Abstract
We isolated seven new iridoid glucosides (valerianairidoids I–VII; 1–3, 6, 7, 9, and 12) and six known compounds from the methanol extract of the dried rhizomes and roots of Valeriana fauriei. Chemical and spectroscopic data were used to elucidate the chemical structures of the seven new iridoid glucosides, and their absolute configurations were determined by comparing their electronic circular dichroism (ECD) spectra with those determined experimentally. Aglycones 1a, 6a, and 9a, which were obtained by enzymatic hydrolysis of the isolated iridoid glucosides, exhibited anti-proliferative activities against cancer stem cells (CSCs) established by a sphere-formation assay using human breast cancer (MDA-MB-231) and human astrocytoma (U-251MG) cells. Interestingly, these iridoids selectively showed anti-proliferative activities against CSCs from MDA-MB-231 cells. These results suggest that the iridoids obtained in this study may have potency as a breast cancer treatment and as preventive agent via exterminating CSCs.
1. Introduction
Valeriana fauriei Briquet (Valerianaceae) is abundant in both Japan and China and has been used for centuries as a sedative and antispasmodic agent []. Iridoids bearing isovaleryl moieties [] and cyclized guaiane-type sesquiterpenes [], among others, have been isolated as V. fauriei constituents. As part of our ongoing research aimed at discovering new cancer-treatment and preventive agents [,,,], we found that cyclized guaiane-type sesquiterpenes and lignans from V. fauriei show cell-death-inducing activities against adriamycin-treated (ADR-treated) HeLa cells by inhibiting heat-shock protein (HSP), and through anti-proliferative effects against cancer stem cells (CSCs) and human astrocytoma cells []. As part of our continuing study, we isolated iridoid glycosides and evaluated their anti-proliferative activities against CSCs.
CSCs have been identified in many types of malignancy—including leukemia and breast, colorectal, and brain cancers []—which are leading causes of cancer recurrence following anti-cancer drug treatment because these cells are resistant to current anti-cancer drugs and radiation therapy, and play important roles in metastasis by acquiring mesenchymal properties, including improved motility and enhanced invasiveness []. Therefore, compounds that are anti-proliferative against CSCs are potentially useful in cancer treatment and as preventive agents. Pyranocoumarin [], lignans [], and sesquiterpenes [] have been reported as naturally occurring agents that are anti-proliferative toward CSCs. In this report, we describe the isolation, structure determination, and anti-proliferative activities of isolated iridoid glycosides and their derivatives against CSCs obtained using a sphere-formation assay with MDA-MB-231 and U-251MG cells.
2. Results and Discussion
2.1. Isolating Constituents
The methanol (MeOH) extract of the dried rhizomes and roots of V. fauriei was partitioned in ethyl acetate–H2O (1:1, v/v) to furnish an ethyl acetate fraction and an aqueous layer. The latter was partitioned with n-BuOH to yield a BuOH fraction (2.4%), which was subsequently separated by normal- and reverse-phase silica-gel column chromatography and high-performance liquid chromatography (HPLC) to yield seven new compounds with the yields (%, weight/weight from the weight of the isolated compounds and dried plant) described later: valerianairidoid I (1, 0.0017%), valerianairidoid II (2, 0.00066%), valerianairidoid III (3, 0.0025%), valerianairidoid IV (6, 0.0012%), valerianairidoid V (7, 0.0027%), valerianairidoid VI (9, 0.00076%), and valerianairidoid VII (12, 0.00011%), together with six known compounds, namely patrinoside (4, 0.012%) [], kanokoside D (5, 0.0022%) [], suspensolide F (8, 0.0027%) [], kanokoside A (10, 0.0020%) [], kanokoside C (11, 0.0036%) [], and 10-isovaleryloxykanokoside C (13, 0.0043%) [] (Figure 1).
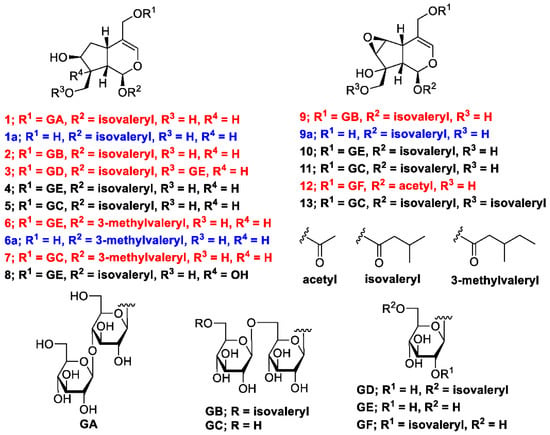
Figure 1.
Chemical structures of constituents 1–13 isolated from V. fauriei and their derivatives 1a, 6a, and 9a.
