Developing 3D Organoid Raft Cultures from Patient-Derived Xenografts as Rapid Models to Screen Efficacy of Experimental Therapeutics
Abstract
1. Introduction
2. Results
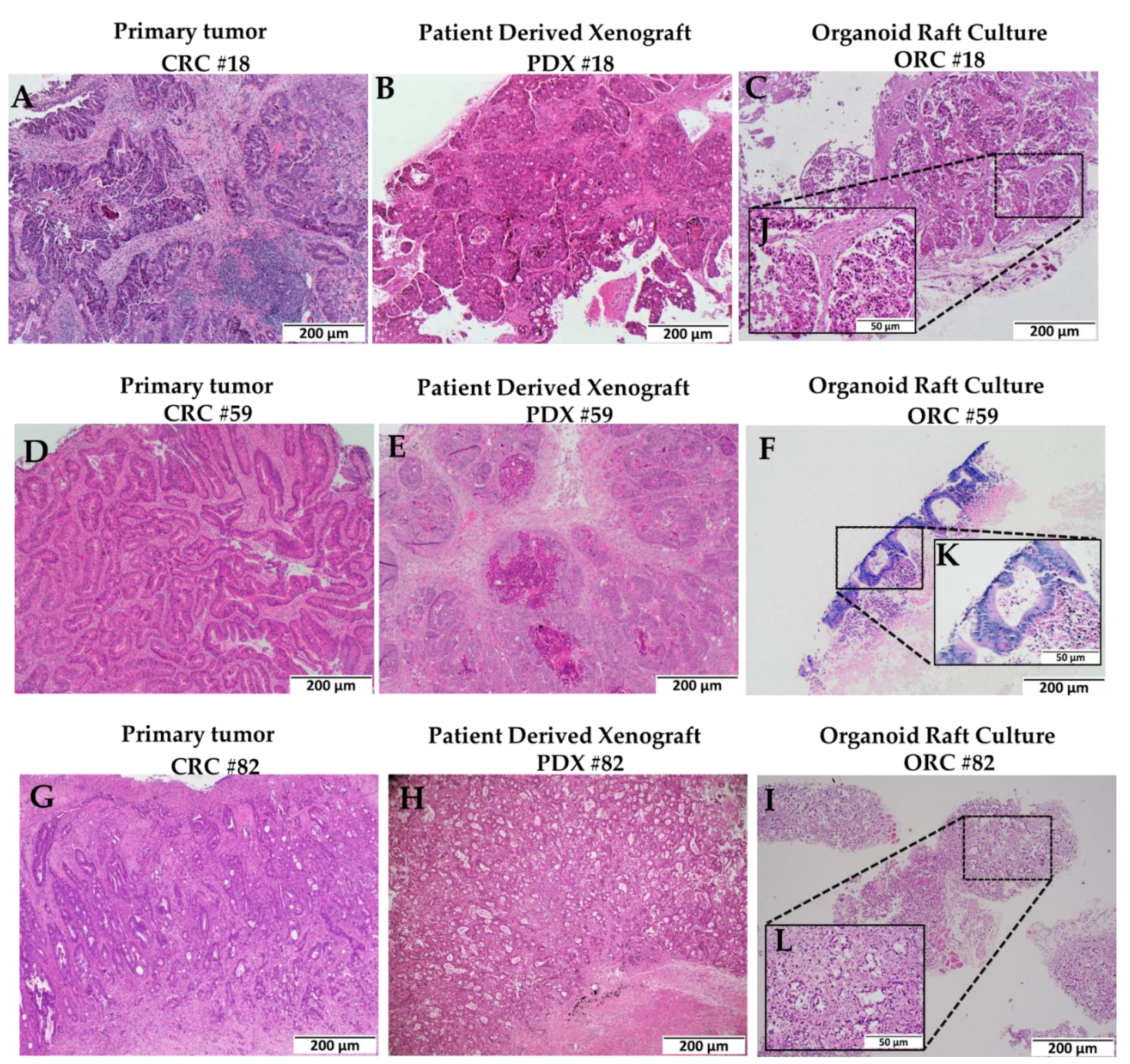
2.1. Organoid Raft Cultures Retain the Morphological Features of Colorectal Cancer
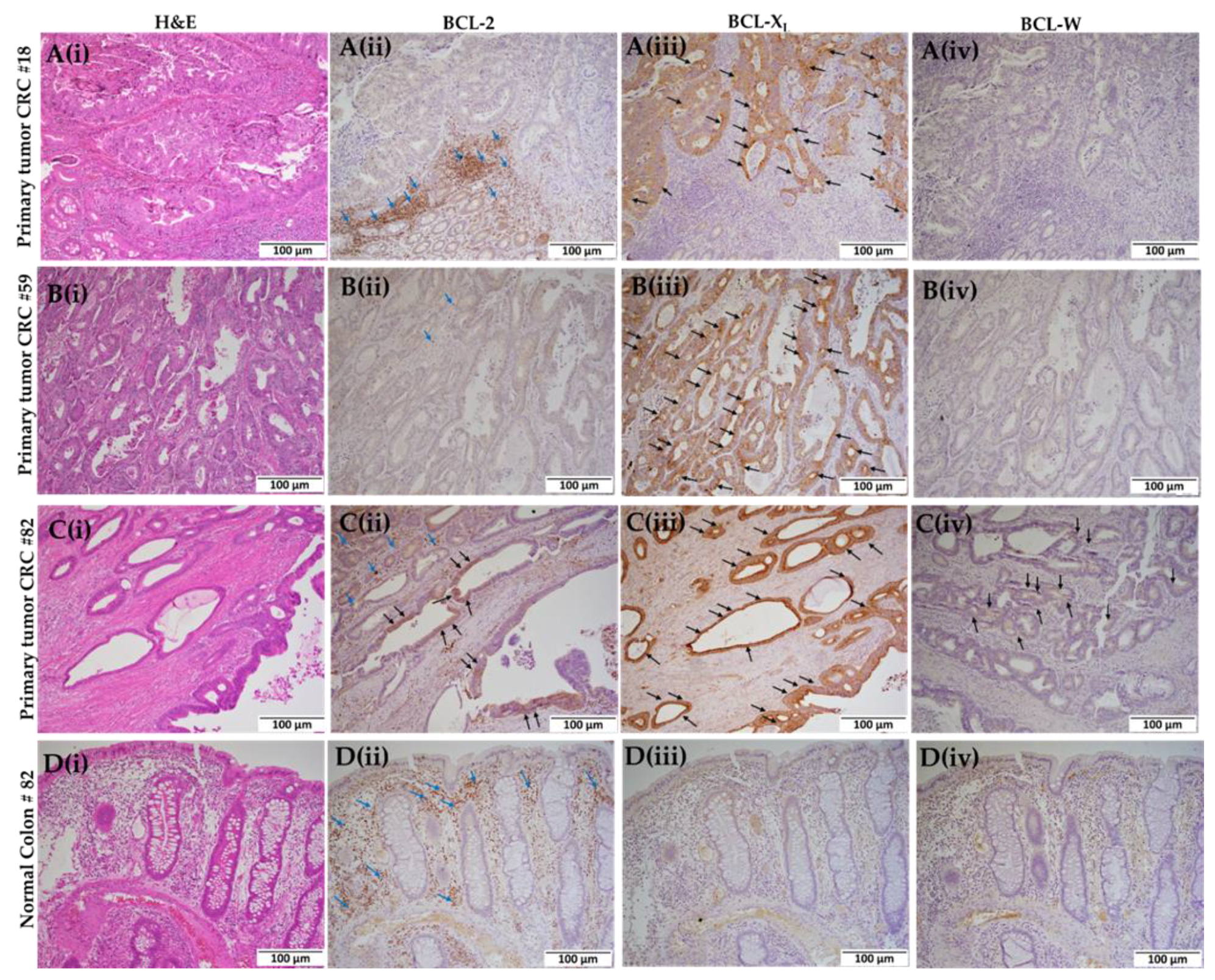
2.2. Colorectal Primary Tissues Express Varying Levels of BCL-2 Family Proteins
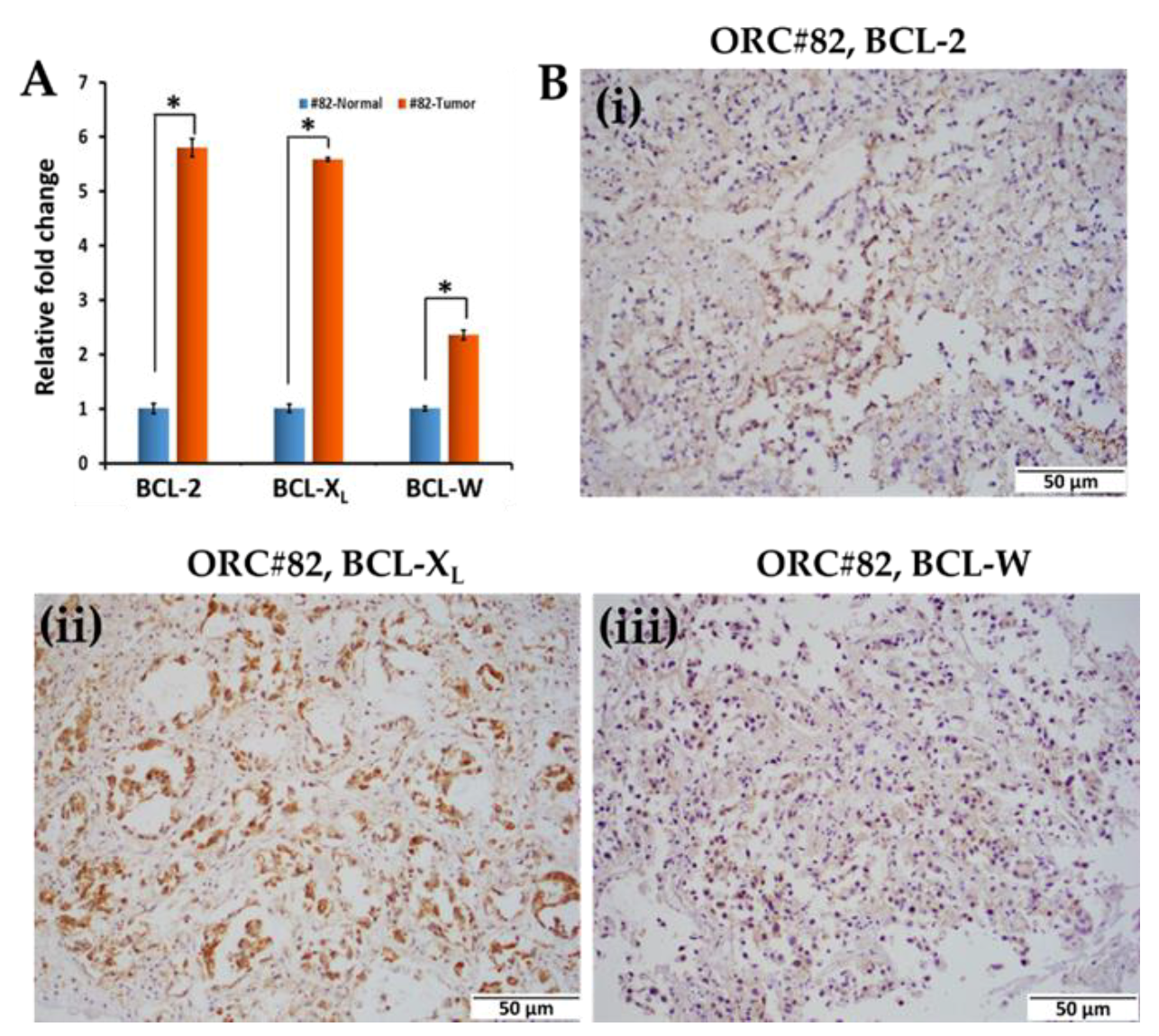
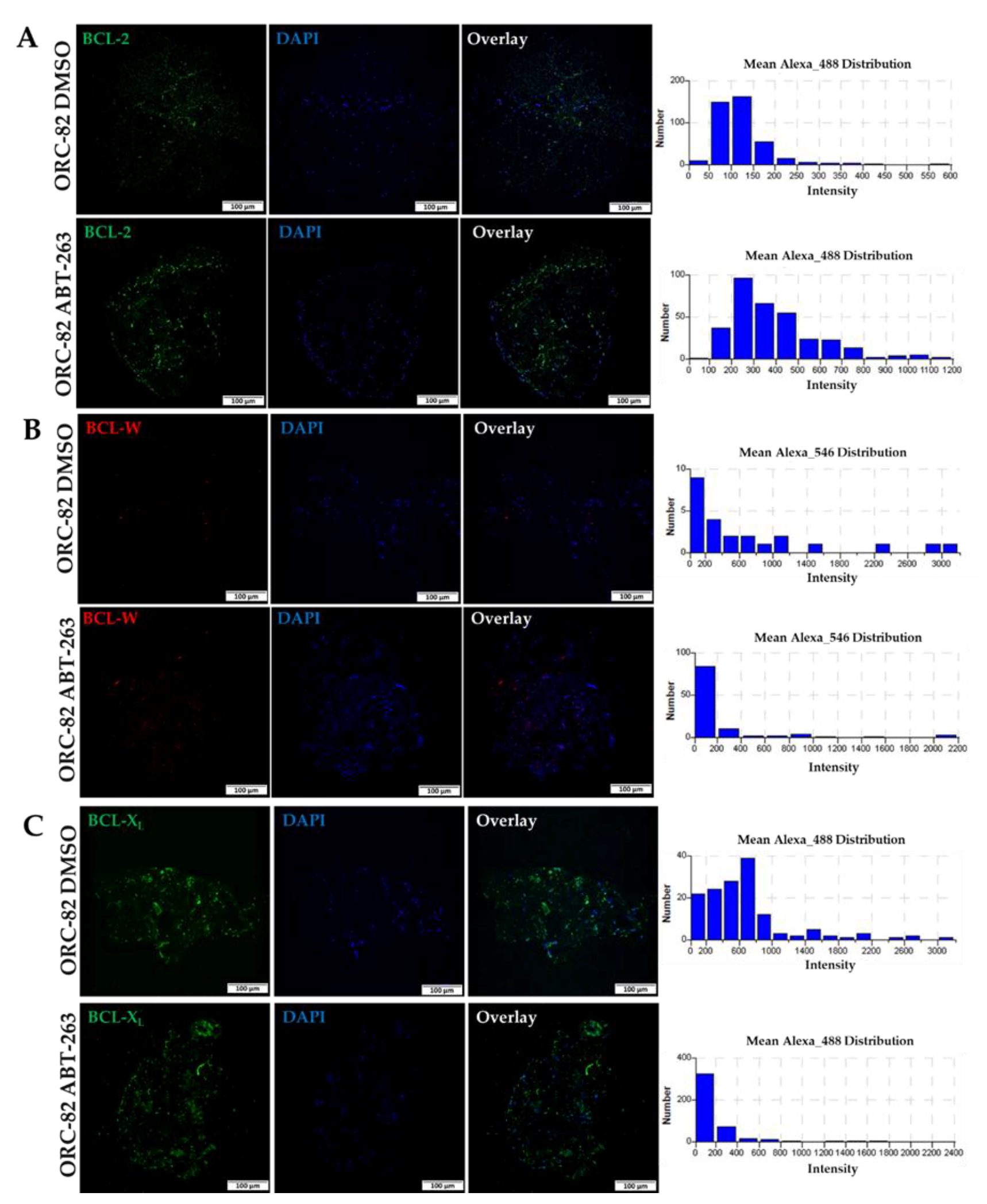
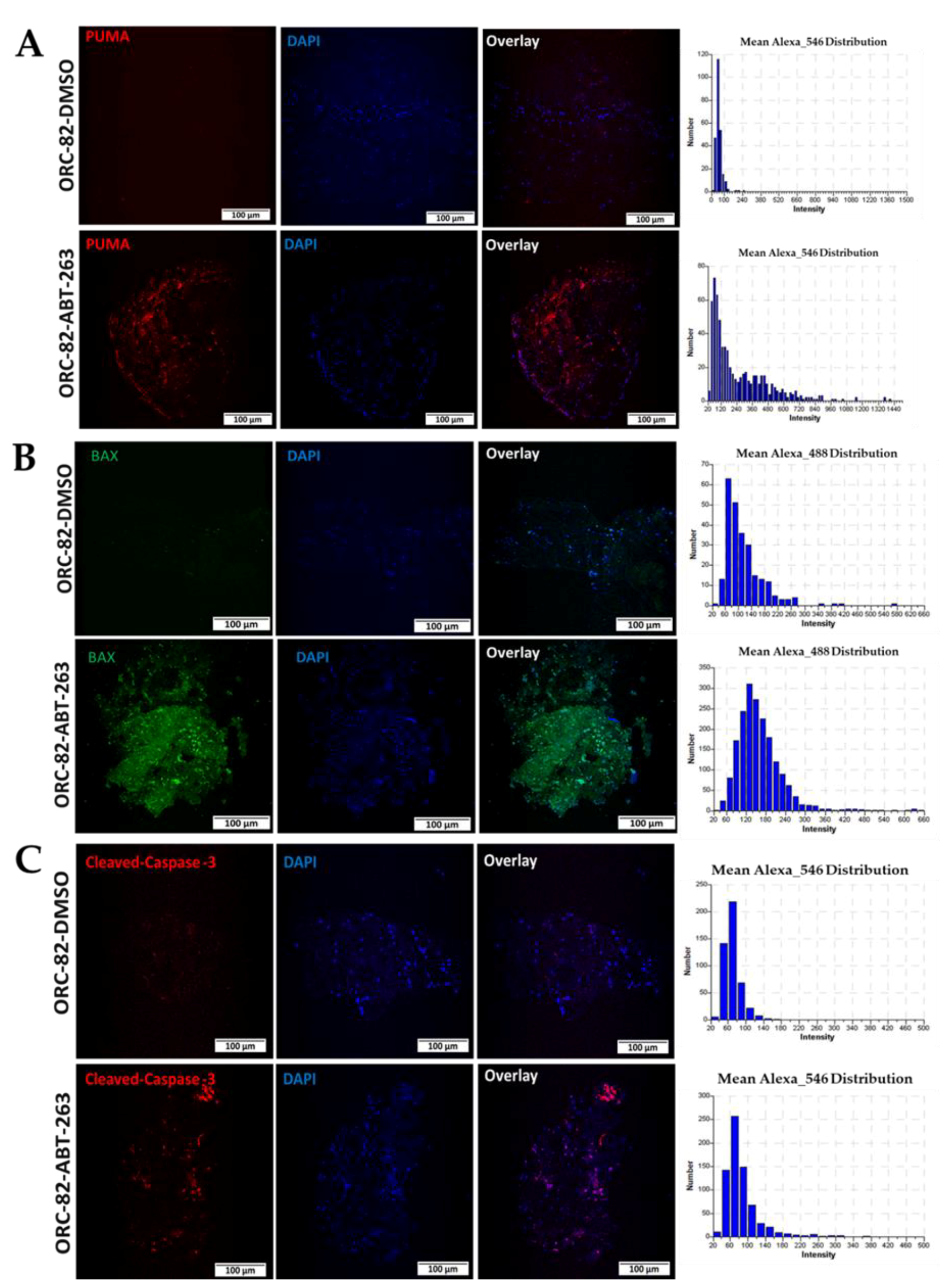
2.3. Effect of ABT-263 Treatment on BCL-2 Family Proteins in Organoid Raft Cultures
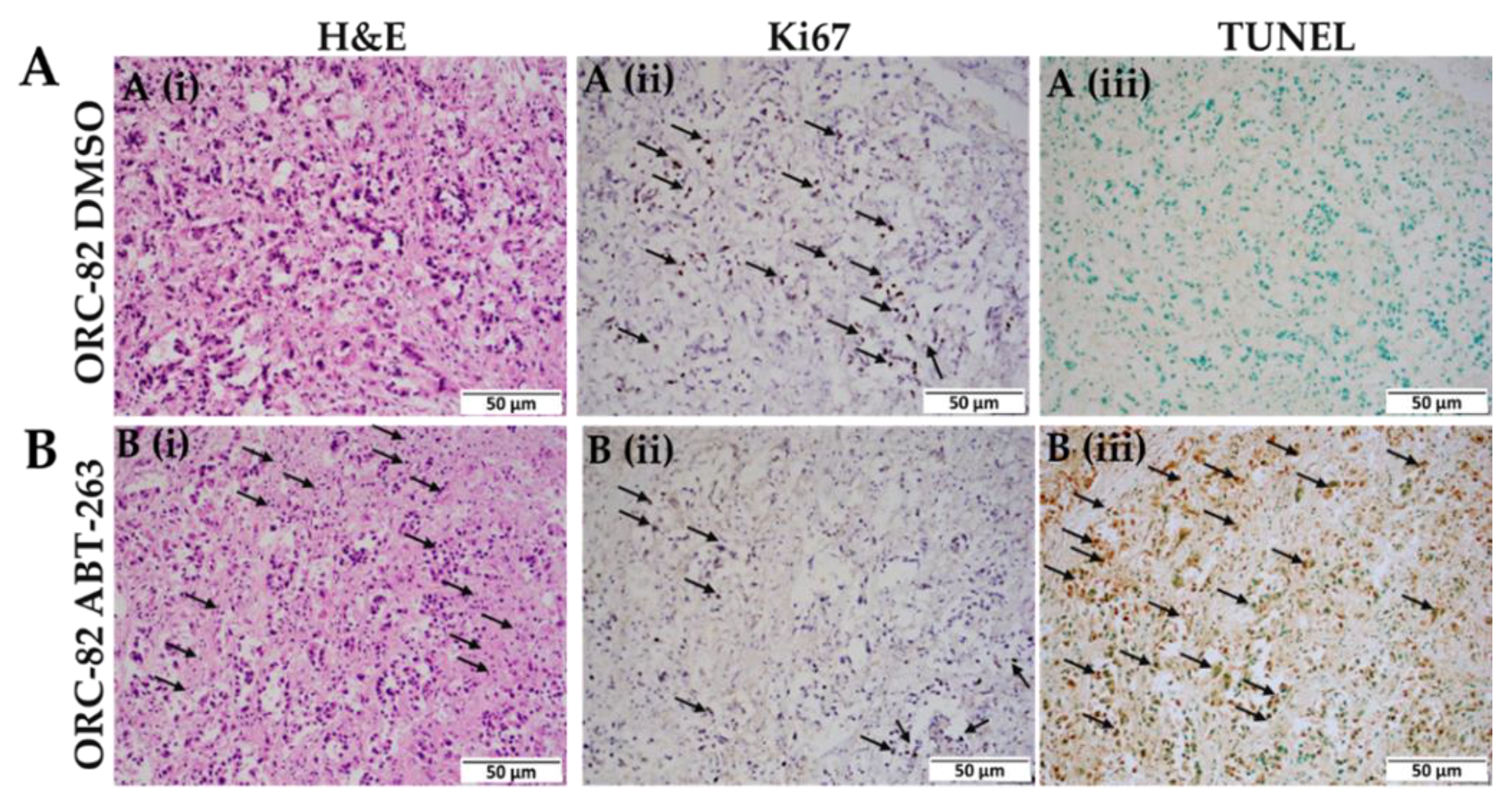
