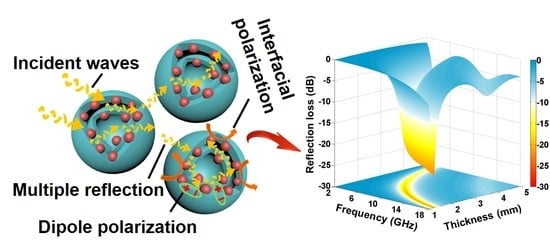
Construction of Dual-Shell Mo2C/C Microsphere towards Efficient Electromagnetic Wave Absorption
Abstract
1. Introduction
2. Results and Discussion
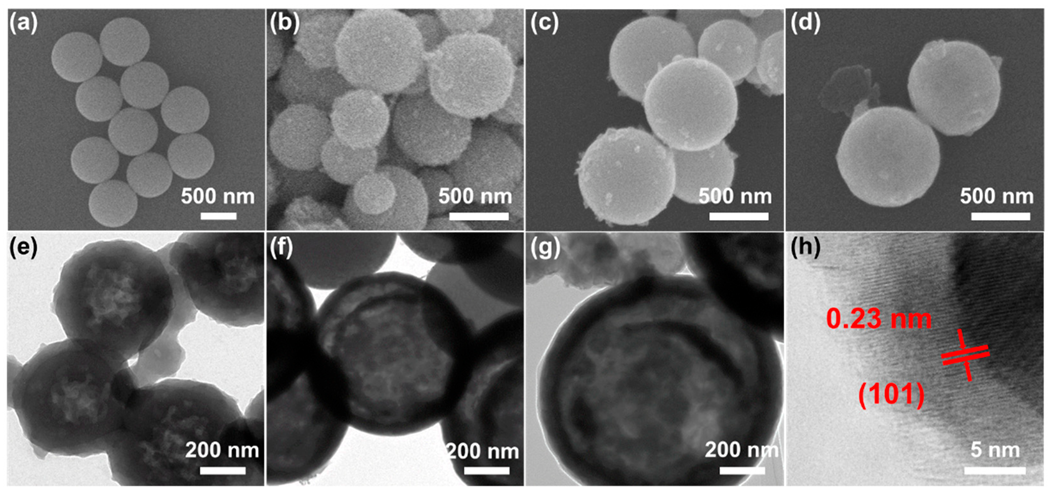
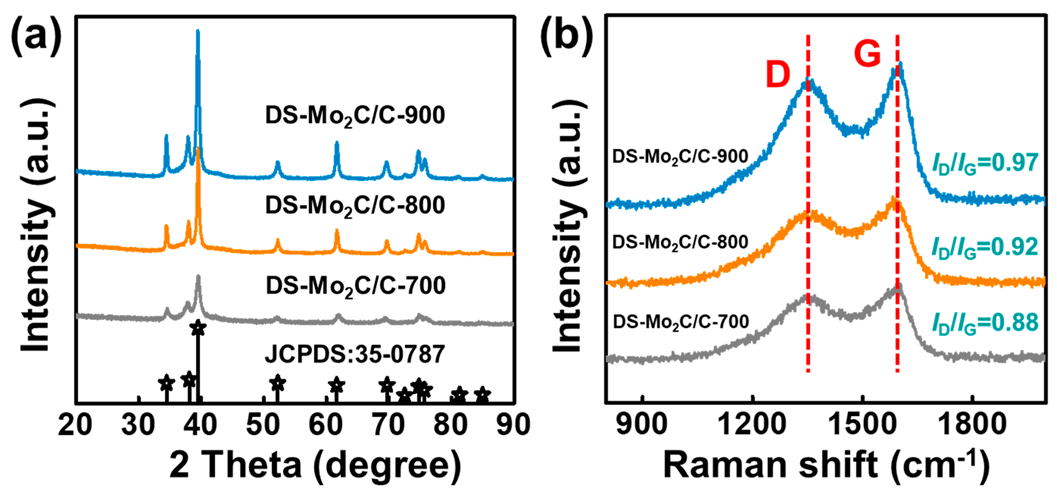
2.1. Material Characteristics
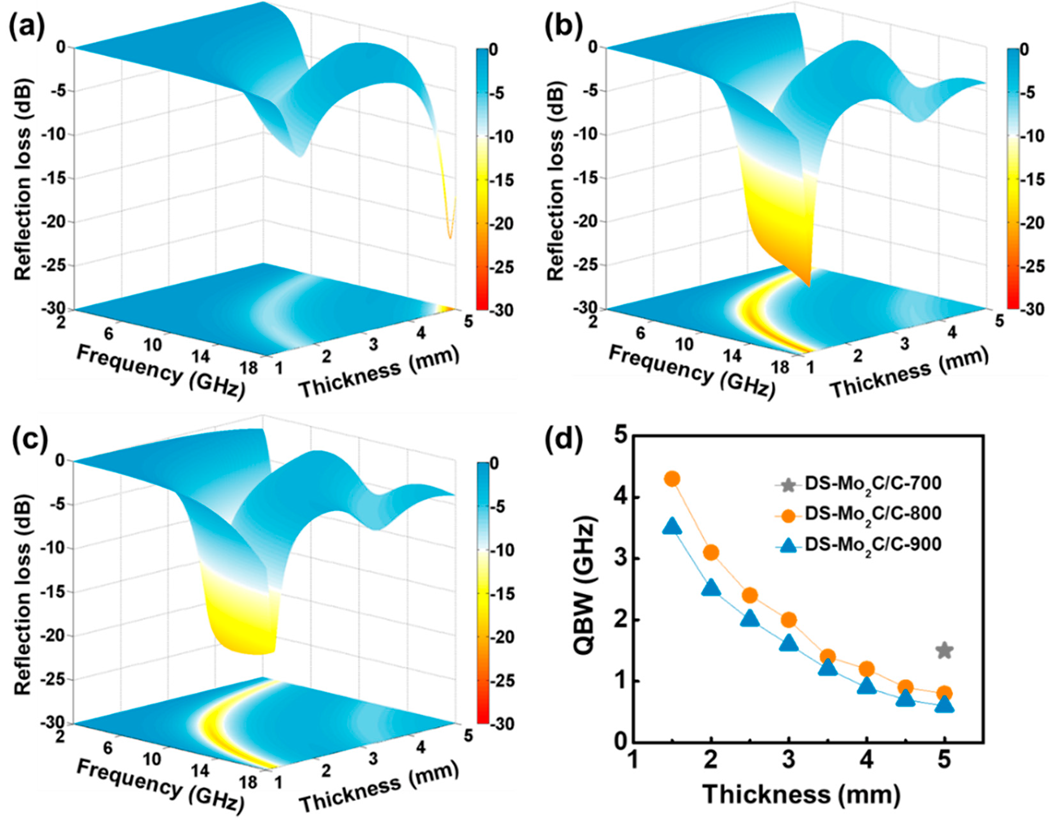
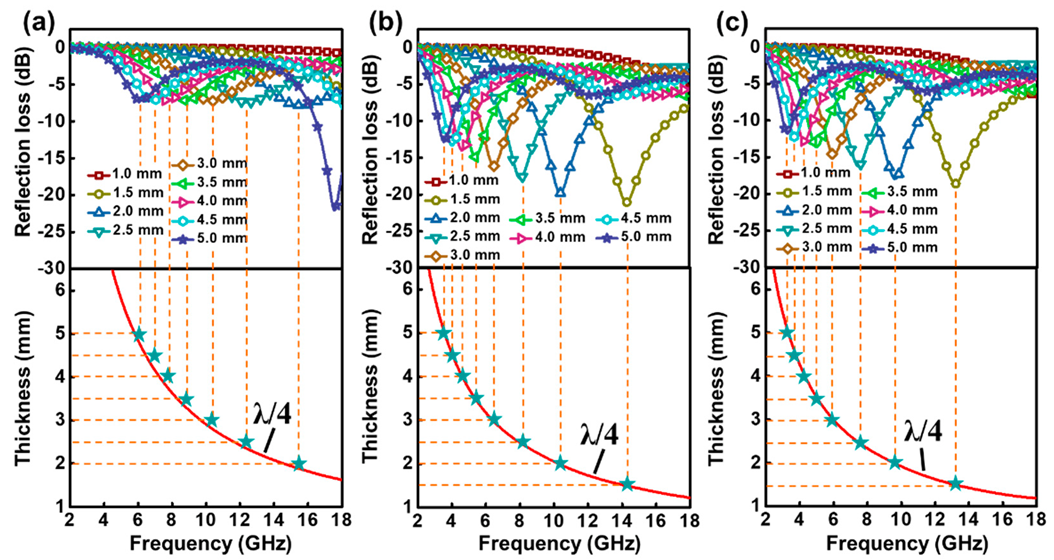
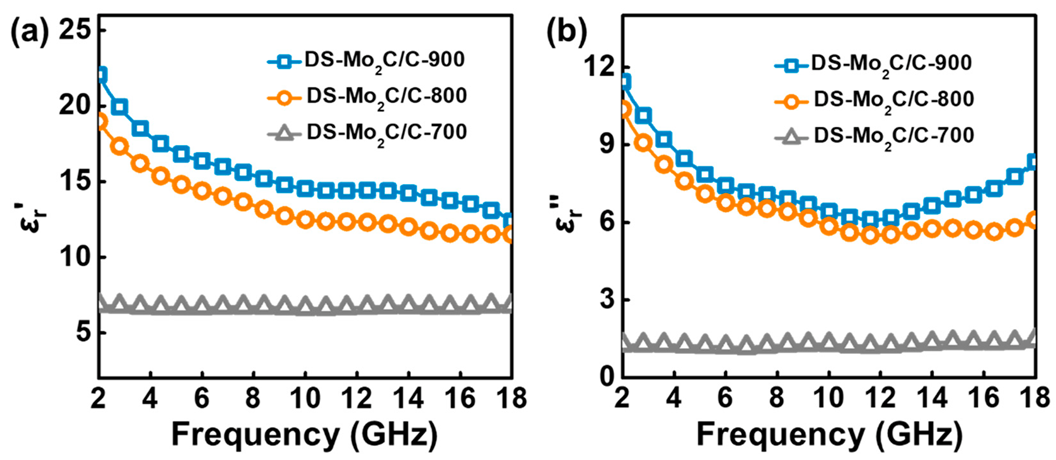
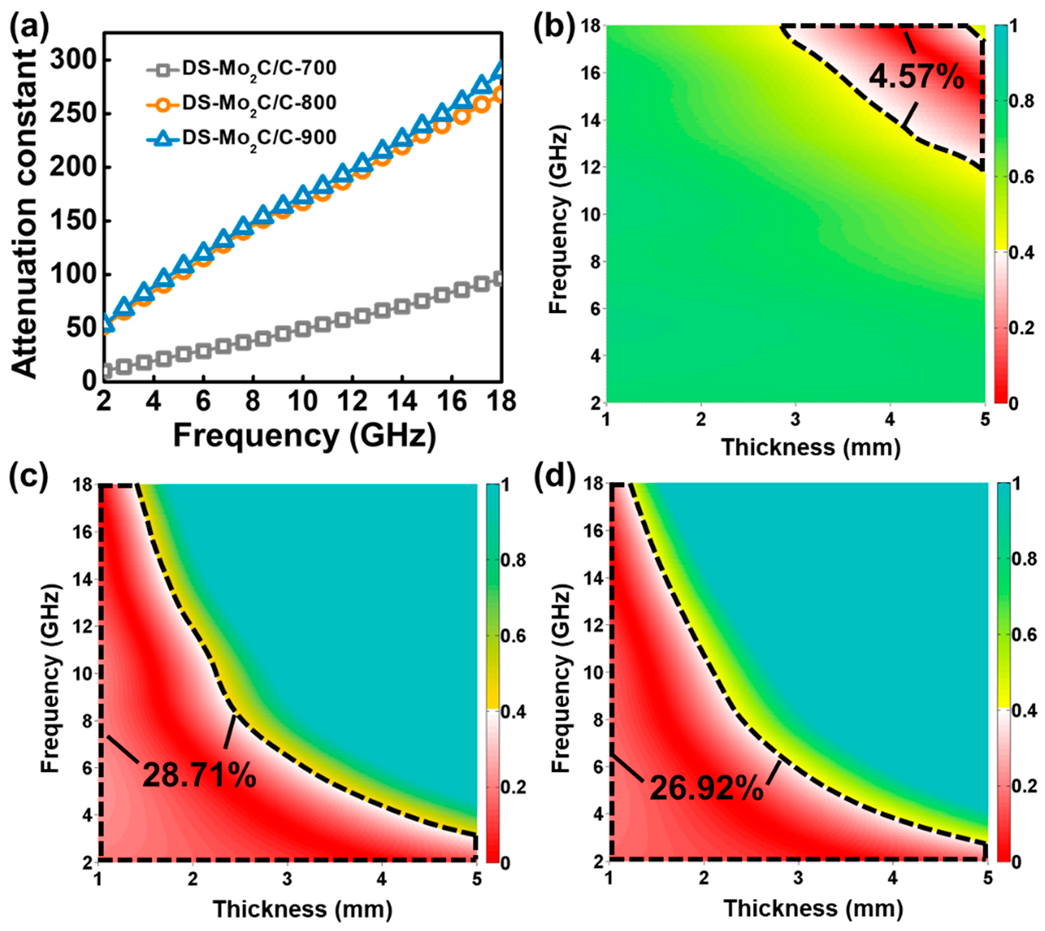
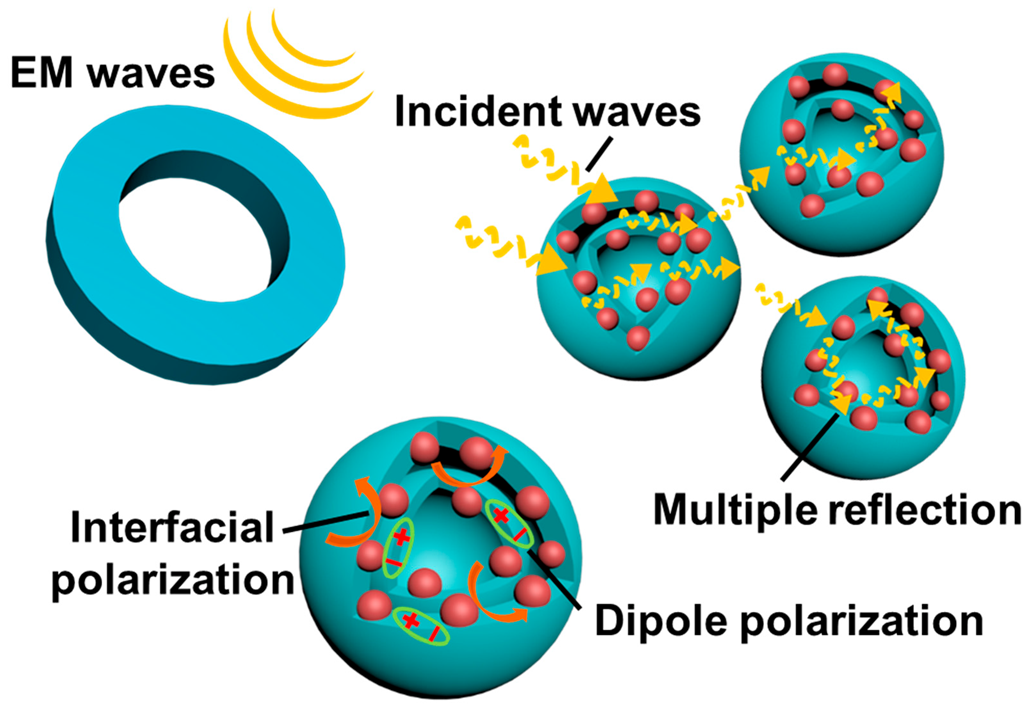
2.2. Electromagnetic Parameters and Performance Analysis
3. Materials and Methods
3.1. Synthesis of Mo-GL Spheres
3.2. Synthesis of Mo-GL Spheres
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Potschke, P.; Pionteck, J.; Voit, B.; Qi, H. Multifunctional Cellulose/rGO/Fe3O4 Composite Aerogels for Electromagnetic Interference Shielding. ACS Appl. Mater. Interfaces 2020, 12, 22088–22098. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Gao, S.; Wang, Y.; Huang, Y.; Wang, Y.; Luo, J. Core-Shell CoNi@Graphitic Carbon Decorated on B,N-Codoped Hollow Carbon Polyhedrons toward Lightweight and High-Efficiency Microwave Attenuation. ACS Appl. Mater. Interfaces 2019, 11, 25624–25635. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Cao, W.Q.; Cao, M.S.; Yuan, J. Assembling Nano-Microarchitecture for Electromagnetic Absorbers and Smart Devices. Adv. Mater. 2020, 32, e2002112. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Jiao, S.; Ma, K.; Lv, J.; Yang, J. Hierarchical Carbon Fiber@MXene@MoS2 Core-sheath Synergistic Microstructure for Tunable and Efficient Microwave Absorption. Adv. Funct. Mater. 2020, 30, 2002595. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2@TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Lv, J.; Liang, X.; Ji, G.; Quan, B.; Liu, W.; Du, Y. Structural and Carbonized Design of 1D FeNi/C Nanofibers with Conductive Network to Optimize Electromagnetic Parameters and Absorption Abilities. ACS Sustain. Chem. Eng. 2018, 6, 7239–7249. [Google Scholar] [CrossRef]
- Li, Z.; Han, X.; Ma, Y.; Liu, D.; Wang, Y.; Xu, P.; Li, C.; Du, Y. MOFs-Derived Hollow Co/C Microspheres with Enhanced Microwave Absorption Performance. ACS Sustain. Chem. Eng. 2018, 6, 8904–8913. [Google Scholar] [CrossRef]
