Reliability of Rodent and Rabbit Models in Preeclampsia Research
Abstract
1. Introduction
2. A Comparison of the Physiology of Pregnancy in Humans and Small Mammals
2.1. Implantation
2.2. Decidualization
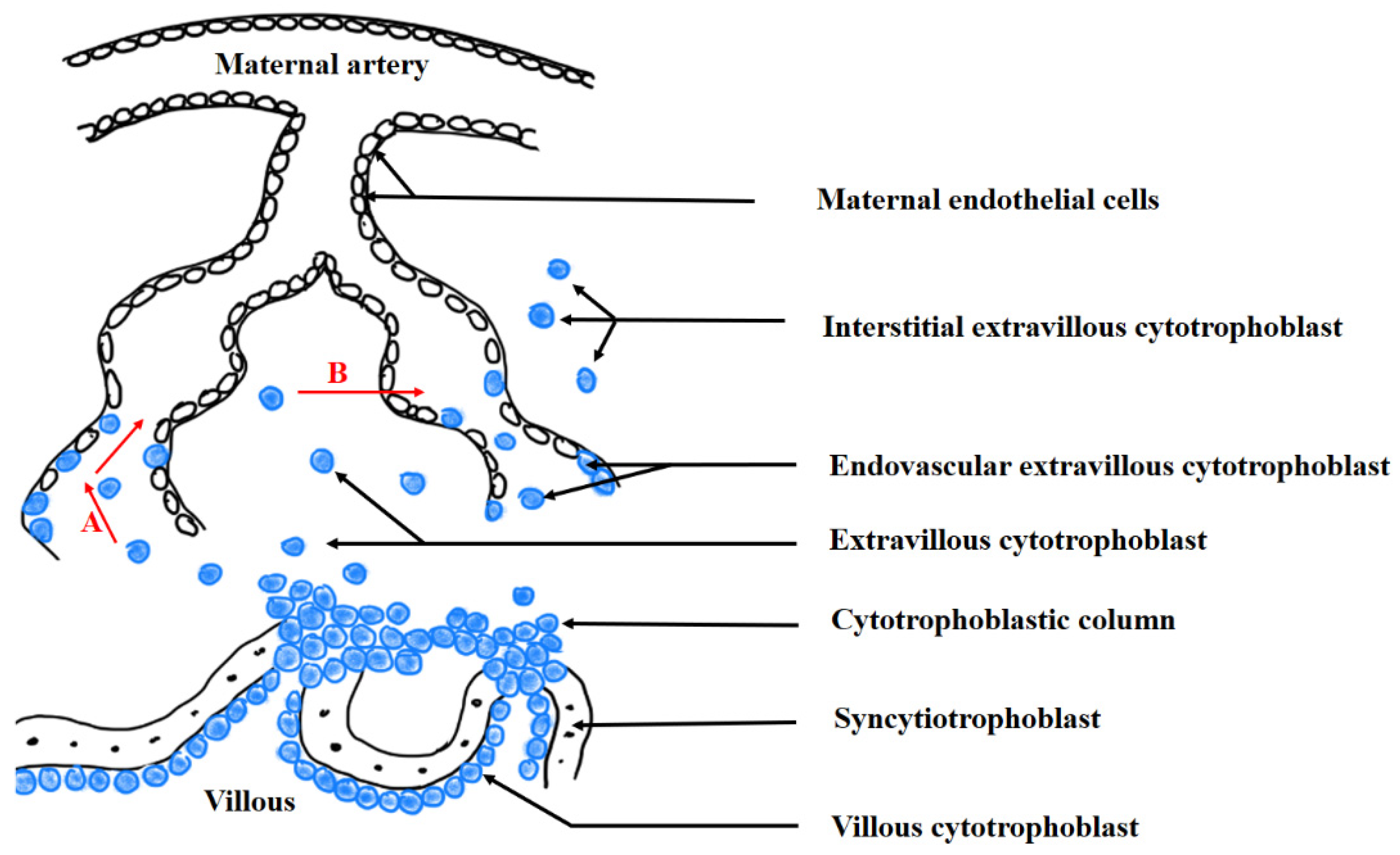
2.3. Trophoblast Invasion
2.4. Maternal–Foetal Blood Barrier
3. Pathomechanism of Preeclampsia
4. Animal Models of Preeclampsia
4.1. An Incorrect Trophoblast Invasion Model of Preeclampsia
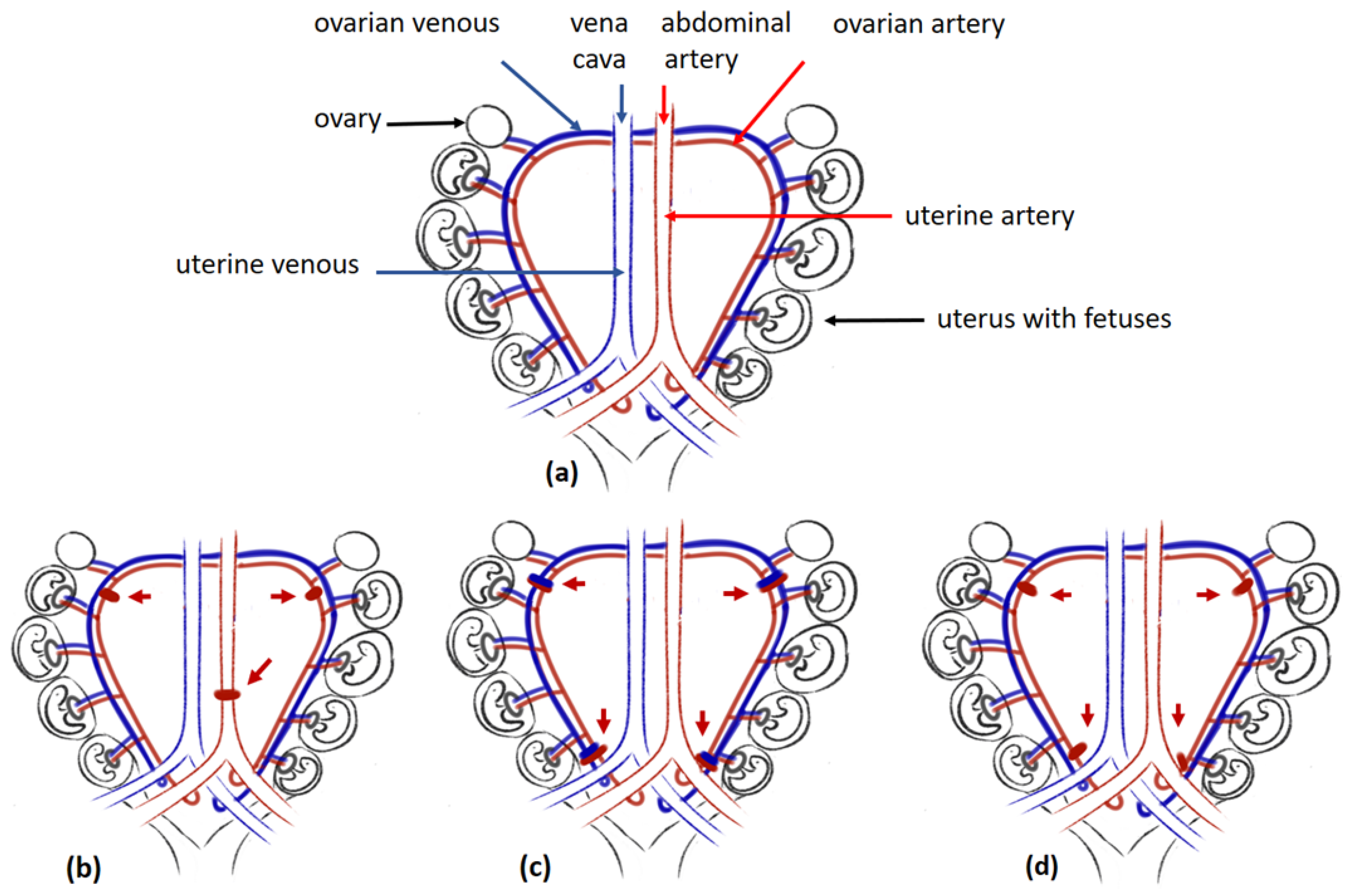
4.2. The Placental Ischaemia in Animal Models for Preeclampsia
4.3. Models of Preeclampsia Employing the Incorrect Angiogenesis
4.4. Inflammatory Factors Exert Preeclampsia in Animal Models
4.5. Endothelial Dysfunction: The Last Step in the Generation of Preeclampsia
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ACOG Practice Bulletin No. 222 Clinical Management Guidelines for Obstetrician—Gynecologists Gestational Hypertension and Preeclampsia. Obstet. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2018, 13, 291–310. [Google Scholar] [CrossRef]
- Socha, M.W.; Malinowski, B.; Puk, O.; Wart, M.; Kazdepka-ziemi, A. The Role of NF- κ B in Uterine Spiral Arteries Remodeling, Insight into the Cornerstone of Preeclampsia. Int. J. Mol. Sci. 2021, 22, 704. [Google Scholar] [CrossRef] [PubMed]
- Opichka, M.A.; Rappelt, M.W.; Gutterman, D.D.; Grobe, J.L.; McIntosh, J.J. Review vascular dysfunction in preeclampsia. Cells 2021, 10, 3055. [Google Scholar] [CrossRef]
- Sankaralingam, S.; Xu, H.; Davidge, S.T. Arginase contributes to endothelial cell oxidative stress in response to plasma from women with preeclampsia. Cardiovasc. Res. 2010, 85, 194–203. [Google Scholar] [CrossRef]
- Guerby, P.; Tasta, O.; Swiader, A.; Pont, F.; Bujold, E.; Parant, O.; Vayssiere, C.; Salvayre, R.; Negre-Salvayre, A. Role of oxidative stress in the dysfunction of the placental endothelial nitric oxide synthase in preeclampsia. Redox Biol. 2021, 40, 101861. [Google Scholar] [CrossRef] [PubMed]
- Fiore, G.; Florio, P.; Micheli, L.; Nencini, C.; Rossi, M.; Cerretani, D.; Ambrosini, G.; Giorgi, G.; Petraglia, F. Endothelin-1 triggers placental oxidative stress pathways: Putative role in preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 4205–4210. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aghajanova, L.; Hamilton, A.E.; Giudice, L.C. Uterine Receptivity to Human Embryonic Implantation: Histology, Biomarkers, and Transcriptomics. Mol. Cell Biochem. 2008, 19, 204–211. [Google Scholar] [CrossRef]
- DeSesso, J.M.; Williams, A.L.; Ahuja, A.; Bowman, C.J.; Hurtt, M.E. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit. Rev. Toxicol. 2012, 42, 185–210. [Google Scholar] [CrossRef]
- Ren-Wei, S.; Fazleabas, A.T. Implantation and Establishment of Pregnancy in Human and Nonhuman Primates. Adv. Anat. Embryol. Cell Biol. 2015, 216, 189–213. [Google Scholar] [CrossRef]
- Abrahamsohn, P.A.; Zorn, T.M.T. Implantation and decidualization in rodents. J. Exp. Zool. 1993, 266, 603–628. [Google Scholar] [CrossRef] [PubMed]
- Thapar, M.; Kumari, G.L.; Shrivastav, T.G.; Pandey, P.K. Hormonal control of implantation in guinea pigs. Steroids 1988, 52, 85–108. [Google Scholar] [CrossRef]
- Kelleher, A.M.; Burns, G.W.; Behura, S.; Wu, G.; Spencer, T.E. Uterine glands impact uterine receptivity, luminal fluid homeostasis and blastocyst implantation. Sci. Rep. 2016, 6, 38078. [Google Scholar] [CrossRef]
- Hefez, E.S.E.; Tsutsumi, Y. Changes in Endometrial Vascularity during Implantation and Pregnancy in the Rabbit. Am. J. Anat. 1966, 18, 249–282. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M. Timing of implantation in New Zealand White rabbits. Congenit. Anom. 2001, 41, 198–203. [Google Scholar] [CrossRef]
- Emera, D.; Romero, R.; Wagner, G. The evolution of menstruation: A new model for genetic assimilation: Explaining molecular origins of maternal responses to fetal invasiveness. BioEssays 2012, 34, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.J.; Chakraborty, D.; Rumi, M.A.K.; Konno, T.; Renaud, S.J. Rat placentation: An experimental model for investigating the hemochorial maternal-fetal interface. Placenta 2012, 33, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Knöfler, M.; Pollheimer, J. Human placental trophoblast invasion and differentiation: A particular focus on Wnt signaling. Front. Genet. 2013, 4, 190. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J. A Review of Mechanisms of Implantation. Dev. Reprod. 2017, 21, 351–359. [Google Scholar] [CrossRef]
- Silva, J.F.; Serakides, R. Intrauterine trophoblast migration: A comparative view of humans and rodents. Cell Adhes. Migr. 2016, 10, 88–110. [Google Scholar] [CrossRef]
- Damsky, C.H.; Fitzgerald, M.L.; Fisher, S.J. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J. Clin. Investig. 1992, 89, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Vićovac, L.; Aplin, J.D. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat. 1996, 156, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.H.; Zhou, Y.; Janatpour, M.; McMaster, M.; Bass, K.; Chun, S.H.; Fisher, S.J. Human cytotrophoblast differentiation/invasion is abnormal in pre-eclampsia. Am. J. Pathol. 1997, 151, 1809–1818. [Google Scholar] [PubMed]
- Burton, G.J.; Jauniaux, E. The cytotrophoblastic shell and complications of pregnancy. Placenta 2017, 60, 134–139. [Google Scholar] [CrossRef]
- Moser, G.; Windsperger, K.; Pollheimer, J.; de Sousa Lopes, S.C.; Huppertz, B. Human trophoblast invasion: New and unexpected routes and functions. Histochem. Cell Biol. 2018, 150, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Trophoblast Invasion: Remodelling of Spiral Arteries and Beyond. In Preeclampsia Basic, Genomic, and Clinical; Saito, S., Ed.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 47–62. ISBN 9789811058905. [Google Scholar]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The Uterine Spiral Arteries in Human Pregnancy: Facts and Controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Phil. Trans. R. Soc. B 2015, 370, 20140070. [Google Scholar] [CrossRef]
- Woods, L.; Perez-Garcia, V.; Hemberger, M. Regulation of Placental Development and Its Impact on Fetal Growth—New Insights From Mouse Models. Front. Endocrinol. 2018, 9, 570. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Robertson, W.B.; Brosens, I.; Dixon, G. Review article: Trophoblast invasion and the establishment of haemochorial placentation in man and laboratory animals. Placenta 1981, 2, 71–91. [Google Scholar] [CrossRef]
