Transgenic Improvement for Biotic Resistance of Crops
Abstract
1. Transgenic Improvement Is Effective to Control Biotic Constraints
1.1. Biotic Constraints to Crops
1.2. Conventional Management
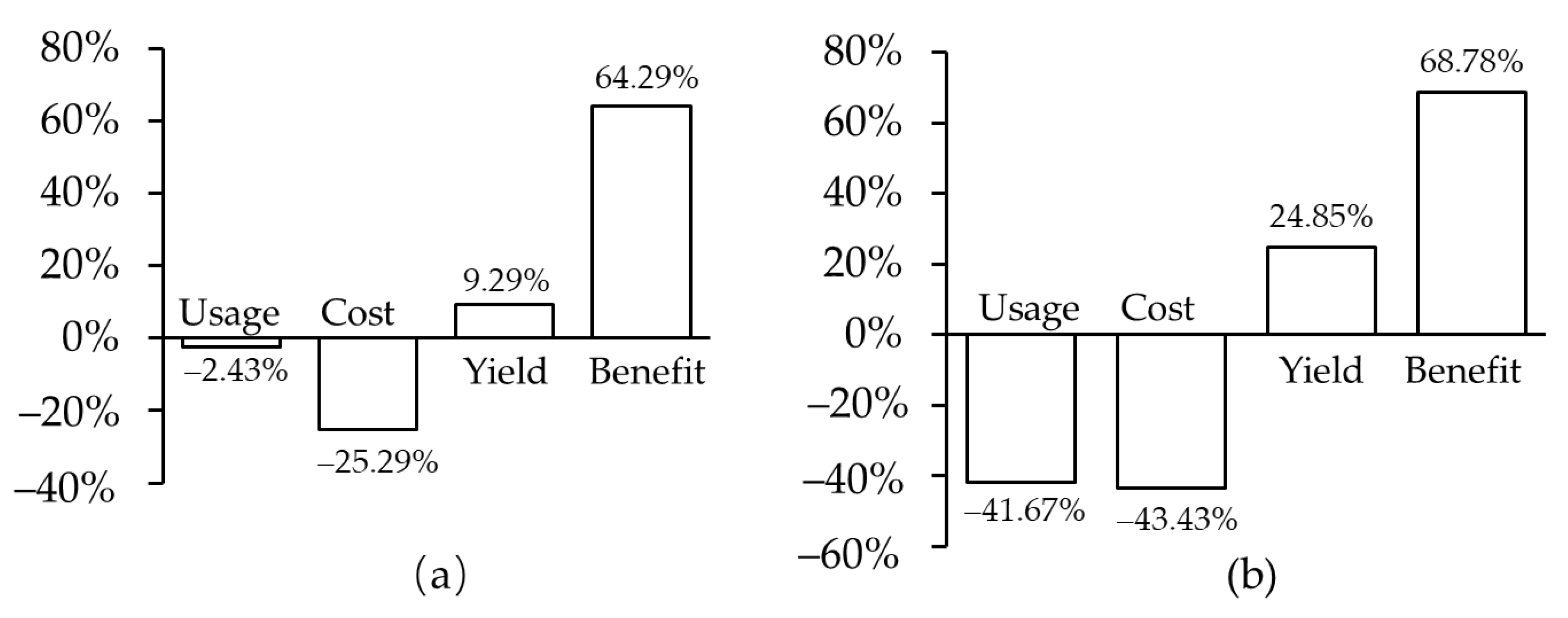
1.3. Transgenic Improvement
2. Commercial Release for Transgenic Resistance to Herbivory Insects, and Pathogenic Viruses, and Fungi
2.1. Resistance to Herbivory Insects
2.2. Resistance to Pathogenic Viruses
2.3. Resistance to Pathogenic Fungi
3. Exploration for Transgenic Resistance to Bacteria and Nematodes
3.1. Resistance to Pathogenic Bacteria
3.2. Resistance to Parasitic Nematodes
4. Strategy Option of Transgenic Improvement
4.1. Three Strategies of Transgenic Manipulation
4.2. Heterologous Expression of Exogenous Genes
4.3. Overexpression of Endogenous Genes
4.4. RNA Interference
4.5. Gene Edition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide research on plant defense against biotic stresses as improvement for sustainable agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- McKenna, T.P.; Koziol, L.; Bever, J.D.; Crews, T.E.; Sikes, B.A. Abiotic and biotic context dependency of perennial crop yield. PLoS ONE 2020, 15, e0234546. [Google Scholar] [CrossRef]
- Nazarov, P.A.; Baleev, D.N.; Ivanova, M.I.; Sokolova, L.M.; Karakozova, M.V. Infectious plant diseases: Etiology, current status, problems and prospects in plant protection. Acta Nat. 2020, 12, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Schumann, G.L.; D’Arcy, C.J. Essential Plant Pathology; APS Press: St. Paul, MN, USA, 2006; pp. 1–338. [Google Scholar]
- Falk, B.W.; Nouri, S. Special issue: Plant virus pathogenesis and disease control. Viruses 2020, 12, 1049. [Google Scholar] [CrossRef]
- Douglas, A.E. Strategies for enhanced crop resistance to insect pests. Annu. Rev. Plant Biol. 2018, 69, 637–660. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.J.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Elling, A.A. Major emerging problems with minor Meloidogyne species. Phytopathology 2013, 103, 1092–1102. [Google Scholar] [CrossRef]
- Ali, M.A.; Azeem, F.; Abbas, A.; Joyia, F.A.; Li, H.; Dababat, A.A. Transgenic strategies for enhancement of nematode resistance in plants. Front. Plant Sci. 2017, 8, 750. [Google Scholar] [CrossRef]
- Jones, D.G.; Dangl, L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Schreiber, K.; Desveaux, D. Message in a bottle: Chemical biology of induced disease resistance in plants. Plant Pathol. J. 2008, 24, 245–268. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, J.; Zipfel, C. Plant pattern recognition receptor complexes at the plasma membrane. Curr. Opin. Plant Biol. 2012, 15, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L. Modeling the impact of crop diseases on global food security. Ann. Rev. Phytopathol. 2020, 8, 24. [Google Scholar] [CrossRef]
- Hampf, A.C.; Nendel, C.; Strey, S.; Strey, R. Biotic yield losses in the Southern Amazon, Brazil: Making use of smartphone–assisted plant disease diagnosis data. Front. Plant Sci. 2021, 12, 621168. [Google Scholar] [CrossRef]
- Fontana, D.C.; de Paula, S.; Torres, A.G.; de Souza, V.H.M.; Pascholati, S.F.; Schmidt, D.; Dourado Neto, D. Endophytic fungi: Biological control and induced resistance to phytopathogens and abiotic stresses. Pathogens 2021, 10, 570. [Google Scholar] [CrossRef]
- Kim, C.; Cho, W.; Kim, H. Yield loss of spring Chinese cabbage as affected by infection time of clubroot disease in fields. Plant Dis. Res. 2000, 6, 23–26. [Google Scholar]
- Shukla, A.K. Estimation of yield losses to Indian mustard (Brassica juncea) due to Sclerotinia stem rot. J. Phytol. Res. 2005, 18, 267–268. [Google Scholar]
- Poveda, J.; Francisco, M.; Cartea, M.E.; Velasco, P. Development of transgenic Brassica crops against biotic stresses caused by pathogens and arthropod pests. Plants 2020, 9, 1664. [Google Scholar] [CrossRef]
- Barrett, C.B. Overcoming global food security challenges through science and solidarity. Am. J. Agric. Econ. 2021, 103, 422–447. [Google Scholar] [CrossRef]
- Roth, M.G.; Webster, R.W.; Mueller, D.S.; Chilvers, M.I.; Faske, T.R.; Mathew, F.M.; Bradley, C.A.; Damicone, J.P.; Kabbage, M.; Smith, D.L. Integrated management of important soybean pathogens of the United States in changing climate. J. Integr. Pest Manag. 2020, 11, 17. [Google Scholar] [CrossRef]
- Yin, K.; Qiu, J.L. Genome editing for plant disease resistance: Applications and perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180322. [Google Scholar] [CrossRef] [PubMed]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- Nelson, R.; Wiesner-Hanks, T.; Wisser, R.; Balint-Kurti, P. Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 2018, 19, 21–33. [Google Scholar] [CrossRef]
- Sharma, A.; Abrahamian, P.; Carvalho, R.; Choudhary, M.; Paret, M.L.; Vallad, G.E.; Jones, J.B. Future of bacterial disease management in crop production. Annu. Rev. Phytopathol. 2022, 60, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Li, C. Breeding crops by design for future agriculture. J. Zhejiang Univ. Sci. B 2020, 21, 423–425. [Google Scholar] [CrossRef]
- Roelfs, A.P. Genetic control of phenotypes in wheat stem rust. Ann. Rev. Phytopathol. 1988, 26, 351–367. [Google Scholar] [CrossRef]
- Elshafei, A.A.; Motawei, M.I.; Esmail, R.M.; Al-Doss, A.A.; Hussien, A.M.; Ibrahim, E.I.; Amer., M.A. Molecular breeding for rust resistance in wheat genotypes. Mol. Biol. Rep. 2021, 48, 731–742. [Google Scholar] [CrossRef]
- Denes, T.E.; Molnar, I.; Rakosy-Tican, E. New insights into the interaction between cultivated potato and Phytophthora infestans. Stud. Univ. Babes-Bolyai Biol. 2015, 60, 165–175. [Google Scholar]
- Joosten, M.H.A.J.; Cozijnsen, T.J.; de Wit, P.J.G.M. Host resistance to fungal tomato pathogen lost by a single base pair change in an avirulence gene. Nature 1994, 367, 384–386. [Google Scholar] [CrossRef]
- Gassmann, W.; Dahlbeck, D.; Chesnokova, O.; Minsavage, G.V.; Jones, J.B.; Staskawicz, B.J. Molecular evolution of virulence in natural field strains of Xanthomonas campestris pv. vesicatoria. J. Bacteriol. 2000, 182, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Stukenbrock, E.H.; McDonald, B.A. Population genetics of fungal and oomycete effectors involved in gene-for-gene interactions. Mol. Plant Microbe Interact. 2009, 22, 371–380. [Google Scholar] [CrossRef]
- Dodds, P.; Thrall, P. Recognition events and host-pathogen co-evolution in gene-for-gene resistance to flax rust. Funct. Plant Biol. 2009, 36, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Solomon-Blackburn, R.M. Progress in breeding potatoes for resistance to virus diseases. Asp. Appl. Biol. 1998, 52, 299–304. [Google Scholar]