2.2. Determining the Structures of Valerianairidoids I–VII (1–3, 6, 7, 9, and 12)
Valerianairidoid I (1) was isolated as an amorphous solid with a negative optical rotation ([α]25D −36.3 in MeOH). Its molecular formula (C27H44O16) was determined by high-resolution mass spectrometry (HRMS) and 13C nuclear magnetic resonance (NMR) spectroscopy (m/z 647 [M+Na]+). The 1H and 13C NMR (CD3OD) spectra of 1 (Table 1) show signals consistent with an iridoid moiety {a methylene [δ H 1.82 (m, H-6 α) and 2.06 (m, H-6 β)], two methylenes, each bearing an oxygen functional group [δ H 3.73 (dd, J = 5.5, 11.0, H-10a), 3.80 (m, H-10b), 4.08 (d, J = 11.0, H-11a), and 4.23 (d, J = 11.0, H-11b)], a methine bearing an oxygen function group [δ H 4.33 (m, H-7)], three methines [δ H 3.01 (dd, J = 7.6, 15.8, H-5), 1.94 (m, H-8), and 2.17 (m, H-9)], an olefinic group [δ C 140.1 (C-3) and 116.4 (C-4)], an acetal moiety [δ C 93.6 (C-1)]}, an isovaleryl moiety {two methyl groups [δ H 0.96 (d, J = 6.9, H-4‴ and 5‴)], a methylene [δ H 2.23 (d, J = 6.9, H-2‴)], a methine [δ H 2.09 (m, H-3‴)], and an ester group [δ C 173.3 (C-1‴)]}, and a cellobiose moiety. The presence and position of the abovementioned cellobiose moiety were determined using double-quantum-filtered homonuclear correlation spectroscopy (DQF COSY) and heteronuclear multiple bond coherence (HMBC) spectroscopy (Figure 2). Long-range H-1′/C-11, H-1″/C-4′, and H-1/C-1‴ correlations were observed, suggesting that the cellobiose moiety was attached at C-11 and the isovaleryl moiety was attached at C-1. The relative configuration was determined using nuclear Overhauser enhancement spectroscopy (NOESY) (Figure 3), which revealed H-1/H-8, H-6α/H-7, and H-7/H-8 NOESY cross-peaks that indicate that H-1, H-6α, H-7, and H-8 are located on the same side in 1. In addition, H-5/H-9, H-5/H-6β, and H-9/H-10 NOESY cross-peaks suggest that H-5, H-6β, H-9, and H-10 are located on the same side. We obtained aglycone 1a by enzymatically hydrolyzing 1. While 1a was identified to be a patrinoside-aglucone by NMR spectroscopy and MS, its absolute configuration was not previously discussed in []. Therefore, we determined the absolute configuration of 1a by calculating its electronic circular dichroism (ECD) spectrum. The calculated ECD data for 1S,5S,7S,8S,9S-configured 1a are in good agreement with the experimental data, whereas the calculated ECD spectrum of ent-1a (1R,5R,7R,8R,9R) is essentially the mirror image of that acquired experimentally (Figure 4). Finally, 1 was subjected to acid hydrolysis using 20% aqueous H2SO4 in 1,4-dioxane, which yielded d-glucose by HPLC separation of its diastereomeric tolylthiocarbamoyl thiazolidine derivative []. The coupling constants (J = 7.6 Hz) for the anomeric position of the two glucoses suggested that they have β-configurations at the glycosidic bonds. We conclude that the chemical structure of valerianairidoid I (1) is as shown in Figure 1, based on the evidence provided above.

Table 1.
13C (150 MHz) and 1H (600 MHz) NMR data for valerianairidoids I–III (1–3) in CD3OD.
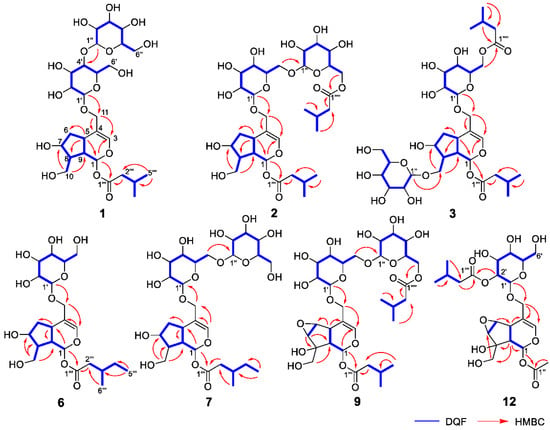
Figure 2.
Important 2D NMR correlations observed for new compounds 1–3, 6, 7, 9, and 12.
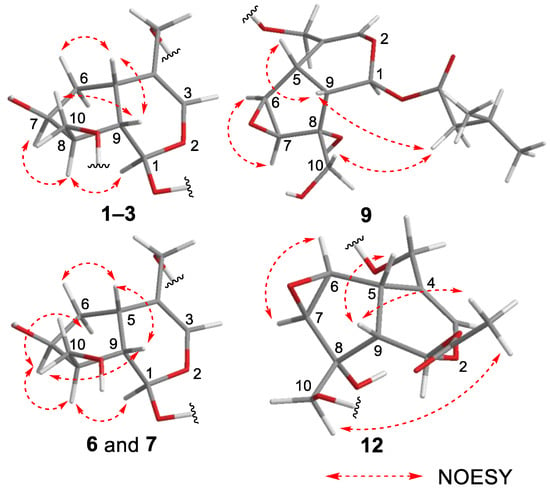
Figure 3.