- Jollie, W.P. Fine structural changes in placental labyrinth of the rat with increasing gestational age. J. Ultrasruct. Res. 1964, 10, 27–47. [Google Scholar] [CrossRef]
- Furukawa, S.; Kuroda, Y.; Sugiyama, A. A comparison of the histological structure of the placenta in experimental animals. J. Toxicol. Pathol. 2014, 27, 11–18. [Google Scholar] [CrossRef]
- Fan, X.; Muruganandan, S.; Shallie, P.D.; Dhal, S.; Petitt, M.; Nayak, N.R. VEGF Maintains Maternal Vascular Space Homeostasis in the Mouse Placenta through Modulation of Trophoblast Giant Cell Functions. Biomolecules 2021, 11, 1062. [Google Scholar] [CrossRef]
- Furukawa, S.; Hayashi, S.; Usuda, K.; Abe, M.; Hagio, S.; Ogawa, I. Toxicological pathology in the rat placenta. J. Toxicol. Pathol. 2011, 24, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Pijnenborg, R.; Vercruysse, L. Animal models of deep trophoblast invasion. In Placental Bed Disorders: Basic Science and Its Translation to Obstetrics; Cambridge University Press: Cambridge, UK, 2010; pp. 127–139. ISBN 9780511750847. [Google Scholar]
- Sutherland, A.E.; Calarco, P.G.; Damsky, C.H. Developmental regulation of integrin expression at the time of implantation in the mouse embryo. Development 1993, 119, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Leonard, S.; Lima, P.D.A.; Croy, B.A.; Murrant, C.L. Gestational modyfication of murine spiral arteries does not reduce their drug-induced vasoconstrictive responses in vivo. Biol. Reprod. 2013, 89, 139. [Google Scholar] [CrossRef] [PubMed]
- Ain, R.; Canham, L.N.; Soares, M.J. Gestation stage-dependent intrauterine trophoblast cell invasion in the rat and mouse: Novel endocrine phenotype and regulation. Dev. Biol. 2003, 260, 176–190. [Google Scholar] [CrossRef]
- Adamson, S.L.; Lu, Y.; Whiteley, K.J.; Holmyard, D.; Hemberger, M.; Pfarrer, C.; Cross, J.C. Interactions between Trophoblast Cells and the Maternal and Fetal Circulation in the Mouse Placenta. Dev. Biol. 2002, 250, 358–373. [Google Scholar] [CrossRef]
- Fischer, B.; Chavatte-Palmer, P.; Viebahn, C.; Santos, A.N.; Duranthon, V. Rabbit as a reproductive model for human health. Reproduction 2012, 144, 1–10. [Google Scholar] [CrossRef]
- Hayes, E.K.; Tessier, D.R.; Percival, M.E.; Holloway, A.C.; Petrik, J.J.; Gruslin, A.; Raha, S. Trophoblast invasion and blood vessel remodeling are altered in a rat model of lifelong maternal obesity. Reprod. Sci. 2014, 21, 648–657. [Google Scholar] [CrossRef]
- Verkeste, C.M.; Slangen, B.F.M.; Daemen, M.; van Straaten, H.; Kohnen, G.; Kaufmann, P.; Peeters, L.L.H. The extent of trophoblast invasion in the preplacental vasculature of the guinea-pig. Placenta 1998, 19, 49–54. [Google Scholar] [CrossRef]
- Rosario, G.X.; Konno, T.; Soares, M.J. Maternal hypoxia activates endovascular trophoblast cell invasion. Dev. Biol. 2008, 314, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Caluwaerts, S.; Vercruysse, L.; Luyten, C.; Pijnenborg, R. Endovascular trophoblast invasion and associated structural changes in uterine spiral arteries of the pregnant rat. Placenta 2005, 26, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Caluwaerts, S.; Luyten, C.; Pijnenborg, R. Interstitial trophoblast invasion in the decidua and mesometrial triangle during the last third of pregnancy in the rat. Placenta 2006, 27, 22–33. [Google Scholar] [CrossRef]
- Sansom, G.S. The Giant Cells in the Placenta of the Rabbit. Proc. R. Soc. B Biol. Sci. 1927, 110, 354–377. [Google Scholar] [CrossRef]
- Blackburn, D.G.; Osteen, K.G.; Winfrey, V.P.; Hoffman, L.H. Obplacental giant cells of the domestic rabbit: Development, morphology, and intermediate filament composition. J. Morphol. 1989, 202, 185–203. [Google Scholar] [CrossRef] [PubMed]
- Burek, J.D.; Goldberg, B.; Hutchins, G.; Strandberg, J.D. The pregnant Syrian hamster as a model to study intravascular trophoblasts and associated maternal blood vessel changes. Vet. Pathol. 1979, 16, 553–566. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Robertson, W.B.; Brosens, I. The arterial migration of trophoblast in the uterus of the golden hamster, Mesocricetus auratus. J. Reprod. Fertil. 1974, 40, 269–280. [Google Scholar] [CrossRef]
- Stafford, E.S. The origin of the blood of the “Placental sign”. Anat. Rec. 1930, 47, 43–57. [Google Scholar] [CrossRef]
- Krichesky, B. Vascular changes in the rabbit uterus and in intraocular endometrial transplants during pregnancy. Anat. Rec. 1943, 87, 221–234. [Google Scholar] [CrossRef]
- Polisca, A.; Scotti, L.; Orlandi, R.; Brecchia, G.; Boiti, C. Doppler evaluation of maternal and fetal vessels during normal gestation in rabbits. Theriogenology 2010, 73, 358–366. [Google Scholar] [CrossRef]
- Tam, W.H.; Burgess, S.M. The developmental changes in the placenta of the guinea-pig. J. Anat. 1977, 123, 601–614. [Google Scholar]
- Ander, S.E.; Diamond, M.S.; Coyne, C.B. Immune responses at the maternal-fetal interface. Sci. Immunol. 2019, 4, eaat6114. [Google Scholar] [CrossRef]
- Elmore, S.A.; Cochran, R.Z.; Bolon, B.; Lubeck, B.; Mahler, B.; Sabio, D.; Ward, J.M. Histology Atlas of the Developing Mouse Placenta. Toxicol. Pathol. 2022, 50, 60–117. [Google Scholar] [CrossRef] [PubMed]
- Ogura, A.; Nishida, T.; Hayashi, Y.; Mochida, K. The development of the Chondrocranium in the golden hamster, Mesocricetus auratus. J. Anat. 1991, 175, 65–77. [Google Scholar] [PubMed]
- Hoffman, L.H.; Winfrey, V.P.; Hoos, P.C. Sites of endometrial vascular leakage during implantation in the rabbit. Anat. Rec. 1990, 227, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Rennie, M.Y.; Whiteley, K.J.; Adamson, S.L.; Sled, J.G. Quantification of gestational changes in the uteroplacental vascular tree reveals vessel specific hemodynamic roles during pregnancy in mice. Biol. Reprod. 2016, 95, 43. [Google Scholar] [CrossRef] [PubMed]
- Nanaev, A.; Chwalisz, K.; Frank, H.-G.; Kohnen, G.; Hegele-Hartung, C.; Kaufmann, P. Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 1995, 282, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Wakeland, A.K.; Parast, M.M. Trophoblast lineage specification, differentiation and their regulation by oxygen tension. J. Endocrinol. 2018, 236, R43–R56. [Google Scholar] [CrossRef]
