ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance
Abstract
1. Introduction
2. Results
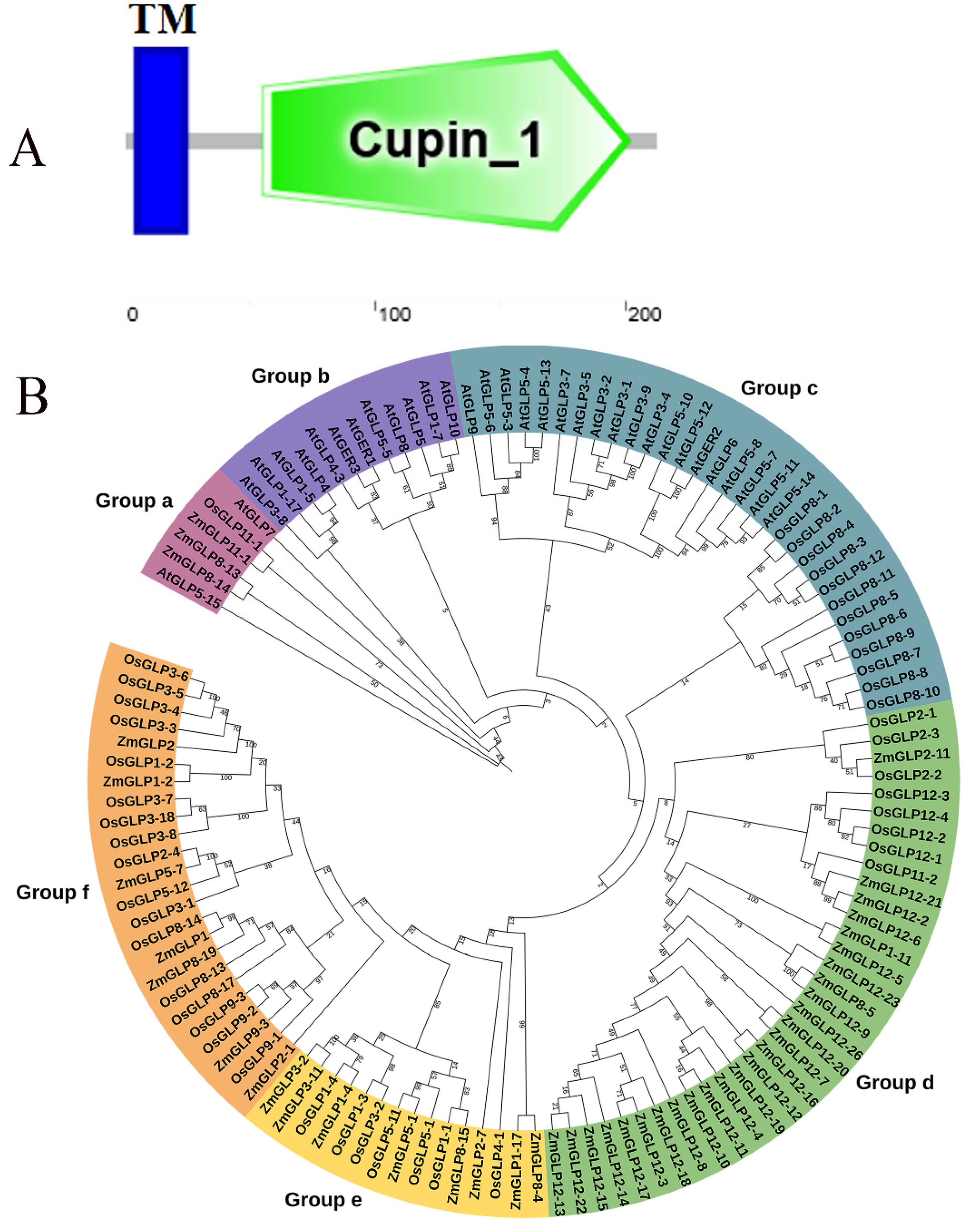
2.1. Identification and Physicochemical Property Analysis of ZmGLP1
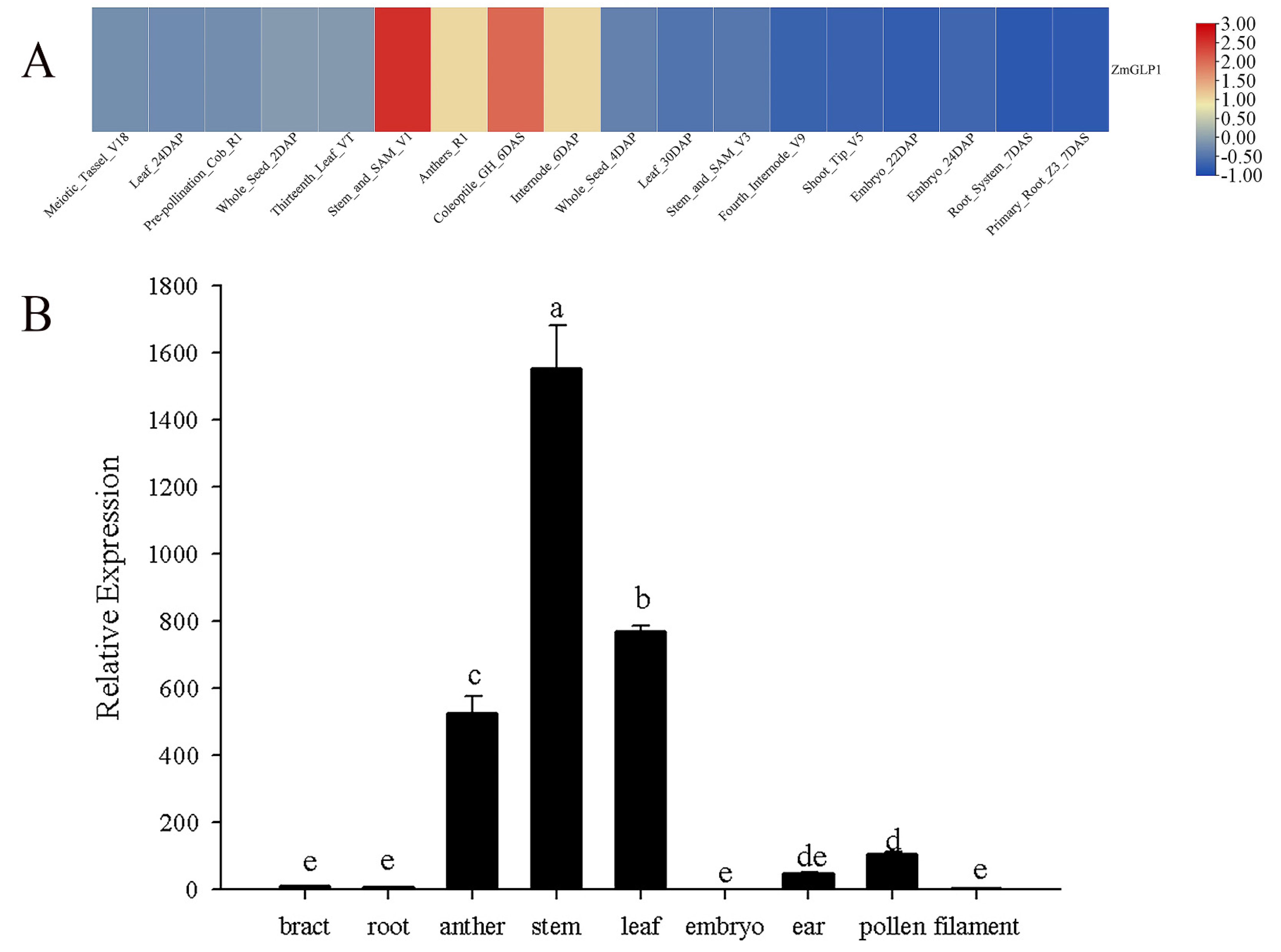
2.2. Expression of ZmGLP1 in Different Maize Tissues
2.3. Analysis of ZmGLP1 Expression Induced by Biotic Stress and Hormone Signals
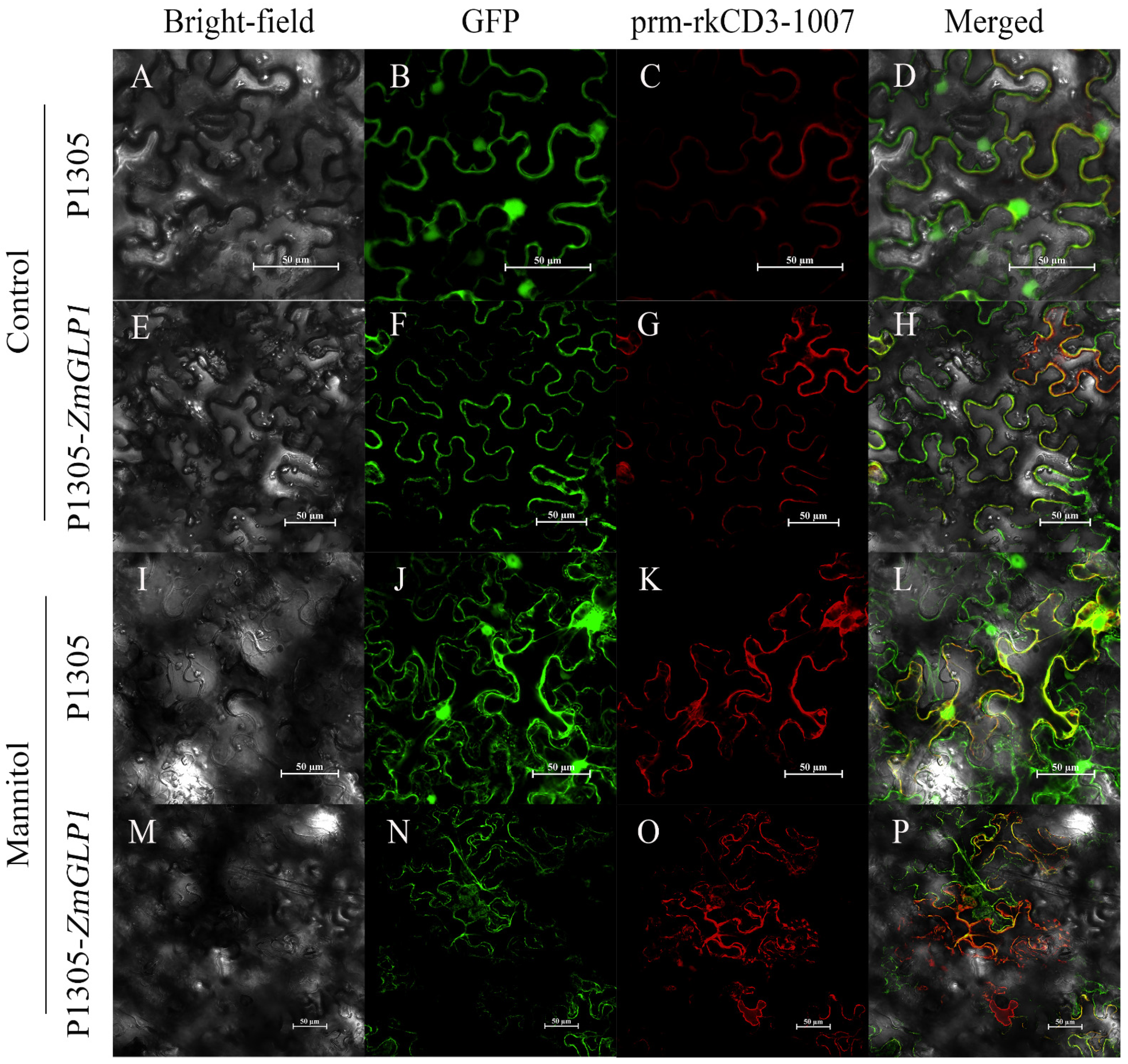
2.4. Subcellular Localization Analysis of ZmGLP1
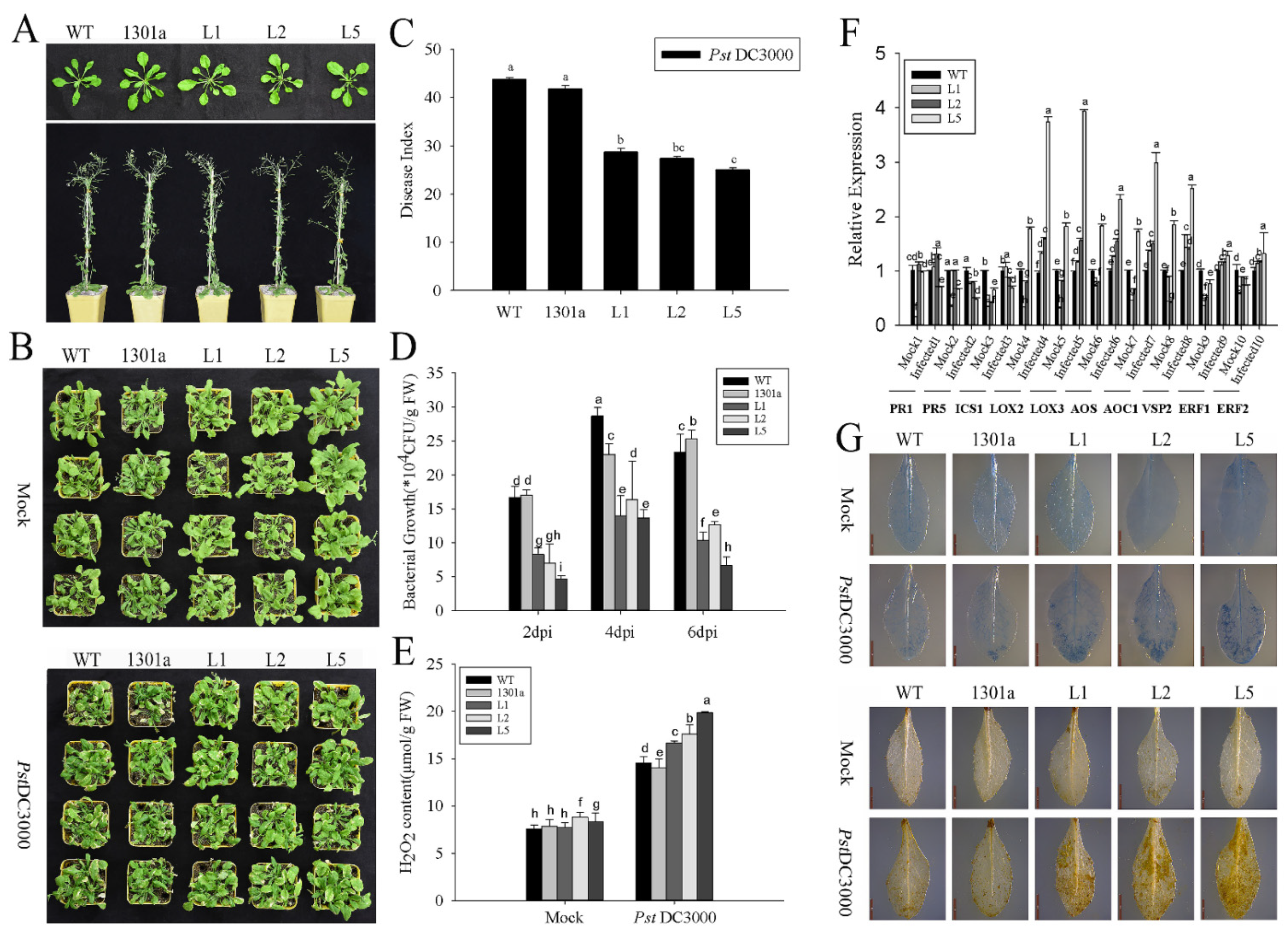
2.5. Expression of ZmGLP1 in Arabidopsis Increases Resistance to PstDC3000
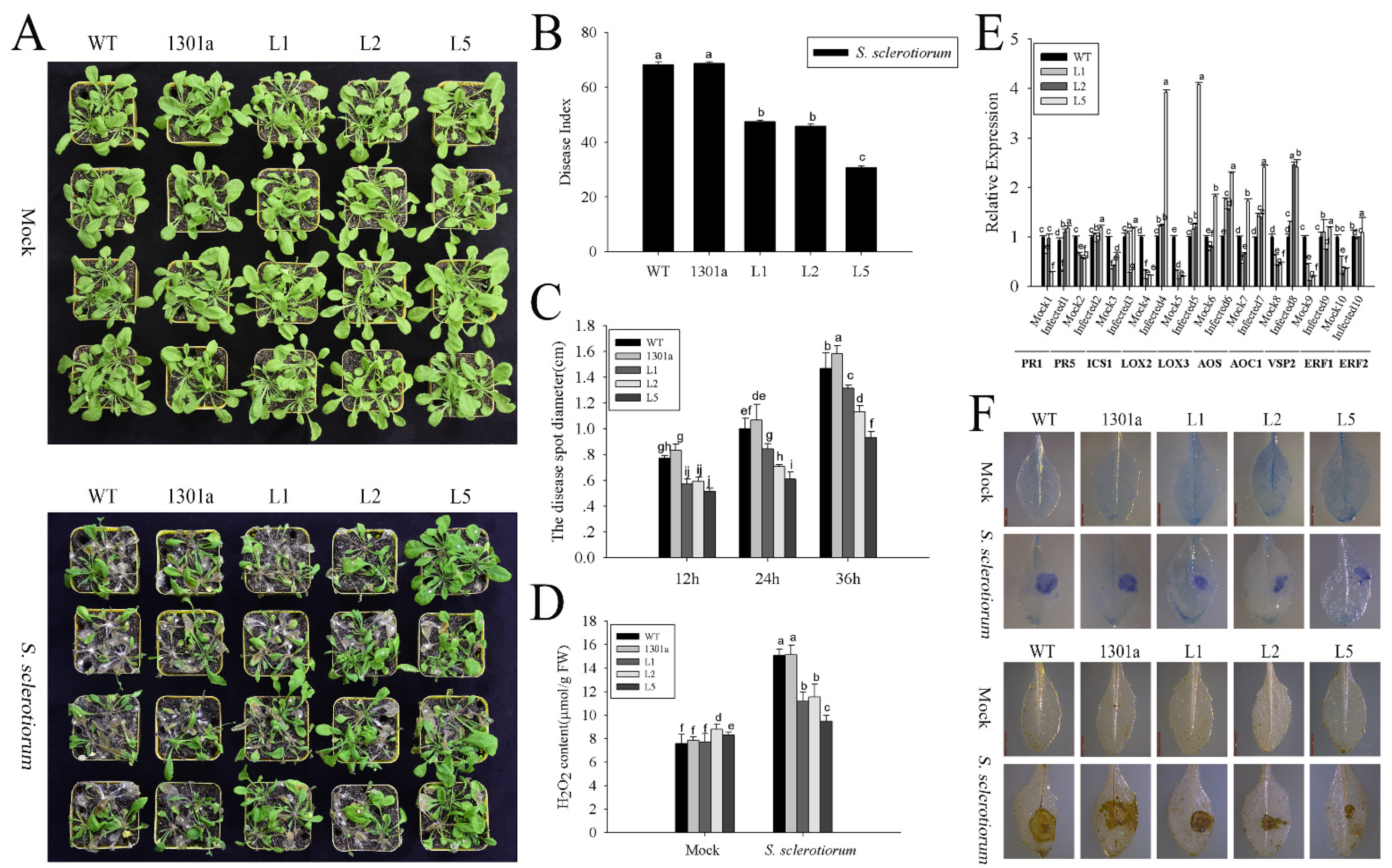
2.6. ZmGLP1 Confers Resistance to S. sclerotiorum in Arabidopsis
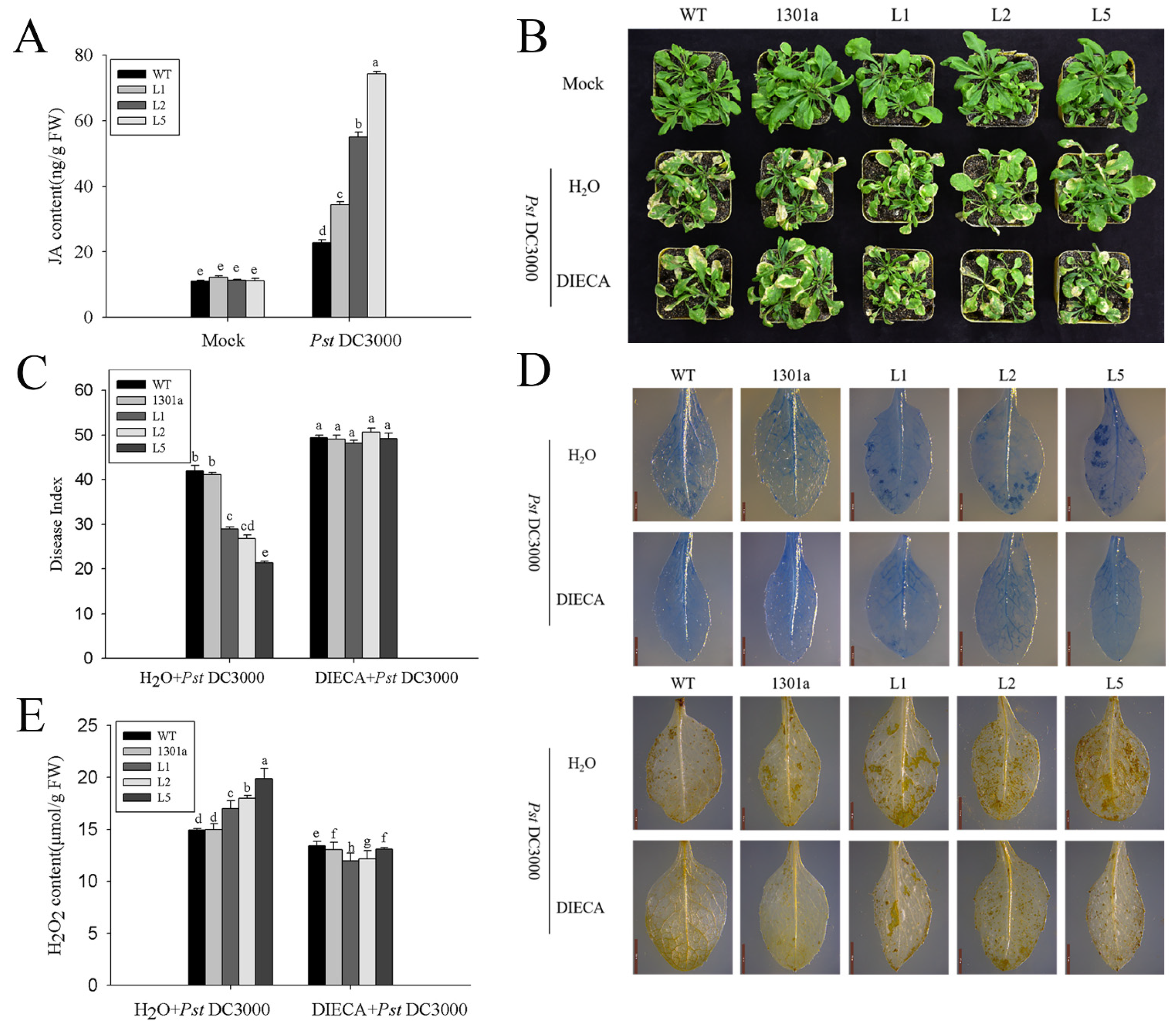
2.7. ZmGLP1-Overexpressing Arabidopsis Exhibits Resistance to PstDC3000 and S. sclerotiorum through the JA-Mediated Signaling Pathway
3. Materials and Methods
3.1. Bioinformatics Analysis of ZmGLP1
3.2. Vector Construction and Arabidopsis Transformation
3.3. Pathogen Cultivation
3.4. Plant Materials and Treatments
3.5. Quantitative PCR
3.6. Subcellular Localization Assay of ZmGLP1
3.7. DAB Staining
3.8. Detection of H2O2
3.9. Trypan Blue Staining
3.10. Detection of JA in ZmGLP1-Overexpressing Arabidopsis