Important NOESY correlations observed for new compounds 1–3, 6, 7, 9, and 12.
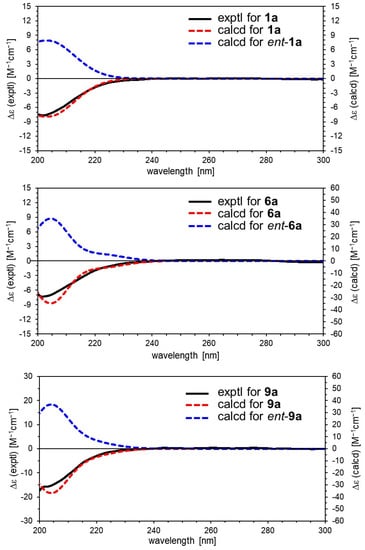
Figure 4.
Experimental and calculated ECD spectra for 1a, 6a, and 9a.
Valerianairidoids II and III (2 and 3) were isolated as amorphous powders with negative optical rotations (2: [α]25D −31.3; 3: [α]25D −20.5 in MeOH). Their molecular formulas (C32H52O17) were determined using HRMS and 13C NMR spectroscopy. A comparison of the NMR data for 1, 2, and 3 reveals that they contain the same aglycone, a glucose moiety attached at C-11, and an isovaleryl moiety attached at C-1. Moreover, 2 and 3 each contain an additional glucose unit and an additional isovaleryl substituent. The HMBC correlations [2: H-6″/C-1′′′′ and H-6′/C-1″] and [3: H-1″/C-10 and H-6′/C-1′′′′] and total correlation spectroscopy (TOCSY) correlation [3: H-1′/H-6′] suggest that the additional glucose moiety is attached at C-6′ in 2 and C-10 in 3, and that the additional isovaleryl moiety is attached at C-6″ in 2 and C-6′ in 3 (Figure 2). Enzymatic hydrolysis of 2 gave 1a, which suggests that 1 and 2 share the same absolute configuration, and the absolute configuration of 3 was deduced to be identical to that of 1 and 2. Based on the above data, we conclude that valerianairidoids II and III (2 and 3, respectively) have the chemical structures shown in Figure 1.
Valerianairidoids IV and V (6 and 7) were isolated as amorphous solids with negative optical rotations (6: [α]25D −21.6; 7: [α]25D −48.9 in MeOH). Their molecular formulas (6: C22H36O11 and 7: C28H46O16) were determined by HRMS, high-resolution electrospray ionization MS (HRESIMS), and 13C NMR spectroscopy. The 1H and 13C NMR (CD3OD) data suggest that valerianairidoid IV (6) contains an iridoid moiety and a glucose moiety at C-11, in a similar manner to 1. In addition, 6 contains a 3-methylvaleryl moiety {two methyl groups [δ H 0.89 (t, J = 7.8, H-5‴) and 0.92 (d, J = 6.8, H-6‴)], two methylene groups [δ H 2.12 (m, H-2‴a), 2.33 (dd, J = 6.0, 15.0, H-2‴b), 1.24 (m, H-4‴a) and 1.37 (m, H-4‴b)], a methine [δ H 1.84 (m, H-3‴)], and an ester moiety [δ C 173.5 (C-1‴)]}. The positions of the 3-methylvaleryl and glucose moieties in 6 were determined from the DQF COSY and HMBC data shown in Figure 2. A comparison of the NMR data for 6 and 7 reveals that 7 contains one more glucose moiety attached at C-6′ compared to 6. The same aglycone (6a) was obtained when 6 and 7 were enzymatically hydrolyzed using β-glucosidase. The planar chemical structure and relative configuration of 6a were elucidated from the NMR and MS data (Table 2). The absolute configuration of 6a was determined to be 1S,5S,7S,8S,9S based on the experimental and calculated ECD spectra, in a similar manner to 1a (Figure 4). We conclude that the chemical structures of valerianairidoids IV and V (6 and 7, respectively) are as shown in Figure 1.

Table 2.
13C (150 MHz) and 1H (600 MHz) NMR data for valerianairidoid IV (6), its aglycone 6a, and valerianairidoid V (7).