- Liu, J.; Liu, D.; Tao, W.; Li, W.; Wang, S.; Chen, P.; Cheng, S.; Gao, D. Molecular marker-facilitated pyramiding of different genes for powdery mildew resistance in wheat. Plant Breed. 2000, 119, 21–24. [Google Scholar] [CrossRef]
- Richardson, K.L.; Vales, M.I.; Kling, J.G.; Mundt, C.C.; Hayes, P.M. Pyramiding and dissecting disease resistance QTL to barley stripe rust. Theor. Appl. Genet. 2006, 113, 485–495. [Google Scholar] [CrossRef]
- Saghai Maroof, M.A.; Jeong, S.C.; Gunduz, I.; Tucker, D.M.; Buss, G.R.; Tolin, S.A. Pyramiding of soybean mosaic virus resistance genes by marker-assisted selection. Crop Sci. 2008, 48, 517–526. [Google Scholar] [CrossRef]
- Keane, P.J. Horizontal or Generalized Resistance to Pathogens in Plants. In Plant Pathology; Cumagun, C.J., Ed.; InTech: Rijeka, Croatia, 2012; pp. 327–362. [Google Scholar]
- Galvez, L.C.; Banerjee, J.; Pinar, H.; Mitra, A. Engineered plant virus resistance. Plant Sci. 2014, 228, 11–25. [Google Scholar] [CrossRef]
- Mendes, B.J.; Filho, F.M.; Farias, P.; Benedito, V. Citrus somatic hybridization with potential for improved blight and CTV resistance. In Vitro Cell. Dev. Biol. Plant 2001, 37, 490–495. [Google Scholar]
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Asea, G.; Vivek, B.S.; Bigirwa, G.; Lipps, P.E.; Pratt, R.C. Validation of consensus quantitative trait loci associated with resistance to multiple foliar pathogens of maize. Phytopathology 2009, 99, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Bohar, R.; Chitkineni, A.; Varshney, R.K. Genetic molecular markers to accelerate genetic gains in crops. Biotechniques 2020, 69, 158–160. [Google Scholar] [CrossRef]
- Webster, R.W.; Roth, M.G.; Reed, H.; Mueller, B.; Groves, C.L.; McCaghey, M.; Chilvers, M.I.; Mueller, D.S.; Kabbage, M.; Smith, D.L. Identification of soybean (Glycine max) check lines for evaluating genetic resistance to sclerotinia stem rot. Plant Dis. 2021, 105, 2189–2195. [Google Scholar] [CrossRef]
- Foria, S.; Magris, G.; Jurman, I.; Schwope, R.; de Candido, M.; de Luca, E.; Ivanisevic, D.; Morgante, M.; Di Gaspero, G. Extent of wild-to-crop interspecific introgression in grapevine (Vitis inifera) as a consequence of resistance breeding and implications for the crop species definition. Hortic. Res. 2022, 9, uhab010. [Google Scholar] [CrossRef]
- Purankar, M.V.; Nikam, A.A.; Devarumath, R.M.; Suprasanna, P. Radiation induced mutagenesis, physio-biochemical profiling and field evaluation of mutants in sugarcane Cv. CoM 0265. Int. J. Radiat. Biol. 2022, 98, 1261–1276. [Google Scholar] [CrossRef]
- Batsa, B.K.; Sharma, R.C.; Rai, S.N. Identifying resistance to banded leaf and sheath blight of maize. Indian Phytopathol. 2005, 58, 121–122. [Google Scholar]
- Kang, B.C.; Yeam, I.; Jahn, M.M. Genetics of plant virus resistance. Annu. Rev. Phytopathol. 2005, 43, 581–621. [Google Scholar] [CrossRef]
- Holbein, J.; Grundler, F.M.; Siddique, S. Plant basal resistance to nematodes: An update. J. Exp. Bot. 2016, 67, 2049–2061. [Google Scholar] [CrossRef]
- Preston, G.M. Profiling the extended phenotype of plant pathogens: Challenges in bacterial molecular plant pathology. Mol. Plant Pathol. 2017, 18, 443–456. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhur, Y.A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef]
- Birkett, M.A.; Pickett, J.A. Prospects of genetic engineering for robust insect resistance. Curr. Opin. Plant Biol. 2014, 19, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Castle, S.; Palumbo, J.; Prabhaker, N. Newer insecticides for plant virus disease management. Virus Res. 2009, 141, 131–139. [Google Scholar] [CrossRef]
- Dababat, A.; Imren, M.; Erginbas-Orakci, G.; Ashrafi, S.; Yavuzaslanoglu, E.; Toktay, H.; Pariyar, S.R.; Elekcioglu, H.I.; Morgounov, A.; Mekete, T. The importance and management strategies of cereal cyst nematodes, Heterodera spp., in Turkey. Euphytica 2015, 202, 173–188. [Google Scholar] [CrossRef]
- Bardin, M.; Ajouzm, S.; Comby, M.; Lopez-Ferber, M.; Graillot, B.; Siegwart, M.; Nicot, P.C. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 2015, 6, 566. [Google Scholar] [CrossRef]
- He, D.C.; He, M.H.; Amalin., D.M.; Liu, W.; Alvindia, D.G.; Zhan, J. Biological control of plant diseases: An evolutionary and eco-economic consideration. Pathogens 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Raymond Park, J.; Mcfarlane, I.; Hartley Phipps, R.; Ceddia, G. The role of transgenic crops in sustainable development. Plant Biotechnol. J. 2011, 9, 2–21. [Google Scholar] [CrossRef]
- Kamthan, A.; Chaudhuri, A.; Kamthan, M.; Datta, A. Genetically modified (GM) crops: Milestones and new advances in crop improvement. Theor. App. Genet. 2016, 129, 1639–1655. [Google Scholar] [CrossRef] [PubMed]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, N.A.; Cominelli, E.; Galbiati, M.; Tonelli, C. The future of science: Food and water for life. Plant Cell 2009, 21, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Farre, G.; Ramessar, K.; Twyman, R.M.; Capell, T.; Christou, P. The humanitarian impact of plant biotechnology: Recent breakthroughs vs bottlenecks for adoption. Curr. Opin. Plant Biol. 2010, 13, 219–225. [Google Scholar] [CrossRef]
- Anjanappa, R.B.; Gruissem, W. Current progress and challenges in crop genetic transformation. J. Plant Physiol. 2021, 261, 153411. [Google Scholar] [CrossRef] [PubMed]
- Georges, F.; Ray, H. Genome editing of crops: A renewed opportunity for food security. GM Crops Food 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Heckel, D.G.; Bock, R. Next-generation insect-resistant plants: RNAi-mediated crop protection. Trends Biotechnol. 2017, 35, 871–882. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi crop protection advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef]
- Xu, R.; Zheng, Z.; Jiao, G. Safety assessment and detection methods of genetically modified organisms. Recent Pat. Food Nutr. Agric. 2014, 6, 27–32. [Google Scholar] [CrossRef]
- Tilgam, J.; Kumar, K.; Jayaswal, D.; Choudhury, S.; Kumar, A.; Jayaswall, K.; Saxena, A.K. Success of microbial genes based transgenic crops: Bt and beyond Bt. Mol. Biol. Rep. 2021, 48, 8111–8122. [Google Scholar] [CrossRef] [PubMed]
- Smyth, S.; Phillips, P.W.B.; Castle, D. Benefits of genetically modified herbicide tolerant canola in Western Canada. Int. J. Biotechnol. 2014, 13, 181. [Google Scholar] [CrossRef]
- Klumper, W.; Qaim, M. A meta-analysis of the impacts of genetically modified crops. PLoS ONE 2014, 9, e111629. [Google Scholar] [CrossRef] [PubMed]
- Pray, C.; Ma, D.; Huang, J.; Qiao, F. Impact of Bt cotton in China. World Dev. 2001, 29, 813–825. [Google Scholar] [CrossRef]
- Showalter, A.M.; Heuberger, S.; Tabashnik, B.E.; Carriere, Y.; Coates, B. A primer for using transgenic insecticidal cotton in developing countries. J. Insect Sci. 2009, 9, 22. [Google Scholar] [CrossRef][Green Version]
