MT1-MMP Expression Levels and Catalytic Functions Dictate LDL Receptor-Related Protein-1 Ligand Internalization Capacity in U87 Glioblastoma Cells
Abstract
1. Introduction
2. Results
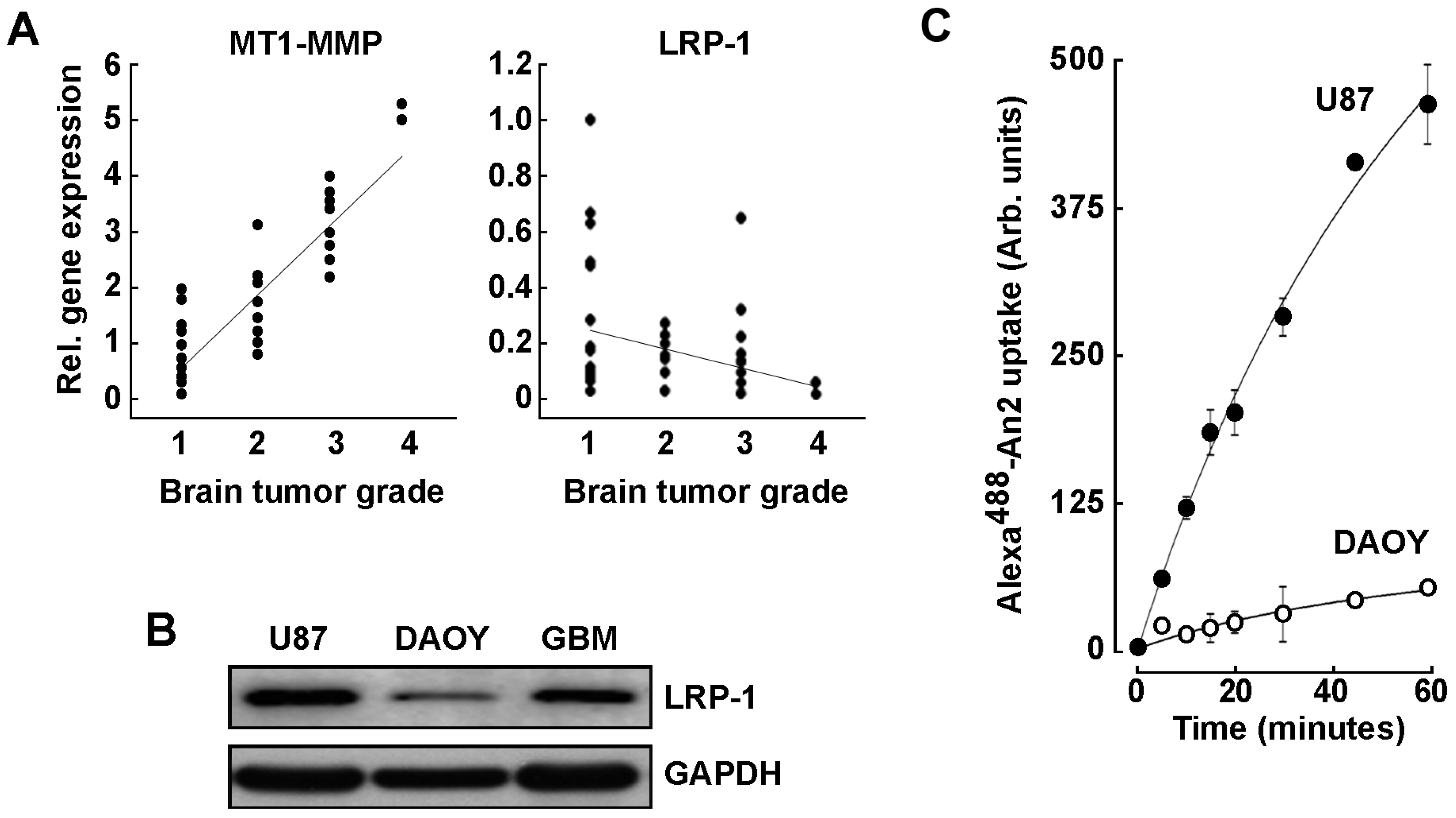
2.1. LRP-1 and MT1-MMP Gene Expression Profiling in Grade 1–4 Brain Tumor Tissues
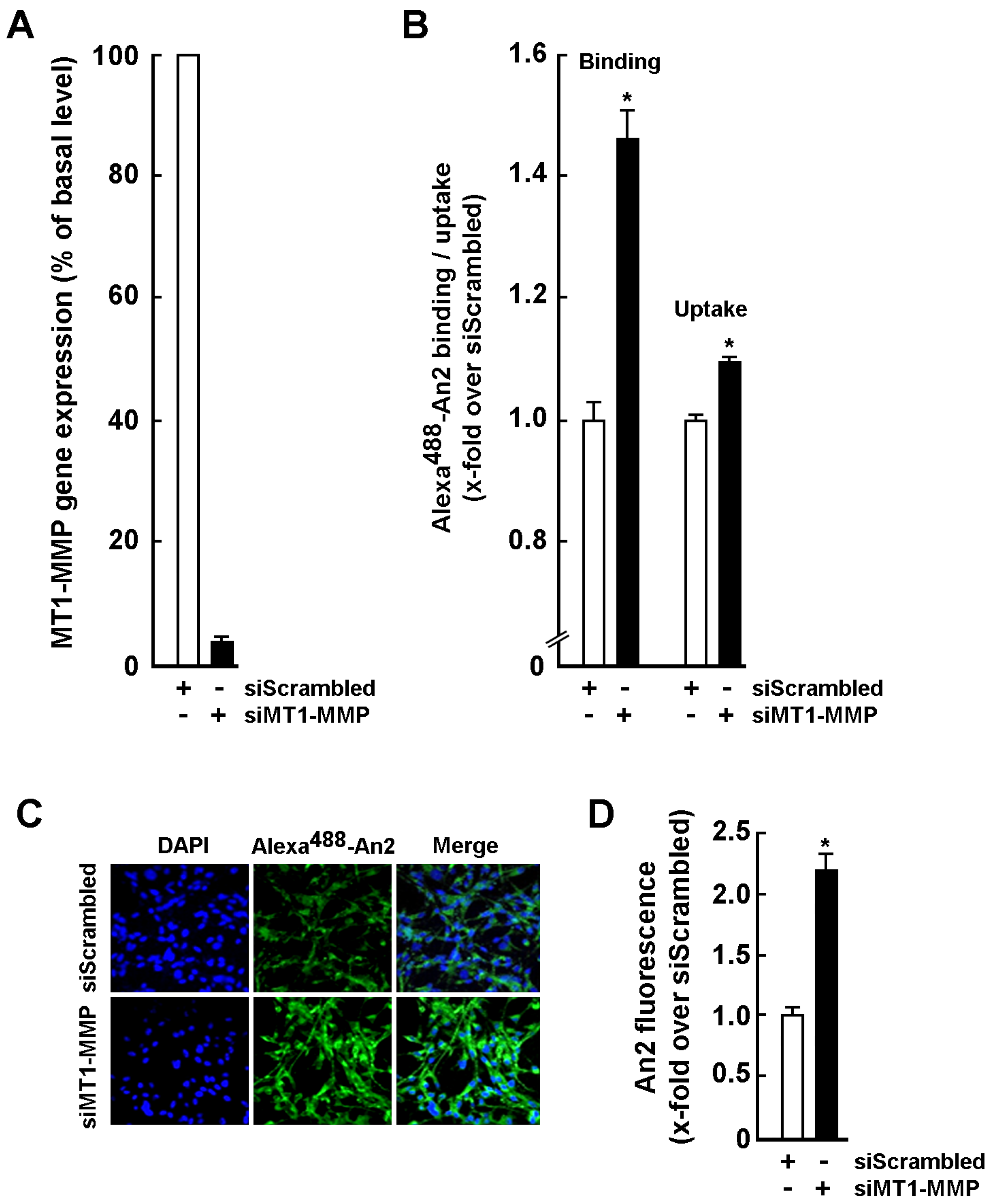
2.2. An2 Internalization in U87 Glioblastoma Cells Is Increased upon MT1-MMP Gene Silencing
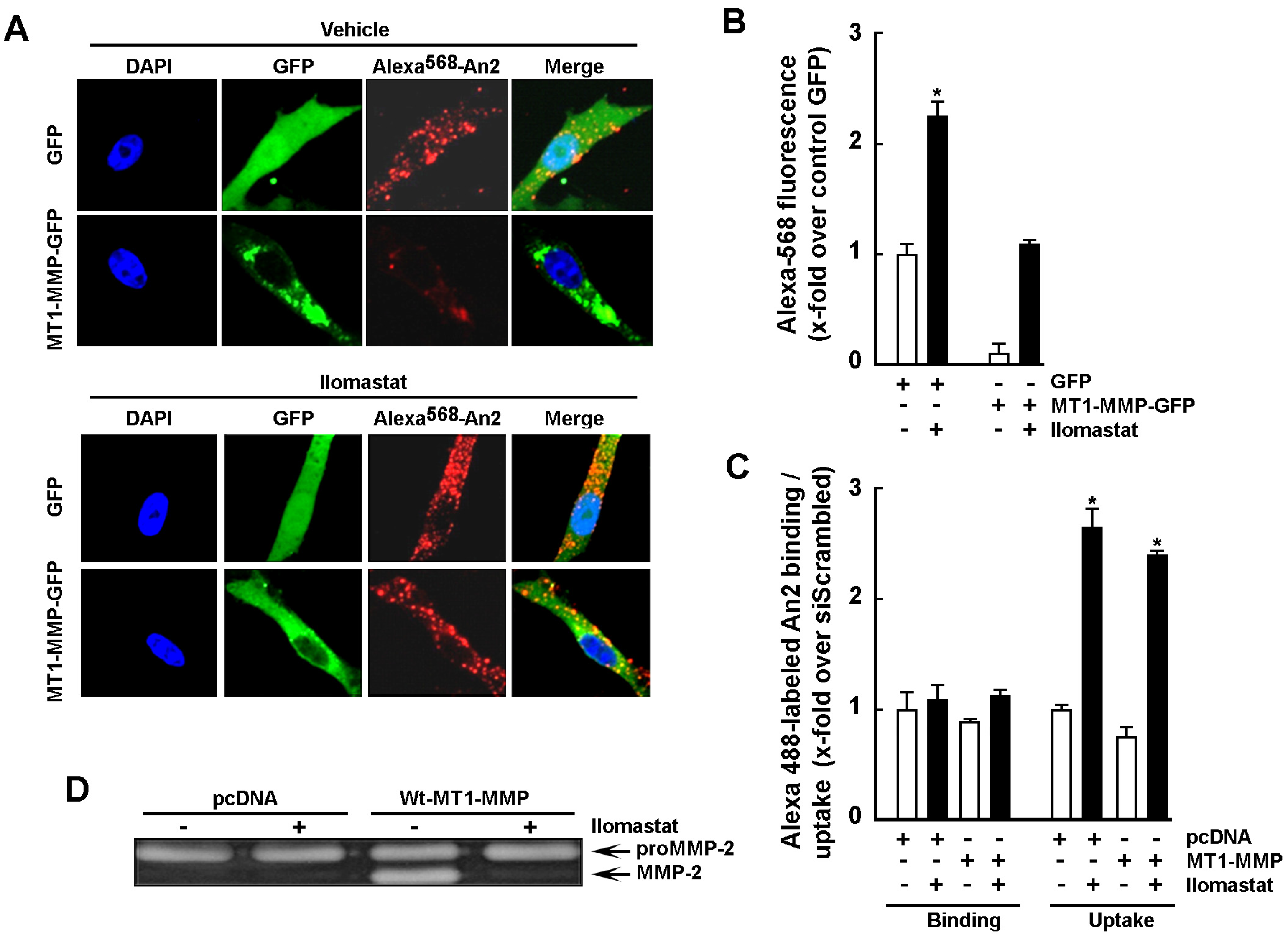
2.3. Increase in Cell Surface MT1-MMP Catalytic Activity Downregulates An2 Internalization
2.4. MT1-MMP Catalytic Activity Is Required for Cell Surface Shedding of LRP-1
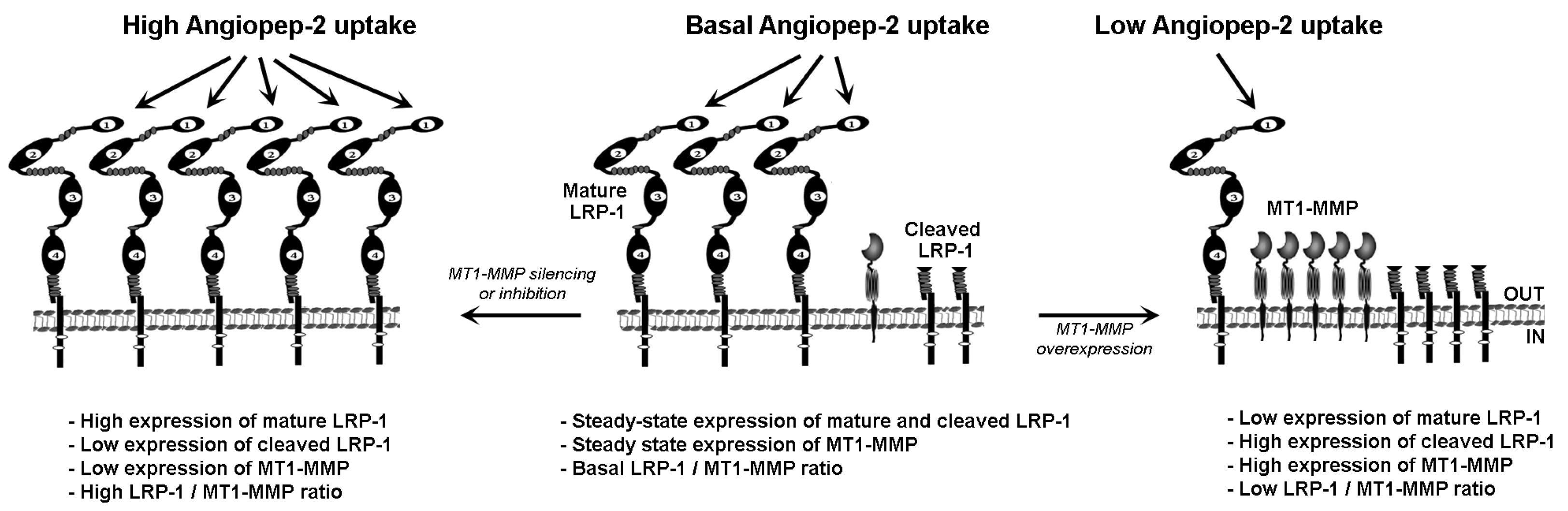
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. TissueScan cDNA Arrays of Grades I-IV Brain Tumor Tissues
4.4. Angiopep-2 Binding and Uptake Assays Using flow Cytometry
4.5. Binding and Uptake Assays of Angiopep-2 Assessed by Confocal Microscopy
4.6. Immunofluorescent Microscopy
4.7. Measurement of LRP-1 Cell-Surface Expression
4.8. Gelatin Zymography
4.9. Total RNA Isolation, cDNA Synthesis and Real-Time Quantitative RT-PCR
4.10. Transfection Method and RNA Interference
4.11. Immunoblotting Procedures
4.12. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [PubMed]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Ajaz, M.; Jefferies, S.; Brazil, L.; Watts, C.; Chalmers, A. Current and investigational drug strategies for glioblastoma. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.L.; Colman, H. Glioma biology and molecular markers. Cancer Treat. Res. 2015, 163, 15–30. [Google Scholar] [PubMed]
- Mou, X.; Ali, Z.; Li, S.; He, N. Applications of magnetic nanoparticles in targeted drug delivery system. J. Nanosci. Nanotechnol. 2015, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Kawami, M.; Aoki, A.; Yumoto, R. Receptor-mediated endocytosis of macromolecules and strategy to enhance their transport in alveolar epithelial cells. Expert Opin. Drug Deliv. 2015, 12, 813–825. [Google Scholar] [CrossRef]
