu, S.F.; Jv, X.M.; Li, J.; Xu, G.J.; Cai, X.Y.; Gao, C.F. Pharmacological characterisation and functional roles for egg-laying of a β-adrenergic-like octopamine receptor in the brown planthopper Nilaparvata lugens. Insect Biochem. Mol. Biol. 2017, 87, 55–64. [Google Scholar] [CrossRef]
- Zheng, L.S.; Liu, X.Q.; Liu, G.G.; Huang, Q.Q.; Wang, J.J.; Jiang, H.B. Knockdown of a β-adrenergic-like octopamine receptor affects locomotion and reproduction of Tribolium castaneum. Int. J. Mol. Sci. 2021, 22, 7252. [Google Scholar] [CrossRef]
- Guo, L.; Fan, X.Y.; Qiao, X.M.; Montell, C.; Huang, J. An octopamine receptor confers selective toxicity of amitraz on honeybees and Varroa mites. eLife 2021, 10, e68268. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Yao, Y.; Huang, J.; Ye, G.Y. Characterization of a β-adrenergic-like octopamine receptor from the rice stem borer (Chilo suppressalis). J. Exp. Biol. 2012, 215, 2646–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.J.; Jiang, L.; Ahamd, S.; Chen, Y.; Zhang, J.Y.; Stanley, D.; Miao, H.; Ge, L.Q. The octopamine receptor, OA2B2, modulates stress resistance and reproduction in Nilaparvata lugens Stål (Hemiptera: Delphacidae). Insect Mol. Biol. 2021, 31, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Balfanz, S.; Strunker, T.; Frings, S.; Baumann, A. A family of octopamine receptors that specifically induce cyclic AMP production or Ca2+ release in Drosophila melanogaster. J. Neurochem. 2005, 93, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ohta, H.; Ozoe, F.; Miyazawa, K.; Huang, J.; Ozoe, Y. Functional and pharmacological characterization of a β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2010, 40, 476–486. [Google Scholar] [CrossRef]
- Huang, J.; Hamasaki, T.; Ozoe, Y. Pharmacological characterization of a Bombyx mori α-adrenergic-like octopamine receptor stably expressed in a mammalian cell line. Arch. Insect Biochem. Physiol. 2010, 73, 74–86. [Google Scholar] [CrossRef]
- Bischof, L.J.; Enan, E.E. Cloning, expression and functional analysis of an octopamine receptor from Periplaneta Americana. Insect Biochem. Mol. Biol. 2004, 34, 511–521. [Google Scholar] [CrossRef]
- Blenau, W.; Bremer, A.S.; Schwietz, Y.; Friedrich, D.; Ragionieri, L.; Predel, R.; Balfanz, S.; Baumann, A. PaOctβ2R: Identification and functional characterization of an octopamine receptor activating adenylyl cyclase activity in the American cockroach Periplaneta americana. Int. J. Mol. Sci. 2022, 23, 1677. [Google Scholar] [CrossRef]
- Grohmann, L.; Blenau, W.; Erber, J.; Ebert, P.R.; Strunker, T.; Baumann, A. Molecular and functional characterization of an octopamine receptor from honeybee (Apis mellifera) brain. J. Neurochem. 2003, 86, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Blenau, W.; Wilms, J.A.; Balfanz, S.; Baumann, A. AmOctα2R: Functional characterization of a honeybee octopamine receptor inhibiting adenylyl cyclase activity. Int. J. Mol. Sci. 2020, 21, 9334. [Google Scholar] [CrossRef]
- Huang, Q.T.; Ma, H.H.; Deng, X.L.; Zhu, H.; Liu, J.; Zhou, Y.; Zhou, X.M. Pharmacological characterization of a β-adrenergic-like octopamine receptor in Plutella xylostella. Arch. Insect Biochem. Physiol. 2018, 98, e21466. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.L.; Guo, L.; Ma, H.H.; Hu, X.P.; Zhou, X.M. Phenyl imidazolidin-2-ones antagonize a β-adrenergic-like octopamine receptor in diamondback moth (Plutella xylostella). Pest Manag. Sci. 2021, 77, 3224–3232. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Utsumi, T.; Ozoe, Y. Amino acid residues involved in interaction with tyramine in the Bombyx mori tyramine receptor. Insect Mol. Biol. 2004, 13, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ohta, H.; Sasaki, K.; Ozoe, F.; Ozoe, Y. Amino acid residues involved in the interaction with the intrinsic agonist (R)-octopamine in the β-adrenergic-like octopamine receptor from the silkworm Bombyx mori. J. Pestic. Sci. 2011, 36, 473–480. [Google Scholar] [CrossRef] [Green Version]