- Blanco, C.A. Heliothis virescens and Bt cotton in the United States. GM Crops Food 2012, 3, 201–212. [Google Scholar] [CrossRef]
- Rocha-Munive, M.G.; Soberon, M.; Castaneda, S.; Niaves, E.; Scheinvar, E.; Eguiarte, L.E.; Mota-Sánchez, D.; Rosales-Robles, E.; Nava-Camberos, U.; Martinez-Carrillo, J.L.; et al. Evaluation of the impact of genetically modified cotton after 20 years of cultivation in Mexico. Front. Bioeng. Biotechnol. 2018, 6, 82. [Google Scholar] [CrossRef]
- Ghareyazie, B.; Alinia, F.; Menguito, C.A.; Rubia, L.G.; de Palma, J.M.; Liwanag, E.A.; Cohen, M.B.; Khush, G.S.; Bennett, J. Enhanced resistance to two stem borers in an aromatic rice containing a synthetic cryIA(b) gene. Mol. Breed. 1997, 3, 401–414. [Google Scholar] [CrossRef]
- Xie, Z.W.; Luo, M.J.; Xu, W.F.; Chi, C.W. Two reactive site locations and structure-function study of the arrowhead proteinase inhibitors, A and B, using mutagenesis. Biochemistry 1997, 36, 5846–5852. [Google Scholar] [CrossRef]
- Hu, J.J.; Tian, Y.C.; Han, Y.F.; Li, Y.; Zhang, B.E. Field evaluation of insect-resistant transgenic Populus nigra trees. Euphytica 2001, 121, 123–127. [Google Scholar] [CrossRef]
- Cui, J.; Luo, J.; van der Werf, W.; Ma, Y.; Xia, J. Effect of pyramiding Bt and CpTI genes on resistance of cotton to Helicoverpa armigera (Lepidoptera: Noctuidae) under laboratory and field conditions. J. Econ. Entomol. 2011, 104, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Ramaseshadri, P.; Segers, G.; Flannagan, R.; Wiggins, E.; Clinton, W.; Ilagan, O.; McNulty, B.; Clark, T.; Bolognesi, R. Physiological and cellular responses caused by RNAi-mediated suppression of Snf7 orthologue in western corn rootworm (Diabrotica virgifera virgifera) larvae. PLoS ONE 2013, 8, e54270. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.C.; Johnston, S.A. The concept of parasite-derived resistance- deriving resistance genes from the parasite’s own genome. J. Theor. Biol. 1985, 113, 395–405. [Google Scholar] [CrossRef]
- Abel, P.P.; Nelson, R.S.; De, B.; Hoffmann, N.; Rogers, S.G.; Fraley, R.T.; Beachy, R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 1986, 232, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Tennant, P.F.; Gonsalves, C.; Ling, K.S.; Fitch, M.; Manshardt, R.; Slightom, L.J.; Gonsalves, D. Differential protection against papaya ringspot virus isolates in coat protein gene transgenic papaya and classically cross-protected papaya. Phytopathology 1994, 84, 1359–1366. [Google Scholar] [CrossRef]
- Mundembe, R.; Matibiri, A.; Sithole-Niang, I. Transgenic plants expressing the coat protein gene of cowpea aphid-borne mosaic potyvirus predominantly convey the delayed symptom development phenotype. Afr. J. Biotechnol. 2009, 8, 2682–2690. [Google Scholar]
- Golemboski, D.B.; Lomonossoff, G.P.; Zaitlin, M. Plants transformed with a tobacco mosaic virus nonstructural gene sequence are resistant to the virus. Proc. Natl. Acad. Sci. USA. 1990, 87, 6311–6315. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, H.; Li, Y.; Su, Y.; Wu, P.; Jiang, Z.; Ming, X.; Tian, J.; Pan, N.; Qu, L.J. Safety assessment for genetically modified sweet pepper and tomato. Toxicology 2003, 188, 297–307. [Google Scholar] [CrossRef]
- Tennant, P.; Souza, M.T.; Fitch, M.M.; Manshardt, R.M.; Slightom, J.L. Line 63-1: A new virus-resistant transgenic papaya. Hortscience 2005, 40, 1196–1199. [Google Scholar] [CrossRef]
- Wu, Z.; Mo, C.; Zhang, S.; Li, H. Characterization of papaya ringspot virus isolates infecting transgenic papaya ‘Huanong No.1’ in South China. Sci. Rep. 2018, 8, 8206. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Tavazza, M.; Noris, E.; Tavazza, R.; Caciagli, P.; Ancora, G.; Crespi, S.; Accotto, G.P. High expression of truncated viral rep protein confers resistance to tomato yellow leaf curl virus in transgenic tomato plants. Mol. Plant Microbe Interact. 1997, 10, 571–579. [Google Scholar] [CrossRef]
- Faria, J.C.; Albino, M.M.C.; Dias, B.B.A.; Cançado, L.J.; Cunha, N.B.D.; Silva, L.D.M.; Vianna, G.R.; Aragão, F.J.L. Partial resistance to bean golden mosaic virus in a transgenic common bean (Phaseolus vulgaris L.) line expressing a mutated rep gene. Plant Sci. 2006, 171, 565–571. [Google Scholar] [CrossRef]
- Aragao, F.J.L.; Nogueira, E.O.P.L.; Tinoco, M.L.P.; Faria, J.C. Molecular characterization of the first commercial transgenic common bean immune to the bean golden mosaic virus. J. Biotechnol. 2013, 166, 42–50. [Google Scholar] [CrossRef]
- Lapidot, M.; Gafny, R.; Ding, B.; Wolf, S.; Lucas, W.J.; Beachy, R.N. A dysfunctional movement protein of tobacco mosaic virus that partially modifies the plasmodesmata and limits virus spread in transgenic plants. Plant J. 1993, 4, 959–970. [Google Scholar] [CrossRef]
- Cooper, B.; Lapidot, M.; Heick, J.A.; Dodds, J.A.; Beachy, R.N. A defective movement protein of TMV in transgenic plants confers resistance to multiple viruses whereas the functional analog increases susceptibility. Virology 1995, 206, 307–313. [Google Scholar] [CrossRef]
- Carvalho, J.L.; De Oliveira Santos, J.; Conte, C.; Pacheco, S.; Nogueira, E.O.; Souza, T.L.; Faria, J.C.; Aragão, F.J. Comparative analysis of nutritional compositions of transgenic RNAi-mediated virus-resistant bean (event EMB-PV051-1) with its non-transgenic counterpart. Transgenic Res. 2015, 24, 813–819. [Google Scholar] [CrossRef]
- Borah, M.; Berbati, M.; Reppa, C.; Holeva, M.; Nath, P.D.; Voloudakis, A. RNA-based vaccination of Bhut Jolokia pepper (Capsicum chinense Jacq.) against cucumber mosaic virus. Virusdisease 2018, 29, 207–211. [Google Scholar] [CrossRef]
- Callahan, A.M.; Dardick, C.D.; Scorza, R. Multilocation comparison of fruit composition for ‘HoneySweet’, an RNAi based plum pox virus resistant plum. PLoS ONE 2019, 14, e0213993. [Google Scholar] [CrossRef]
- Halterman, D.A.; Kramer, L.C.; Wielgus, S.; Jiang, J. Performance of transgenic potato containing the late blight resistance gene RB. Plant Dis. 2008, 92, 339–343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaidi, S.S.; Tashkandi, M.; Mansoor, S.; Mahfouz, M.M. Engineering plant immunity: Using CRISPR/Cas9 to generate virus resistance. Front. Plant Sci. 2016, 7, 1673. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Amin, I.; Hameed, A.; Mansoor, S. CRISPR-Cas13a: Prospects for plant virus resistance. Trends Biotechnol. 2018, 36, 1207–1210. [Google Scholar] [CrossRef] [PubMed]
- Noureen, A.; Khan, M.Z.; Amin, I.; Zainab, T.; Ahmad, N.; Haider, S.; Mansoor, S. Broad-spectrum resistance against multiple PVY strains by CRSIPR/Cas13 system in Solanum tuberosum crop. GM Crops Food 2022, 13, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Stuiver, M.H.; Custers, J.H. Engineering disease resistance in plants. Nature 2001, 411, 865–868. [Google Scholar] [CrossRef]