3.11. Statistical Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, X.; Liu, J.; Ren, W.; Yang, Q.; Chai, Z.; Chen, R.; Wang, L.; Zhao, J.; Lang, Z.; Wang, H.; et al. Gene-indexed mutations in maize. Mol. Plant 2018, 11, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Song, F.; Sun, S.; Guo, C.; Zhu, Z.; Wang, X. Characterization and molecular mapping of two novel genes resistant to pythium stalk rot in maize. Phytopathology 2019, 109, 804–809. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, X.; Wang, W.; Liu, X.; Zhao, H.; Wu, C.; Chang, X.; Zhang, M.; Chen, H.; Gong, G. Etiology and symptoms of maize leaf spot caused by Bipolaris spp. in Sichuan, 2020, China. Pathogens 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z. Dynamic analysis on the disease progression of maize leaf blight between Xianyu335 and improved Xianyu335 in Western Region of Heilongjiang Province. J. Maize Sci. 2013, 21, 119–123. [Google Scholar] [CrossRef]
- Ilyas, M.; Naqvi, S.M.S.; Mahmood, T. In silico analysis of transcription factor binding sites in promoters of germin-like protein genes in rice. Arch. Biol. Sci. 2016, 68, 863–876. [Google Scholar] [CrossRef]
- Li, L.; Xu, X.; Chen, C.; Shen, Z. Genome-wide characterization and expression analysis of the germin-like protein family in rice and Arabidopsis. Int. J. Mol. Sci. 2016, 17, 1622. [Google Scholar] [CrossRef]
- Zimmermann, G.; Baumlein, H.; Mock, H.P.; Himmelbach, A.; Schweizer, P. The multigene family encoding germin-like proteins of barley. Regulation and function in Basal host resistance. Plant Physiol. 2006, 142, 181–192. [Google Scholar] [CrossRef]
