Morusin Protected Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammation through Inhibiting EGFR-AKT/NF-κB Signaling and Improving Barrier Functions
Abstract
1. Introduction
2. Results
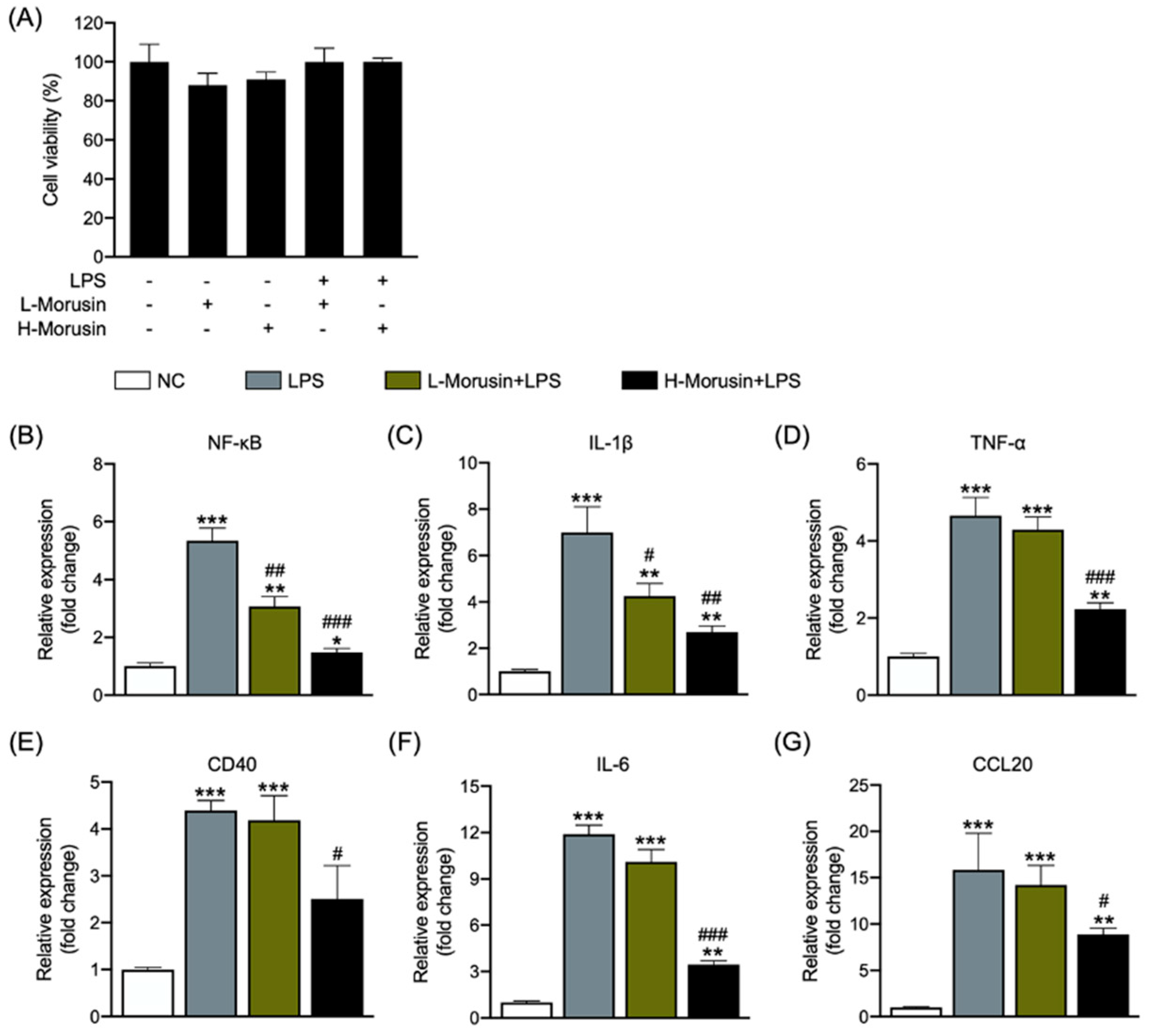
2.1. Morusin Alleviated LPS-Induced RECs’ Inflammation in a Concentration-Dependent Manner
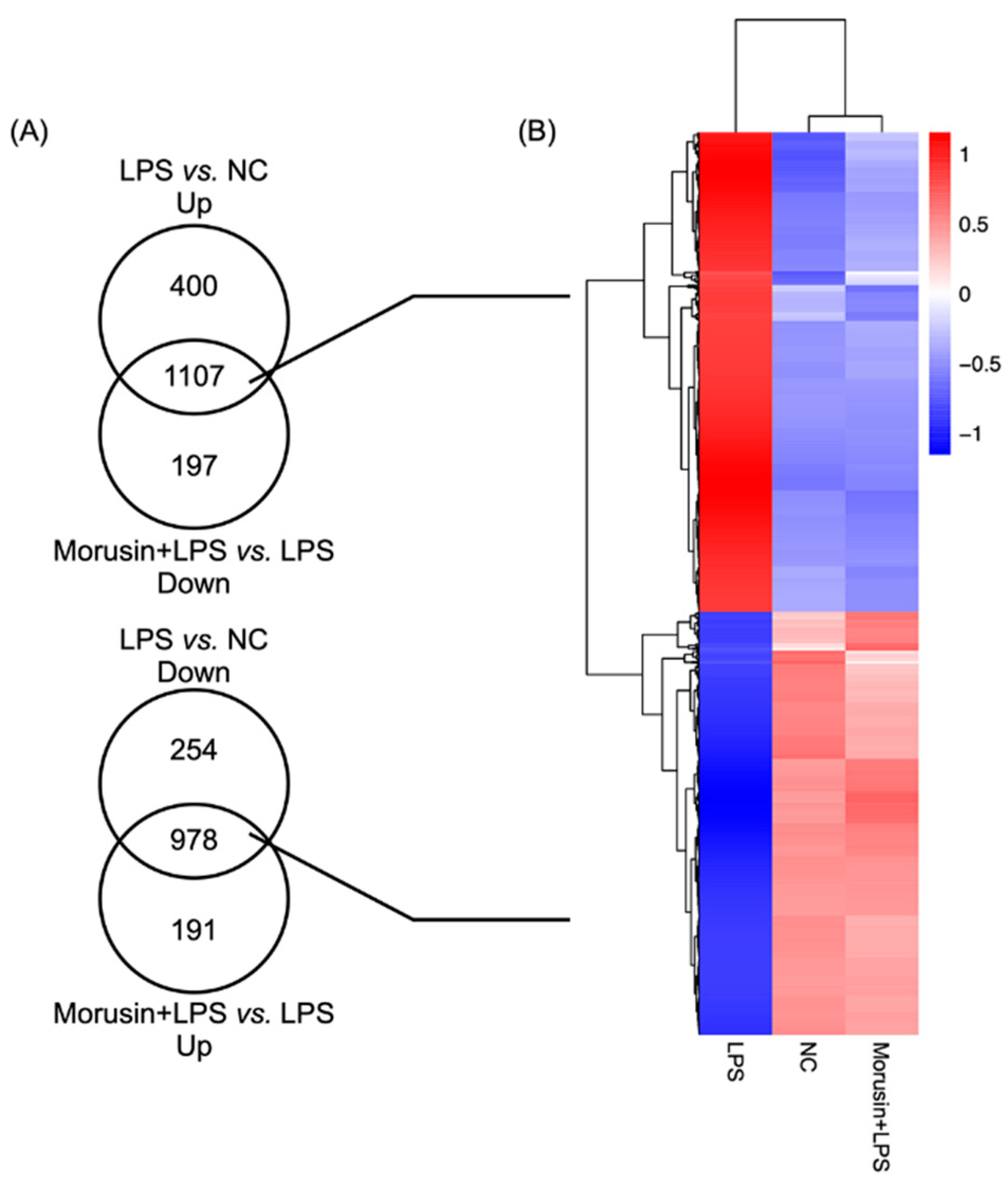
2.2. Overview of the Transcriptome in RECs among Different Treatments
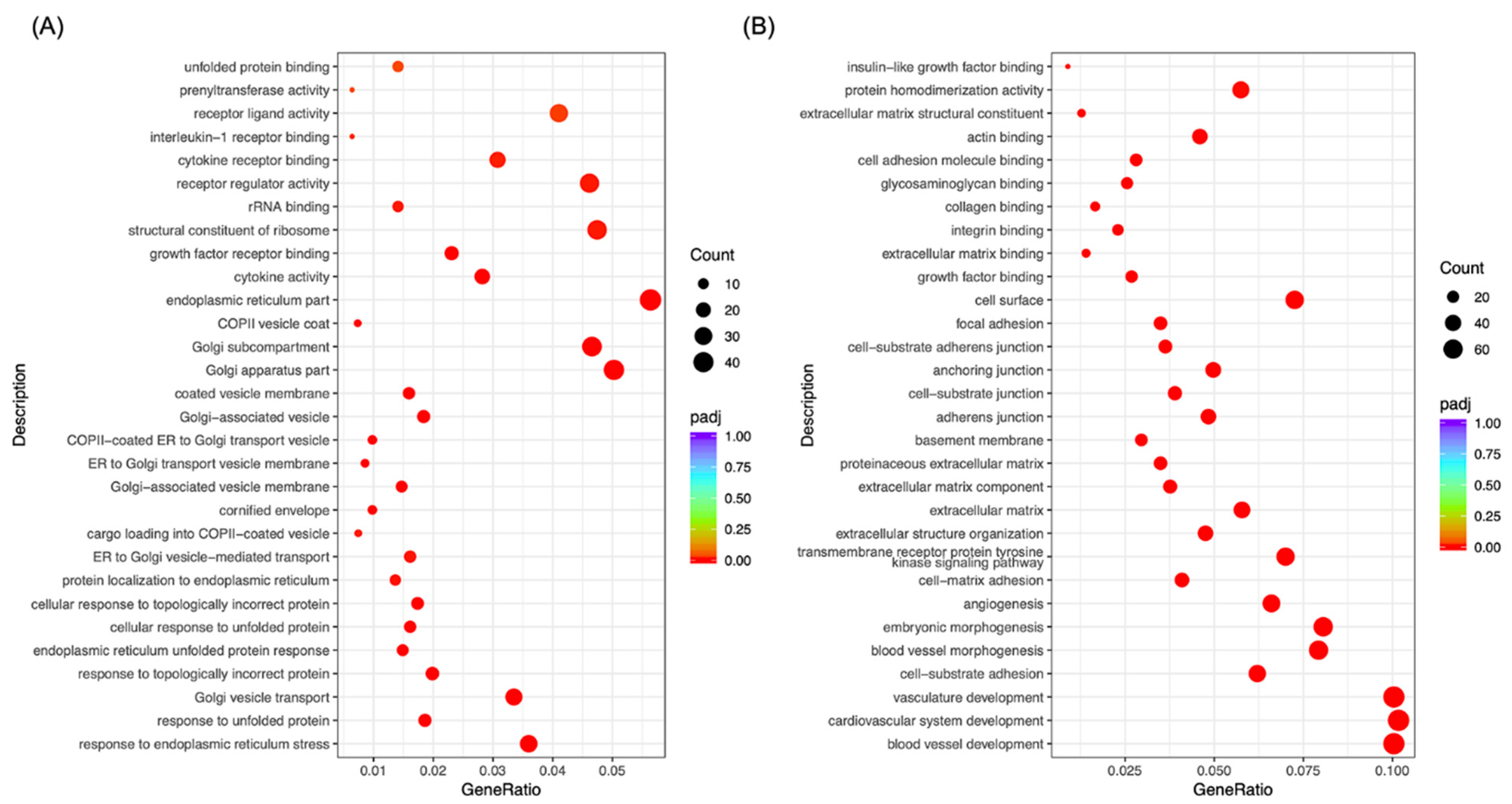
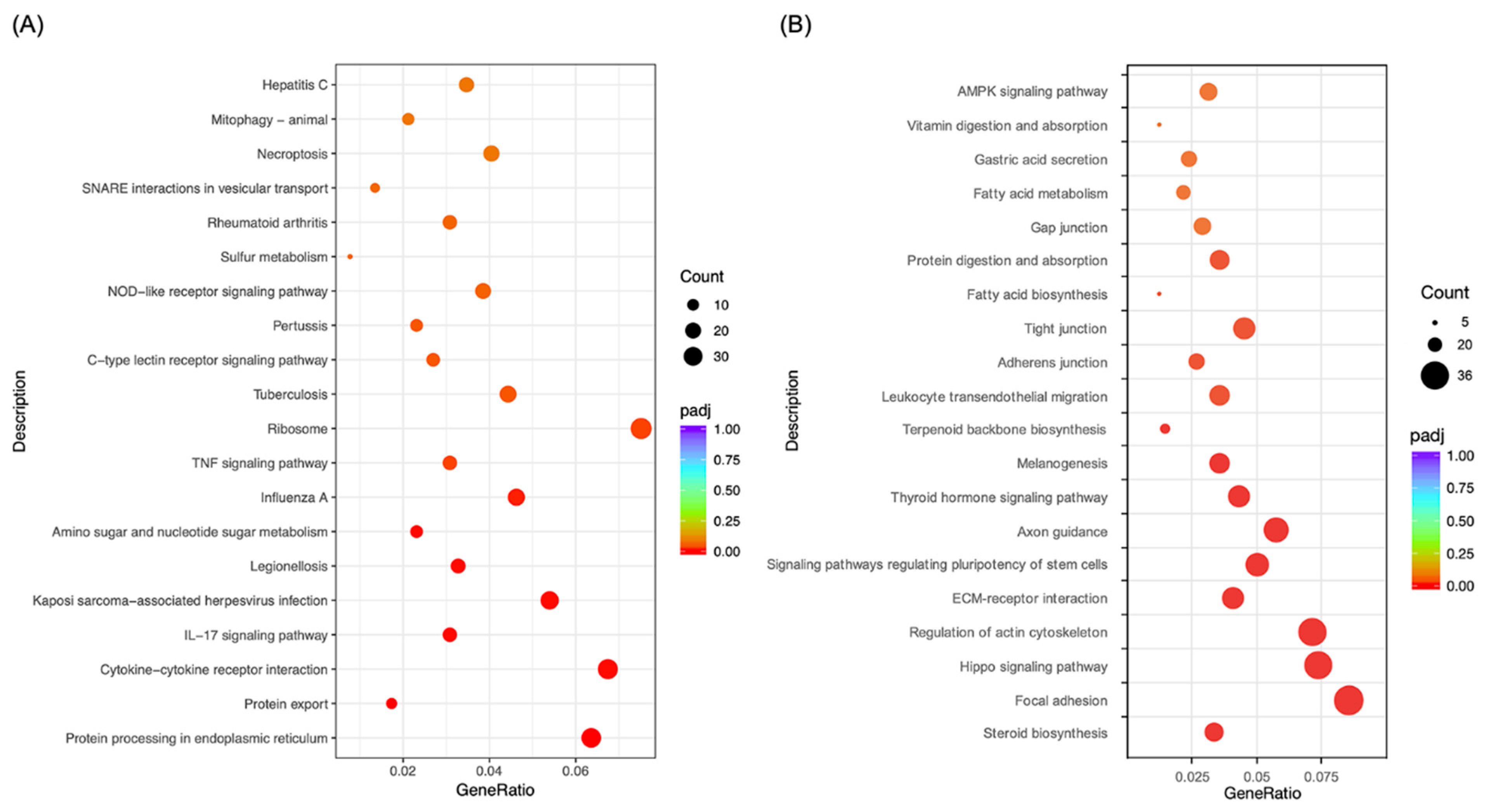
2.3. Morusin Altered Cell Signaling to Regulate LPS-Induced Inflammation in RECs
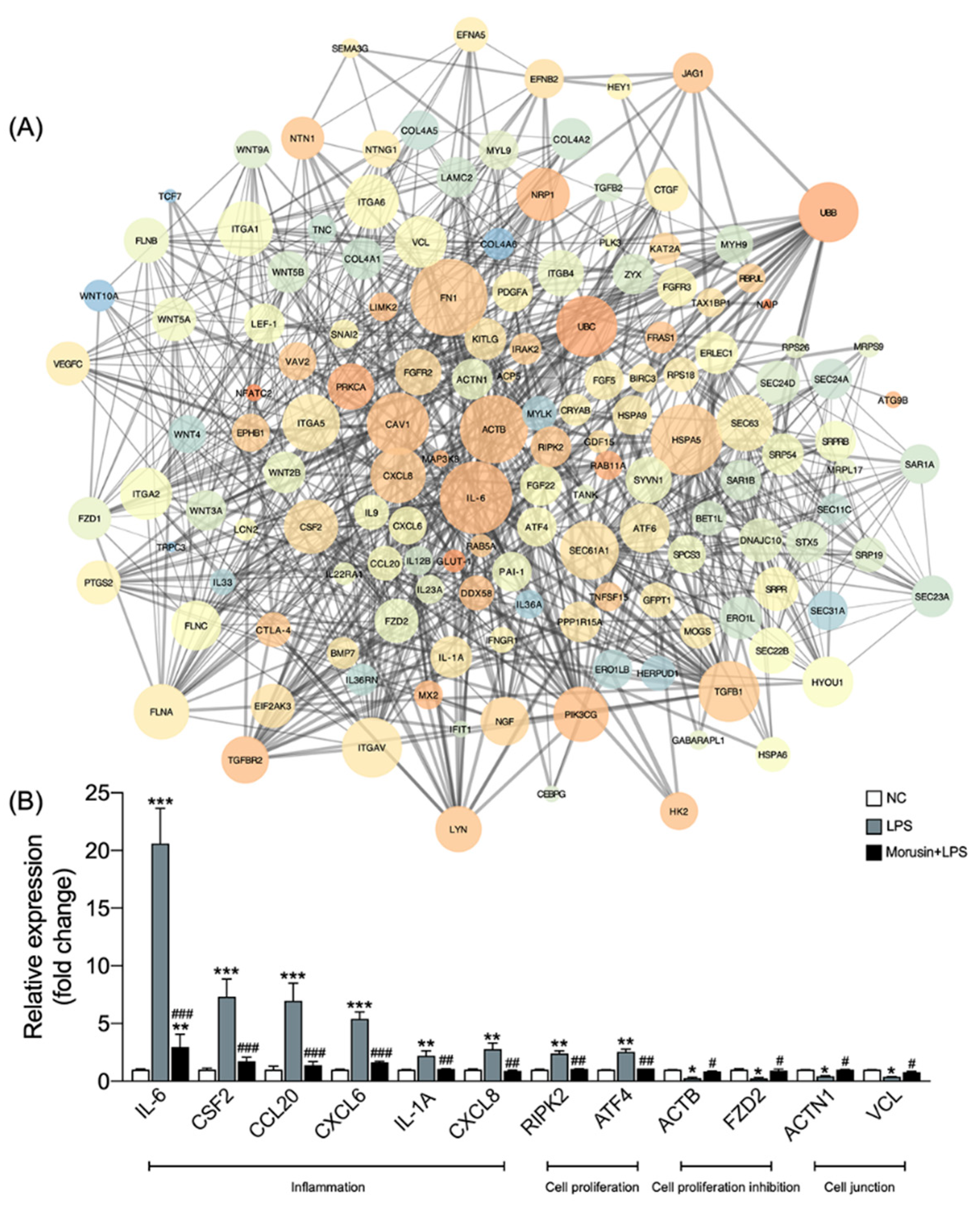
2.4. Morusin Altered the Expression of Hub Genes Involved in Inflammation, Cell Proliferation, and Cell Junction Processes in RECs
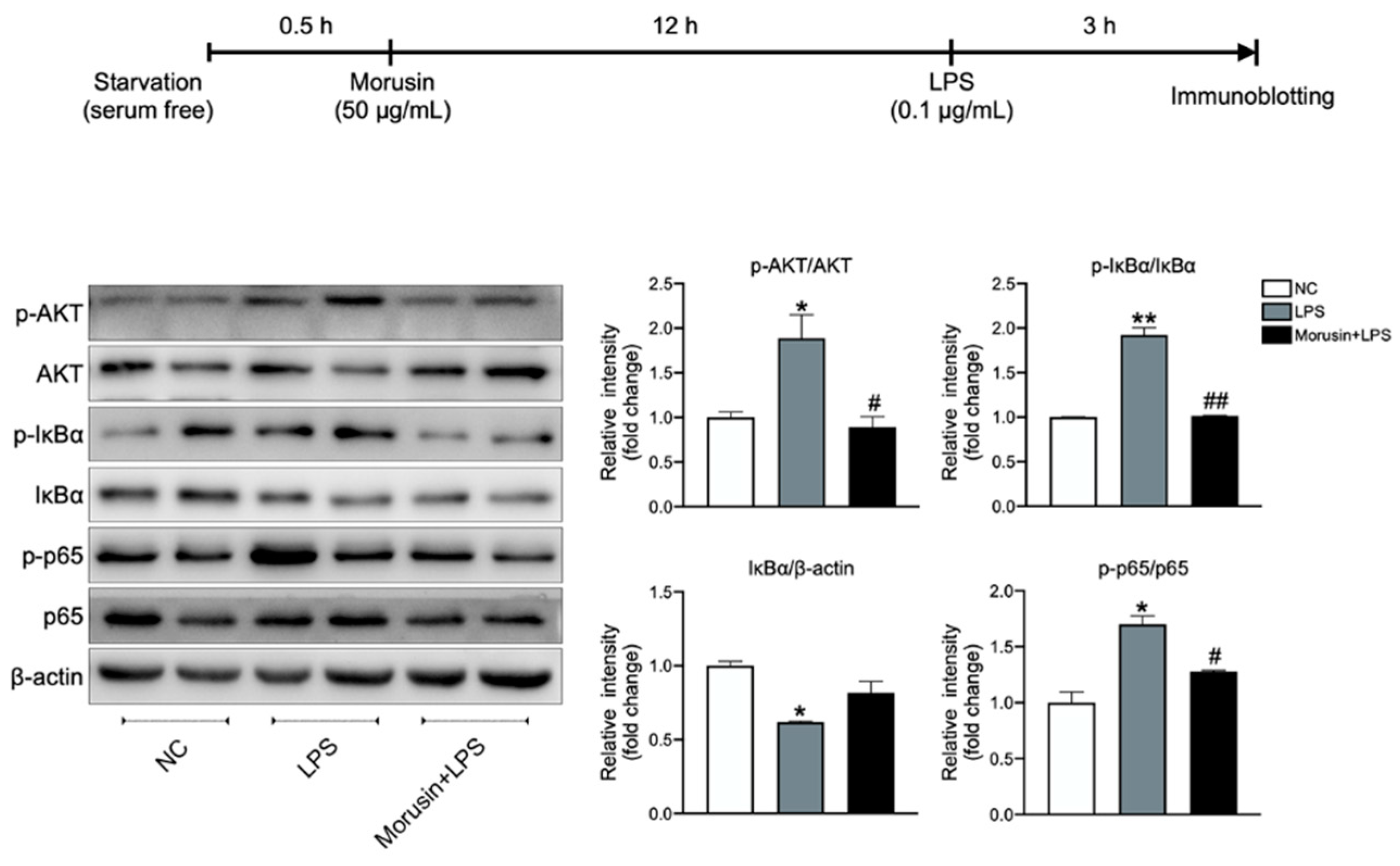
2.5. Morusin Inhibited the Activation of AKT and NF-κB Signaling in RECs upon LPS Stimulation
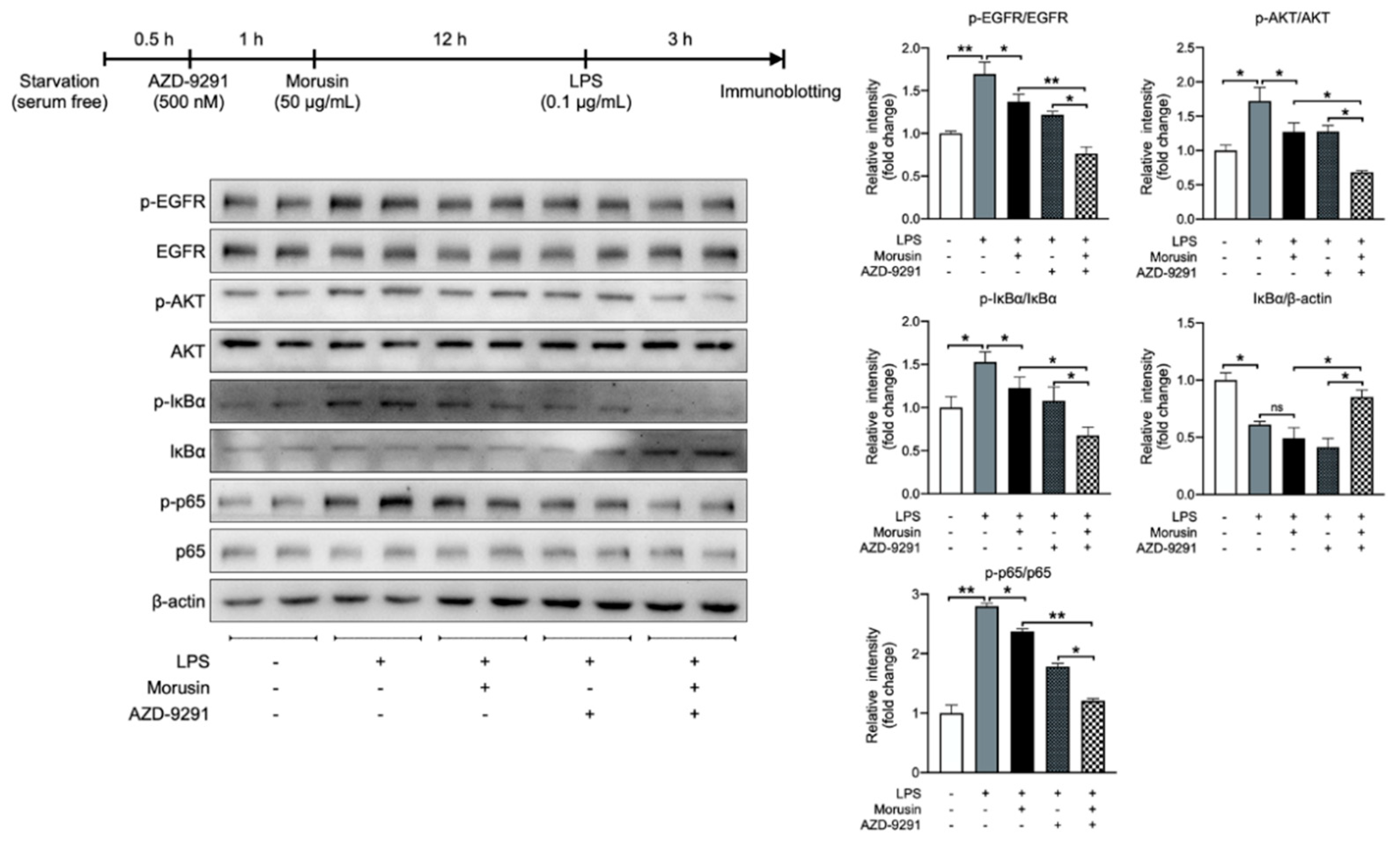
2.6. Morusin Suppressed LPS-Induced AKT and NF-κB Activation by Inhibiting EGFR-Mediated Signaling
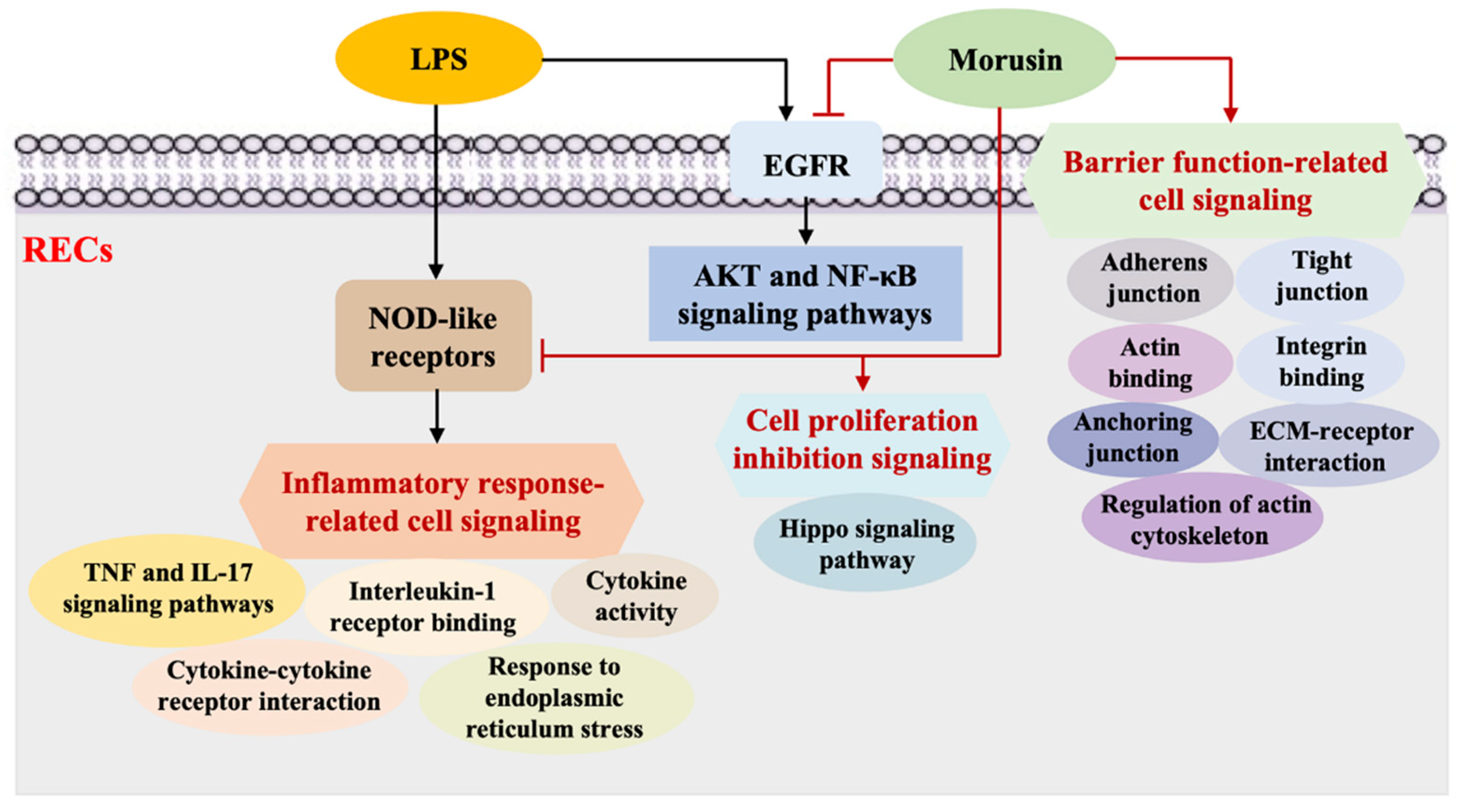
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Viability Analysis
4.3. RNA Extraction and Quantitative Real-Time PCR (qPCR)
4.4. Transcriptome Sequencing
4.5. Bioinformatics Analysis
4.6. Immunoblotting Analysis
4.7. Analysis of EGFR Inhibition on the Phosphorylation of AKT and NF-κB p65
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baaske, L.; Gabel, G.; Dengler, F. Ruminal epithelium: A checkpoint for cattle health. J. Dairy Res. 2020, 87, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.F.; Faciola, A.P. Ruminal acidosis, bacterial changes, and lipopolysaccharides. J. Anim. Sci. 2020, 98, skaa248. [Google Scholar] [CrossRef]