- Wally, O.; Punja, Z.K. Genetic engineering for increasing fungal and bacterial disease resistance in crop plants. GM Crops 2010, 1, 199–206. [Google Scholar] [CrossRef]
- Hwang, B.H.; Bae, H.; Lim, H.S.; Kim, K.B.; Kim, S.J.; Im, M.H.; Park, B.S.; Kim, J. Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell Tissue Organ Cult. 2010, 103, 293–305. [Google Scholar] [CrossRef]
- Wang, Z.; Fang, H.; Chen, Y.; Chen, K.; Li, G.; Gu, S.; Tan, X. Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol. Plant Pathol. 2014, 15, 677–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Huai, D.; Yang, Q.; Cheng, Y.; Ma, M.; Kliebenstein, D.J.; Zhou, Y. Overexpression of three glucosinolate iosynthesis genes in Brassica napus identifies enhanced resistance to Sclerotinia sclerotiorum and Botrytis cinerea. PLoS ONE 2015, 10, e0140491. [Google Scholar]
- Fang, Y.; Tyler, B.M. Efficient disruption and replacement of an effector gene in the oomycete Phytophthora sojae using CRISPR/Cas9. Mol. Plant Pathol. 2016, 17, 127–139. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Mehari, H.; Meena, R.P.; Bhat, A.; Yadav, P.; Papalou, P.; Rawat, S.; Grover, A. Overexpression of NPR1 in Brassica juncea confers road spectrum resistance to fungal pathogens. Front. Plant Sci. 2017, 8, 1693. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Jiang, N.; Zhou, X.; Hou, X.; Yang, G.; Meng, J.; Luan, Y. Tomato MYB49 enhances resistance to Phytophthora infestans and tolerance to water deficit and salt stress. Planta 2018, 248, 1487–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, T.; Kan, J.; Yao, Y.; Guo, D.; Yang, Y.; Ling, X.; Wang, J.; Zhang, B. The GhMYB36 transcription factor confers resistance to biotic and abiotic stress by enhancing PR1 gene expression in plants. Plant Biotechnol. J. 2022, 20, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Zhou, T.; Trick, H.N.; Gill, B.S.; Bockus, W.W.; Muthukrishnan, S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum. J. Exp. Bot. 2003, 54, 1101–1111. [Google Scholar] [CrossRef]
- Yang, S.; Gao, M.; Xu, C.; Gao, J.; Deshpande, S.; Lin, S.; Roe, B.A.; Zhu, H. Alfalfa benefits from Medicago truncatula: The RCT1 gene from M. truncatula confers broad spectrum resistance to anthracnose in alfalfa. Proc. Natl. Acad. Sci. USA 2008, 105, 12164–12169. [Google Scholar] [CrossRef]
- Alexander, D.; Goodman, R.M.; Gut-Rella, M.; Glascock, C.; Weymann, K.; Friedrich, L.; Maddox, D.; Ahl-Goy, P.; Luntz, T.; Ward, E.; et al. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc. Natl. Acad. Sci. USA 1993, 90, 7327–7331. [Google Scholar] [CrossRef]
- Doares, S.H.; Narvaez-Vazquez, J.; Conconi, A.; Ryan, C.A. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995, 108, 1741–1746. [Google Scholar] [CrossRef]
- Albert, I.; Böhm, H.; Albert, M.; Feiler, C.E.; Imkampe, J.; Wallmeroth, N.; Brancato, C.; Raaymakers, T.M.; Oome, S.; Zhang, H.; et al. An RLP23-SOBIR1-BAK1 complex mediates NLP-triggered immunity. Nat. Plants 2015, 1, 15140. [Google Scholar] [CrossRef]
- Song, Y.; Liu, L.; Wang, Y.; Valkenburg, D.J.; Zhang, X.; Zhu, L.; Thomma, B.P.H.J. Transfer of tomato immune receptor Ve1 confers Ave1-dependent Verticillium resistance in tobacco and cotton. Plant Biotechnol. J. 2018, 16, 638–648. [Google Scholar] [CrossRef]
- Du, J.; Verzaux, E.; Chaparro-Garcia, A.; Bijsterbosch, G.; Keizer, L.C.P.; Zhou, J.; Liebrand, T.W.H.; Xie, C.; Govers, F.; Robatzek, S.; et al. Elicitin recognition confers enhanced resistance to Phytophthora infestans in potato. Nat. Plants 2015, 1, 15034. [Google Scholar] [CrossRef]
- Foster, S.J.; Park, T.H.; Pel, M.; Brigneti, G.; Sliwka, J.; Jagger, L.; van der Vossen, E.; Jones, J.D. Rpi-vnt1.1, a Tm-2(2) homolog from Solanum venturii, confers resistance to potato late blight. Mol. Plant Microbe Interact. 2009, 22, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.L.; Xie, S.; Chakraborty, A.; Wang, O.; Matny, M.; Jugovich, J.A.; Kolmer, T.; Richardson, D.; Bhatt, M.; Hoque, M.; et al. A five-transgene cassette confers broad-spectrum resistance to a fungal rust pathogen in wheat. Nat. Biotechnol. 2021, 39, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Mourgues, F.; Brisset, M.N.; Chevreau, E. Strategies to improve plant resistance to bacterial diseases through genetic engineering. Trends Biotechnol. 1998, 16, 203–210. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Xu, J.L.; Zhou, S.C.; Yu, J.; Xie, X.W.; Xu, M.R.; Sun, Y.; Zhu, L.H.; Fu, B.Y.; Gao, Y.M.; et al. Pyramiding Xa23 and Rxo1 for resistance to two bacterial diseases into an elite indica rice variety using molecular approaches. Mol. Breed. 2009, 23, 279–287. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Nagpala, P.; Destéfano-Beltrán, L.; Huang, J.H.; Kim, J.H.; Denny, T.; Cetiner, S. Expression of a cecropin B lytic peptide analog in transgenic tobacco confers enhanced resistance to bacterial wilt caused by Pseudomonas solanacearum. Plant Sci. 1993, 89, 43–53. [Google Scholar] [CrossRef]
- Huang, Y.; Nordeen, R.O.M.D.; Di, M.; Owens, L.D.; Mcbeath, J.H. Expression of an engineered cecropin gene cassette in transgenic tobacco plants confers disease resistance to Pseudomonas syringae pv. tabaci. Phytopathology 1997, 87, 494–499. [Google Scholar] [CrossRef]
- Mitra, A.; Zhang, Z. Expression of a human lactoferrin cDNA in tobacco cells produces antibacterial protein(s). Plant Physiol. 1994, 106, 977–981. [Google Scholar] [CrossRef]
- Trudel, J.; Potvin, C.; Asselin, A. Secreted hen lysozyme in transgenic tobacco: Recovery of bound enzyme and in vitro growth inhibition of plant pathogens. Plant Sci. 1995, 106, 55–62. [Google Scholar] [CrossRef]
- Nakajima, H.; Muranaka, T.; Ishige, F.; Akutsu, K.; Oeda, K. Fungal and bacterial disease resistance in transgenic plants expressing human lysozyme. Plant Cell Rep. 1997, 16, 674–679. [Google Scholar] [CrossRef]
- Zhang, Z.; Coyne, D.P.; Vidaver, A.K.; Mitra, A. Expression of human lactoferrin cDNA confers resistance to Ralstonia solanacearum in transgenic tobacco plants. Phytopathology 1998, 88, 730–734. [Google Scholar] [CrossRef]