2.4. ABT-263 Treatment of Organoid Raft Cultures Decreases Cell Proliferation and Increases Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials Required for Organoid Raft Cultures (ORCs)
- Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal bovine serum and 1% Penicillin Streptomycin Antibiotic (100×, Corning, Cat # 30-002-CI, Fisher Scientific, Hampton, NH) was used for culturing feeder cells.
- We used Ham’s F12:F12 mix (1X) (1 L, package, GIBCO, Cat # 21700-0075, Thermo Fisher Scientific, Waltham, MA, USA). For reconstitution, directions on the package were followed, and 1.176 g of sodium bicarbonate was added. The pH was adjusted to 7.0 with 1 N HCl before filtration. F12, not more than a month old, should be used, as the pH fluctuates swiftly.
- Insulin stock (1000X) (Sigma–Aldrich, Cat # I-1882, Millipore Sigma, Burlington, MA, USA) was made by dissolving 100 mg in 20 mL sterile ultrapure water inside a cell culture hood. Aliquots were stored at −20 °C.
- Apo-transferrin stock (1000X) (Sigma–Aldrich, Cat # T1147, Millipore Sigma, Burlington, MA, USA) was made by dissolving 100 mg in 20 mL of sterile ultrapure water inside a cell culture hood. Aliquots were stored at −20 °C.
- Hydrocortisone-21-hemisuccinate stock (10,000X) (Sigma–Aldrich, Cat # H-488, Millipore Sigma, Burlington, MA, USA) was made by dissolving 100 mg in 25 mL of 100% ethanol inside a cell culture hood. Aliquots were stored at −20 °C.