- Harisa, G.I.; Alanazi, F.K. Low density lipoprotein bionanoparticles: From cholesterol transport to delivery of anti-cancer drugs. Saudi Pharm. J. 2014, 22, 504–515. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Fu, C.; Liu, H.; Tan, L.; Meng, X. Multifunctional silica-based nanocomposites for cancer nanotheranostics. J. Biomed. Nanotechnol. 2014, 10, 1784–1809. [Google Scholar] [CrossRef]
- Rosenkranz, A.A.; Ulasov, A.V.; Slastnikova, T.A.; Khramtsov, Y.V.; Sobolev, A.S. Use of intracellular transport processes for targeted drug delivery into a specified cellular compartment. Biochemistry 2014, 79, 928–946. [Google Scholar] [CrossRef]
- Polo, L.; Valduga, G.; Jori, G.; Reddi, E. Low-density lipoprotein receptors in the uptake of tumour photosensitizers by human and rat transformed fibroblasts. Int. J. Biochem. Cell Biol. 2002, 34, 10–23. [Google Scholar] [CrossRef]
- Singh, P.; Prasuhn, D.; Yeh, R.M.; Destito, G.; Rae, C.S.; Osborn, K.; Finn, M.G.; Manchester, M. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J. Control. Release 2007, 120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ye, G.; Li, J.; Wang, Y. Recent advance in molecular angiogenesis in glioblastoma: The challenge and hope for anti-angiogenic therapy. Brain Tumor Pathol. 2015, 32, 229–236. [Google Scholar] [CrossRef]
- Van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sega, E.I.; Low, P.S. Tumor detection using folate receptor-targeted imaging agents. Cancer Metastasis Rev. 2008, 27, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Maletínská, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human glioblastoma cell lines: Levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar]
- Lopes, M.B.; Bogaev, C.A.; Gonias, S.L.; VandenBerg, S.R. Expression of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein is increased in reactive and neoplastic glial cells. FEBS Lett. 1994, 338, 301–305. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Lee, J.; Schwartz, A.L.; Bu, G. Low-density lipoprotein receptor-related protein 1 promotes cancer cell migration and invasion by inducing the expression of matrix metalloproteinases 2 and 9. Cancer Res. 2009, 69, 879–886. [Google Scholar] [CrossRef]