Valerianairidoid VI (9) was isolated as an amorphous solid with a negative optical rotation ([α]25D −15.4 in MeOH), and its molecular formula (C32H50O18) was determined by HRESIMS and 13C NMR spectroscopy. The 1H and 13C NMR (CD3OD) spectra suggest that 9 contains an iridoid moiety, a gentiobiose moiety, and two isovaleryl moieties (Table 3); their positions were determined by DQF COSY and HMBC NMR spectroscopy (Figure 2), and the relative stereochemistry of 9 was determined by NOESY spectroscopy (Figure 3). Aglycone 9a was obtained by the enzymatic hydrolysis of 9 using cellulase and identified as the aglycone of kanokoside A []; however, its absolute configuration was not determined previously. The absolute configuration of 9 was further established as 1S,5S,6S,7S,8R,9S by comparing the experimental and calculated ECD spectra of 9a. We conclude that the chemical structure of valerianairidoid VI (9) is as shown in Figure 1.

Table 3.
13C (150 MHz) and 1H NMR (600 MHz) data for valerianairidoids VI and VII (9 and 12) recorded in CD3OD.
Valerianairidoid VII (12) was isolated as an amorphous powder with a negative optical rotation ([α]25D −47.8 in MeOH); its molecular formula (C23H34O13) was determined by HRMS and 13C NMR spectroscopy, and its 1H and 13C NMR (CD3OD) spectra suggest that 12 contains iridoid and glucose moieties in a similar manner to 9. In addition, 12 contains isovaleryl and acetyl moieties {a methyl [δ H 2.05 (s, H-2″) and an ester [δ C 172.4 (C-1″)]} (Table 3). The positions of the isovaleryl and acetyl moieties were determined by DQF COSY and HMBC NMR spectroscopy (Figure 2). The absolute configuration of the iridoid moiety was determined to be that same as in 9. We conclude that the chemical structure of valerianairidoid VII (12) is as shown in Figure 1.
2.3. Evaluating Anti-Proliferative Activities against Non-CSCs and CSCs
The antiproliferative activities of isolated compounds 1–13 and aglycones 1a, 6a, and 9a against MDA-MB-231 and U-251MG cells (non-CSCs) and their CSCs were evaluated. The CSCs used in this study were established using a sphere-formation assay, with sphere formation confirmed by cell morphology and the expression of the Nanog stem-cell marker in both MDA-MB-231 and U-251MG cells []. ADR was used as the positive control, and cell viability was evaluated using the CellTiter-Glo® 3D cell viability assay. None of the isolated compounds showed any anti-proliferative activity against either the non-CSC or CSC cell line [1–13; IC50 > 100 μM]. On the other hand, aglycones 1a, 6a, and 9a showed significant anti-proliferative activities against CSCs from MDA-MB-231 [IC50; 1a (45.0 ± 5.3 μM), 6a (17.6 ± 1.0 μM), and 9a (47.7 ± 3.9 μM)]. Interestingly, 1a, 6a, and 9a showed anti-proliferative activities against CSCs with lower IC50 values than non-CSCs of MDA-MB-231 [IC50; 1a (>100 μM), 6a (47.7 ± 4.2 μM), and 9a (>100 μM)] cells (full experimental data applied for IC50 calculations are described in Supplementary Materials S5). On the other hand, 1a, 6a, and 9a showed no activity (IC50 > 100 μM) against either non-CSCs or CSCs from U-251MG cells. Therefore, these aglycones may have an inhibitory effect on the characteristic signal pathway(s) in CSCs from MDA-MB-231 cells.
3. Materials and Methods
3.1. General Experimental Procedures
Specific rotations were measured using a P-2200 digital polarimeter (l = 5 cm; JASCO, Tokyo, Japan). IR spectra were measured using a JASCO FT/IR-4600 typeA spectrometer; ECD spectroscopy was performed using a JASCO J-1500 spectrometer. ESI and high-resolution ESI mass spectra were recorded on a Shimadzu LCMS-IT-TOF instrument. FAB and HRFABMS data were recorded using a JASCO SX-102A mass spectrometer. 1H NMR spectra were recorded on JEOL ECS400 (400 MHz) and JNM-ECA 600 (600 MHz) spectrometers (JEOL). 13C NMR spectroscopy was performed on a JEOL JNM-ECA 600 (150 MHz) spectrometer. 2D-NMR experiments were carried out on a JEOL JNM-ECA 600 (600 MHz) spectrometer (JEOL).
Normal-phase silica-gel column chromatography was performed using silica gel 60 (63–210 μm; Kanto Chemical Co., Tokyo, Japan). Reverse-phase silica-gel column chromatography was performed using C18-OPN gel (140 μm; Nacalai Tesque, Kyoto, Japan). HPLC was performed using an SPD-M10Avp UV-vis detector (Shimadzu, Kyoto, Japan).
3.2. Plant Material
The dried rhizomes and roots of V. fauriei from Hokkaido (Japan) were purchased from Tochimoto Tenkaido (Osaka Prefecture, Japan) in August 2020.
