Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment
Abstract
1. Background
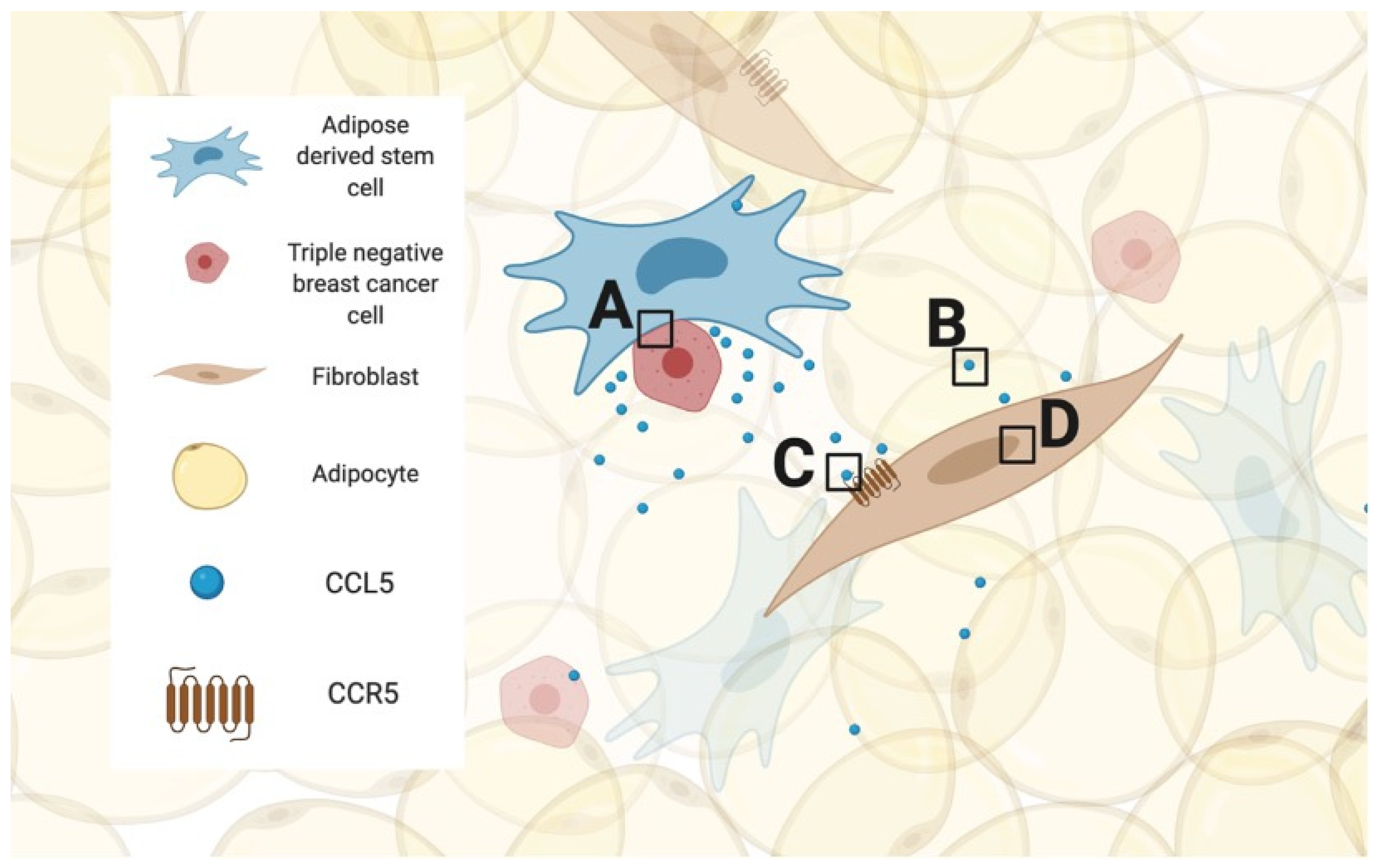
2. Breast Cancer and the Importance of the Tumor Microenvironment
3. The Molecule CCL5, the Receptor CCR5 and Its Structural Mutation That Confers HIV Resistance
4. The CCL5/CCR5 Axis in Human Diseases and Its Related Downstream and Upstream Pathways
5. Hurdle #1: What Is the Result of Blocking CCR5 in Cancer?
6. The Importance of Maraviroc and Other CCR5 Inhibitors
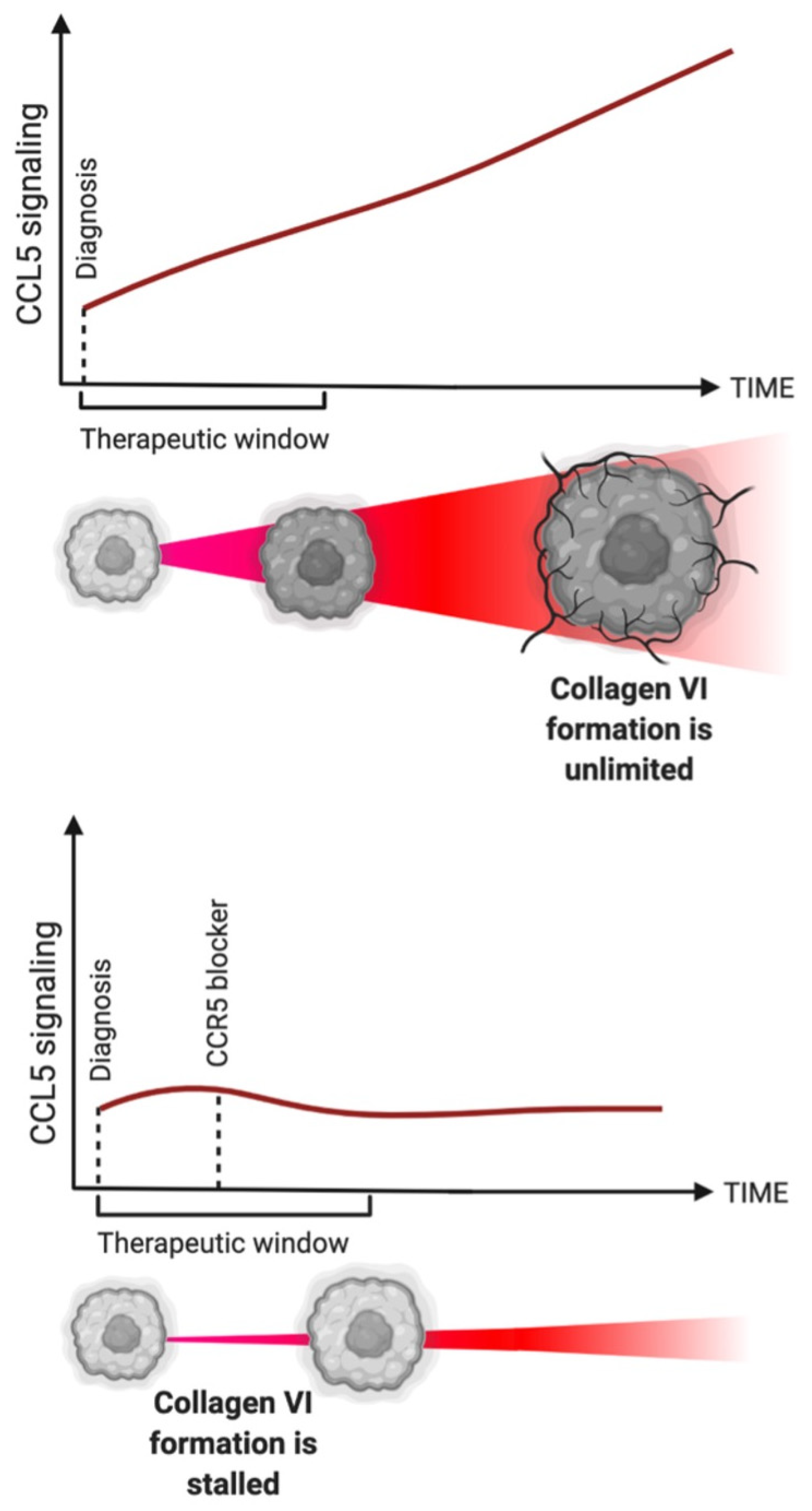
7. Hurdle #2: What Is the Theerapeutic Window?
8. Hurdle #3: What Should Be Targeted and Why?
9. mRNA as the New Frontier
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired immunodeficiency syndrome |
| CCL5 | C-C chemokine ligand 5 |
| CCR5 | C-C chemokine receptor 5 |
| CK | Chemokine |
| HIV | Human immunovirus |
| ILC | Innate lymphoid cells |
| MDSC | Myeloid-derived suppressor cell |
| MSC | Mesenchymal stem cells |
| RANTES | Regulated upon Activation, Normal T-Cell Expressed and Secreted |
| TACS | Tumor-associated signatures |
| TALEN | Transcription activator-like effector nuclease |
| TAM | Tumor-associated macrophages |
| TIL | Tumor-infiltrating lymphocyte |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
References
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef]
- Gnant, M.; Harbeck, N.; Thomssen, C. St. Gallen/Vienna 2017: A brief summary of the consensus discussion about escalation and de-escalation of primary breast cancer treatment. Breast Care 2017, 12, 101–106. [Google Scholar] [CrossRef]