- Demeule, M.; Regina, A.; Che, C.; Poirier, J.; Nguyen, T.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Identification and design of peptides as a new drug delivery system for the brain. J. Pharmacol. Exp. Ther. 2007, 324, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector Angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Che, C.; Yang, G.; Thiot, C.; Lacoste, M.C.; Currie, J.C.; Demeule, M.; Regina, A.; Béliveau, R.; Castaigne, J.P. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J. Med. Chem. 2010, 53, 2814–2824. [Google Scholar] [CrossRef]
- Bertrand, Y.; Currie, J.C.; Poirier, J.; Demeule, M.; Abulrob, A.; Fatehi, D.; Stanimirovic, D.; Sartelet, H.; Castaigne, J.P.; Béliveau, R. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br. J. Cancer 2011, 105, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Tang, S.C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef]
- Pahwa, S.; Stawikowski, M.J.; Fields, G.B. Monitoring and inhibiting MT1-MMP during cancer initiation and progression. Cancers 2014, 6, 416–435. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015, 44–46, 207–223. [Google Scholar] [CrossRef]
- Sina, A.; Proulx-Bonneau, S.; Roy, A.; Poliquin, L.; Cao, J.; Annabi, B. The lectin concanavalin-A signals MT1-MMP catalytic independent induction of COX-2 through an IKKgamma/NF-kappaB-dependent pathway. J. Cell Commun. Signal. 2010, 4, 31–38. [Google Scholar] [CrossRef]
- Proulx-Bonneau, S.; Pratt, J.; Annabi, B. A role for MT1-MMP as a cell death sensor/effector through the regulation of endoplasmic reticulum stress in U87 glioblastoma cells. J. Neurooncol. 2011, 104, 33–43. [Google Scholar] [CrossRef]
- Pratt, J.; Annabi, B. Induction of autophagy biomarker BNIP3 requires a JAK2/STAT3 and MT1-MMP signaling interplay in Concanavalin-A-activated U87 glioblastoma cells. Cell Signal. 2014, 26, 917–924. [Google Scholar] [CrossRef]