- Tian, C.; Du, Y.; Xu, P.; Qiang, R.; Wang, Y.; Ding, D.; Xue, J.; Ma, J.; Zhao, H.; Han, X. Constructing Uniform Core-Shell PPy@PANI Composites with Tunable Shell Thickness toward Enhancement in Microwave Absorption. ACS Appl. Mater. Interfaces 2015, 7, 20090–20099. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Tian, R.; Li, H.; Zhou, Y.; Duan, H.; Liu, H. Porous Co-C Core-Shell Nanocomposites Derived from Co-MOF-74 with Enhanced Electromagnetic Wave Absorption Performance. ACS Appl. Mater. Interfaces 2018, 10, 11333–11342. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Zeng, X.; Cui, T.; Zhao, Y.; Li, Y.; Tong, G. Facile Hydrothermal Synthesis of Fe3O4/C Core-Shell Nanorings for Efficient Low-Frequency Microwave Absorption. ACS Appl. Mater. Interfaces 2016, 8, 7370–7380. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, F.; Cui, L.; Xu, X.; Han, X.; Du, Y. Composition Optimization and Microstructure Design in MOFs-Derived Magnetic Carbon-Based Microwave Absorbers: A Review. Nano-Micro Lett. 2021, 13, 208. [Google Scholar] [CrossRef]
- Guo, T.; Huang, B.; Li, C.; Lou, Y.; Tang, X.-Z.; Huang, X.; Yue, J. Magnetic sputtering of FeNi/C bilayer film on SiC fibers for effective microwave absorption in the low-frequency region. Ceram. Int. 2021, 47, 5221–5226. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Feng, H.; Shui, H.; Yu, R. Metal organic framework-derived Fe/carbon porous composite with low Fe content for lightweight and highly efficient electromagnetic wave absorber. Chem. Eng. J. 2017, 314, 320–327. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Li, H.; Lin, Y.; Jiang, Z.; Xie, Z.; Kuang, Q.; Zheng, L. MOF-Derived Porous Co/C Nanocomposites with Excellent Electromagnetic Wave Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 13604–13611. [Google Scholar] [CrossRef]
- Tian, X.; Meng, F.; Meng, F.; Chen, X.; Guo, Y.; Wang, Y.; Zhu, W.; Zhou, Z. Synergistic Enhancement of Microwave Absorption Using Hybridized Polyaniline@helical CNTs with Dual Chirality. ACS Appl. Mater. Interfaces 2017, 9, 15711–15718. [Google Scholar] [CrossRef]
- Chen, H.; Huang, Z.; Huang, Y.; Zhang, Y.; Ge, Z.; Qin, B.; Liu, Z.; Shi, Q.; Xiao, P.; Yang, Y.; et al. Synergistically assembled MWCNT/graphene foam with highly efficient microwave absorption in both C and X bands. Carbon 2017, 124, 506–514. [Google Scholar] [CrossRef]
- Zhao, B.; Deng, J.; Liang, L.; Zuo, C.; Bai, Z.; Guo, X.; Zhang, R. Lightweight porous Co3O4 and Co/CoO nanofibers with tunable impedance match and configuration-dependent microwave absorption properties. Cryst. Eng. Comm. 2017, 19, 6095–6106. [Google Scholar] [CrossRef]