- Liu, Y.J.; Jia, M.X.; Zhou, H.; Zhang, L.; Liu, C.; Si, H.L.; Liu, Y.Q.; Gu, S.Q.; Gong, X.D.; Dong, J.G. Genome-wide Identification of GLP Gene Family in Maize (Zea mays) and It’s Expression Analysis When Maize is Exposed to Setosphaeria turcica. Chin. J. Agric. Biotechnol. 2021, 29, 13. [Google Scholar] [CrossRef]
- Breen, J.; Bellgard, M. Germin-like proteins (GLPs) in cereal genomes: Gene clustering and dynamic roles in plant defence. Funct. Integr. Genom. 2010, 10, 463–476. [Google Scholar] [CrossRef]
- Majeed, N.; Javaid, B.; Deeba, F.; Naqvi, S.; Douches, D. Enhanced Fusarium oxysporum f. sp. tuberosi Resistance in Transgenic Potato Expressing a Rice GLP Superoxide Dismutase Gene. Am. J. Potato Res. 2018, 95, 383–394. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Zhu, F.; Li, H.; Li, L.; Yang, Q. Characterization of peanut gennin-like proteins, AhGLPs inplant development and defense. PLoS ONE 2013, 8, e61722. [Google Scholar] [CrossRef]
- Zhang, N.; Guan, R.; Yang, Y.; Bai, Z.; Ge, F.; Liu, D. Isolation and characterization of a Fusarium oxysporum-resistant gene LrGLP1 from Lilium regale Wilson. In Vitro Cell Dev. Biol.-Plant 2017, 53, 461–468. [Google Scholar] [CrossRef]