- Kent-Dennis, C.; Aschenbach, J.R.; Griebel, P.J.; Penner, G.B. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J. Dairy Sci. 2020, 103, 9587–9603. [Google Scholar] [CrossRef] [PubMed]
- Stoica, C.; Cox, G. Old problems and new solutions: Antibiotic alternatives in food animal production. Can. J. Microbiol. 2021, 67, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Dey, A.; Rose, M.K.; Dahiya, S.S. Impact of dietary phytogenic composite feed additives on immune response, antioxidant status, methane production, growth performance and nutrient utilization of Buffalo (Bubalus bubalis) calves. Antioxidants 2022, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Park, J.H.; Lee, Y.G.; Seo, K.H.; Oh, E.J.; Lee, D.Y.; Lim, D.W.; Han, D.; Baek, N.I. Three new isoprenylated flavonoids from the root bark of Morus alba. Molecules 2016, 21, 1112. [Google Scholar] [CrossRef]
- Cao, Y.G.; Zheng, X.K.; Yang, F.F.; Li, F.; Qi, M.; Zhang, Y.L.; Zhao, X.; Kuang, H.X.; Feng, W.S. Two new phenolic constituents from the root bark of Morus alba L. and their cardioprotective activity. Nat. Prod. Res. 2018, 32, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Cho, S.W.; Lee, S.G.; Choi, C.Y. The beneficial effects of Morusin, an isoprene flavonoid isolated from the root bark of Morus. Int. J. Mol. Sci. 2020, 21, 6514. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.E.; Ha, H.; Shin, H.K.; Seo, C.S. Anti-allergic and anti-inflammatory effects of Kuwanon G and Morusin on MC/9 mast cells and HaCaT keratinocytes. Molecules 2019, 24, 265. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, Q.; Chen, J.; Han, J. Morusin inhibits human osteosarcoma via the PI3K-AKT signaling pathway. Curr. Pharm. Biotechnol. 2020, 21, 1402–1409. [Google Scholar] [CrossRef]