- Pratt, J.; Iddir, M.; Bourgault, S.; Annabi, B. Evidence of MTCBP-1 interaction with the cytoplasmic domain of MT1-MMP: Implications in the autophagy cell index of high-grade glioblastoma. Mol. Carcinog. 2016, 55, 148–160. [Google Scholar] [CrossRef]
- He, L.; Chu, D.; Li, X.; Zheng, J.; Liu, S.; Li, J.; Zhao, Q.; Ji, G. Matrix metalloproteinase-14 is a negative prognostic marker for patients with gastric cancer. Dig. Dis. Sci. 2013, 58, 1264–1270. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wu, K.P.; Wu, A.B.; Yang, Z.C.; Li, J.M.; Mo, Y.L.; Xu, M.; Wu, B.; Yang, Z.X. MMP-14 overexpression correlates with poor prognosis in non-small cell lung cancer. Tumour Biol. 2014, 35, 9815–9821. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xue, Y.X.; Liu, L.B.; Wang, P.; Liu, Y.H.; Ying, H.Q. Expressions of matrix metalloproteinase-7 and matrix metalloproteinase-14 associated with the activation of extracellular signal-regulated kinase1/2 in human brain gliomas of different pathological grades. Med. Oncol. 2011, 28 (Suppl. 1), S433–S438. [Google Scholar] [CrossRef] [PubMed]
- Nanni, S.B.; Pratt, J.; Beauchemin, D.; Haidara, K.; Annabi, B. Impact of Concanavalin-A-mediated cytoskeleton disruption on low-density lipoprotein receptor-related protein-1 internalization and cell surface expression in glioblastomas. Biomark. Cancer 2016, 8, 77–87. [Google Scholar] [CrossRef]
- Quinn, K.A.; Pye, V.J.; Dai, Y.P.; Chesterman, C.N.; Owensby, D.A. Characterization of the soluble form of the low density lipoprotein receptor-related protein (LRP). Exp. Cell Res. 1999, 251, 433–441. [Google Scholar] [CrossRef]
- Rozanov, D.V.; Hahn-Dantona, E.; Strickland, D.K.; Strongin, A.Y. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J. Biol. Chem. 2004, 279, 4260–4268. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, J.; Tran, H.; Verbeek, M.M.; Reiss, K.; Estus, S.; Bu, G. LRP1 shedding in human brain: Roles of ADAM10 and ADAM17. Mol. Neurodegener. 