- Dai, S.; Cheng, Y.; Quan, B.; Liang, X.; Liu, W.; Yang, Z.; Ji, G.; Du, Y. Porous-Carbon-Based Mo2C Nanocomposites as Excellent Microwave Absorber: A New Exploration. Nanoscale 2018, 10, 6945–6953. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Li, X.; Anasori, B.; Zhang, L.; Cheng, L.; Gogotsi, Y. Laminated and Two-Dimensional Carbon-Supported Microwave Absorbers Derived from MXenes. ACS Appl. Mater. Interfaces 2017, 9, 20038–20045. [Google Scholar] [CrossRef]
- Tong, Y.; He, M.; Zhou, Y.; Nie, S.; Zhong, X.; Fan, L.; Huang, T.; Liao, Q.; Wang, Y. Three-Dimensional Hierarchical Architecture of the TiO2/Ti3C2Tx/RGO Ternary Composite Aerogel for Enhanced Electromagnetic Wave Absorption. ACS Sustain. Chem. Eng. 2018, 6, 8212–8222. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Han, X.; Liu, D.; Zhao, H.; Li, Z.; Xu, P.; Du, Y. Ultrasmall Mo2C Nanoparticle-Decorated Carbon Polyhedrons for Enhanced Microwave Absorption. ACS Appl. Nano Mater. 2018, 1, 5366–5376. [Google Scholar] [CrossRef]
- Wang, Y.H.; Han, X.J.; Xu, P.; Liu, D.W.; Cui, L.R.; Zhao, H.H.; Du, Y.C. Synthesis of Pomegranate-Like Mo2C@C Microsphere for Highly Efficient Microwave Absorption. Chem. Eng. J. 2019, 372, 312–320. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Peng, Z.; Zhang, Z.; Zhou, H.; Zhang, X.; Yakobson, B.I.; Goddard, W.A.; Guo, X.; Hauge, R.H.; et al. Atomic H-Induced Mo2C Hybrid as an Active and Stable Bifunctional Electrocatalyst. ACS Nano 2017, 11, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Gong, Q.; Song, X.; Feng, K.; Nie, K.; Zhao, F.; Wang, Y.; Zeng, M.; Zhong, J.; Li, Y. Mo2C Nanoparticles Dispersed on Hierarchical Carbon Microflowers for Efficient Electrocatalytic Hydrogen Evolution. ACS Nano 2016, 10, 11337–11343. [Google Scholar] [CrossRef]
- Zhou, P.; Chen, J.H.; Liu, M.; Jiang, P.; Li, B.; Hou, X.M. Microwave Absorption Properties of SiC@SiO2@Fe3O4 Hybrids in the 2–18 GHz Range. Int. J. Min. Met. Mater. 2017, 24, 804–813. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, G.; Wu, H.; Liu, B.; Fang, X.; Zhang, D.; Zhu, J. Double-layer Microwave Absorber based on Nanocrystalline CoFe2O4 and CoFe2O4/PANI Multi-Core/Shell Composites. Mater. Sci. 2017, 35, 94–104. [Google Scholar] [CrossRef]
- Li, X.-P.; Deng, Z.; Li, Y.; Zhang, H.-B.; Zhao, S.; Zhang, Y.; Wu, X.-Y.; Yu, Z.-Z. Controllable synthesis of hollow microspheres with Fe@Carbon dual-shells for broad bandwidth microwave absorption. Carbon 2019, 147, 172–181. [Google Scholar] [CrossRef]