- Sledge, G.W.; Mamounas, E.P.; Hortobagyi, G.N.; Burstein, H.J.; Goodwin, P.J.; Wolff, A.C. Past, present, and future challenges in breast cancer treatment. J. Clin. Oncol. 2014, 32, 1979. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast cancer treatment: A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.L.; Czerniecki, B.J. Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Weitzenfeld, P.; Ben-Baruch, A. The chemokine system, and its CCR5 and CXCR4 receptors, as potential targets for personalized therapy in cancer. Cancer Lett. 2014, 352, 36–53. [Google Scholar] [CrossRef] [PubMed]
- Cocchi, F.; DeVico, A.L.; Garzino-Demo, A.; Arya, S.K.; Gallo, R.C.; Lusso, P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 1995, 270, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, S.; Lasorsa, V.A.; Russo, R.; Andolfo, I.; D’alterio, G.; Rosato, B.E.; Frisso, G.; Abete, P.; Cassese, G.M.; Servillo, G.; et al. Regulatory noncoding and predicted pathogenic coding variants of CCR5 predispose to severe COVID-19. Int. J. Mol. Sci. 2021, 22, 5372. [Google Scholar] [CrossRef] [PubMed]
- Soria, G.; Ben-Baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Michie, C.A.; Tantscher, E.; Schall, T.; Rot, A. Physiological secretion of chemokines in human breast milk. Eur. Cytokine Netw. 1998, 9, 123–129. [Google Scholar]