2009, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Selvais, C.; Gaide Chevronnay, H.P.; Lemoine, P.; Dedieu, S.; Henriet, P.; Courtoy, P.J.; Marbaix, E.; Emonard, H. Metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 ectodomain decreases endocytic clearance of endometrial matrix metalloproteinase-2 and -9 at menstruation. Endocrinology 2009, 150, 3792–3799. [Google Scholar] [CrossRef]
- Emonard, H.; Theret, L.; Bennasroune, A.H.; Dedieu, S. Regulation of LRP-1 expression: Make the point. Pathol. Biol. 2014, 62, 84–90. [Google Scholar] [CrossRef]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef]
- Amalinei, C.; Caruntu, I.D.; Giusca, S.E.; Balan, R.A. Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. 2010, 51, 215–228. [Google Scholar]
- Fields, G.B. New strategies for targeting matrix metalloproteinases. Matrix Biol. 2015, 44–46, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Lehti, K.; Rose, N.F.; Valavaara, S.; Weiss, S.J.; Keski-Oja, J. MT1-MMP promotes vascular smooth muscle dedifferentiation through LRP1 processing. J. Cell Sci. 2009, 122, 126–315. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Lucchiari, C.; Botturi, A.; Silvani, A.; Lamperti, E.; Gaviani, P.; Innocenti, A.; Finocchiaro, C.Y.; Masiero, M.; Pravettoni, G. Cognitive strategies and quality of life of patients with high-grade glioma. Support. Care Cancer 2015, 23, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Ulasov, I.; Thaci, B.; Sarvaiya, P.; Yi, R.; Guo, D.; Auffinger, B.; Pytel, P.; Zhang, L.; Kim, C.K.; Borovjagin, A.; et al. Inhibition of MMP14 potentiates the therapeutic effect of temozolomide and radiation in gliomas. Cancer Med. 2013, 2, 457–467. [Google Scholar] [CrossRef]
- Ulasov, I.; Borovjagin, A.V.; Kaverina, N.; Schroeder, B.; Shah, N.; Lin, B.; Baryshnikov, A.; Cobbs, C. MT1-MMP silencing by an shRNA-armed glioma-targeted conditionally replicative adenovirus (CRAd) improves its anti-glioma efficacy in vitro and in vivo. Cancer Lett. 2015, 365, 240–250. [Google Scholar] [CrossRef]