- An, Z.; Liu, R.; Zhang, J. Carbon/Carbon-Ag-Fe3O4 Dual Shell Hollow Microspheres: High Efficient Pyrolysis Synthesis and Broad Band Microwave Absorption. J. Alloys Compd. 2022, 905, 164254. [Google Scholar] [CrossRef]
- Xu, L.; Tao, J.; Zhang, X.; Yao, Z.; Zavabeti, A.; Zhou, J. Co@N-doped Double-Shell Hollow Carbon via Self-Templating-Polymerization Strategy for Microwave Absorption. Carbon 2022, 188, 34–44. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhao, H.B.; Cheng, J.B.; Liu, B.W.; Fu, Q.; Wang, Y.Z. Hierarchical Ti3C2Tx@ZnO Hollow Spheres with Excellent Microwave Absorption Inspired by the Visual Phenomenon of Eyeless Urchins. Nano-Micro Lett. 2022, 14, 76. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, L.; Lou, X.W. Formation of Triple-Shelled Molybdenum-Polydopamine Hollow Spheres and Their Conversion into MoO2/Carbon Composite Hollow Spheres for Lithium-Ion Batteries. Angew Chem. Int. Ed. Eng. 2016, 55, 14668–14672. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Han, X.; Xu, P.; Cui, L.; Zhao, H.; Liu, D.; Wang, F.; Du, Y. Ternary Mo2C/Co/C Composites with Enhanced Electromagnetic Waves Absorption. Chem. Eng. J. 2020, 387, 124159. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, M.H.; Deng, X.S.; Li, Z.G.; Chen, Z.S.; Shi, J.M.; Han, X.J.; Du, Y.C. Reduced Graphene Oxide Aerogel Decorated with Mo2C Nanoparticles Towards Multifunctional Properties of Hydrophobicity, Thermal Insulation, and Microwave Absorption. Int. J. Miner. Metall. Mater. 2022. [Google Scholar] [CrossRef]
- Gao, Q.; Zhao, X.; Xiao, Y.; Zhao, D.; Cao, M. A Mild Route to Mesoporous Mo2C-C Hybrid Microsphere for High Performance Lithium-ion Batteries. Nanoscale 2014, 6, 6151. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Ouyang, Y.; Gao, Q.; Ouyang, L.; Hu, R.; Liu, J.; Zhu, M. Hierarchical MoO2/Mo2C/C Hybrid Nanowires as High-Rate and Long-Life Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 19987–19993. [Google Scholar] [CrossRef]
- Ge, R.; Huo, J.; Sun, M.; Zhu, M.; Li, Y.; Chou, S.; Li, W. Surface and Interface Engineering: Molybdenum Carbide-Based Nanomaterials for Electrochemical Energy Conversion. Small 2021, 17, e1903380. [Google Scholar] [CrossRef]
- Qutaish, H.; Tanaka, S.; Kaneti, Y.V.; Lin, J.; Bando, Y.; Alshehri, A.A.; Yusa, S.I.; Yamauchi, Y.; Hossain, M.S.A.; Kim, J. Soft-Templated Synthesis of Mesoporous Nickel Oxide using Poly(styrene-block-acrylic acid-block-ethylene glycol) Block Copolymers. Micropor. Mesopor. Mat. 2018, 271, 16–22. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Cai, L.J.; Lu, W.; Chai, Y. Phase and Facet Control of Molybdenum Carbide Nanosheet Observed by In Situ TEM. Small 2017, 13, 1700051. [Google Scholar]