- Vochyanova, Z.; Pokorna, M.; Rotrekl, D.; Smekal, V.; Fictum, P.; Suchy, P.; Gajdziok, J.; Smejkal, K.; Hosek, J. Prenylated fla- vonoid morusin protects against TNBS-induced colitis in rats. PLoS ONE 2017, 12, e0182464. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Min, T.R.; Chi, G.Y.; Choi, Y.H.; Park, S.H. Induction of apoptosis by morusin in human non-small cell lung cancer cells by suppression of EGFR/STAT3 activation. Biochem. Biophys. Res. Commun. 2018, 505, 194–200. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, M.; Su, X.; Zhan, K.; Zhang, C.; Zhao, G. Effects of alfalfa flavonoids on the production performance, immune system, and ruminal fermentation of dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 1416–1424. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, G.; Wang, Y.; Zhang, Y. Moringa oleifera leaf flavonoids protect bovine mammary epithelial cells from hydrogen peroxide-induced oxidative stress in vitro. Reprod. Domest. Anim. 2020, 55, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Masoudzadeh, S.H.; Mohammadabadi, M.; Khezri, A.; Stavetska, R.V.; Oleshko, V.P.; Babenko, O.I.; Yemets, Z.; Kalashnik, O.M. Effects of diets with different levels of fennel (Foeniculum vulgare) seed powder on DLK1 gene expression in brain, adipose tissue, femur muscle and rumen of Kermani lambs. Small Rumin. Res. 2020, 193, 106276. [Google Scholar] [CrossRef]