- Christensen, A.B.; Thordal-Christensen, H.; Zimmermann, G.; Gjetting, T.; Lyngkjær, M.F.; Dudler, R.; Schweizer, P. The germinlike protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley. Mol. Plant-Microbe Interact. 2004, 17, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Li, X.; Zhu, Y.; Ge, X.; Sun, Y.; Liu, N. Gh ABP19, a novel germin-like protein from Gossypium hirsutum, plays an important role in the regulation of resistance to Verticillium and Fusarium wilt pathogens. Front. Plant Sci. 2019, 10, 583. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Su, B.; Chen, X.; Xie, S.; Gu, S.; Wang, Q.; Huang, D.; Jiang, H. An Endophytic Bacterial Strain Isolated from Eucommia ulmoides Inhibits Southern Corn Leaf Blight. Front. Microbiol. 2017, 8, 903–915. [Google Scholar] [CrossRef]
- Zhou, Q.; Gu, S.Y.; Xu, L.; Tan, G.J. Effects of endophyte DZSY21 colonization on DNA methylation levels in Maize. J. Hefei Norm. Univ. 2019, 3, 32–37. [Google Scholar] [CrossRef]
- Bari, R.; Jones, J. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Dickman, M.B.; Fluhr, R. Centrality of Host Cell Death in Plant-Microbe Interactions. Annu. Rev. Phytopathol. 2013, 51, 543–570. [Google Scholar] [CrossRef]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Dong, A.X. Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in Rlanguage. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef]