- Tuuli, M.G.; Longtine, M.S.; Nelson, D.M. Review: Oxygen and trophoblast biology—A source of controversy. Placenta 2011, 32, S109–S118. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Oxygen and development of the human placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B.; Weiss, G.; Moser, G. Trophoblast invasion and oxygenation of the placenta: Measurements versus presumptions. J. Reprod. Immunol. 2014, 101–102, 74–79. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.S.; Norwitz, E.R.; Panyavatthanasinh, S.; Kim, S.M.; Lee, J.; Park, C.-W.; Kim, B.J.; Jun, J.K. Levels of Adipokines in Amniotic Fluid and Cord Blood Collected from Dichorionic-Diamniotic Twins Discordant for Fetal Growth. PLoS ONE 2016, 11, e0154537. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhou, Y.; Wei, H. Roles of HLA-G in the Maternal-Fetal Immune Microenvironment. Front. Immunol. 2020, 11, 592010. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhou, Y.; Ni, X.; Tong, X.; Xu, X.; Dong, Z.; Sun, R.; Tian, Z.; Wei, H. Natural Killer Cells Promote Fetal Development through the Secretion of Growth-Promoting Factors. Immunity 2017, 47, 1100–1113.e6. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S. HLA-G-mediated NK cell senescence promotes vascular remodeling: Implications for reproduction. Cell. Mol. Immunol. 2014, 11, 460–466. [Google Scholar] [CrossRef]
- Leiser, R.; Beier, H.M. Morphological studies of lacunar formation in the early rabbit placenta. Trophobl. Res. 1988, 3, 97–110. [Google Scholar]
- Kaufmann, P.; Davidoff, M. The Guinea-Pig Placenta; Springer: Berlin/Heidelberg, Germany, 1977; ISBN 9783642666186. [Google Scholar]
- Han, L.W.; Gao, C.; Mao, Q. An update on expression and function of p-gp/abcb1 and bcrp/abcg2 in the placenta and fetus. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 817–829. [Google Scholar] [CrossRef]
- Silva Carmona, A.; Mendieta Zerón, H. NF-κB and SOD expression in preeclamptic placentas. Turk. J. Med. Sci. 2016, 46, 783–788. [Google Scholar] [CrossRef]
- Podjarny, E.; Losonczy, G.; Baylis, C. Animal Models of Preeclampsia. Semin. Nephrol. 2004, 24, 596–606. [Google Scholar] [CrossRef][Green Version]
- Guzin, K.; Tomruk, S.; Tuncay, A.Y.; Naki, M.; Sezginsoy, S.; Zemheri, E.; Yucel, N.; Kanadikirik, F. The relation of increased uterine artery blood flow resistance and impaired trophoblast invasion in pre-eclamptic pregnancies. Arch. Gynecol. Obstet. 2005, 272, 283–288. [Google Scholar] [CrossRef]
- Roberts, J.M.; Escudero, C. The placenta in preeclampsia. Pregnancy Hypertens. 2012, 2, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wang, S.; Li, D. The integrative roles of chemokines at the maternal—Fetal interface in early pregnancy. Cell. Mol. Immunol. 2014, 11, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Deb, K.; Chaturvedi, M.M.; Jaiswal, Y.K. A “minimum dose” of lipopolisaccharide required for implantation failure: Assessment of its effect on the maternal reproductive organs and interleukin-1a expression in the mouse. Reproduction 2004, 128, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Damsky, C.H.; Fisher, S.J. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J. Clin. Investig. 1997, 99, 2152–2164. [Google Scholar] [CrossRef]
- Zhou, Y.; Damsky, C.H.; Chiu, K.; Roberts, J.M.; Fisher, S.J. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J. Clin. Investig. 1993, 91, 950–960. [Google Scholar] [CrossRef]
- Zanoli, L.; Briet, M.; Empana, P.J.; Cunha, G.P.; Mäki-Petäjä, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.; Spronck, B.; et al. Vascular consequences of inflammation: A position statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682–1698. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, C.; Wang, P.; Gao, J.; Liu, X.; Li, Y.; Yan, S.; Shi, Y. HIF-1 α affects trophoblastic apoptosis involved in the onset of preeclampsia by regulating FOXO3a under hypoxic conditions. Mol. Med. Rep. 2020, 21, 2484–2492. [Google Scholar] [CrossRef]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of anti-angiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Sezer, S.D.; Kucuk, M.; Doger, F.K.; Yuksel, H.; Odabasi, A.R.; Turkmen, M.K.; Cakmak, B.C.; Omurlu, I.K.; Kinas, M.G. VEGF, PIGF and HIF-1 a in placentas of early- and late-onset pre-eclamptic patients. Gynecol. Endocrinol. 2013, 29, 797–800. [Google Scholar] [CrossRef]
- Bates, D.O. An unexpected tail of VEGF and PlGF in pre-eclampsia. Biochem. Soc. Trans. 2011, 39, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Aydin, S.; Uludag, S.; Vildan, O.; Tolun, N. Maternal plasma levels of cytokines in normal and preeclamptic pregnancies and their relationship with diastolic blood pressure and fibronectin levels. Acta Obstet. Gynecol. Scand. 2003, 82, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Syngelaki, A.; Sarquis, R.; Zvanca, M.; Nicolaides, K.H. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat. Diagn. 2011, 31, 66–74. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, J.; Kwak, S.-N.; Lee, K.-S.; Lee, D.-K.; Ha, K.-S.; Won, M.-H.; Jeoung, D.; Lee, H.; Kwon, Y.-G.; et al. Functional role of NF-kB in expression of human endothelial nitric oxide synthase. Biochem. Biophys. Res. Commun. 2014, 448, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- LaMarca, B. Endothelial dysfunction; an important mediator in the Pathophysiology of Hypertension during Preeclampsia. Minerva Ginecol. 2012, 64, 309–320. [Google Scholar]
- Sánchez-Aranguren, L.C.; Prada, C.E.; Riaño-medina, C.E.; Lopez, M. Endothelial dysfunction and preeclampsia: Role of oxidative stress. Front. Physiol. 2014, 5, 372. [Google Scholar] [CrossRef]
- Kumasawa, K. Animal Models in Preeclampsia. In Preeclampsia Basic, Genomic, and Clinical; Saito, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 141–155. ISBN 9789811058912. [Google Scholar]
- Bakrania, B.A.; George, E.M.; Granger, J.P. Animal models of preeclampsia: Investigating pathophysiology and therapeutic targets. Am. J. Obstet. Gynecol. 2022, 226, S973–S987. [Google Scholar] [CrossRef]