- Zhang, Q.; Du, Z.; Hou, M.; Ding, Z.; Huang, X.; Chen, A.; Ma, Y.; Lu, S.; Tang, X.-Z. Ultralight, Anisotropic, and Self-Supported Graphene/MWCNT Aerogel with High-Performance Microwave Absorption. Carbon 2022, 188, 442–452. [Google Scholar] [CrossRef]
- Du, Y.; Liu, T.; Yu, B.; Gao, H.; Xu, P.; Wang, J.; Wang, X.; Han, X. The Electromagnetic Properties and Microwave Absorption of Mesoporous Carbon. Mater. Chem. Phys. 2012, 135, 884–891. [Google Scholar] [CrossRef]
- Li, Y.; Liao, Y.; Ji, L.; Hu, C.; Zhang, Z.; Zhang, Z.; Zhao, R.; Rong, H.; Qin, G.; Zhang, X. Quinary High-Entropy-Alloy@Graphite Nanocapsules with Tunable Interfacial Impedance Matching for Optimizing Microwave Absorption. Small 2022, 18, e2107265. [Google Scholar] [CrossRef]
- Qiang, R.; Du, Y.; Wang, Y.; Wang, N.; Tian, C.; Ma, J.; Xu, P.; Han, X. Rational Design of Yolk-Shell C@C Microspheres for the Effective Enhancement in Microwave Absorption. Carbon 2016, 98, 599–606. [Google Scholar] [CrossRef]
- Du, Y.; Wang, J.; Cui, C.; Liu, X.; Wang, X.; Han, X. Pure carbon microwave absorbers from anion-exchange resin pyrolysis. Synth. Met. 2010, 160, 2191–2196. [Google Scholar] [CrossRef]
- Bi, N.; Liu, X.; Wu, N.; Cui, C.; Sun, Y. Improved Electrochemical Performance of Onion-Like Carbon Coated Magnetite Nanocapsules as Electromagnetic Absorptive Anode Materials for Lithium-Ion Batteries. RSC Adv. 2015, 5, 32452–32459. [Google Scholar] [CrossRef]
- Duan, W.; Li, X.; Wang, Y.; Qiang, R.; Tian, C.; Wang, N.; Han, X.; Du, Y. Surface Functionalization of Carbonyl Iron with Aluminum Phosphate Coating Toward Enhanced Anti-Oxidative Ability and Microwave Absorption Properties. Appl. Surf. Sci. 2018, 427, 594–602. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, G.; Liu, W.; Quan, B.; Liang, X.; Shang, C.; Cheng, Y.; Du, Y. Thermal Conversion of an Fe3O4@Metal-Organic Framework: A New Method for an Efficient Fe-Co/Nanoporous Carbon Microwave Absorbing Material. Nanoscale 2015, 7, 12932–12942. [Google Scholar] [CrossRef]
- Lv, H.; Ji, G.; Zhang, H.; Du, Y. Facile Synthesis of a CNT@Fe@SiO2 Ternary Composite with Enhanced Microwave Absorption Performance. RSC Adv. 2015, 5, 76836–76843. [Google Scholar] [CrossRef]
- Li, X.; Yin, X.; Han, M.; Song, C.; Xu, H.; Hou, Z.; Zhang, L.; Cheng, L. Ti3C2MXenes Modified with in situ Grown Carbon Nanotubes for Enhanced Electromagnetic Wave Absorption Properties. J. Mater. Chem. C 2017, 5, 4068–4074. [Google Scholar] [CrossRef]