- Hang, T.; Ling, X.; He, C.; Xie, S.; Ding, T. Isolation of the zmers4 gene from maize and its functional analysis in transgenic plants. Front. Microbiol. 2021, 12, 632908. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.H. Chitosan Oligosaccharide-Induced Resistance to Pseudomonas syringaepv Tomato DC3000 in Arabidopsis and a Preliminary Study on the Resistance Mechanism; Dalian Ocean University: Dalian, China, 2016. [Google Scholar]
- Gao, X.N. Control of Sclerotinia sclerotiorum in Rapeseed by Endophytic Bacterial Strain Em7; Northwest Sci-Tech University: Shenzhen, China, 2012. [Google Scholar]
- Katagiri, F.; Thilmony, R.; He, S.Y. The Arabidopsis thaliana-Pseudomonas syringae interaction. Arab. Book 2002, 1. [Google Scholar] [CrossRef] [PubMed]
- Zha, K.; Xie, H.; Ge, M.; Wang, Z.; Wang, Y.; Si, W.; Gu, L. Expression of Maize MADS Transcription Factor ZmES22 Negatively Modulates Starch Accumulation in Rice Endosperm. Int. J. Mol. Sci. 2019, 20, 483–499. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Cai, X.; Nebenfuhr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Bhadauria, V.; Miraz, P.; Kennedy, R.; Banniza, S.; Wei, Y. Dual trypan-aniline blue fluorescence staining methods for studying fungus-plant interactions. Biotech. Histochem. 2010, 85, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Maiti, M.K. Functional role of rice germin-like protein 1 in regulation of plant height and disease resistance. Biochem. Biophys. Res. Commun. 2010, 394, 178–183. [Google Scholar] [CrossRef]