- Bogdan, S.; Luca, V.; Ober, C.; Melega, I.; Pestean, C.; Codea, R.; Oana, L. Comparison among different methods for blood pressure monitoring in rats: Literature review. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2019, 76, 5. [Google Scholar] [CrossRef]
- Chau, K.; Welsh, M.; Makris, A.; Hennessy, A. Progress in preeclampsia: The contribution of animal models. J. Hum. Hypertens. 2022, 36, 705–710. [Google Scholar] [CrossRef]
- Gong, P.; Liu, M.; Hong, G.; Li, Y.; Xue, P.; Zheng, M.; Wu, M.; Shen, L.; Yang, M.; Diao, Z.; et al. Curcumin improves LPS-induced preeclampsia-like phenotype in rat by inhibiting the TLR4 signaling pathway. Placenta 2016, 41, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zheng, M.; Gong, P.; Lin, C.; Zhou, J.; Li, Y.; Shen, L.; Diao, Z.; Yan, G.; Sun, H.; et al. Single administration of ultra-low-dose lipopolysaccharide in rat early pregnancy induces TLR4 activation in the placenta contributing to preeclampsia. PLoS ONE 2015, 10, e0124001. [Google Scholar] [CrossRef] [PubMed]
- Geusens, N.; Verlohren, S.; Luyten, C.; Taube, M.; Hering, L.; Vercruysse, L.; Hanssens, M.; Dudenhausen, J.W.; Dechend, R.; Pijnenborg, R. Endovascular Trophoblast Invasion, Spiral Artery Remodelling and Uteroplacental Haemodynamics in a Transgenic Rat Model of Pre-eclampsia. Placenta 2008, 29, 614–623. [Google Scholar] [CrossRef]
- Dechend, R.; Gratze, P.; Wallukat, G.; Shagdarsuren, E.; Plehm, R.; Bräsen, J.H.; Fiebeler, A.; Schneider, W.; Caluwaerts, S.; Vercruysse, L.; et al. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension 2005, 45, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, G.; Pussetto, M.; Rose, M.; Staff, A.C.; Blois, S.M.; Toblli, J.E. Defective trophoblast invasion underlies fetal growth restriction and preeclampsia-like symptoms in the stroke-prone spontaneously hypertensive rat. Mol. Hum. Reprod. 2017, 23, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ma, L.; Lin, L.; Wang, Y.L.; Yang, H. The intervention effect of aspirin on a lipopolysaccharide-induced preeclampsia-like mouse model by inhibiting the nuclear factor-κB pathway. Biol. Reprod. 2018, 99, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Sones, J.L.; Yarborough, C.C.; Besso, V.O.; Lemenze, A.; Douglas, N.C. Genotypic analysis of the female BPH/5 mouse, a model of superimposed preeclampsia. PLoS ONE 2021, 16, e0253453. [Google Scholar] [CrossRef]
- Thompson, L.P.; Pence, L.; Pinkas, G.; Song, H.; Telugu, B.P. Placental Hypoxia During Early Pregnancy Causes Maternal Hypertension and Placental Insufficiency in the Hypoxic Guinea Pig Model. Biol. Reprod. 2016, 95, 128. [Google Scholar] [CrossRef]
- Gilbert, J.S.; Babcock, S.A.; Granger, J.P. Hypertension Produced by Reduced Uterine Perfusion in Fms-Like Tyrosine Kinase-1 Expression. Pregnancy Hypertens. 2007, 50, 1142–1147. [Google Scholar] [CrossRef]
- Alexander, B.T.; Kassab, S.E.; Miller, M.T.; Abram, S.R.; Reckelhoff, J.F.; Bennett, W.A.; Granger, J.P. Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 2001, 37, 1191–1195. [Google Scholar] [CrossRef]
- Morton, J.S.; Levasseur, J.; Ganguly, E.; Quon, A.; Kirschenman, R.; Dyck, J.R.B. Characterisation of the Selective Reduced Uteroplacental Perfusion (sRUPP) Model of Preeclampsia. Sci. Rep. 2019, 9, 9565. [Google Scholar] [CrossRef] [PubMed]
- Fushima, T.; Sekimoto, A.; Minato, T.; Ito, T.; Oe, Y.; Kisu, K.; Sato, E.; Funamoto, K.; Hayase, T.; Kimura, Y.; et al. Reduced uterine perfusion pressure (RUPP) model of preeclampsia in Mice. PLoS ONE 2016, 11, e0155426. [Google Scholar] [CrossRef]
- Losonczy, G.; Brown, G.; Venuto, R.C. Reduced Uterine Perfusion Pressure Hypertension in Pregnant Rabbits. Am. J. Med. Sci. 1992, 303, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.; Yuan, H.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Carlstrom, M.; Wentzel, P.; Skøtt, O.; Persson, A.E.G.; Carlstro, M.; Eriksson, U.J. Angiogenesis inhibition causes hypertension and placental dysfunction in a rat model of preeclampsia. J. Hypertens. 2009, 27, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Tal, R.; Shaish, A.; Barshack, I.; Polak-Charcon, S.; Afek, A.; Volkov, A.; Feldman, B.; Avivi, C.; Harats, D. Effects of hypoxia-inducible factor-1α overexpression in pregnant mice: Possible implications for preeclampsia and intrauterine growth restriction. Am. J. Pathol. 2010, 177, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Yao, J.; He, Q.; Liu, M.; Duan, T.; Wang, K. Exosomes from Women with Preeclampsia Induced Vascular Dysfunction by Delivering sFlt (Soluble Fms-Like Tyrosine Kinase)-1 and sEng (Soluble Endoglin) to Endothelial Cells. Hypertension 2018, 72, 1381–1390. [Google Scholar] [CrossRef]
- Bergmann, A.; Ahmad, S.; Cudmore, M.; Gruber, A.D.; Wittschen, P.; Gröne, H.; Ahmed, A.; Weich, H.A. Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J. Cell. Mol. Med. 2010, 14, 1857–1867. [Google Scholar] [CrossRef]
- Lu, F.; Longo, M.; Tamayo, E.; Maner, W.; Al-hendy, A.; Anderson, G.D.; Hankins, G.D.V.; Saade, G.R. The effect of over-expression of sFlt-1 on blood pressure and the occurrence of other manifestations of preeclampsia in unrestrained conscious pregnant mice. Am. J. Obstet. Gynecol. 2007, 196, 396.e1–396.e7. [Google Scholar] [CrossRef]
- Kumasawa, K.; Ikawa, M.; Kidoya, H.; Hasuwa, H.; Saito-Fujita, T.; Morioka, Y.; Takakura, N.; Kimura, T.; Okabe, M. Pravastatin induces placental growth factor (PGF) and ameliorates preeclampsia in a mouse model. Proc. Natl. Acad. Sci. USA 2011, 108, 1451–1455. [Google Scholar] [CrossRef] [PubMed]
- Warrington, J.P.; Drummond, H.A.; Granger, J.P.; Ryan, M.J. Placental ischemia-induced increases in brain water content and cerebrovascular permeability: Role of TNF-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1425–R1431. [Google Scholar] [CrossRef] [PubMed]
- George, E.M.; Stout, J.M.; Stec, D.E.; Granger, J.P. Heme oxygenase induction attenuates TNF-α-induced hypertension in pregnant rodents. Front. Pharmacol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- LaMarca, B.; Speed, J.; Ray, L.F.; Cockrell, K.; Wallukat, G.; Dechend, R.; Granger, J. Hypertension in response to IL-6 during pregnancy: Role of AT1-receptor activation. Int. J. Interf. Cytokine Mediat. Res. 2011, 3, 65–70. [Google Scholar] [CrossRef]