- Yang, R.; Wang, B.; Xiang, J.; Mu, C.; Zhang, C.; Wen, F.; Wang, C.; Su, C.; Liu, Z. Fabrication of NiCo-Anchored Graphene Nanosheets by Liquid-Phase Exfoliation for Excellent Microwave Absorbers. ACS Appl. Mater. Interfaces 2017, 9, 12673–12679. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, F.; Liu, Z. Investigation on Microwave Absorption Properties for Multiwalled Carbon Nanotubes/Fe/Co/Ni Nanopowders as Lightweight Absorbers. J. Phys. Chem. C 2011, 115, 14025–14030. [Google Scholar] [CrossRef]
- Shu, R.; Wang, M.; Yang, Y.; Chang, S.; Zhang, G.; Gan, Y.; Shi, J.; He, J. Solvothermal Synthesis of Reduced Graphene Oxide/Ferroferric Oxide Hybrid Composites with Enhanced Microwave Absorption Properties. Nano 2017, 12, 1750144. [Google Scholar] [CrossRef]
- Zhang, P.; Han, X.; Kang, L.; Qiang, R.; Liu, W.; Du, Y. Synthesis and Characterization of Polyaniline Nanoparticles with Enhanced Microwave Absorption. RSC Adv. 2013, 3, 12694–12701. [Google Scholar] [CrossRef]
- Lv, H.; Liang, X.; Ji, G.; Zhang, H.; Du, Y. Porous Three-Dimensional Flower-like Co/CoO and Its Excellent Electromagnetic Absorption Properties. ACS Appl. Mater. Interfaces 2015, 7, 9776–9783. [Google Scholar] [CrossRef]
- Qi, X.; Hu, Q.; Cai, H.; Xie, R.; Bai, Z.; Jiang, Y.; Qin, S.; Zhong, W.; Du, Y. Heteronanostructured Co@Carbon Nanotubes-Graphene Ternary Hybrids: Synthesis, Electromagnetic and Excellent Microwave Absorption Properties. Sci. Rep. 2016, 6, 37972. [Google Scholar] [CrossRef]
- Cao, Y.; Su, Q.; Che, R.; Du, G.; Xu, B. One-Step Chemical Vapor Synthesis of Ni/Graphene Nanocomposites with Excellent Electromagnetic and Eectrocatalytic Properties. Synth. Met. 2012, 162, 968–973. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, Y.; Liu, Y.-J.; Sui, G.-X. Lightweight Spongy Bone-Like Graphene@SiC Aerogel Composites for High-Performance Microwave Absorption. Chem. Eng. J. 2018, 337, 522–531. [Google Scholar] [CrossRef]
- Delfini, A.; Albano, M.; Vricella, A.; Santoni, F.; Rubini, G.; Pastore, R.; Marchetti, M. Advanced Radar Absorbing Ceramic-Based Materials for Multifunctional Applications in Space Environment. Materials 2018, 11, 1730. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Z.; Li, Y.; Dai, S.; Ji, G.; Zhao, H.; Cao, J.; Du, Y. Rationally Regulating Complex Dielectric Parameters of Mesoporous Carbon Hollow Spheres to Carry out Efficient Microwave Absorption. Carbon 2018, 127, 643–652. [Google Scholar] [CrossRef]