- Banerjee, J.; Das, N.; Dey, P.; Maiti, M.K. Transgenically expressed rice germin-like protein1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components. Biochem. Biophys. Res. Commun. 2010, 402, 637–643. [Google Scholar] [CrossRef]
- Ilyas, M.; Rasheed, A.; Mahmood, T. Functional characterization of germin and germin-like protein genes in various plant species using transgenic approaches. Biotechnol. Lett. 2016, 38, 1405–1421. [Google Scholar] [CrossRef]
- Feys, B.J.F.; Benedetti, C.E.; Turner, P.J.G. Arabidopsis mutants selected for resistance to the phytotoxin coronatine are male sterile, insensitive to methyl jasmonate, and resistant to a bacterial pathogen. Plant Cell 1994, 6, 751–759. [Google Scholar] [CrossRef]
- Kloek, A.P.; Verbsky, M.L.; Sharma, S.B.; Schoelz, J.E.; Vogel, J.; Klessig, D.F.; Kunkel, B.N. Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J. 2001, 26, 509–522. [Google Scholar] [CrossRef]
- de Torres Zabala, M.; Zhai, B.; Jayaraman, S.; Eleftheriadou, G.; Winsbury, R.; Yang, R.; Truman, W.; Tang, S.; Smirnoff, N.; Grant, M. Novel JAZ co-operativity and unexpected JA dynamics underpin Arabidopsis defence responses to Pseudomonas syringae infection. New Phytol. 