- Gouwy, M.; Struyf, S.; Proost, P.; Van Damme, J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine Growth Factor Rev. 2005, 16, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Adcock, I.M.; Abedini, A.; Kiani, A.; Kazempour-Dizaji, M.; Movassaghi, M.; Garssen, J. The role of pattern recog- nition receptors in lung sarcoidosis. Eur. J. Pharmacol. 2017, 808, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chavez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, H.; Wu, Q.; Li, F.; Wang, Y.; Liu, S.; Wang, J. Sestrin 2 protects against LPS-induced acute lung injury by inducing mitophagy in alveolar macrophages. Life Sci. 2021, 267, 118941. [Google Scholar] [CrossRef]
- Wree, A.; McGeough, M.D.; Inzaugarat, M.E.; Eguchi, A.; Schuster, S.; Johnson, C.D.; Pena, C.A.; Geisler, L.J.; Papouchado, B.G.; Hoffman, H.M.; et al. NLRP3 inflammasome driven liver injury and fibrosis: Roles of IL-17 and TNF in mice. Hepatology 2018, 67, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef]
- Odenwald, M.A.; Turner, J.R. The intestinal epithelial barrier: A therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xing, J.; Lu, F.; Chang, W.; Liang, N.; Li, J.; Wang, Y.; Li, X.; Zhao, X.; Hou, R.; et al. Psoriatic dermal-derived mesenchymal stem cells reduce keratinocyte junctions, and increase glycolysis. Acta Derm. Venereol. 2020, 100, adv00122. [Google Scholar] [CrossRef]
- Batissoco, A.C.; Salazar-Silva, R.; Oiticica, J.; Bento, R.F.; Mingroni-Netto, R.C.; Haddad, L.A. A cell junctional protein net- work associated with connexin-26. Int. J. Mol. Sci. 2018, 19, 2535. [Google Scholar] [CrossRef]