3.3. Extracting and Isolating Compounds
The methanol extract (1150 g) of the dried rhizomes and roots of V. fauriei (6 kg) was prepared as previously described []. The methanol extract was partitioned into ethyl acetate–H2O (1:1, v/v). The H2O layer was mixed at a 1:1 v/v ratio with n-BuOH to provide a BuOH-soluble fraction (Fr. B, 146.25 g, 2.4%). A portion of the BuOH-soluble fraction (69.56 g) was separated using normal-phase silica-gel column chromatography [CHCl3–MeOH (1:0 → 50:1 → 20:1 → 10:1 → 5:1 → 3:1 → 1:1 v/v)] to yield eight fractions (B1–B8). Fraction B6 (5844.6 mg) was separated by reverse-phase silica-gel column chromatography into ten fractions (B6-1–10). Fraction B6-4 (1830.55 mg) was purified by HPLC {COSMOSIL 5C18-MS-II (250 × 4.6 mm i.d. and 250 × 20 mm i.d.); mobile phase: H2O–CH3CN (41:9, v/v)} to yield 4 (354.9 mg). Fraction B6-5 (598.34 mg) was purified by HPLC {COSMOSIL 5C18-MS-II (250 × 4.6 mm i.d. and 250 × 20 mm i.d.); mobile phase: H2O–CH3CN (41:9, v/v)} to yield 6 (34.76 mg) and 12 (3.06 mg). Fraction B7 (7048.6 mg) was separated by reverse-phase silica-gel column chromatography into seven fractions (B7-1–7). Fraction B7-3 (2.8577 g) was purified by HPLC {COSMOSIL 5C18-MS-II (250 × 4.6 mm i.d. and 250 × 20 mm i.d.); mobile phase: H2O–CH3CN (7:3, v/v)} to yield 8 (76.69 mg) and 10 (57.57 mg). Fraction B7-5 (514.02 mg) was purified by HPLC {COSMOSIL 5C18-MS-II (250 × 4.6 mm i.d. and 250 × 20 mm i.d.); mobile phase: H2O–CH3CN (7:3, v/v)} to yield 2 (18.95 mg), 3 (72.15 mg), 9 (21.58 mg), and 13 (123.42 mg). Fraction B8 (17.87 g) was separated by reverse-phase silica-gel column chromatography to yield seven fractions (B8-1–7). Fraction B8-6 (1185.29 mg) was purified by HPLC {YMC-Actus Triart C18 (250 × 4.6 mm i.d. and 250 × 20 mm i.d.); mobile phase: H2O–CH3CN (3:2, v/v)} to yield 1 (48.23 mg), 5 (61.96 mg), 7 (77.77 mg), and 11 (102.70 mg).
3.4. Valerianairidoid I (1)
Amorphous solid, [α]25D −36.3 (c 0.3, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 1), IR (ATR) νmax 3360, 2931, 1745,1665, 1375, and 1073 cm−1, ESI-MS m/z 647 [M+Na]+, HRESIMS m/z 647.2534 [M+Na]+ (calcd. for C27H44O16Na, 647.2522).
3.5. Valerianairidoid II (2)
Amorphous solid, [α]25D −31.3 (c 0.1, MeOH), 1H NMR (CD3OD, 600 MHz), 13C NMR (150 MHz) (Table 1), IR (ATR) νmax 3359, 2957, 1731, 1369, 1294, and 1033 cm−1, FABMS m/z 731 [M+Na]+, HRFABMS m/z 731.3092 [M+Na]+ (calcd. for C32H52O17Na, 731.3102).
3.6. Valerianairidoid III (3)
Amorphous solid, [α]25D −20.5 (c 0.5, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 1), IR (ATR) νmax 3363, 2960, 1734, 1367, 1294, and 1018 cm−1, FABMS m/z 731 [M+Na]+, HRFABMS m/z 731.3105 [M+Na]+ (calcd. for C32H52O17Na, 731.3102).
3.7. Valerianairidoid IV (6)
Amorphous solid, [α]25D −21.6 (c 0.5, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 2), IR (ATR) νmax 3363, 2927, 1748, 1664, 1362, and 1076 cm−1, ECD (MeOH) [201.0 nm (Δε −18.8)], FABMS m/z 499 [M+Na]+, HRFABMS m/z 499.2159 [M+Na]+ (calcd. for C22H36O11Na, 499.2155).
3.8. Valerianairidoid V (7)
Amorphous solid, [α]25D −48.9 (c 0.3, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 2); IR (ATR) νmax 3336, 2923, 1741, 1362, 1267, and 1032 cm−1, ESI-MS m/z 661 [M+Na]+, HRESIMS m/z 661.2681 [M+Na]+ (calcd. for C28H46O16Na, 661.2678).
3.9. Valerianairidoid VI (9)
Amorphous solid, [α]25D −15.4 (c 0.1, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 3), IR (ATR) νmax 3366, 2958, 1731, 1370, 1294, and 1032 cm−1, FABMS m/z 745 [M+Na]+, HRFABMS m/z 745.2881 [M+Na]+ (calcd. for C32H50O18Na, 745.2895).