- Human epidermal growth factor stock (10,000X) MilliporeSigma Calbiochem, Cat # 32-483-1200UG, Fisher Scientific, Hampton, NH, USA) was made by diluting 100 µL in 20 mL of sterile ultrapure water inside a cell culture hood. Aliquots were stored at −20 °C.
- Cholera toxin stock (10,000X, 1 mM) (Cayman Chemical, Cat # NC1425699, Fisher Scientific, Hampton, NH) was made by dissolving 1 mg in 2 mL of sterile ultrapure water inside a cell culture hood. Of this solution, 174 µL was added to 826 µL of sterile ultrapure water. Aliquots were stored at 4 °C. This solution should not be frozen.
- We used Type I Rat Tail Collagen (Corning, Cat # CB-40236, Fisher Scientific, Hampton, NH, USA).
- F12 stock (10X) (GIBCO, Cat # 21700-0075, Thermo Fisher Scientific, Waltham, MA, USA) was made by dissolving one 1-L package of Ham’s F12 medium in 100 mL of sterile ultrapure water. In this stock, pH was not adjusted, and sodium bicarbonate was not added. Filter-sterilized aliquots were then stored at −20 °C.
- We used phosphate-buffered saline (PBS) (10X) (Fisher BioReagents, Cat # BP39920, Fisher Scientific, Hampton, NH, USA).
- We used Antibiotic and Antimycotic mixture (Anti–Anti) (100X) (GIBCO, Cat # 15240062 Thermo Fisher Scientific, Waltham, MA, USA.