- Cao, M.S.; Yang, J.; Song, W.L.; Zhang, D.Q.; Wen, B.; Jin, H.B.; Hou, Z.L.; Yuan, J. Ferroferric Oxide/Multiwalled Carbon Nanotube vs Polyaniline/Ferroferric Oxide/Multiwalled Carbon Nanotube Multiheterostructures for Highly Effective Microwave Absorption. ACS Appl. Mater. Interfaces 2012, 4, 6949–6956. [Google Scholar] [CrossRef]
- Guan, X.; Yang, Z.; Zhou, M.; Yang, L.; Bagher Aslibeiki, R.; Ji, G. 2D MXene nanomaterials: Synthesis, mechanism, and multifunctional applications in microwave absorption. Small Struct. 2022, 3, 2200102. [Google Scholar] [CrossRef]
- Zhou, W.; Long, L.; Xiao, P.; Li, Y.; Luo, H.; Hu, W.-D.; Yin, R.-M. Silicon Carbide Nano-Fibers in-situ Grown on Carbon Fibers for Enhanced Microwave Absorption Properties. Ceram. Int. 2017, 43, 5628–5634. [Google Scholar] [CrossRef]
- Micheli, D.; Vricella, A.; Pastore, R.; Marchetti, M. Synthesis and Electromagnetic Characterization of Frequency Selective Radar Absorbing Materials Using Carbon Nanopowders. Carbon 2014, 77, 756–774. [Google Scholar] [CrossRef]
- Liu, W.; Shao, Q.; Ji, G.; Liang, X.; Cheng, Y.; Quan, B.; Du, Y. Metal–Organic-Frameworks Derived Porous Carbon-Wrapped Ni Composites with Optimized Impedance Matching as Excellent Lightweight Electromagnetic Wave Absorber. Chem. Eng. J. 2017, 313, 734–744. [Google Scholar] [CrossRef]
- Tao, J.Q.; Zhou, J.T.; Yao, Z.J.; Jiao, Z.B.; Wei, B.; Tan, R.Y.; Li, Z. Multi-Shell Hollow Porous Carbon Nanoparticles with Excellent Microwave Absorption Properties. Carbon 2021, 172, 542–555. [Google Scholar] [CrossRef]
- Wang, B.L.; Wu, Q.; Fu, Y.G.; Liu, T. A Review on Carbon/Magnetic Metal Composites for Microwave Absorption. J. Mater. Sci. Technol. 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Wen, B.; Ma, L.; Lin, Y. Facile Synthesis of Nickel/Carbon Nanotubes Hybrid Derived from Metal Organic Framework as a Lightweight, Strong and Efficient Microwave Absorber. J. Colloid Interface Sci. 2021, 590, 561–570. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, J.; Gu, W.; Zhao, H.; Zheng, J.; Zhang, B.; Ji, G. Cellulose-Chitosan Framework/Polyailine Hybrid Aerogel Toward Thermal Insulation and Microwave Absorbing Application. Chem. Eng. J. 2020, 395, 125190. [Google Scholar] [CrossRef]