- Kobilka, B.K. G protein coupled receptor structure and activation. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 794–807. [Google Scholar] [CrossRef] [Green Version]
- Hauser, F.; Cazzamali, G.; Williamson, M.; Blenau, W.; Grimmelikhuijzen, C.J. A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog. Neurobiol. 2006, 80, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Kaya-Zeeb, S.; Delac, S.; Wolf, L.; Marante, A.L.; Scherf-Clavel, O.; Thamm, M. Robustness of the honeybee neuro-muscular octopaminergic system in the face of cold stress. Front. Physiol. 2022, 13, 1002740. [Google Scholar] [CrossRef]
- Guo, Y.F.; Qiu, J.R.; Chen, T.; Gao, S.J.; Bu, S.H.; Wang, R.; Wang, J.D. Characterization and functional analysis of a β-adrenergic-like octopamine receptor from the oriental armyworm (Mythimna separata Walker). Arch. Insect Biochem. Physiol. 2021, 106, e21772. [Google Scholar] [CrossRef]
- El-Kholy, S.; Stephano, F.; Li, Y.; Bhandari, A.; Fink, C.; Roeder, T. Expression analysis of octopamine and tyramine receptors in Drosophila. Cell Tissue Res. 2015, 361, 669–684. [Google Scholar] [CrossRef]
- Xu, G.; Chang, X.F.; Gu, G.X.; Jia, W.X.; Guo, L.; Huang, J.; Ye, G.Y. Molecular and pharmacological characterization of a β-adrenergic-like octopamine receptor from the green rice leafhopper Nephotettix cincticeps. Insect Biochem. Mol. Biol. 2020, 120, 103337. [Google Scholar] [CrossRef]
- Zhukovskaya, M.I.; Polyanovsky, A.D. Biogenic amines in insect antennae. Front. Syst. Neurosci. 2017, 11, 45. [Google Scholar] [CrossRef] [PubMed]
- Hillier, N.K.; Kavanagh, R.M. Differential octopaminergic modulation of olfactory receptor neuron responses to sex pheromones in Heliothis virescens. PLoS ONE 2015, 10, e0143179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflüger, H.J.; Duch, C. Dynamic neural control of insect muscle metabolism related to motor behavior. Physiology 2011, 26, 293–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blenau, W.; Balfanz, S.; Baumann, A. PeaTAR1B: Characterization of a second type 1 tyramine receptor of the american cockroach, Periplaneta americana. Int. J. Mol. Sci. 2017, 18, 2279. [Google Scholar] [CrossRef] [Green Version]
- Reim, T.; Balfanz, S.; Baumann, A.; Blenau, W.; Thamm, M.; Scheiner, R. AmTAR2: Functional characterization of a honeybee tyramine receptor stimulating adenylyl cyclase activity. Insect Biochem. Mol. Biol. 2017, 80, 91–100. [Google Scholar] [CrossRef]
- Vergoz, V.; Roussel, E.; Sandoz, J.C.; Giurfa, M. Aversive learning in honeybees revealed by the olfactory conditioning of the sting extension reflex. PLoS ONE 2007, 2, e288. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, M.; Giannoni Guzman, M.; Morales-Matos, C.; Del Valle Diaz, R.A.; Abramson, C.I.; Giray, T. Dopamine and octopamine influence avoidance learning of honey bees in a place preference assay. PLoS ONE 2011, 6, e25371. [Google Scholar] [CrossRef]
- Xu, G.; Wu, S.F.; Gu, G.X.; Teng, Z.W.; Ye, G.Y.; Huang, J. Pharmacological characterization of dopamine receptors in the rice striped stem borer, Chilo suppressalis. Insect Biochem. Mol. Biol. 2017, 83, 80–93. [Google Scholar] [CrossRef]
- Nuss, A.B.; Ejendal, K.F.; Doyle, T.B.; Meyer, J.M.; Lang, E.G.; Watts, V.J.; Hill, C.A. Dopamine receptor antagonists as new mode-of-action insecticide leads for control of Aedes and Culex mosquito vectors. PLoS Negl. Trop. Dis. 2015, 9, e0003515. [Google Scholar] [CrossRef]