- We used 3T3-J2 mouse fibroblasts cells (Cat#EF303, Kerafast, Boston, MA, USA).
- Stainless steel grids (Edward J. Darby Inc, Philadelphia, PA, USA) measuring 1”× 1” (L × B) consisted of squares of wire mesh with square pores (size, 0.25 mm). The corners of the wired mesh were bent to make rafts/boats (approximate height 2 mm).
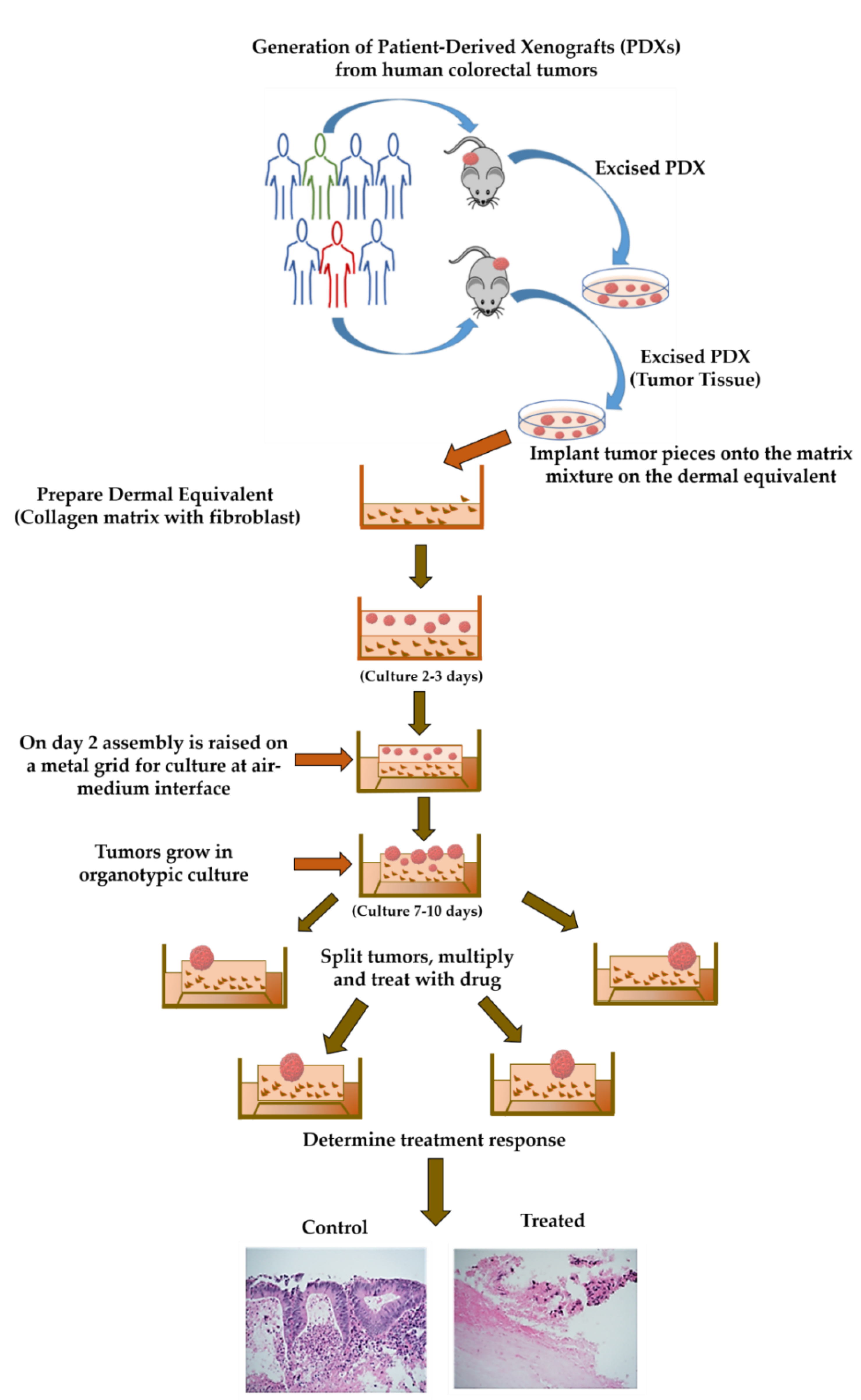
4.2. Generation of Patient-Derived Xenografts (PDXs)
4.3. Collection of the Patient-Derived Xenograft Tissue
4.4. Preparing Collagen Dermal Equivalent
- 400 µL of 10× Reconstitution Buffer (RB Buffer: 2.2 gm sodium bicarbonate, 0.2 gm sodium hydroxide (Sigma, Cat # 1310-73-2, Millipore Sigma, Burlington, MA, USA), and 4.76 gm HEPES (free acid; Sigma, Cat# H 3375) per 100 mL. Aliquots of 10 mL were stored at −20 °C);
- 400 µL 10× F12;
- 4 mL Type-1 Rat tail collagen.