- Proulx-Bonneau, S.; Guezguez, A.; Annabi, B. A concerted HIF-1α/MT1-MMP signalling axis regulates the expression of the 3BP2 adaptor protein in hypoxic mesenchymal stromal cells. PLoS ONE 2011, 6, e21511. [Google Scholar] [CrossRef]
- Annabi, B.; Laflamme, C.; Sina, A.; Lachambre, M.P.; Béliveau, R. A MT1-MMP/NF-kappaB signaling axis as a checkpoint controller of COX-2 expression in CD133+ U87 glioblastoma cells. J. Neuroinflammation 2009, 6, 8. [Google Scholar] [CrossRef]
- Fortier, S.; Labelle, D.; Sina, A.; Moreau, R.; Annabi, B. Silencing of the MT1-MMP/ G6PT axis suppresses calcium mobilization by sphingosine-1-phosphate in glioblastoma cells. FEBS Lett. 2008, 582, 799–804. [Google Scholar] [CrossRef] [PubMed]
- Ohkawara, H.; Ishibashi, T.; Sugimoto, K.; Ikeda, K.; Ogawa, K.; Takeishi, Y. Membrane type 1-matrix metalloproteinase/Akt signaling axis modulates TNF-α-induced procoagulant activity and apoptosis in endothelial cells. PLoS ONE 2014, 9, e105697. [Google Scholar] [CrossRef] [PubMed]
- Mahimkar, R.; Alfonso-Jaume, M.A.; Cape, L.M.; Dahiya, R.; Lovett, D.H. Graded activation of the MEK1/MT1-MMP axis determines renal epithelial cell tumor phenotype. Carcinogenesis 2011, 32, 1806–1814. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Ishibashi, T.; Sawamura, T.; Inoue, N.; Kamioka, M.; Uekita, H.; Ohkawara, H.; Sakamoto, T.; Sakamoto, N.; Okamoto, Y.; et al. LOX-1-MT1-MMP axis is crucial for RhoA and Rac1 activation induced by oxidized low-density lipoprotein in endothelial cells. Cardiovasc. Res. 2009, 84, 127–136. [Google Scholar] [CrossRef]
- Jiang, A.; Lehti, K.; Wang, X.; Weiss, S.J.; Keski-Oja, J.; Pei, D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc. Natl. Acad. Sci. USA 2001, 98, 13693–13698. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, C. RAB38 confers a poor prognosis, associated with malignant progression and subtype preference in glioma. Oncol. Rep. 2013, 30, 2350–2356. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Zhang, C.; Li, M.; Jiang, C.; Li, Y. Rab27a was identified as a prognostic biomaker by mRNA profiling, correlated with malignant progression and subtype preference in gliomas. PLoS ONE 2014, 9, e89782. [Google Scholar] [CrossRef]