3.10. Valerianairidoid VII (12)
Amorphous solid, [α]25D −47.8 (c 0.2, MeOH), 1H NMR (CD3OD, 600 MHz) and 13C NMR (150 MHz) (Table 3), IR (ATR) νmax 3356, 2924, 1745, 1670, 1362, and 1032 cm−1, ESIMS m/z 541 [M+Na]+, HRESIMS m/z 541.1893 [M+Na]+ (calcd. for C23H34O13Na, 541.1892).
3.11. Enzymatic Hydrolysis of Valerianairidoids I, II, IV, V, and VI (1, 2, 6, 7, and 9)
Enzymes [1, 6, and 7: β-glucosidase (5 mg, from sweet almond); 2 and 9: cellulase (10 mg, from Aspergillus niger)] were added to a solution of the valerianairidoid [1 and 7: 5 mg in 100 mM acetate buffer (5.0 mL, pH 5.5); 2: 5 mg in 50 mM citric acid–NaOH buffer (5.0 mL, pH 4.0); 6: 10 mg in 100 mM acetate buffer (5.0 mL, pH 5.5); 9: 10 mg in 50 mM citric acid–NaOH buffer (5.0 mL, pH 4.0)] and the mixture was stirred at 35 °C for 24 h. The supernatant solution was concentrated under vacuum to obtain a residue, which was subjected to HPLC {COSMOSIL 5C18-MS-II (250 × 4.6 mm), mobile phase: H2O–CH3CN (7:3, v/v)} to obtain the aglycone [1: 1a (0.26 mg); 2: 1a (0.85 mg); 6: 6a (3.56 mg); 7: 6a (0.88 mg); 9: 9a (3.15 mg)].
3.12. Aglycone of Valerianairidoid I (1a)
Amorphous solid, [α]25D −73.1 (c 0.1, MeOH), 1H NMR (CDCl3, 600 MHz) and 13C NMR (150 MHz) are identical to patrinoside-aglucone [], FABMS m/z 323 [M+Na]+, HRFABMS m/z 323.1484 [M+Na]+ (calcd. for C15H24O6Na, 323.1471).
3.13. Aglycone of Valerianairidoid IV (6a)
Amorphous solid, [α]25D −51.9 (c 0.1, MeOH), 1H NMR (CDCl3, 600 MHz), and 13C NMR (150 MHz) spectra (Table 2), FABMS m/z 337 [M+Na]+, HRFABMS m/z 337.1637 [M+Na]+ (calcd. for C16H26O6Na, 337.1627)
3.14. Aglycone of Valerianairidoid VI (9a)
Amorphous solid, [α]25D −139.5 (c 0.1, MeOH), 1H NMR (CDCl3, 600 MHz) and 13C NMR (150 MHz) are identical with the aglycone of kanokoside A [], FABMS m/z 337 [M+Na]+, HRFABMS m/z 337.1275 [M+Na]+ (calcd. for C16H26O6Na, 337.1263)
3.15. Calculating the Theoretical ECD Spectra of 1a, 6a, and 9a
The initial geometries of the conformers of 1S,5S,7S,8S,9S-1a were generated and their geometries optimized under vacuum conditions using the Merck molecular force field (MMFF) as implemented in the Spartan ’16 program []. Stable conformers with Boltzmann distributions over 1% were further optimized at the CAM-B3LYP/def2-TZVP level of density functional theory (DFT). Normal mode analysis was performed at the same level to confirm that none of the conformers show any imaginary frequencies and to obtain Gibbs free energies (G) [,]. Low free-energy conformers with Boltzmann distributions over 1% (Supplementary Materials) were subjected to ECD calculations using time-dependent DFT (TD-DFT) at the CAM-B3LYP/def2-TZVPP level. All DFT and TD-DFT calculations were performed using the integral equation formalism polarizable continuum model (IEFPCM) in MeOH within Gaussian 16 []. The resultant rotatory strengths of the lowest 30 excited states of each conformer were converted into Gaussian-type curves with half-bands (0.30 eV) using SpecDis v1.71 []. Theoretical ECD spectra were obtained after correction based on the Boltzmann distribution of the conformers and their relative free energies (ΔG). The ECD spectra of 1S,5S,7S,8S,9S-6a and 1S,5S,6S,7S,8R,9S-9a were also calculated according to the procedure described above (Figure 4).
3.16. Acid Hydrolyses of 1–3, 6, 7, 9, and 12
We used the method reported by Tanaka et al. [] with slight modifications to determine the absolute configurations of the monosaccharide constituents of 1–3, 6, 7, 9, and 12. A sample of 1–3, 6, 7, 9, or 12 (0.5 mg) was dissolved in 5% aqueous H2SO4–1,4-dioxane (1:1, v/v, 6.0 mL) and heated at 90 °C for 3 h. The solution was partitioned into ethyl acetate–H2O and the H2O fraction was neutralized. After drying in vacuo, the residue was dissolved in pyridine (0.5 mL) containing l-cysteine methyl ester hydrochloride (1.0 mg), and the mixture was heated at 60 °C for 1 h, after which phenyl isothiocyanate was added, and the mixture was heated at 60 °C for 1 h. The mixture was analyzed by reverse-phase HPLC {COSMOSIL 5C18-AR-II (250 × 4.6 mm i.d.), mobile phase: 0.3% CH3COOH–CH3CN (8:2, v/v); detection: UV (254 nm), flow rate: 1.0 mL/min, column temperature: 25 °C} to identify the d-glucose derivative by comparing the retention time with that of authentic samples (retention time: d-glucose, 33.4 min; l-glucose, 31.5 min).