4.5. Seeding on Collagen Bed/Boat
4.6. Generation of Organoid Raft Cultures (ORCs)
4.7. Treatment with Navitoclax (ABT-263)
4.8. Harvesting the Organoid Raft Cultures (ORCs)
4.9. Immunohistochemical (IHC) Analysis
4.10. RNA Extraction and Quantitative-PCR
4.11. TUNEL Staining
4.12. Immunofluorescence Microscopy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29692415 (accessed on 4 April 2022). [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31597751 (accessed on 4 April 2022). [CrossRef]
- Sato, T.; Stange, D.E.; Ferrante, M.; Vries, R.G.; Van Es, J.H.; Van den Brink, S.; Van Houdt, W.J.; Pronk, A.; Van Gorp, J.; Siersema, P.D.; et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 2011, 141, 1762–1772. Available online: https://www.ncbi.nlm.nih.gov/pubmed/21889923 (accessed on 13 May 2022). [CrossRef]
- Weeber, F.; van de Wetering, M.; Hoogstraat, M.; Dijkstra, K.K.; Krijgsman, O.; Kuilman, T.; Gadellaa-van Hooijdonk, C.G.; van der Velden, D.L.; Peeper, D.S.; Cuppen, E.P.; et al. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc. Natl. Acad. Sci. USA 2015, 112, 13308–13311. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26460009 (accessed on 14 April 2022). [CrossRef] [PubMed]
- Meyers, B.M.; Cosby, R.; Quereshy, F.; Jonker, D. Adjuvant systemic chemotherapy for stages II and III colon cancer after complete resection: A clinical practice guideline. Curr. Oncol. 2016, 23, 418–424. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5176375/ (accessed on 4 April 2022). [CrossRef] [PubMed]
- Sobrero, A.; Lonardi, S.; Rosati, G.; Di Bartolomeo, M.; Ronzoni, M.; Pella, N.; Scartozzi, M.; Banzi, M.; Zampino, M.G.; Pasini, F.; et al. FOLFOX or CAPOX in Stage II to III Colon Cancer: Efficacy Results of the Italian Three or Six Colon Adjuvant Trial. J. Clin. Oncol. 2018, 36, 1478–1485. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29620994 (accessed on 4 April 2022). [CrossRef] [PubMed]
- Aparo, S.; Goel, S. Evolvement of the treatment paradigm for metastatic colon cancer. From chemotherapy to targeted therapy. Crit. Rev. Oncol./Hematol. 2012, 83, 47–58. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3849106/ (accessed on 4 April 2022). [CrossRef]
- Gonzalo Recondo, E.D.-C., Jr.; de la Vega, M.; Greco, M.; Gonzalo Recondo, M.E.V., Sr. Advances and new perspectives in the treatment of metastatic colon cancer. World J. Gastrointest. Oncol. 2014, 6, 211. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4092338/ (accessed on 4 April 2022). [CrossRef] [PubMed]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis 2020, 25, 305–320. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7244464/ (accessed on 4 April 2022). [CrossRef] [PubMed]
- Bedi, A.; Pasricha, P.J.; Akhtar, A.J.; Barber, J.P.; Bedi, G.C.; Giardiello, F.M.; Zehnbauer, B.A.; Hamilton, S.R.; Jones, R.J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995, 55, 1811–1816. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7728743 (accessed on 4 April 2022). [PubMed]
- Reed, J. Bcl-2 and the regulation of programmed cell death. J. Cell Biol. 1994, 124, 1–6. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8294493 (accessed on 4 April 2022). [CrossRef] [PubMed]
- Manne, U.; Myers, R.B.; Moron, C.; Poczatek, R.B.; Dillard, S.; Weiss, H.; Brown, D.; Srivastava, S.; Grizzle, W.E. Prognostic significance of Bcl-2 expression and p53 nuclear accumulation in colorectal adenocarcinoma. Int. J. Cancer 1997, 74, 346–358. Available online: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9221816 (accessed on 14 April 2022). [CrossRef]
- Corcoran, R.B.; Cheng, K.A.; Hata, A.N.; Faber, A.C.; Ebi, H.; Coffee, E.M.; Greninger, P.; Brown, R.D.; Godfrey, J.T.; Cohoon, T.J.; et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 2013, 23, 121–128. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23245996 (accessed on 4 April 2022). [CrossRef] [PubMed]
- Shao, H.; Jing, K.; Mahmoud, E.; Huang, H.; Fang, X.; Yu, C. Apigenin Sensitizes Colon Cancer Cells to Antitumor Activity of ABT-263. Mol. Cancer Ther. 2013, 12, 2640–2650. Available online: http://mct.aacrjournals.org/content/molcanther/12/12/2640.full.pdf (accessed on 4 April 2022). [CrossRef] [PubMed]
- Balakrishnan, K.; Gandhi, V. Bcl-2 antagonists: A proof of concept for CLL therapy. Investig. New Drugs 2013, 31, 1384–1394. Available online: https://www.ncbi.nlm.nih.gov/pubmed/23907405 (accessed on 4 April 2022). [CrossRef] [PubMed]
- Tse, C.; Shoemaker, A.R.; Adickes, J.; Anderson, M.G.; Chen, J.; Jin, S.; Johnson, E.F.; Marsh, K.C.; Mitten, M.J.; Nimmer, P.; et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008, 68, 3421–3428. Available online: https://www.ncbi.nlm.nih.gov/pubmed/18451170 (accessed on 14 April 2022). [CrossRef] [PubMed]
- Chen, Q.; Song, S.; Wei, S.; Liu, B.; Honjo, S.; Scott, A.; Jin, J.; Ma, L.; Zhu, H.; Skinner, H.D.; et al. ABT-263 induces apoptosis and synergizes with chemotherapy by targeting stemness pathways in esophageal cancer. Oncotarget 2015, 6, 25883–25896. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4694873/ (accessed on 4 April 2022). [CrossRef]
- Rysanek, D.; Vasicova, P.; Kolla, J.N.; Sedlak, D.; Andera, L.; Bartek, J.; Hodny, Z. Synergism of BCL-2 family inhibitors facilitates selective elimination of senescent cells. Aging 2022, 14, 6381–6414. Available online: https://www.ncbi.nlm.nih.gov/pubmed/35951353 (accessed on 14 April 2022). [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5699948/ (accessed on 4 April 2022). [CrossRef]
- Kety, S.S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol. Rev. 1951, 3, 1–41. Available online: https://pharmrev.aspetjournals.org/content/pharmrev/3/1/1.full.pdf (accessed on 4 April 2022).