- Lopes Pires, M.E.; Clarke, S.R.; Marcondes, S.; Gibbins, J.M. Lipopolysaccharide potentiates platelet responses via toll-like receptor 4-stimulated Akt-Erk-PLA2 signalling. PLoS ONE 2017, 12, e0186981. [Google Scholar] [CrossRef] [PubMed]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-kappaB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef]
- Han, S.W.; Roman, J. Fibronectin induces cell proliferation and inhibits apoptosis in human bronchial epithelial cells: Pro-oncogenic effects mediated by PI3-kinase and NF-kappa B. Oncogene 2006, 25, 4341–4349. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-kappaB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Zeng, Y.; Huang, X.; Chen, H.; Wang, S.; Wu, J.; Li, Q.; Zhu, W.; Li, H.; et al. A novel phytochemical, DIM, inhibits proliferation, migration, invasion and TNF-alpha induced inflammatory cytokine production of synovial fibroblasts from rheumatoid arthritis patients by targeting MAPK and AKT/mTOR signal pathway. Front. Immunol. 2019, 10, 1620. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Upregulation of Akt/NF-kappaB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carno- sic acid nanoparticle. Int. J. Nanomed. 2016, 11, 6401–6420. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, A.L.; Yoon, C.; Huffman, L.D.; Duncker, P.C.; Kohen, R.; Passino, R.; Hafner, H.; Johnson, C.; Kawaguchi, R.; Carbajal, K.S.; et al. Analysis of the immune response to sciatic nerve injury identifies efferocytosis as a key mechanism of nerve debridement. eLife 2020, 9, e60223. [Google Scholar] [CrossRef] [PubMed]
- Lepennetier, G.; Hracsko, Z.; Unger, M.; Van Griensven, M.; Grummel, V.; Krumbholz, M.; Berthele, A.; Hemmer, B.; Kowarik, M.C. Cytokine and immune cell profiling in the cerebrospinal fluid of patients with neuro-inflammatory diseases. J. Neuroinflammation 2019, 16, 219. [Google Scholar] [CrossRef]