- Wang, H.J.; Gao, Y.; Chen, L.; Li, Y.L.; Jiang, C.L. RAB34 was a progression- and prognosis-associated biomarker in gliomas. Tumour Biol. 2015, 36, 1573–1578. [Google Scholar] [CrossRef]
- Markovic, D.S.; Vinnakota, K.; Chirasani, S.; Synowitz, M.; Raguet, H.; Stock, K.; Sliwa, M.; Lehmann, S.; Kalin, R.; van Rooijen, N.; et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl. Acad. Sci. USA 2009, 106, 12530–12535. [Google Scholar] [CrossRef]
- Gingras, D.; Bousquet-Gagnon, N.; Langlois, S.; Lachambre, M.P.; Annabi, B.; Béliveau, R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP). FEBS Lett. 2001, 507, 231–236. [Google Scholar] [CrossRef]
- Belkaid, A.; Fortier, S.; Cao, J.; Annabi, B. Necrosis induction in glioblastoma cells reveals a new “bioswitch” function for the MT1-MMP/G6PT signaling axis in proMMP-2 activation versus cell death decision. Neoplasia 2007, 9, 332–340. [Google Scholar] [CrossRef][Green Version]
- Annabi, B.; Bouzeghrane, M.; Moumdjian, R.; Moghrabi, A.; Béliveau, R. Probing the infiltrating character of brain tumors: Inhibition of RhoA/ROK-mediated CD44 cell surface shedding from glioma cells by the green tea catechin EGCg. J. Neurochem. 2005, 94, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The role of CD44 in disease pathophysiology and targeted treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Orian-Rousseau, V.; Ponta, H. Perspectives of CD44 targeting therapies. Arch. Toxicol. 2015, 89, 3–14. [Google Scholar] [CrossRef]
- Bukowska, B.; Marczak, A. Innovative therapy of ovarian cancer based on overexpression of CD44 receptor. Ginekol. Pol. 2015, 86, 388–391. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, S.; Yang, X.; Zhai, G. Current research on hyaluronic acid-drug bioconjugates. Eur. J. Med. Chem. 2014, 86, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Winer, A.; Adams, S.; Mignatti, P. Matrix Metalloproteinase Inhibitors in Cancer Therapy: Turning Past Failures into Future Successes. Mol. Cancer Ther. 2018, 17, 1147–1155. [Google Scholar] [CrossRef]