- Krogh, A. The rate of diffusion of gases through animal tissues, with some remarks on the coefficient of invasion. J. Physiol. 1919, 52, 391–408. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1402717/ (accessed on 4 April 2022). [CrossRef] [PubMed]
- Peniche Silva, C.J.; Liebsch, G.; Meier, R.J.; Gutbrod, M.S.; Balmayor, E.R.; van Griensven, M. A New Non-invasive Technique for Measuring 3D-Oxygen Gradients in Wells During Mammalian Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 595. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2020.00595 (accessed on 14 April 2022). [CrossRef] [PubMed]
- Al-Ani, A.; Toms, D.; Kondro, D.; Thundathil, J.; Yu, Y.; Ungrin, M. Oxygenation in cell culture: Critical parameters for reproducibility are routinely not reported. PLoS ONE 2018, 13, e0204269. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6191109/ (accessed on 4 April 2022). [CrossRef] [PubMed]
- Wenger, R.H. Mammalian oxygen sensing, signalling and gene regulation. J. Exp. Biol. 2000, 203 Pt 8, 1253–1263. Available online: https://journals.biologists.com/jeb/article/203/8/1253/8520/Mammalian-oxygen-sensing-signalling-and-gene (accessed on 4 April 2022). [CrossRef]
| CRC Patient # | Sex | Age | Body Mass Index | Sample Origin | Tumor (T), Nodes (N), and Metastases (M) | Degree of Differentiation | |
|---|---|---|---|---|---|---|---|
| 1 | CRC-18 | Female | 73 | 21.92 | Colon | T3N0Mx | Moderate |
| 2 | CRC-59 | Male | 40 | 26.42 | Sigmoid Colon | T3N0 | Moderate |
| 3 | CRC-82 | Male | 65 | 34.94 | Ascending Colon | T3N1 | Moderate |
| Genes | Primer Sequence (5′→ 3′) | |
|---|---|---|
| 1 | BCL2-F | GGA TTG TGG CCT TCT TTG AG |
| 2 | BCL2-R | GCC GGT TCA GGT ACT CAG TC |
| 3 | BCL-XL-F | AGT TTG AAC TGC GGT ACC GG |
| 4 | BCL-XL-R | GCA TTG TTC CCA TAG AGT TC |
| 5 | BCL W-F | GCG GAG TTC ACAGCT CTA TAC |
| 6 | BCL W-R | AAA AGG CCC CTA CAG TTA CCA |
| 7 | β-Actin-F | CTG CTT GCT GAT CCA CAT CTG |
| 8 | β-Actin-R | ATC AAG ATC ATT GCT CCT CCT GAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajpai, P.; Banerjee, N.S.; Moore, D.W.; Kim, H.-G.; Afaq, F.; Contreras, C.M.; Heslin, M.J.; Reddy, V.B.; Peter, S.; Varambally, S.; et al. Developing 3D Organoid Raft Cultures from Patient-Derived Xenografts as Rapid Models to Screen Efficacy of Experimental Therapeutics. Int. J. Mol. Sci. 2022, 23, 14392. https://doi.org/10.3390/ijms232214392
Bajpai P, Banerjee NS, Moore DW, Kim H-G, Afaq F, Contreras CM, Heslin MJ, Reddy VB, Peter S, Varambally S, et al. Developing 3D Organoid Raft Cultures from Patient-Derived Xenografts as Rapid Models to Screen Efficacy of Experimental Therapeutics. International Journal of Molecular Sciences. 2022; 23(22):14392. https://doi.org/10.3390/ijms232214392
Chicago/Turabian StyleBajpai, Prachi, Nilam Sanjib Banerjee, Dianne W. Moore, Hyung-Gyoon Kim, Farrukh Afaq, Carlo M. Contreras, Martin J. Heslin, Vishnu B. Reddy, Shajan Peter, Sooryanarayana Varambally, and et al. 2022. "Developing 3D Organoid Raft Cultures from Patient-Derived Xenografts as Rapid Models to Screen Efficacy of Experimental Therapeutics" International Journal of Molecular Sciences 23, no. 22: 14392. https://doi.org/10.3390/ijms232214392
APA StyleBajpai, P., Banerjee, N. S., Moore, D. W., Kim, H.-G., Afaq, F., Contreras, C. M., Heslin, M. J., Reddy, V. B., Peter, S., Varambally, S., Al Diffalha, S., & Manne, U. (2022). Developing 3D Organoid Raft Cultures from Patient-Derived Xenografts as Rapid Models to Screen Efficacy of Experimental Therapeutics. International Journal of Molecular Sciences, 23(22), 14392. https://doi.org/10.3390/ijms232214392