- Yan, Y.; Zhou, B.; Qian, C.; Vasquez, A.; Kamra, M.; Chatterjee, A.; Lee, Y.J.; Yuan, X.; Ellis, L.; Di Vizio, D.; et al. Receptor-interacting protein kinase 2 (RIPK2) stabilizes c-Myc and is a therapeutic target in prostate cancer metastasis. Nat. Commun. 2022, 13, 669. [Google Scholar] [CrossRef]
- Du, J.; Liu, H.; Mao, X.; Qin, Y.; Fan, C. ATF4 promotes lung cancer cell proliferation and invasion partially through regulating Wnt/beta-catenin signaling. Int. J. Med. Sci. 2021, 18, 1442–1448. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, C.W.; Ayscough, K.R. A role for actin in aging and apoptosis. Biochem. Soc. Trans. 2005, 33, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.C.; Huang, X.Y.; Zheng, F.F.; Xie, J.; She, L.; Feng, Y.; Su, B.H.; Zheng, D.L.; Lu, Y.G. FZD2 inhibits the cell growth and migration of salivary adenoid cystic carcinomas. Oncol. Rep. 2016, 35, 1006–1012. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Tarozzi, A.; Marchetti, C.; Nicolini, B.; D’Amico, M.; Ticchi, N.; Pruccoli, L.; Tumiatti, V.; Simoni, E.; Lodola, A.; Mor, M.; et al. Combined inhibition of the EGFR/AKT pathways by a novel conjugate of quinazoline with isothiocyanate. Eur. J. Med. Chem. 2016, 117, 283–291. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on epidermal growth factor receptor (EGFR) structure, signaling pathways, interactions, and recent updates of EGFR inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef]