2016, 209, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Qi, C.H.; Jiang, H.; Zheng, P.F.; Hao, Y.J. Functional identification of apple on MdHIR4 in biotic stress. Plant Sci. 2018, 283, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Khan, N.U.; Wang, N.; Yang, X.; Qiu, D. The protein elicitor PevD1 enhances resistance to pathogens and promotes growth in Arabidopsis. Int. J. Biol. Sci. 2016, 12, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Averyanov, A. Oxidative burst and plant disease resistance. Front. Biosci. 2008, 1, 142. [Google Scholar] [CrossRef]
- Munir, F.; Hayashi, S.; Batley, J.; Naqvi, S.M.S.; Mahmood, T. Germin-like protein 2 gene promoter from rice is responsive to fungal pathogens in transgenic potato plants. Funct. Integr. Genom. 2016, 16, 19–27. [Google Scholar] [CrossRef]
- Li, Q.Y. The 919-Position Glutamate of RBOHD Regulates the Molecular Mechanism of Lipopolysaccharide-Induced Reactive Oxygen Species Production; Zhejiang University: Hangzhou, China, 2019. [Google Scholar]
- Li, P.; Zhao, L.; Qi, F.; Htwe, N.M.; Li, Q.; Zhang, D.; Lin, F.; Shang-Guan, K.; Liang, Y. The receptor-like cytoplasmic kinase ripk regulates broad-spectrum ros signaling in multiple layers of plant immune system. Mol. Plant 2021, 14, 10. [Google Scholar] [CrossRef]
- Dickman, M.B.; Park, Y.K.; Oltersdorf, T.; Li, W.; Clemente, T.; French, R. Abrogation of disease development in plants expressing animal antiapoptotic genes. Proc. Natl. Acad. Sci. USA 2001, 98, 6957–6962. [Google Scholar] [CrossRef]