- Irani, R.A.; Zhang, Y.; Zhou, C.C.; Blackwell, S.C.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Autoantibody-mediated angiotensin receptor activation contributes to preeclampsia through tumor necrosis factor-α signaling. Hypertension 2010, 55, 1246–1253. [Google Scholar] [CrossRef]
- Bobek, G.; Surmon, L.; Mirabito, K.M.; Makris, A.; Hennessy, A. Placental Regulation of Inflammation and Hypoxia after TNF- a Infusion in Mice. Am. J. Reprod. Immunol. 2015, 74, 407–418. [Google Scholar] [CrossRef]
- Chatterjee, P.; Chiasson, V.L.; Kopriva, S.E.; Young, K.J.; Chatterjee, V.; Jones, K.A.; Mitchell, B.M. Interleukin 10 deficiency exacerbates toll-like receptor 3-induced preeclampsia-like symptoms in mice. Hypertension 2011, 58, 489–496. [Google Scholar] [CrossRef]
- Heyward, C.; Sones, J.L.; Lob, H.; Yuen, L.; Abbott, K.; Huang, W.; Begun, Z.; Butler, S.; August, A.; Davisson, R. The decidua of preeclamptic-like BPH/5 mi. J. Reprod. Immunol. 2017, 120, 27–33. [Google Scholar] [CrossRef]
- Zuo, J.; Jiang, Z. Melatonin attenuates hypertension and oxidative stress in a rat model of L-NAME-induced gestational hypertension. Vasc. Med. 2020, 25, 295–301. [Google Scholar] [CrossRef]
- Shu, W.E.N.; Li, H.; Gong, H.A.O.; Zhang, M.E.I.; Niu, X.; Ma, Y.; Zhang, X.I.N.; Cai, W.E.I.; Yang, G.; Wei, M.; et al. Evaluation of blood vessel injury, oxidative stress and circulating inflammatory factors in an L—NAME—Induced preeclampsia-like rat model. Exp. Ther. Med. 2018, 16, 585–594. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Wang, X.; Zheng, Y.; Jin, Z.; Zhi, J. Role of agonistic autoantibodies against type-1 angiotensin II receptor in the pathogenesis of retinopathy in preeclampsia. Sci. Rep. 2016, 6, 29036. [Google Scholar] [CrossRef] [PubMed]
- Huai, J.; Yang, Z.; Yi, Y.H.; Wang, G.J. Different Effects of Pravastatin on Preeclampsia—Like Symptoms in Different Mouse Models. Chin. Med. J. 2018, 131, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.C.; Zhang, Y.; Irani, R.A.; Zhang, H.; Mi, T.; Popek, E.J.; Hicks, M.J.; Ramin, S.M.; Kellems, R.E.; Xia, Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 2008, 14, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Kusinski, L.C.; Stanley, J.L.; Dilworth, M.R.; Hirt, C.J.; Andersson, I.J.; Renshall, L.J.; Baker, B.C.; Baker, P.N.; Sibley, C.P.; Wareing, M.; et al. eNOS knockout mouse as a model of fetal growth restriction with an impaired uterine artery function and placental transport phenotype. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R86–R93. [Google Scholar] [CrossRef]
- Xue, L.U.; Xie, K.; Wu, L.A.N.; Yu, X.; Long, W.E.I.; Li, C.; Jia, R.; Ding, H. A novel peptide relieves endothelial cell dysfunction in preeclampsia by regulating the PI3K/mTOR/HIF1 α pathway. Int. J. Mol. Med. 2021, 47, 276–288. [Google Scholar] [CrossRef]
- Cotechini, T.; Komisarenko, M.; Sperou, A.; Macdonald-Goodfellow, S.; Adams, M.A.; Graham, C.H. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J. Exp. Med. 2014, 211, 165–179. [Google Scholar] [CrossRef]
- LaMarca, B.D.; Ryan, M.J.; Granger, J.P. Pathophysiology of Hypertension Inflammatory Cytokines During Preeclampsia: Role of Inflammatory Cytokines. Curr. Hypertens. Rev. 2007, 3, 69–74. [Google Scholar] [CrossRef]
- Boggess, K.A.; Berggren, E.K.; Koskenoja, V.; Urlaub, D.; Lorenz, C. Severe preeclampsia and maternal self-report of oral health, hygiene, and dental care. J. Periodontol. 2013, 84, 143–151. [Google Scholar] [CrossRef]
- Varshney, S.; Gautam, A. Poor periodontal health as a risk factor for development of pre-eclampsia in pregnant women. J. Indian Soc. Periodontol. 2014, 18, 321–325. [Google Scholar] [CrossRef]
- Zhuo, J.; Xiao, D.; Hu, Y.; Wang, Z.; Paradis, A.; MataGreenwood, E.; Zhang, L. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension 2013, 62, 599–607. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, Y.; Gao, Y.; Liang, G.; Chen, Q.; Jiang, S.; Yang, X.; Fan, C.; Wang, H.; Wang, J.; et al. Distinct placental molecular processes associated with early-onset and late-onset preeclampsia. Theranostics 2021, 11, 5028–5044. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [PubMed]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Marshall, S.A.; Hannan, N.J.; Jelinic, M.; Nguyen, T.P.H.; Girling, J.E.; Parry, L.J. Animal models of preeclampsia: Translational failings and why. Am. J. Physiol. Integr. Comp. Physiol. 2018, 314, R499–R508. [Google Scholar] [CrossRef] [PubMed]
- Yart, L.; Roset Bahmanyar, E.; Cohen, M.; Martinez de Tejada, B. Role of the Uteroplacental Renin-Angiotensin System in Placental Development and Function, and Its Implication in the Preeclampsia Pathogenesis. Biomedicines 2021, 9, 1332. [Google Scholar] [CrossRef]
- Rodriguez, M.; Moreno, J.; Hasbun, J. RAS in Pregnancy and Preeclampsia and Eclampsia. Int. J. Hypertens. 2012, 2012, 739274. [Google Scholar] [CrossRef]
- Procopciuc, L.M.; Nemeti, G.; Buzdugan, E.; Iancu, M.; Stamatian, F.; Caracostea, G. Renin-angiotensin system gene variants and risk of early- and late-onset preeclampsia: A single center case-control study. Pregnancy Hypertens. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Li, J.; LaMarca, B.; Reckelhoff, J.F. A model of preeclampsia in rats: The reduced uterine perfusion pressure (RUPP) model. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1–H8. [Google Scholar] [CrossRef]
- Saif, J.; Ahmad, S.; Rezai, H.; Litvinova, K.; Sparatore, A.; Alzahrani, F.A.; Wang, K.; Ahmed, A. Redox Biology Hydrogen sulfide releasing molecule MZe786 inhibits soluble Flt-1 and prevents preeclampsia in a refined RUPP mouse model. Redox Biol. 2021, 38, 101814. [Google Scholar] [CrossRef]
- Abitbol, M.M. Simplified technique to produce toxemia in the rat: Consideration on cause of toxemia. Clin. Exp. Hypertens. Hypertens. Pregnancy 1982, 1, 93–103. [Google Scholar] [CrossRef]
- Zhu, M.; Ren, Z.; Possomato-vieira, J.S.; Khalil, R.A. Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy. Am. J. Physiol. Integr. Comp. Physiol. 2016, 311, R505–R521. [Google Scholar] [CrossRef] [PubMed]