- Rivero, M.; Furman, N.; Mencacci, N.; Picca, P.; Toum, L.; Lentz, E.; Bravo-Almonacid, F.; Mentaberry, A. Stacking of antimicrobial genes in potato transgenic plants confers increased resistance to bacterial and fungal pathogens. J. Biotechnol. 2012, 157, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, D.K.; Natarajan, S.; Mandal, S.; Mitra, A. Lactoferrin-derived resistance against plant pathogens in transgenic plants. J. Agric. Food Chem. 2013, 61, 11730–11735. [Google Scholar] [CrossRef] [PubMed]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dangl, J.L.; Dietrich, R.A. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 2000, 26, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Kunze, G.; Chinchilla, D.; Caniard, A.; Jones, J.D.G.; Boller, T.; Felix, G. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts agrobacterium-mediated transformation. Cell 2006, 125, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Lacombe, S.; Rougon-Cardoso, A.; Sherwood, E.; Peeters, N.; Dahlbeck, D.; van Esse, H.P.; Smoker, M.; Rallapalli, G.; Thomma, B.P.H.J.; Staskawicz, B.; et al. Interfamily transfer of a plant pattern-recognition receptor confers broad-spectrum bacterial resistance. Nat. Biotechnol. 2010, 28, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Schwessinger, B.; Bahar, O.; Thomas, N.; Holton, N.; Nekrasov, V.; Ruan, D.; Canlas, P.E.; Daudi, A.; Petzold, C.J.; Singan, V.R.; et al. Transgenic expression of the dicotyledonous pattern recognition receptor EFR in rice leads to ligand-dependent activation of defense responses. PLoS Pathog. 2015, 11, e1004809. [Google Scholar]
- Schoonbeek, H.; Wang, H.H.; Stefanato, F.L.; Craze, M.; Bowden, S.; Wallington, E.; Zipfel, C.; Ridout, C.J. Arabidopsis EF-Tu receptor enhances bacterial disease resistance in transgenic wheat. New Phytol. 2015, 206, 606–613. [Google Scholar] [CrossRef]
- Lu, F.; Wang, H.; Wang, S.; Jiang, W.; Shan, C.; Li, B.; Yang, J.; Zhang, S.; Sun, W. Enhancement of innate immune system in monocot rice by transferring the dicotyledonous elongation factor Tu receptor EFR: EFR confers bacterial disease resistance in monocot rice. J. Integr. Plant. Biol. 2015, 57, 641–652. [Google Scholar] [CrossRef]
- Boschi, F.; Schvartzman, C.; Murchio, S.; Ferreira, V.; Siri, M.I.; Galván, G.A.; Smoker, M.; Stransfeld, L.; Zipfel, C.; Vilaró, F.L.; et al. Enhanced bacterial wilt resistance in potato through expression of Arabidopsis EFR and introgression of quantitative resistance from Solanum commersonii. Front. Plant Sci. 2017, 8, 1642. [Google Scholar] [CrossRef]
- Pfeilmeier, S.; George, J.; Morel, A.; Roy, S.; Smoker, M.; Stransfeld, L.; Downie, J.A.; Peeters, N.; Malone, J.G.; Zipfel, C. Expression of the Arabidopsis thaliana immune receptor EFR in Medicago truncatula reduces infection by a root pathogenic bacterium, but not nitrogen-fixing rhizobial symbiosis. Plant Biotechnol. J. 2019, 17, 569–579. [Google Scholar] [CrossRef]
- Lilley, C.J.; Devlin, F.; Urwin, P.E.; Atkinson, H.J. Parasitic nematodes, proteinases and transgenic plants. Parasitol. Today 1999, 15, 414–417. [Google Scholar] [CrossRef]
- Bakhetia, M.; Charlton, W.L.; Urwin, P.E.; McPherson, M.J.; Atkinson, H.J. RNA interference and plant parasitic nematodes. Trends Plant Sci. 2005, 10, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Rosso, M.N.; Dubrana, M.P.; Cimbolini, N.; Jaubert, S.; Abad, P. Application of RNA interference to root-knot nematode genes encoding esophageal gland proteins. Mol. Plant Microbe Interact. 2005, 18, 615–620. [Google Scholar] [CrossRef]
- Gheysen, G.; Kyndt, T.; Haegeman, A.; Joseph, S.; Remy, S.; Swennen, R. RNAi for research and applications in plant-nematode interactions. In Vitro Cell. Dev. Biol. Anim. 2010, 46, S28–S29. [Google Scholar]
- Bakhetia, M.; Charlton, W.; Atkinson, H.J.; McPherson, M.J. RNA interference of dual oxidase in the plant nematode Meloidogyne incognita. Mol. Plant Microb. Interact. 2005, 18, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.C.; Veluthambi, K.; Subramaniam, K. Host generated double stranded RNA induces RNAi in plant parasitic nematodes and protects the host from infection. Mol. Biochem. Parasitol. 2006, 148, 219–222. [Google Scholar] [CrossRef]
- Shingles, J.; Lilley, C.J.; Atkinson, H.J.; Urwin, P.E. Meloidogyne incognita: Molecular and biochemical characterisation of a cathepsin L cysteine proteinase and the effect on parasitism following RNAi. Exp. Parasitol. 2007, 115, 114–120. [Google Scholar] [CrossRef]
- Charlton, W.L.; Harel, H.Y.M.; Bakhetia, M.; Hibbard, J.K.; Atkinson, H.J.; Mcpherson, M.J. Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int. J. Parasitol. 2010, 40, 855–864. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Alkharouf, N.W.; Meyer, S.L.; Aly, M.A.; Gamal, E.A.K. Post-transcriptional gene silencing of root-knot nematode in transformed soybean roots. Exp. Parasitol. 2011, 127, 90–99. [Google Scholar] [CrossRef]
- Niu, J.H.; Jian, H.; Xu, J.; Chen, C.; Guo, Q. RNAi silencing of the Meloidogyne incognita Rpn7 gene reduces nematode parasitic success. Euro. J. Plant Pathol. 2012, 134, 131–144. [Google Scholar] [CrossRef]
- Papolu, P.K.; Gantasala, N.P.; Kamaraju, D.; Banakar, P.; Sreevathsa, R.; Rao, U. Utility of host delivered RNAi of two FMRF amide like peptides, flp-14 and flp-18, for the management of root-knot nematode, Meloidogyne incognita. PLoS ONE 2013, 8, e80603. [Google Scholar] [CrossRef] [PubMed]
- Antonino de Souza, J.D., Jr.; Ramos Coelho, R.; Tristan Lourenço, I.; da Rocha Fragoso, R.; Barbosa Viana, A.A.; Lima Pepino de Macedo, L.; Mattar da Silva, M.C.; Gomes Carneiro, R.M.; Engler, G.; de Almeida-Engler, J.; et al. Knocking- down Meloidogyne incognita proteases by plant-delivered dsRNA has negative pleiotropic effect on nematode vigor. PLoS ONE 2013, 8, e85364. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.K.; Papolu, P.K.; Banakar, P.; Choudhary, D.; Sirohi, A.; Rao, U. Tomato transgenic plants expressing hairpin construct of a nematode protease gene conferred enhanced resistance to root-knot nematodes. Front. Microbiol. 2015, 6, 260. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.K.; Banakar, P.; Rao, U. The status of RNAi-based transgenic research in plant nematology. Front. Microbiol. 2015, 5, 760. [Google Scholar] [CrossRef]
- Walawage, S.L.; Britton, M.T.; Leslie, C.A.; Uratsu, S.L.; Li, Y.; Dandekar, M. Stacking resistance to crown gall and nematodes in walnut rootstocks. BMC Genomics 2013, 14, 668. [Google Scholar] [CrossRef]
- Dinh, P.T.Y.; Zhang, L.; Brown, C.R.; Elling, A.A. Plant-mediated RNA interference of effector gene Mc16D10L confers resistance against Meloidogyne chitwoodi in diverse genetic backgrounds of potato and reduces pathogenicity of nematode offspring. Nematology 2014, 6, 669–682. [Google Scholar] [CrossRef]
- Klink, V.P.; Kim, K.H.; Martins, V.; Macdonald, M.H.; Beard, H.S.; Alkharouf, N.W.; Lee, S.K.; Park, S.C.; Matthews, B.F. A correlation between host-mediated expression of parasite genes as tandem inverted repeats and abrogation of development of female Heterodera glycines cyst formation during infection of Glycine max. Planta 2009, 230, 53–71. [Google Scholar] [CrossRef]
- Li, J.; Todd, T.C.; Oakley, T.R.; Lee, J.L.; Trick, H.N. Host-derived suppression of nematode reproductive and fitness genes decreases fecundity of Heterodera glycines Ichinohe. Planta 2010, 232, 775–785. [Google Scholar] [CrossRef]