- Eri, M.G.; Alex, L. The hypersensitive response facilitates plant infection by the necrotrophic pathogen botrytis cinerea. Curr. Biol. 2000, 10, 13. [Google Scholar] [CrossRef]
- Elad, Y.; Williamson, B.; Tudzynski, P.; Delen, N. Botrytis: Biology, Pathology, and Control; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; pp. 119–141. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, L.; Ge, L.; Ye, X.; Xu, L.; Si, W.; Ding, T. ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance. Int. J. Mol. Sci. 2022, 23, 14316. https://doi.org/10.3390/ijms232214316
Mao L, Ge L, Ye X, Xu L, Si W, Ding T. ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance. International Journal of Molecular Sciences. 2022; 23(22):14316. https://doi.org/10.3390/ijms232214316
Chicago/Turabian StyleMao, Lixue, Lijie Ge, Xinchun Ye, Li Xu, Weina Si, and Ting Ding. 2022. "ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance" International Journal of Molecular Sciences 23, no. 22: 14316. https://doi.org/10.3390/ijms232214316
APA StyleMao, L., Ge, L., Ye, X., Xu, L., Si, W., & Ding, T. (2022). ZmGLP1, a Germin-like Protein from Maize, Plays an Important Role in the Regulation of Pathogen Resistance. International Journal of Molecular Sciences, 23(22), 14316. https://doi.org/10.3390/ijms232214316