- Gutkowska, J.; Granger, J.P.; LaMarca, B.B.; Danalache, B.A.; Wang, D.; Jankowski, M. Changes in cardiac structure in hypertension produced by placental ischemia in pregnant rats: Effect of tumor necrosis factor blockade. J. Hypertens. 2011, 29, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Gadonski, G.; LaMarca, B.B.D.; Sullivan, E.; Bennett, W.; Chandler, D.; Granger, J.P. Pregnancy and IL-6 Hypertension Produced by Reductions in Uterine Perfusion in the Pregnant Rat Role of Interleukin 6. Hypertension 2006, 48, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Waker, C.A.; Kaufman, M.R.; Brown, T.L. Current State of Preeclampsia Mouse Models: Approaches, Relevance, and Standardization. Front. Physiol. 2021, 12, 681632. [Google Scholar] [CrossRef]
- LaMarca, B.; Speed, J.; Fournier, L.; Babcock, S.A.; Berry, H.; Granger, J.P. Hypertension in Response to Chronic Reductions in Uterine Perfusion in Pregnant Rats: Effect of Tumor Necrosis Factor-α Blockade. Hypertension 2008, 52, 1161–1167. [Google Scholar] [CrossRef]
- Bridges, J.P.; Gilbert, J.S.; Colson, D.; Gilbert, S.A.; Dukes, M.P.; Ryan, M.J.; Granger, J.P. Oxidative stress contributes to soluble fms-like tyrosine kinase-1 induced vascular dysfunction in pregnant rats. Am. J. Hypertens. 2009, 22, 564–568. [Google Scholar] [CrossRef]
- Szalai, G.; Romero, R.; Chaiworapongsa, T.; Xu, Y.; Wang, B.; Ahn, H.; Xu, Z.; Chiang, P.J.; Sundell, B.; Wang, R.; et al. Full-Length Human Placental sFlt-1-e15a Isoform Induces Distinct Maternal Phenotypes of Preeclampsia in Mice. PLoS ONE 2015, 10, e0119547. [Google Scholar] [CrossRef]
- Murphy, S.R.; LaMarca, B.; Cockrell, K.; Arany, M.; Granger, J.P. L-Arginine supplementation abolishes the blood pressure and endothelin response to chronic increases in plasma sFlt-1 in pregnant rats. Am. J. Physiol. Integr. Comp. Physiol. 2012, 302, 259–263. [Google Scholar] [CrossRef]
- Turanov, A.A.; Lo, A.; Hassler, M.R.; Makris, A.; Ashar-patel, A.; Alterman, J.F.; Coles, A.H.; Haraszti, R.A.; Roux, L.; Godinho, B.M.D.C.; et al. Articles RNAi modulation of placental sFLT1 for the treatment of preeclampsia. Nat. Biotechnol. 2018, 36, 1164–1175. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Ma, J.Y.; Kapoun, A.M.; Shao, Q.; Kerr, I.; Lam, A.; Young, G.O.; Sannajust, F.; Stathis, P.; et al. Recombinant Vascular Endothelial Growth Factor 121 Attenuates Hypertension and Improves Kidney Damage in a Rat Model of Preeclampsia. Hypertension 2007, 50, 686–692. [Google Scholar] [CrossRef]
- Eddy, A.C.; Iii, G.L.B.; George, E.M. Pro-angiogenic therapeutics for preeclampsia. Biol. Sex Differ. 2018, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Matsubara, Y.; Uchikura, Y.; Sugiyama, T. Pathophysiology of Preeclampsia: The Role of Exosomes. Int. J. Mol. Sci. 2021, 22, 2572. [Google Scholar] [CrossRef]
- Solomon, C.G.; Seely, E.W. Preeclampsia—Searching for the Cause. N. Engl. J. Med. 2004, 350, 641–642. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.R.; LaMarca, B.B.D.; Parrish, M.; Cockrell, K.; Granger, J.P. Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: Role of tumor necrosis factor-α. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R130–R135. [Google Scholar] [CrossRef]
- Philogene, M.C.; Han, D.; Alvarado, F.; Fedarko, N.S.; Zonderman, A.B.; Evans, M.K.; Crews, D.C. Prevalence of Angiotensin II Type 1 Receptor Antibodies in Persons with Hypertension and Relation to Blood Pressure and Medication. Am. J. Hypertens. 2020, 33, 734–740. [Google Scholar] [CrossRef]
- Campbell, N.; LaMarca, B.; Cunningham, M.W.J. The role of Agonistic Autoantibodies to the Angiotensin II Type 1 Receptor (AT1-AA) in Pathophysiology of Preeclampsia. Curr. Pharm. Biotechnol. 2018, 19, 781–785. [Google Scholar] [CrossRef]
- LaMarca, B.D.; Cockrell, K.; Sullivan, E.; Bennett, W.; Granger, J.P. Role of Endothelin in Mediating Tumor Necrosis Factor-Induced Hypertension in Pregnant Rats. Hypertension 2005, 46, 82–87. [Google Scholar] [CrossRef]
- Giardina, J.B.; Green, G.M.; Cockrell, K.L.; Granger, J.P.; Khalil, R.A.; Jena, B.; Green, G.M.; Kathy, L.; Granger, J.P.; Tnf-, R.A.K. TNF-a enhances contraction and inhibits endothelial NO-cGMP relaxation in systemic vessels of pregnant rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R130–R143. [Google Scholar] [CrossRef]
- Shesely, E.G.; Gilbert, C.; Granderson, G.; Carretero, C.D.; Carretero, O.A.; Beierwaltes, W.H. Nitric oxide synthase gene knockout mice do not become hypertensive during pregnancy. Am. J. Obstet. Gynecol. 2001, 185, 1198–1203. [Google Scholar] [CrossRef]
- Kulandavelu, S.; Whiteley, K.J.; Qu, D.; Mu, J.; Bainbridge, S.A.; Adamson, S.L. Endothelial nitric oxide synthase deficiency reduces uterine blood flow, spiral artery elongation, and placental oxygenation in pregnant mice. Hypertension 2012, 60, 231–238. [Google Scholar] [CrossRef]
- Nathan, L.; Cuevas, J.; Chaudhuri, G. The role of nitric oxide in the altered vascular reactivity of pregnancy in the rat. Br. J. Pharmacol. 1995, 114, 955–960. [Google Scholar] [CrossRef]
- Ma, X.P.; Liu, C.D.; Cao, G.M.; Zhang, Z.Y. Transthyretin increases migration and invasion of rat placental trophoblast cells. FEBS Openbio 2020, 10, 1568–1576. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, W.; Hu, R.; Wang, H.; Ma, D.; Li, X. The effect of pre-eclampsia-like syndrome induced by L-NAME on learning and memory and hippocampal glucocorticoid receptor expression: A rat model. Hypertens. Pregnancy 2017, 36, 36–43. [Google Scholar] [CrossRef]
- De Alwis, N.; Binder, N.K.; Beard, S.; Mangwiro, Y.T.M.; Kadife, E.; Cuffe, J.S.M.; Keenan, E.; Fato, B.R.; Kaitu, T.J.; Brownfoot, F.C.; et al. The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health. Life Sci. Alliance 2022, 5, e202201517. [Google Scholar] [CrossRef]
- Losonczy, G.; Mucha, I.; Muller, V.; Kriston, T.; Ungvari, Z.; Tornoci, L.; Rosivall, L.; Venuto, R. The vasoconstrictor effects of L-NAME, a nitric oxide synthase inhibitor, in pregnant rabbits. Br. J. Pharmacol. 1996, 118, 1012–1018. [Google Scholar] [CrossRef][Green Version]
- Yoshikawa, K.; Umekawa, T.; Maki, S.; Kubo, M.; Nii, M.; Tanaka, K.; Tanaka, H.; Osato, K.; Kamimoto, Y.; Kondo, E.; et al. Tadalafil Improves L-NG-Nitroarginine Methyl Ester-Induced Preeclampsia with Fetal Growth Restriction-Like Symptoms in Pregnant Mice. Am. J. Hypertens. 2018, 31, 89–96. [Google Scholar] [CrossRef]