- Feng, J.; Zong, Y.; Sun, Y.; Zhang, Y.; Yang, X.; Long, G.; Wang, Y.; Li, X.; Zheng, X. Optimization of Porous FeNi/N-GN Composites with Superior Microwave Absorption Performance. Chem. Eng. J. 2018, 345, 441–451. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Apollo, C.; Marchetti, M.; Gradoni, G.; Moglie, F.; Primiani, V.M. Carbon Based Nanomaterial Composites in RAM and Microwave Shielding Applications. In Proceedings of the 2009 9th IEEE Conference on Nanotechnology (IEEE-NANO), Genoa, Italy, 26–30 July 2009; pp. 226–235. [Google Scholar]
- Qiang, R.; Du, Y.; Chen, D.; Ma, W.; Wang, Y.; Xu, P.; Ma, J.; Zhao, H.; Han, X. Electromagnetic Functionalized Co/C Composites by in situ Pyrolysis of Metal-Organic Frameworks (ZIF-67). J. Alloys Compd. 2016, 681, 384–393. [Google Scholar] [CrossRef]
- Meng, F.; Wei, W.; Chen, X.; Xu, X.; Jiang, M.; Jun, L.; Wang, Y.; Zhou, Z. Design of Porous C@Fe3O4 Hybrid Nanotubes with Excellent Microwave Absorption. Phys. Chem. Chem. Phys. 2016, 18, 2510–2516. [Google Scholar] [CrossRef]
- Jiang, J.; Li, D.; Geng, D.; An, J.; He, J.; Liu, W.; Zhang, Z. Microwave Absorption Properties of Core Double-Shell FeCo/C/BaTiO3 Nanocomposites. Nanoscale 2014, 6, 3967–3971. [Google Scholar] [CrossRef]
- Liu, D.; Qiang, R.; Du, Y.; Wang, Y.; Tian, C.; Han, X. Prussian Blue Analogues Derived Magnetic FeCo Alloy/Carbon Composites with Tunable Chemical Composition and Enhanced Microwave Absorption. J. Colloid Interface Sci. 2018, 514, 10–20. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Wang, Y.; Ma, L.; Li, Z.; Chen, Z.; Lv, X.; Chang, Y.; Liu, Y.; Shi, J. Construction of Dual-Shell Mo2C/C Microsphere towards Efficient Electromagnetic Wave Absorption. Int. J. Mol. Sci. 2022, 23, 14502. https://doi.org/10.3390/ijms232314502
Deng X, Wang Y, Ma L, Li Z, Chen Z, Lv X, Chang Y, Liu Y, Shi J. Construction of Dual-Shell Mo2C/C Microsphere towards Efficient Electromagnetic Wave Absorption. International Journal of Molecular Sciences. 2022; 23(23):14502. https://doi.org/10.3390/ijms232314502
Chicago/Turabian StyleDeng, Xuesong, Yahui Wang, Lifang Ma, Zhigang Li, Zongsheng Chen, Xiangyin Lv, Yajing Chang, Yi Liu, and Jiaming Shi. 2022. "Construction of Dual-Shell Mo2C/C Microsphere towards Efficient Electromagnetic Wave Absorption" International Journal of Molecular Sciences 23, no. 23: 14502. https://doi.org/10.3390/ijms232314502
APA StyleDeng, X., Wang, Y., Ma, L., Li, Z., Chen, Z., Lv, X., Chang, Y., Liu, Y., & Shi, J. (2022). Construction of Dual-Shell Mo2C/C Microsphere towards Efficient Electromagnetic Wave Absorption. International Journal of Molecular Sciences, 23(23), 14502. https://doi.org/10.3390/ijms232314502