- Habibovic, A.; Hristova, M.; Heppner, D.E.; Danyal, K.; Ather, J.L.; Janssen-Heininger, Y.M.; Irvin, C.G.; Poynter, M.E.; Lundblad, L.K.; Dixon, A.E.; et al. DUOX1 mediates persistent epithelial EGFR activation, mucous cell metaplasia, and airway remodeling during allergic asthma. JCI Insight 2016, 1, e88811. [Google Scholar] [CrossRef]
- Kelly, F.L.; Weinberg, K.E.; Nagler, A.E.; Nixon, A.B.; Star, M.D.; Todd, J.L.; Brass, D.M.; Palmer, S.M. EGFR-dependent IL8 production by airway epithelial cells after exposure to the food flavoring chemical 2,3-butanedione. Toxicol. Sci. 2019, 169, 534–542. [Google Scholar] [CrossRef]
- Heijink, I.H.; van Oosterhout, A.; Kapus, A. Epidermal growth factor receptor signalling contributes to house dust mite-induced epithelial barrier dysfunction. Eur. Respir. J. 2010, 36, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Alberti, C.; Pinciroli, P.; Valeri, B.; Ferri, R.; Ditto, A.; Umezawa, K.; Sensi, M.; Canevari, S.; Tomassetti, A. Ligand-dependent EGFR activation induces the co-expression of IL-6 and PAI-1 via the NFkB pathway in advanced-stage epithelial ovarian cancer. Oncogene 2012, 31, 4139–4149. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lan, W.; Ye, S.; Zhu, B.; Fu, Z. Transcriptomic analyses reveal the protective immune regulation of conjugated linoleic acids in sheep ruminal epithelial cells. Front. Physiol. 2020, 11, 588082. [Google Scholar] [CrossRef]
- Yang, C.; Zhu, B.; Ye, S.; Fu, Z.; Li, J. Isomer-specific effects of cis-9,trans-11- and trans-10,cis-12-CLA on immune regulation in ruminal epithelial cells. Animals 2021, 11, 1169. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene ontology analysis for RNA-seq: Accounting for selection bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. S2), W316–W322. [Google Scholar] [CrossRef]