- Selivanova, E.K.; Shvetsova, A.A.; Borzykh, A.A.; Gaynullina, D.K.; Kiryukhina, O.O.; Lukoshkova, E.V.; Potekhina, V.M.; Kuzmin, V.S.; Tarasova, O.S. Intrauterine L-NAME Exposure Weakens the Development of Sympathetic Innervation and Induces the Remodeling of Arterial Vessels in Two-Week-Old Rats. Int. J. Mol. Med. 2021, 22, 12327. [Google Scholar] [CrossRef]
- Teng, R.; Wu, T.; Sharma, R.; Garrison, R.D.; Hudak, M.L. Early neonatal hypotension in premature infants born to preeclamptic mothers. J. Perinatol. 2006, 26, 471–475. [Google Scholar] [CrossRef]
- Danielson, L.A.; Conrad, K.P. Acute Blockade of Nitric Oxide Synthase Inhibits Renal Vasodilation and Hyperfiltration During Pregnancy in Chronically Instrumented Conscious Rats. J. Clin. Investig. 1995, 96, 482–490. [Google Scholar] [CrossRef]
- Seligman, S.P.; Buyon, J.P.; Clancy, R.M.; Young, B.K.; Abramson, S.B. The role of nitric oxide in the pathogenesis of preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 944–948. [Google Scholar] [CrossRef]
- Cushen, S.C.; Goulopoulou, S. New Models of Pregnancy-Associated Hypertension. Am. J. Hypertens. 2017, 30, 1053–1062. [Google Scholar] [CrossRef]
- Reese, J.; Wang, H.; Ding, T.; Paria, B.C. The Hamster as a Model for Embryo Implantation: Insights into a Multifaceted Process Jeff. Semin. Cell Dev. Biol. 2008, 19, 194–203. [Google Scholar] [CrossRef]
- De Rijk, E.P.C.T.; van Esch, E.; Flik, G. Pregnancy Dating in the Rat: Placental Morphology and Maternal. Toxicol. Pathol. 2002, 30, 271–282. [Google Scholar] [CrossRef]
- Carter, A.M. Evolution of Placental Hormones: Implications for Animal Models. Front. Endocrynol. 2022, 13, 891927. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wu, G.; Johnson, G.A. Pregnancy recognition signals in mammals: The roles of interferons and estrogens. Anim. Reprod. 2017, 14, 7–29. [Google Scholar] [CrossRef]
- Hilliard, J. Corpus Luteum Function Mice in Guinea and Pigs, Hamsters, Rats. Biol. Reprod. 1973, 8, 203–221. [Google Scholar] [CrossRef]
- Phillips, C.A.; Poyser, N.L. Implantation in the Rat. J. Reprod. Fertil. 1981, 62, 73–81. [Google Scholar] [CrossRef]
- Orsini, M.W.; Donovan, B.T. Implantation and Induced Decidualization of the Uterus in the Guinea Pig, as Indicated by Pontamine Blue. Biol. Reprod. 1971, 5, 270–281. [Google Scholar] [CrossRef][Green Version]
- Blandau, R.J. Observations on implantation of the guinea pig ovum. Anat Rec 1949, 103, 19–47. [Google Scholar] [CrossRef]
- Bridgman, J. A morphological study of the development of the placenta of the rat. II. An histological and cytological study of the development of the chorioallantoic placenta of the white rat. J. Morphol. 1848, 83, 195–223. [Google Scholar] [CrossRef]
- Shukla, V.; Soares, M.J. Modeling Trophoblast Cell-Guided Uterine Spiral Artery Transformation in the Rat. Int. J. Mol. Sci. 2022, 23, 2947. [Google Scholar] [CrossRef]
- Orsini, M.W. The trophoblastic giant cells and endovascular cells associated with pregnancy in the hamster, Cricetus auratus. Am. J. Anat. 1954, 94, 273–331. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Robertson, W.B.; Brosens, I. The role of ovarian steroids in placental development and endovascular trophoblast migration in the golden hamster. J. Reprod. Fertil. 1975, 44, 43–51. [Google Scholar] [CrossRef]
- Lopez-Tello, J.; Arias-Alvarez, M.; Gonzalez-Bulnes, A.; Sferuzzi-Perri, A.N. Models of Intrauterine growth restriction and fetal programming in rabbits. Mol. Reprod. Dev. 2019, 86, 1781–1809. [Google Scholar] [CrossRef]
- Varberg, K.M.; Iqbal, K.; Muto, M.; Simon, M.E.; Scott, R.L.; Kozai, K.; Choudhury, R.H.; Aplin, J.D.; Biswell, R.; Gibson, M.; et al. ASCL2 reciprocally controls key trophoblast lineage decisions during hemochorial placenta development. Proc. Natl. Acad. Sci. USA 2021, 118, e2016517118. [Google Scholar] [CrossRef]
- Bibeau, K.; Sicotte, B.; Béland, M.; Bhat, M.; Gaboury, L.; Couture, R.; St-Louis, J.; Brochu, M. Placental underperfusion in a rat model of intrauterine growth restriction induced by a reduced plasma volume expansion. PLoS ONE 2016, 11, e0145982. [Google Scholar] [CrossRef]
- Mossman, H.W. The rabbit placenta and the problem of placental transmission. Am. J. Anat. 1926, 37, 433–497. [Google Scholar] [CrossRef]
- Takata, K.; Fujikura, K.; Shin, B. Ultrastructure of the Rodent Placental Labyrinth: A Site of Barrier and Transport. J. Reprod. Dev. 1997, 43, 13–24. [Google Scholar] [CrossRef]
- Jauniaux, E.; Moscoso, J.G. Morphology and Significance of the Human Yolk Sac. In The First Twelve Weeks of Gestation; Springer: Berlin/Heidelberg, Germany, 1992; pp. 192–213. [Google Scholar]
- Lu, H.; Gong, L.; Xu, H.; Zhou, Q.; Zhao, H.; Wu, S.; Hu, R.; Li, X. Environmental Enrichment Protects Offspring of a Rat Model of Preeclampsia from Cognitive Decline. Cell. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Bytautiene, E.; Bulayeva, N.; Bhat, G.; Li, L.; Rosenblatt, K.P.; Saade, G.R. Long-term alterations in maternal plasma proteome after sFlt1-induced preeclampsia in mice. Am. J. Obstet. Gynecol. 2013, 208, 388.e1–388.e10. [Google Scholar] [CrossRef]
- Gatford, K.L.; Andraweera, P.H.; Roberts, C.T.; Care, A.S. Animal Models of Preeclampsia: Causes, Consequences, and Interventions. Hypertension 2020, 75, 1363–1381. [Google Scholar] [CrossRef]

| Species | Inducing Factor | Time of PE Induction | HA | P or A/C Ratio | Ref |
|---|---|---|---|---|---|
| Incorrect trophoblast invasion | |||||
| Rat | 0.5 µg/kg LPS | 5 GD | + | + | [95,96] |
| Rat | Transgenic rats hAngiotensinogen female x hRenin male | spontaneously | + | + | [97,98] |
| Rat | Stroke-prone spontaneously hypertensive rat (SHRSP) | spontaneously | + | + | [99] |
| Mouse | LPS 20 µg/kg | 7.5–17.5 GD | + | + | [100] |
| Mouse | BPH5 mouse | spontaneously | + | + | [101] |
| Guinea pig | Exposure to hypoxia 10.5% O2 | 28–30 GD up to term | + | ns | [102] |
| Placental ischaemia | |||||
| Rat | RUPP model | 14 GD | + | -, ns | [103,104] |
| Rat | Selective RUPP model | 14 GD | + | ns | [105] |
| Mouse | RUPP model | 14.5 GD | + | + | [106] |
| Rabbit | RUPP model | 25 GD | + | - | [107] |
| Incorrect angiogenesis | |||||
| Rat | Injection—adenovirus expressing sFlt-1 | 8 or 9 GD | + | + | [108] |
| Rat | Injection—adenovirus sFtl-1 and/or sEng | 8 or 9 GD | + | + | [109] |
| Rat | Injection—Suramin (an inhibitor of angiogenesis) | 10 and 11 GD | + | - | [110] |