- Danchin, E.G.J.; Arguel, M.J.; Campan-Fournier, A.; Perfus-Barbeoch, L.; Magliano, M.; Rosso, M.N.; Da Rocha, M.; Da Silva, C.; Nottet, N.; Labadie, K.; et al. Identification of novel target genes for safer and more specific control of root-knot nematodes from a pan-genome mining. PLoS Pathogen. 2013, 9, e1003745. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Q.; Fu, F.; Li, W. Three strategies of transgenic manipulation for crop improvement. Front. Plant Sci. 2022, 13, 948518. [Google Scholar] [CrossRef]
- Liu, D. Design of gene constructs for transgenic maize. Meth. Mol. Biol. 2009, 526, 3–20. [Google Scholar]
- Parvathy, S.T.; Udayasuriyan, V.; Bhadana, V. Codon usage bias. Mol. Biol. Rep. 2022, 49, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, J.J.; Meredith, W.R. Genetic basis for variability of Cry1Ac expression among commercial transgenic Bacillus thuringiensis (Bt) cotton cultivars in the United States. J. Cotton Sci. 2004, 8, 17–23. [Google Scholar]
- Poongothai, S.; Iiavarasan, R.; Karrunakaran, C.M. Cry1Ac levels and biochemical variations in Bt cotton as influenced by tissue maturity and senescence. J. Plant Breed. Crop Sci. 2010, 2, 96–103. [Google Scholar]
- Chen, Y.; Li, Y.; Zhou, M.; Cai, Z.; Tambel, L.I.M.; Zhang, X.; Chen, Y.; Chen, D. Nitrogen deficit decreases seed Cry1Ac endotoxin expression in Bt transgenic cotton. Plant Physiol. Biochem. 2019, 141, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, J.J.; Sumerford, D.V. Potential factors impacting season-long expression of Cry1Ac in 13 commercial varieties of Bollgard cotton. J. Insect Sci. 2001, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, J.J.; Hardee, D.D.; Adams, L.C.; Sumerford, D.V. Correlating differences in larval survival and development of bollworms (Lepidoptera: Noctuidae) and fall armyworms (Lepidoptera: Noctuidae) to differential expression of Cry1A(c) δ–endotoxin in various plant parts among commercial cultivars of transgenic Bacillus thuringiensis cotton. J. Econ. Entomol. 2001, 94, 284–290. [Google Scholar] [PubMed]
- Adamczyk, J.J.; Perera, O.; Meredith, W.R. Production of mRNA from the Cry1Ac transgene differs among Bollgard lines which correlates to the level of subsequent protein. Transgenic Res. 2009, 18, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shen, W.; Fang, Z.; Liu, B. Cry1ab/c in different stages of growth in transgenic rice Bt-shanyou63. Front. Biosci. 2016, 21, 447–454. [Google Scholar]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef]
- Palma, L.; Muñoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Melo, A.L.; Soccol, V.T.; Soccol, C.R. Bacillus thuringiensis: Mechanism of action, resistance, and new applications: A review. Crit. Rev. Biotechnol. 2016, 36, 317–326. [Google Scholar] [CrossRef] [PubMed]
- de Wit, P.J.G.M. Molecular characterization of gene-for-gene systems in plant-fungus interactions and the application of avirulence genes in control of plant pathogens. Annu. Rev. Phytopathol. 1992, 30, 391–418. [Google Scholar] [CrossRef]
- Kamoun, S.; van West, P.; Vleeshouwers, V.G.A.A.; de Groot, K.E.; Govers, F. Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell 1998, 10, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Pamboukdjian, N.; Ponchet, M.; Poupet, A.; Delon, R.; Verrier, J.L.; Roby, D.; Ricci, P. Pathogen induced elicitin production in transgenic tobacco generates a hypersensitive response and nonspecific disease resistance. Plant Cell 1999, 11, 223–235. [Google Scholar] [CrossRef]
- Heath, M.C. Nonhost resistance and non-specific plant defenses. Curr. Opin. Plant Biol. 2000, 3, 315–319. [Google Scholar] [CrossRef]
- Stalker, D.M.; Mcbride, K.E.; Malyj, L.D. Herbicide resistance in transgenic plants expressing a bacterial detoxification gene. Science 1988, 242, 419–423. [Google Scholar] [CrossRef]
- Matten, S.R.; Reynolds, A.H. Current resistance management requirements for Bt cotton in the United States. J. New Seeds 2003, 5, 137–178. [Google Scholar] [CrossRef]
- Fraser, M.J., Jr. Insect transgenesis: Current applications and future prospects. Annu. Rev. Entomol. 2012, 57, 267–289. [Google Scholar] [CrossRef]
- Davis, M.J.; Ying, Z. Development of papaya breeding lines with transgenic resistance to papaya ringspot virus. Plant Dis. 2004, 88, 352–358. [Google Scholar] [CrossRef]
- Ilardi, V.; Nicola-Negri, E.D. Genetically engineered resistance to plum pox virus infection in herbaceous and stone fruit hosts. GM Crop 2011, 2, 24–33. [Google Scholar] [CrossRef]
- Orbegozo, J.; Solorzano, D.; Cuellar, W.J.; Bartolini, I.; Roman, M.L.; Ghislain, M.; Kreuze, J. Marker-free PLRV resistant potato mediated by Cre-loxP excision and RNAi. Transgenic Res. 2016, 25, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Chiozza, M.V.; Burachik, M.; Miranda, P.V. Compositional analysis of soybean event IND–ØØ41Ø–5. GM Crops Food 2020, 11, 154–163. [Google Scholar] [CrossRef]
- Demaneche, S.; Sanguin, H.; Pote, J.; Navarro, E.; Bernillon, D.; Mavingui, P.; Wildi, W.; Vogel, T.M.; Simonet, P. Antibiotic-resistant soil bacteria in transgenic plant fields. Proc. Natl. Acad. Sci. USA 2008, 105, 3957–3962. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Bradeen, J.M.; Naess, S.K.; Raasch, J.A.; Wielgus, S.M.; Haberlach, G.T.; Liu, J.; Kuang, H.; Austin-Phillips, S.; Buell, C.R.; et al. Gene RB cloned from Solanum bulbocastanum confers broad spectrum resistance to potato late blight. Proc. Natl. Acad. Sci. USA. 2003, 100, 9128–9133. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Hennig, J.; Szwacka, M.; Malepszy, S. Tobacco PR-2d promoter is induced in transgenic cucumber in response to biotic and abiotic stimuli. J. Plant Physiol. 2004, 161, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lim, S.; Yoda, H.; Choi, Y.E.; Sano, H. Simultaneous activation of salicylate production and fungal resistance in transgenic Chrysanthemum producing caffeine. Plant Signal Behav. 2011, 6, 409–412. [Google Scholar] [CrossRef]
- Jisha, V.; Dampanaboina, L.; Vadassery, J.; Mithöfer, A.; Kappara, S.; Ramanan, R. Overexpression of an AP2/ERF type transcription factor OsEREBP1 confers biotic and abiotic stress tolerance in rice. PLoS ONE 2015, 10, e0127831. [Google Scholar] [CrossRef]
- Coppola, M.; Corrado, G.; Coppola, V.; Cascone, P.; Martinelli, R.; Digilio, M.C.; Pennacchio, F.; Rao, R. Prosystemin overexpression in tomato enhances resistance to different biotic stresses by activating genes of multiple signaling pathways. Plant Mol. Biol. Rep. 2015, 33, 1270–1285. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Tang, Y.; Chen, J.; Ding, W. Overexpression of NtWRKY50 increases resistance to Ralstonia solanacearum and alters salicylic acid and jasmonic acid production in tobacco. Front. Plant Sci. 2017, 8, 1710. [Google Scholar] [CrossRef]