- Otasek, D.; Morris, J.H.; Boucas, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef]
- Ma, L.; Ni, Y.; Wang, Z.; Tu, W.; Ni, L.; Zhuge, F.; Zheng, A.; Hu, L.; Zhao, Y.; Zheng, L.; et al. Spermidine improves gut barrier integrity and gut microbiota function in diet-induced obese mice. Gut Microbes 2020, 12, 1832857. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Deng, X.; Wu, L.; Jiang, T.; Fu, Z.; Li, J. Morusin Protected Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammation through Inhibiting EGFR-AKT/NF-κB Signaling and Improving Barrier Functions. Int. J. Mol. Sci. 2022, 23, 14428. https://doi.org/10.3390/ijms232214428
Yang C, Deng X, Wu L, Jiang T, Fu Z, Li J. Morusin Protected Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammation through Inhibiting EGFR-AKT/NF-κB Signaling and Improving Barrier Functions. International Journal of Molecular Sciences. 2022; 23(22):14428. https://doi.org/10.3390/ijms232214428
Chicago/Turabian StyleYang, Chunlei, Xiangfei Deng, Linjun Wu, Tianrui Jiang, Zhengwei Fu, and Jinjun Li. 2022. "Morusin Protected Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammation through Inhibiting EGFR-AKT/NF-κB Signaling and Improving Barrier Functions" International Journal of Molecular Sciences 23, no. 22: 14428. https://doi.org/10.3390/ijms232214428
APA StyleYang, C., Deng, X., Wu, L., Jiang, T., Fu, Z., & Li, J. (2022). Morusin Protected Ruminal Epithelial Cells against Lipopolysaccharide-Induced Inflammation through Inhibiting EGFR-AKT/NF-κB Signaling and Improving Barrier Functions. International Journal of Molecular Sciences, 23(22), 14428. https://doi.org/10.3390/ijms232214428