| Mouse | Injection—adenovirus expressing stabilised HIF-1α | 8 GD | + | + | [111] |
| Mouse | Injection—exosomes of preeclamptic women | 5.5; 10.5; 15.5 GD | + | + | [112] |
| Mouse | Injection—adenovirus carrying sFlt-1 | 4; 8; 9 GD | + | + | [113] |
| Mouse | Injection—adenovirus carrying sFlt-1 | 8 GD | + | + | [114] |
| Mouse | Injection into blastocyst lentivirus carrying human sFlt-1 | in vitro | + | + | [115] |
| Inflammation | |||||
| Rat | TNFα infusion | 14–19 GD | + | ns | [116] |
| Rat | TNFα infusion | 14 GD | + | - | [117] |
| Rat | IL6 infusion | 14–19 GD | + | - | [118] |
| Mouse | Injection of purified IgG from PE women | 13 and 14 GD | + | + | [119] |
| Mouse | TNFα injection | 13 GD | + | + | [120] |
| Mouse | IL10 deficient mouse | spontaneously | + | + | [121] |
| Mouse | BPH5 mouse | spontaneously | + | + | [122] |
| Endothelial dysfunction | |||||
| Rat | L-NAME in drinking water | 8–19 GD | + | + | [123] |
| Rat | L-NAME injection | 9-20 GD or 10-20 GD | + | + | [124] |
| Rat | AT1-AA antibody injection | 13 and 14 GD | + | + | [125] |
| Mouse | L-NAME injection | 7–18 GD | + | + | [126] |
| Mouse | AT1-AA antibody injection | 13 GD | + | + | [127] |
| Mouse | eNOS knockout mice | spontaneously | + | + | [128] |
| Human | Mouse | Rat | Hamster | Guinea Pig | Rabbit | Ref | |
|---|---|---|---|---|---|---|---|
| Gestation days | 280 days | 20–21 days | About 21 days | About 16 days | 59–72 days | 28–31 days | [14,55,70,176,177] |
| Maternal recognition of pregnancy | Luteotrophic factor (choronic gonadotrophin) | Vaginal stimulation + luteotrophic factor (pituitary prolactin) | Vaginal stimulation + luteotrophic factor (pituitary prolactin) | Vaginal stimulation + luteotrophic factor (pituitary prolactin) | Luteotrophic factor from pituitary | Vaginal stimulation + luteotrophic factor (oestrogen) | [17,178,179,180] |
| Signal for decidualization | Hormonal stimulus | Hormonal and implantation stimulus | Hormonal and implantation stimulus | Hormonal and implantation stimulus | Hormonal and implantation stimulus | Hormonal and implantation stimulus | [17] |
| Implantation | 5–6 after ovulation | 4–5 GD | 5–6 GD | 4–5 GD | 7–8 GD | 7–9 GD | [12,15,59,176,181,182] |
| Model of implantation | Interstitial | Eccentric/ interstitial | Eccentric/ interstitial | Eccentric/ interstitial | Interstitial | Superficial | [9,183] |
| Trophoblast invasion | Post implantation—9 weeks (interstitial) 9–22 weeks (endovascular) | 14 GD | 12.5–13.5 GD | Before 12 GD | 10–30 GD | 8–9 GD | [17,39,58,60,184,185,186,187,188,189] |
| Way of invasion | I/E | I | I/E | E | I/E | [36] | |
| Deepness of trophoblast invasion | Myometrial | Decidual | Mesometrium | Mesometrium | Mesometrium and far beyond uterus | Mesometrium | [36,49] |
| Trophoblastic plug | + | - | - | + | - | - | [50] |
| Spiral arteries remodelling | Before pregnancy | 8–12 GD | 6.5–13.5 GD | 5–6 GD | Before 30 GD | About 8 GD | [3,17,38,57,58,60] |
| Maternal blood influx into sinus/lacunae | 10–12 W | 9.5–14.5 GD | 12–15 GD | 12 GD | About 18 GD | About 10 GD | [51,52,53,54,55,56,186,190,191,192] |
| Type of foetal/ maternal barrier | Haemomonochorial (1) Foetal blood (2) FE (3) BM (4) SYN (5) Maternal blood | Haemotrichorial (1) Foetal blood (2) FE (3) BM (4) SYN (5) SYN (6) CYT (7) Maternal blood | Haemotrichorial (1) Foetal blood (2) FE (3) BM (4) SYN (5) SYN (6) CYT (7) Maternal blood | Haemotrichorial (1) Foetal blood (2) FE (3) BM (4) SYN (5) SYN (6) CYT (7) Maternal blood | Haemomonochorial (1) Foetal blood (2) FE (3) BM (4) SYN (5) Maternal blood | Haemodichorial (1) Foetal blood (2) FE (3) BM (4) CYT (5) SYN (6) Maternal blood | [9,71] |
| Modification of chorionic surface | Villi | Labyrinth | Labyrinth | Labyrinth | Labyrinth | Labyrinth | [9,70] |
| Placenta Foetal side | (1) Villous trophoblast—placental–foetal interface. (2) Extravillous trophoblast cells—uterine–placental interface. | (1) Labyrinth corresponding to human villous trophoblast. (2) Junctional zone (known as basal zone) corresponding to human extravillous trophoblast. | (1) Labyrinth corresponding to human villous trophoblast. (2) Junctional zone (known as basal zone) corresponding to human extravillous trophoblast. | Labyrinth corresponding to human villous trophoblast and including (2) junctional zone (known as basal zone) corresponding to human extravillous trophoblast. | (1) Main placenta, including: (a) labyrinth; (b) interlobium; (c) yolk sac placenta. (2) Placenta-seam, including: (a) Subplacenta. (b) Junctional zone. | (1) Labyrinth corresponding to human villous trophoblast and (2) junctional zone. | [17,33,50,70] |
| Placenta Maternal side | (1) Maternal decidua. (2) Maternal myometrium | (1) Mesometrial decidua. (2) Mesometrial triangle including maternal gland. | (1) Mesometrial decidua. (2) Mesometrial triangle including maternal gland. | (1) Mesometrial decidua. (2) Mesometrial triangle including maternal gland. | (1) Decidua. (2) Mesometrium. | (1) Deciua including necrosis zone and separation zone). (2) Mesometrium. | [33] |
| Shape of placenta | Discoid | Discoid | Discoid | Discoid | Discoid | Bidiscoid | [9,33] |
| Yolk sac placentation | 12 weeks/20 weeks | Inverted/at term | Inverted/at term | Inverted/at term | Inverted/at term | Inverted/at term | [193] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakowicz, A.; Bralewska, M.; Kamola, P.; Pietrucha, T. Reliability of Rodent and Rabbit Models in Preeclampsia Research. Int. J. Mol. Sci. 2022, 23, 14344. https://doi.org/10.3390/ijms232214344
Sakowicz A, Bralewska M, Kamola P, Pietrucha T. Reliability of Rodent and Rabbit Models in Preeclampsia Research. International Journal of Molecular Sciences. 2022; 23(22):14344. https://doi.org/10.3390/ijms232214344
Chicago/Turabian StyleSakowicz, Agata, Michalina Bralewska, Piotr Kamola, and Tadeusz Pietrucha. 2022. "Reliability of Rodent and Rabbit Models in Preeclampsia Research" International Journal of Molecular Sciences 23, no. 22: 14344. https://doi.org/10.3390/ijms232214344
APA StyleSakowicz, A., Bralewska, M., Kamola, P., & Pietrucha, T. (2022). Reliability of Rodent and Rabbit Models in Preeclampsia Research. International Journal of Molecular Sciences, 23(22), 14344. https://doi.org/10.3390/ijms232214344