- Klee, H.J. Ripening physiology of fruit from transgenic tomato (Lycopersicon esculentum) plants with reduced ethylene synthesis. Plant Physiol. 1993, 102, 911–916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taning, C.N.T.; Arpaia, S.; Christiaens, O.; Dietz-Pfeilstetter, A.; Jones, H.; Mezzetti, B.; Sabbadini, S.; Sorteberg, H.G.; Sweet, J.; Ventura, V.; et al. RNA-based biocontrol compounds: Current status and perspectives to reach the market. Pest Manag. Sci. 2020, 76, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Giudice, G.; Moffa, L.; Varotto, S.; Cardone, M.F.; Bergamini, C.; de Lorenzis, G.; Velasco, R.; Nerva, L.; Chitarra, W. Novel and emerging biotechnological crop protection approaches. Plant Biotechnol. J. 2021, 19, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Rajam, M.V. RNA silencing technology: A boon for crop improvement. J. Biosci. 2020, 45, 118. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.; Xu, Z.P. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Mysore, K.S. Caveat of RNAi in plants: The off-target effect. Methods Mol. Biol. 2011, 744, 13–25. [Google Scholar]
- Neumeier, J.; Meister, G. siRNA Specificity: RNAi mechanisms and strategies to reduce off-target effects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Reddy Palli, S.; Han, Z. Off-target effects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef]
- Pandey, P.; Mysore, K.S.; Senthil-Kumar, M. Recent advances in plant gene silencing methods. Methods Mol. Biol. 2022, 2408, 1–22. [Google Scholar]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Daub, M.E.; Ehrenshaft, M. The photoactivated Cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 2000, 38, 461–490. [Google Scholar] [CrossRef]
- Cessna, S.G.; Sear, V.E.; Dickman, M.B.; Low, P.S. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell 2000, 12, 2191–2200. [Google Scholar] [CrossRef]
- Gaj, T.; Gersbach, C.A.; Barbas, C.F., III. ZFN, TALEN, and CRISPR/Cas–based methods for genome engineering. Trends Biotechnol. 2013, 31, 397–405. [Google Scholar] [CrossRef]
- Leibowitz, M.L.; Papathanasiou, S.; Doerfler, P.A.; Blaine, L.J.; Sun, L.; Yao, Y.; Zhang, C.Z.; Weiss, M.J.; Pellman, D. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat. Genet. 2021, 53, 889–905. [Google Scholar] [CrossRef] [PubMed]
- Molla, K.A.; Sretenovic, S.; Bansal, K.C.; Qi, Y. Precise plant genome editing using base editors and prime editors. Nat. Plants 2021, 7, 1166–1187. [Google Scholar] [CrossRef] [PubMed]
- Nerkar, G.; Devarumath, S.; Purankar, M.; Kumar, A.; Valarmathi, R.; Devarumath, R.; Appunu, C. Advances in crop breeding through precision genome editing. Front. Genet. 2022, 13, 880195. [Google Scholar] [CrossRef] [PubMed]
- Chilcoat, D.; Liu, Z.B.; Sander, J. Use of CRISPR/Cas9 for crop improvement in maize and soybean. Prog. Mol. Biol. Transl. Sci. 2017, 149, 27–46. [Google Scholar]
- Sánchez-León, S.; Gil-Humanes, J.; Ozuna, C.V.; Giménez, M.J.; Sousa, C.; Voytas, D.F.; Barro, F. Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol. J. 2018, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, D.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana enthamiana using Cas9 RNA–guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Shan, Q.; Wang, Y.; Li, J.; Zhang, Y.; Chen, K.; Liang, Z.; Zhang, K.; Liu, J.; Xi, J.J.; Qiu, J.L.; et al. Targeted genome modification of crop plants using a CRISPR/Cas system. Nat. Biotechnol. 2013, 31, 686–688. [Google Scholar] [CrossRef]
- van der Oost, J.; Westra, E.R.; Jackson, R.N.; Wiedenheft, B. Unravelling the structural and mechanistic basis of CRISPR–Cas systems. Nat Rev Microbiol. 2014, 12, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Lawrenson, T.; Shorinola, O.; Stacey, N.; Li, C.; Østergaard, L.; Patron, N.; Uauy, C.; Harwood, W. Induction of targeted, heritable mutations in barley and Brassica oleracea using RNA–guided Cas9 nuclease. Genome Biol. 2015, 16, 258. [Google Scholar] [CrossRef] [PubMed]
- Malnoy, M.; Viola, R.; Jung, M.H.; Koo, O.J.; Kim, S.; Kim, J.S.; Velasco, R.; Nagamangala Kanchiswamy, C.D. DNA-free genetically edited grapevine and apple protoplast using CRISPR/Cas9 ribonucleoproteins. Front. Plant Sci. 2016, 7, 1904. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, C.; Fu, Y.; Liu, Q.; Jiao, X.; Wang, K. Expanding the range of CRISPR/Cas9 genome editing in rice. Mol. Plant 2016, 9, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I.J.C. Latest developed strategies to minimize the off-target effects in CRISPR-Cas mediated genome editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef] [PubMed]
- Honée, G. Engineered resistance against fungal plant pathogens. Eur. J. Plant Pathol. 1999, 105, 319–326. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Wei, P.; Zhang, B.; Gou, F.; Feng, Z.; Mao, Y.; Yang, L.; Zhang, H.; Xu, N.; et al. The CRISPR/Cas9 system produces specific and homozygous targeted gene editing in rice in one generation. Plant Biotechnol. J. 2014, 12, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, X.; Shan, Q.; Zhang, Y.; Liu, J.; Gao, C.; Qiu, J.L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 2014, 32, 947–951. [Google Scholar] [CrossRef]
- Zhu, J.; Song, N.; Sun, S.; Yang, W.; Zhao, H.; Song, W.; Lai, J. Efficiency and inheritance of targeted mutagenesis in maize using CRISPR-Cas9. J. Genet. Genom. 2016, 43, 25–36. [Google Scholar] [CrossRef]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016, 532, 293. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, Z.; Wu, P.; Fang, M.; Pan, X.; Lin, Q.; Luo, W.; Wu, G.; Li, H. Reassessment of the four yield–related genes Gn1a, DEP1, GS3, and IPA1 in rice using a CRISPR/Cas9 system. Front. Plant Sci. 2016, 7, 377. [Google Scholar] [CrossRef] [PubMed]
- Waltz, E. CRISPR-edited crops free to enter market, skip regulation. Nat. Biotechnol. 2016, 34, 582. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a CRISPR/Cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Braatz, J.; Harloff, H.J.; Mascher, M.; Stein, N.; Himmelbach, A.; Jung, C. CRISPR-Cas9 targeted mutagenesis leads to simultaneous modification of different homoeologous gene copies in polyploid oilseed rape (Brassica napus). Plant Physiol. 2017, 174, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, A.; Ogawa, T.; Koizuka, C.; Kaneko, K.; Inaba, M.; Imamura, J.; Koizuka, N. CRISPR/Cas9-mediated genome editing of the fatty acid desaturase 2 gene in Brassica napus. Plant Physiol. Biochem. 2018, 131, 63–69. [Google Scholar] [CrossRef]
- Langner, T.; Kamoun, S.; Belhaj, K. CRISPR crops: Plant genome editing toward disease resistance. Ann. Rev. Phytopathol. 2018, 56, 479–512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ge, X.; Luo, X.; Wang, P.; Fan, Q.; Hu, G.; Xiao, J.; Li, F.; Wu, J. Simultaneous editing of two copies of Gh14–3–3d confers enhanced transgene-clean plant defense against Verticillium dahliae in allotetraploid upland cotton. Front. Plant Sci. 2018, 9, 842. [Google Scholar] [CrossRef]