- Kurzrock, R.; Gabrail, N.; Chandhasin, C.; Moulder, S.; Smith, C.; Brenner, A.; Sankhala, K.; Mita, A.; Elian, K.; Bouchard, D.; et al. Safety, pharmacokinetics, and activity of GRN1005, a novel conjugate of angiopep-2, a peptide facilitating brain penetration, and paclitaxel, in patients with advanced solid tumors. Mol. Cancer Ther. 2012, 11, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Regina, A.; Demeule, M.; Tripathy, S.; Lord-Dufour, S.; Currie, J.C.; Iddir, M.; Annabi, B.; Castaigne, J.P.; Lachowicz, J.E. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 2015, 14, 129–140. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratt, J.; Haidara, K.; Annabi, B. MT1-MMP Expression Levels and Catalytic Functions Dictate LDL Receptor-Related Protein-1 Ligand Internalization Capacity in U87 Glioblastoma Cells. Int. J. Mol. Sci. 2022, 23, 14214. https://doi.org/10.3390/ijms232214214
Pratt J, Haidara K, Annabi B. MT1-MMP Expression Levels and Catalytic Functions Dictate LDL Receptor-Related Protein-1 Ligand Internalization Capacity in U87 Glioblastoma Cells. International Journal of Molecular Sciences. 2022; 23(22):14214. https://doi.org/10.3390/ijms232214214
Chicago/Turabian StylePratt, Jonathan, Khadidja Haidara, and Borhane Annabi. 2022. "MT1-MMP Expression Levels and Catalytic Functions Dictate LDL Receptor-Related Protein-1 Ligand Internalization Capacity in U87 Glioblastoma Cells" International Journal of Molecular Sciences 23, no. 22: 14214. https://doi.org/10.3390/ijms232214214
APA StylePratt, J., Haidara, K., & Annabi, B. (2022). MT1-MMP Expression Levels and Catalytic Functions Dictate LDL Receptor-Related Protein-1 Ligand Internalization Capacity in U87 Glioblastoma Cells. International Journal of Molecular Sciences, 23(22), 14214. https://doi.org/10.3390/ijms232214214