3.17. Cells
Breast cancer (MDA-MB-231) and glioblastoma (U-251 MG) cells (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM/high glucose and DMEM/low glucose (Wako, Osaka, Japan), respectively, containing 10% fetal bovine serum (FBS; Sigma–Aldrich, St. Louis, MO, USA) and 1% penicillin/streptomycin (PC/SM; Wako).
3.18. Cell Viability Assay for non-CSCs
MDA-MB-231 and U-251 MG cells were seeded at a density of 3.0 × 103 cells/90 μL per well in 96-well plates (3596; Corning, NY, USA) and treated with the test compounds (10 μL per well) 24 h after seeding. After 3 d, CellTiter-Glo® 3D Reagent (Promega, Madison, WI, USA) was added to each well at an equivalent volume to the cell culture medium in the well, mixed by shaking for 5 min at room temperature, and incubated for 25 min at 37 °C under 5% CO2. The 100 μL supernatants were then transferred in technical replicates to 96-well white plates (136101; Thermo Fisher Scientific), and their luminescence was measured using a luminometer (GloMax® Discover System; Promega).
3.19. Cell Viability Assay for CSCs
CSCs of MDA-MB-231 and U-251 MG were prepared using a sphere-formation assay, as described previously []. Briefly, cells were cultured in DMEM/F12 (Thermo Fisher Scientific, Waltham, MA, USA) containing 1% PC/SM, 2% B-27 [B-27® Serum-Free Supplement (50×); Thermo Fisher Scientific], 20 ng/mL epidermal growth factor (EGF; Peprotech, Rocky Hill, NJ, USA), and 20 ng/mL basic fibroblast growth factor (b-FGF; Peprotech) for 7 d in ultra-low attachment six-well plates (Corning International, Corning, NY, USA). Cell viability was evaluated using the CellTiter-Glo® 3D Reagent in the same manner as described for the non-CSCs.
3.20. Statistics
Statistical analyses were performed using GraphPad Prism 8.21 software using one-way Analysis Of Variance (ANOVA) followed by the Dunnett’s test to analyze differences between the treatment groups. Differences were considered significant at: * p < 0.05, ** p <0.01, or *** p < 0.001.
4. Conclusions
We isolated seven new iridoid glucosides, namely the valerianairidoids I–VII (1–3, 6, 7, 9, and 12), and six known compounds from the MeOH extract of the dried rhizomes and roots of V. fauriei. Chemical structures, including the absolute configurations of the new compounds, were elucidated by NMR, MS, and ECD spectroscopy, and derivatization. The isolated iridoid glycosides did not show any anti-proliferative activity against MDA-MB-231 and U-251MG cells or their CSCs. On the other hand, aglycones 1a, 6a, and 9a showed selective anti-proliferative activities against CSCs from MDA-MB-231 cells. Therefore, these aglycones have potential as breast cancer and preventive agents that may inhibit the characteristic signaling pathway(s) in CSCs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232214206/s1.