- Teng, Z.W.; Xiong, S.J.; Xu, G.; Gan, S.Y.; Chen, X.; Stanley, D.; Yan, Z.C.; Ye, G.Y.; Fang, Q. Protein discovery: Combined transcriptomic and proteomic analyses of venom from the endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). Toxins 2017, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.W.; Wu, H.Z.; Ye, X.H.; Xiong, S.J.; Xu, G.; Wang, F.; Fang, Q.; Ye, G.Y. An ovarian protein involved in passive avoidance of an endoparasitoid to evade its host immune response. J. Proteome Res. 2019, 18, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 1987, 196, 947–950. [Google Scholar] [CrossRef]





| Agonist | CcOctβ1R | CcOctβ2R | CcOctβ3R | |||
|---|---|---|---|---|---|---|
| EC50 (M) | LogEC50 | EC50 (M) | LogEC50 | EC50 (M) | LogEC50 | |
| OA | 3.90 × 10−8 | −7.41 ± 0.053 | 6.69 × 10−9 | −8.18 ± 0.043 | 3.36 × 10−8 | −7.47 ± 0.133 |
| TA | 1.53 × 10−6 | −5.81 ± 0.080 | 7.59 × 10−7 | −6.12 ± 0.062 | 2.99 × 10−6 | −5.52 ± 0.039 |
| Naphazoline | 2.31 × 10−10 | −9.64 ± 0.172 | 1.57 × 10−11 | −10.8 ± 0.631 | 5.06 × 10−9 | −8.30 ± 0.364 |
| Clonidine | 4.75 × 10−7 | −6.32 ± 0.063 | 1.42 × 10−7 | −6.85 ± 0.045 | 5.34 × 10−7 | −6.27 ± 0.090 |
| Tolazoline | 1.30 × 10−6 | −5.89 ± 0.078 | 4.85 × 10−7 | −6.31 ± 0.027 | 5.04 × 10−6 | −5.30 ± 0.082 |
| Medetomidine | 3.65 × 10−7 | −6.44 ± 0.055 | 2.03 × 10−8 | −7.69 ± 0.031 | 4.18 × 10−8 | −7.38 ± 0.094 |
| Lisuride | 5.94 × 10−8 | −7.23 ± 0.060 | 4.49 × 10−8 | −7.35 ± 0.030 | 4.60 × 10−9 | −8.34 ± 0.090 |
| Amitraz | 1.65 × 10−8 | −7.78 ± 0.121 | 3.27 × 10−9 | −8.49 ± 0.047 | 5.81 × 10−9 | −8.24 ± 0.130 |
| DPMF | 9.25 × 10−9 | −8.03 ± 0.078 | 2.37 × 10−9 | −8.63 ± 0.046 | 4.35 × 10−9 | −8.36 ± 0.089 |
| Antagonist | CcOctβ1R | CcOctβ2R | CcOctβ3R | |||
|---|---|---|---|---|---|---|
| IC50 (M) | LogIC50 | IC50 (M) | LogIC50 | IC50 (M) | LogIC50 | |
| Chlorpromazine | 2.86 × 10−5 | −4.54 ± 1.209 | 7.45 × 10−6 | −5.13 ± 0.053 | 2.38 × 10−6 | −5.62 ± 0.158 |
| Cyproheptadine | 3.95 × 10−6 | −5.40 ± 0.483 | 3.80 × 10−7 | −6.42 ± 0.024 | 2.31 × 10−7 | −6.64 ± 0.261 |
| Metoclopramide | 1.48 × 10−6 | −5.83 ± 0.134 | 2.03 × 10−6 | −5.69 ± 0.048 | 2.32 × 10−7 | −6.64 ± 0.084 |
| Mianserin | 1.57 × 10−7 | −6.80 ± 0.080 | 7.58 × 10−8 | −7.12 ± 0.022 | 4.06 × 10−8 | −7.39 ± 0.094 |
| Phentolamine | 1.25 × 10−6 | −5.91 ± 0.149 | 2.26 × 10−7 | −6.65 ± 0.027 | 3.67 × 10−8 | −7.44 ± 0.121 |
| Epinastine | 3.76 × 10−8 | −7.43 ± 0.080 | 1.44 × 10−8 | −7.84 ± 0.037 | 7.34 × 10−9 | −8.13 ± 0.323 |
| Methiothepin | n.a. | n.a. | 1.47 × 10−6 | −5.83 ± 0.043 | 1.63 × 10−6 | −5.79 ± 0.734 |
| Clozapine | n.a. | n.a. | 4.69 × 10−7 | −6.33 ± 0.024 | 2.55 × 10−7 | −6.59 ± 0.089 |
| Asenapine | 1.70 × 10−7 | −6.77 ± 0.036 | 2.34 × 10−8 | −7.63 ± 0.029 | 1.13 × 10−8 | −7.95 ± 0.119 |
| Amitriptyline | 9.92 × 10−6 | −5.00 ± 0.029 | 1.95 × 10−6 | −5.71 ± 0.064 | 9.03 × 10−7 | −6.04 ± 0.090 |
| Chlorprothixene | n.a. | n.a. | 1.91 × 10−6 | −5.72 ± 0.157 | 2.82 × 10−7 | −6.55 ± 0.381 |
| Doxepin | 2.69 × 10−6 | −5.57 ± 0.290 | 2.11 × 10−6 | −5.68 ± 0.161 | 1.99 × 10−7 | −6.70 ± 0.147 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Zhang, Y.-Y.; Gu, G.-X.; Yang, G.-Q.; Ye, G.-Y. Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). Int. J. Mol. Sci. 2022, 23, 14513. https://doi.org/10.3390/ijms232314513
Xu G, Zhang Y-Y, Gu G-X, Yang G-Q, Ye G-Y. Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). International Journal of Molecular Sciences. 2022; 23(23):14513. https://doi.org/10.3390/ijms232314513
Chicago/Turabian StyleXu, Gang, Yuan-Yuan Zhang, Gui-Xiang Gu, Guo-Qing Yang, and Gong-Yin Ye. 2022. "Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae)" International Journal of Molecular Sciences 23, no. 23: 14513. https://doi.org/10.3390/ijms232314513
APA StyleXu, G., Zhang, Y.-Y., Gu, G.-X., Yang, G.-Q., & Ye, G.-Y. (2022). Molecular and Pharmacological Characterization of β-Adrenergic-like Octopamine Receptors in the Endoparasitoid Cotesia chilonis (Hymenoptera: Braconidae). International Journal of Molecular Sciences, 23(23), 14513. https://doi.org/10.3390/ijms232314513