- Zaidi, S.S.; Mukhtar, M.S.; Mansoor, S. Genome editing: Targeting susceptibility genes for plant disease resistance. Trends Biotechnol. 2018, 36, 898–906. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, S.; Tian, S.; Zhang, J.; Ren, Y.; Li, M.; Gong, G.; Zhang, H.; Xu, Y. CRISPR/Cas9-mediated mutagenesis of ClBG1 decreased seed size and promoted seed germination in watermelon. Hortic. Res. 2021, 8, 1–12. [Google Scholar] [CrossRef]
- Hinge, V.R.; Chavhan, R.L.; Kale, S.P.; Suprasanna, P.; Kadam, U.S. Engineering resistance against viruses in field crops using CRISPR-Cas9. Curr. Genom. 2021, 22, 214–231. [Google Scholar] [CrossRef]
- Usman, B.; Nawaz, G.; Zhao, N.; Liao, S.; Qin, B.; Liu, F.; Liu, Y.; Li, R. Programmed editing of rice (Oryza sativa L.) OsSPL16 gene using CRISPR/Cas9 improves grain yield by modulating the expression of pyruvate enzymes and cell cycle proteins. Int. J. Mol. Sci. 2021, 22, 249. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liao, H.; Chern, M.; Yin, J.; Chen, Y.; Wang, J.; Zhu, X.; Chen, Z.; Yuan, C.; Zhao, W.; et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 3174–3179. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; Ji, C.; Atienza-Grande, G.; Huguet-Tapia, J.C.; Perez-Quintero, A.; Li, T.; Eom, J.S.; Li, C.; Nguyen, H.; Liu, B.; et al. Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat. Biotechnol. 2019, 37, 1344–1350. [Google Scholar] [CrossRef]
- Jia, H.; Zhang, Y.; Orbović, V.; Xu, J.; White, F.F.; Jones, J.B.; Wang, N. Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol. J. 2017, 15, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Gomez, M.A.; Lin, Z.D.; Moll, T.; Chauhan, R.D.; Hayden, L.; Renninger, K.; Beyene, G.; Taylor, N.J.; Carrington, J.C.; Staskawicz, B.J.; et al. Simultaneous CRISPR/Cas9-mediated editing of cassava eIF4E isoforms nCBP-1 and nCBP-2 reduces cassava brown streak disease symptom severity and incidence. Plant Biotechnol. J. 2019, 17, 421–434. [Google Scholar] [CrossRef]
- Chandrasekaran, J.; Brumin, M.; Wolf, D.; Leibman, D.; Klap, C.; Pearlsman, M.; Sherman, A.; Arazi, T.; Gal-On, A. Development of broad virus resistance in non-transgenic cucumber using CRISPR/Cas9 technology. Mol. Plant Pathol. 2016, 17, 1140–1153. [Google Scholar] [CrossRef]
- Wang, X.; Tu, M.; Wang, D.; Liu, J.; Li, Y.; Li, Z.; Wang, Y.; Wang, X. CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol. J. 2018, 16, 844–855. [Google Scholar] [CrossRef]
- Santillán Martínez, M.I.; Bracuto, V.; Koseoglou, E.; Appiano, M.; Jacobsen, E.; Visser, R.G.F.; Wolters, A.A.; Bai, Y. CRISPR/Cas9-targeted mutagenesis of the tomato susceptibility gene PMR4 for resistance against powdery mildew. BMC Plant Biol. 2020, 19, 284. [Google Scholar] [CrossRef]
- Ortigosa, A.; Gimenez-Ibanez, S.; Leonhardt, N.; Solano, R. Design of a bacterial speck resistant tomato by CRISPR/Cas9-mediated editing of SlJAZ2. Plant Biotechnol. J. 2019, 17, 665–673. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, Y.; Wu, G.; Zou, S.; Chen, Y.; Gao, C.; Tang, D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J. 2017, 91, 714–724. [Google Scholar] [CrossRef]
- Khang, B. The regulatory status of genome-edited crops. Plant Biotechnol. J. 2016, 14, 510–518. [Google Scholar]
- Cox, D.B.T.; Gootenberg, J.S.; Abudayyeh, O.O.; Franklin, B.; Kellner, M.J.; Joung, J.; Zhang, F. RNA editing with CRISPR-Cas13. Science 2017, 358, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Ali, Z.; Butt, H.; Mahas, A.; Aljedaani, F.; Khan, M.Z.; Ding, S.; Mahfouz, M. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol. 2018, 19, 1. [Google Scholar] [CrossRef] [PubMed]

| Crop | Gene Introduced | Doner Species | Target Insect | Released Event | ||
|---|---|---|---|---|---|---|
| Lepidopteran | Coleopteran | Hemipteran | ||||
| Cotton | Cry | B. thuringiensis | 23 (1) | 1 | 25 | |
| vip3 | 1 | |||||
| CpTI | V. unguiculata | (1) | ||||
| Cowpea | Cry | B. thuringiensis | 1 | 1 | ||
| Eggplant | Cry | B. thuringiensis | 1 | 1 | ||
| Maize | Cry | B. thuringiensis | 15 (2) | 8 (3) | 25 | |
| vip3 | B. thuringiensis | 2 (1) | ||||
| RNAi of Snf7 | D. virgifera | (1) | ||||
| Potato | Cry | B. thuringiensis | 30 | 30 | ||
| Rice | Cry | B. thuringiensis | 3 (2) | 3 | ||
| Soybean | Cry | B. thuringiensis | 4 (2) | 4 | ||
| Sugarcane | Cry | B. thuringiensis | 3 | 3 | ||
| Tomato | Cry | B. thuringiensis | 1 | 1 | ||
| Total | 54 | 38 | 1 | 93 | ||
| Crop | Gene Introduced | Target Virus | Released Event |
|---|---|---|---|
| Bean | Replicase gene bgmv_ac1 | Bean golden mosaic virus | 1 |
| Papaya | Coat protein gene prsv_cp | Papaya ringspot virus | 3 |
| Replicase gene prsv_rep | 1 | ||
| Plum | Coat protein gene ppv_cp | Plum pox virus | 1 |
| Potato | Coat protein gene pvy_cp | Potato virus Y | 8 |
| Replicase gene plrv_orf1 | Potato leaf roll virus | 7 | |
| Helicase gene plrv_orf2 | (7) | ||
| Squash | Coat protein gene zymv_cp | Zucchini yellow mosaic virus | 2 |
| Coat protein gene cmv_cp | Cucumber mosaic virus | [1] | |
| Coat protein gene wmv_cp | Watermelon mosaic virus 2 | [2] | |
| Sweet pepper | Coat protein gene cmv_cp | Cucumber mosaic virus | 1 |
| Tomato | Coat protein gene cmv_cp | Cucumber mosaic virus | 1 |
| Total | 25 | ||
| Crop | Gene Modified | Resistance Improved | Reference |
|---|---|---|---|
| Citrus | CsLOB1 | Citrus canker | [236] |
| Cassava | nCBP-1, nCBP-2 | Cassava brown streak disease | [237] |
| Cucumber | eIF4E | Cucumber vein yellowing virus | [238] |
| Grape | VvWRKY52 | Botrytis cinerea | [239] |
| Rice | Bsr-k1 | Broad spectrum resistance | [234] |
| OsSWEET11, 13, 14 | Bacterial blight | [235] | |
| Tomato | Pmr4 | Powdery mildew | [240] |
| Jaz2 | Bacterial speck disease | [241] | |
| Wheat | TaMlo-A1, -B1, -D1 | Powdery mildew | [220] |
| TaEdr1 (three homologs) | [242] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Wang, Y.; Fu, F.; Li, W. Transgenic Improvement for Biotic Resistance of Crops. Int. J. Mol. Sci. 2022, 23, 14370. https://doi.org/10.3390/ijms232214370
Yu H, Wang Y, Fu F, Li W. Transgenic Improvement for Biotic Resistance of Crops. International Journal of Molecular Sciences. 2022; 23(22):14370. https://doi.org/10.3390/ijms232214370
Chicago/Turabian StyleYu, Haoqiang, Yingge Wang, Fengling Fu, and Wanchen Li. 2022. "Transgenic Improvement for Biotic Resistance of Crops" International Journal of Molecular Sciences 23, no. 22: 14370. https://doi.org/10.3390/ijms232214370
APA StyleYu, H., Wang, Y., Fu, F., & Li, W. (2022). Transgenic Improvement for Biotic Resistance of Crops. International Journal of Molecular Sciences, 23(22), 14370. https://doi.org/10.3390/ijms232214370