Author Contributions
Conceptualization, T.M.; methodology, T.M. and T.Y.; investigation, H.Y., T.M., T.K., M.O., T.Y. and T.O.; formal analysis, T.K., M.O., T.Y. and T.O.; resources and software T.M., T.Y. and T.W.; data curation, H.Y., T.M., T.K., T.O. and T.Y. writing–original draft preparation, H.Y. and T.M.; writing–review and editing, funding acquisition, project administration, and validation, T.M.; visualization, H.Y., T.M. and T.O.; supervision and funding acquisition, T.M. and T.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by JSPS KAKENHI (grant number JP20H03397).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, P.C.; Ran, X.H.; Luo, H.R.; Hu, J.M.; Chen, R.; Ma, Q.Y.; Dai, H.F.; Liu, Y.Q.; Xie, M.J.; Zhou, J.; et al. Volvalerelactones A and B, Two New Sesquiterpenoid Lactones with an Unprecedented Skeleton from Valeriana officinalis var. latifolia. Org. Lett. 2011, 13, 3036–3039. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, J.; Li, Y.; Watanabe, R.; Oshima, Y.; Yamakuni, T.; Ohizumi, Y. Iridoids and Sesquiterpenoids with NGF-Potentiating Activity from the Rhizomes and Roots of Valeriana fauriei. Chem. Pharm. Bull. 2006, 54, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Matsuoka, S.; Ohizumi, Y. Antidepressant Principles of Valeriana fauriei Roots. Chem. Pharm. Bull. 1995, 43, 169–170. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kitagawa, T.; Imahori, D.; Matsuzaki, A.; Saito, Y.; Ohta, T.; Yoshida, T.; Nakayama, Y.; Ashihara, E.; Watanabe, T. Linderapyrone: A Wnt Signal Inhibitor Isolated from Lindera umbellate. Bioorg. Med. Chem. Lett. 2021, 45, 128161. [Google Scholar] [CrossRef]
- Matsumoto, T.; Imahori, D.; Achiwa, K.; Saito, Y.; Ohta, T.; Yoshida, T.; Kojima, N.; Yamashita, M.; Nakayama, Y.; Watanabe, T. Chemical Structures and Cytotoxic Activities of the Constituents Isolated from Hibiscus tiliaceus. Fitoterapia 2020, 142, 104524. [Google Scholar] [CrossRef] [PubMed]
- Imahori, D.; Matsumoto, T.; Saito, Y.; Ohta, T.; Yoshida, T.; Nakayama, Y.; Watanabe, T. Cell Death-Inducing Activities via P-Glycoprotein Inhibition of the Constituents Isolated from Fruits of Nandina domestica. Fitoterapia 2021, 154, 105023. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Imahori, D.; Ohnishi, E.; Okayama, M.; Kitagawa, T.; Ohta, T.; Yoshida, T.; Kojima, N.; Yamashita, M.; Watanabe, T. Chemical Structures and Induction of Cell Death via Heat Shock Protein Inhibition of the Prenylated Phloroglucinol Derivatives Isolated from Hypericum erectum. Fitoterapia 2022, 156, 105097. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kitagawa, T.; Imahori, D.; Yoshikawa, H.; Okayama, M.; Kobayashi, M.; Kojima, N.; Yamashita, M.; Watanabe, T. Cell Death-Inducing Activities via Hsp Inhibition of the Sesquiterpenes Isolated from Valeriana fauriei. J. Nat. Med. 2021, 75, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Clevers, H. Cancer Stem Cells Revisited. Nat. Med. 2017, 23, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.Y.; Le, H.T.; Min, H.-Y.; Pei, H.; Lim, Y.; Song, I.; Nguyen, Y.T.K.; Hong, S.; Han, B.W.; Lee, H.Y. Evodiamine Inhibits Both Stem Cell and Non-Stem-Cell Populations in Human Cancer Cells by Targeting Heat Shock Protein 70. Theranostics 2021, 11, 2932–2952. [Google Scholar] [CrossRef] [PubMed]
- Cebrián-Torrejón, G.; Assad Kahn, S.; Lagarde, N.; Castellano, F.; Leblanc, K.; Rodrigo, J.; Molinier-Frenkel, V.; Rojas de Arias, A.; Ferreira, M.E.; Thirant, C.; et al. Antiproliferative Activity of Trans-Avicennol from Zanthoxylum chiloperone var. angustifolium against Human Cancer Stem Cells. J. Nat. Prod. 2012, 75, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, A.; Nishi, M.; Yoshida, S.; Hasegawa, M.; Yasuma, C.; Ryo, A.; Suzuki, Y. Eucommicin A, a β-Truxinate Lignan from Eucommia ulmoides, Is a Selective Inhibitor of Cancer Stem Cells. Phytochemistry 2016, 122, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Imahori, D.; Saito, Y.; Zhang, W.; Ohta, T.; Yoshida, T.; Nakayama, Y.; Ashihara, E.; Watanabe, T. Cytotoxic Activities of Sesquiterpenoids from the Aerial Parts of Petasites japonicus against Cancer Stem Cells. J. Nat. Med. 2020, 74, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Nishiya, K.; Kimura, T.; Takeya, K.; Itokawa, H. Sesquiterpenoids and Iridoid Glycosides from Valeriana fauriei. Phytochemistry 1992, 31, 3511–3514. [Google Scholar] [CrossRef]
- Iwagawa, T.; Yaguchi, S.; Hase, T. Iridoid Glucosides from Viburnum suspensum. Phytochemistry 1990, 29, 310–312. [Google Scholar] [CrossRef]
- Gering, B.; Wichtl, M. Phytochemical Investigations on Penstemon hirsutus. J. Nat. Prod. 1987, 50, 1048–1054. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile Discrimination of Aldose Enantiomers by Reversed-Phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, Y.; Gong, Z.F.; Duan, X.Y.; Liu, Y.W. Two New Terpenoids from Valeriana officinalis. Chin. J. Nat. Med. 2009, 7, 270–273. [Google Scholar] [CrossRef]
- Wavefunction Inc. Available online: https://www.wavefun.com/ (accessed on 15 November 2022).
- Pescitelli, G.; Bruhn, T. Good Computational Practice in the Assignment of Absolute Configurations by TDDFT Calculations of ECD Spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian16, Revision A.03; Gaussian, Incorp.: Wallingford, UK, 2016. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).