- Khalid, A.; Wolfram, J.; Ferrari, I.; Mu, C.; Mai, J.; Yang, Z.; Zhao, Y.; Ferrari, M.; Ma, X.; Shen, H. Recent Advances in Discovering the Role of CCL5 in Metastatic Breast Cancer. Mini Rev. Med. Chem. 2015, 15, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Mishra, M.K.; Eltoum, I.A.; Bae, S.; Lillard, J.W., Jr.; Singh, R. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci. Rep. 2018, 8, 1323. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Xie, R.; Xiang, T.; Zhao, Z.; Lin, S.; Liang, Z.; Chen, Z.; Zhu, B. Autocrine CCL5 signaling promotes invasion and migration of CD133+ ovarian cancer stem-like cells via NF-κB-mediated MMP-9 upregulation. Stem Cells 2012, 30, 2309–2319. [Google Scholar] [CrossRef]
- Huang, R.; Wang, S.; Wang, N.; Zheng, Y.; Zhou, J.; Yang, B.; Wang, X.; Zhang, J.; Guo, L.; Wang, S.; et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed]
- Kranjc, M.K.; Novak, M.; Pestell, R.G.; Lah, T.T. Cytokine CCL5 and receptor CCR5 axis in glioblastoma multiforme. Radiol. Oncol. 2019, 53, 397–406. [Google Scholar] [CrossRef]
- Lee, N.J.; Choi, D.Y.; Song, J.K.; Jung, Y.Y.; Kim, D.H.; Kim, T.M.; Kim, D.J.; Kwon, S.M.; Kim, K.B.; Choi, K.E.; et al. Deficiency of C-C chemokine receptor 5 suppresses tumor development via inactivation of NF-κB and inhibition of monocyte chemoattractant protein-1 in urethane-induced lung tumor model. Carcinogenesis 2012, 33, 2520–2528. [Google Scholar] [CrossRef][Green Version]
- Che, L.F.; Shao, S.F.; Wang, L.X. Downregulation of CCR5 inhibits the proliferation and invasion of cervical cancer cells and is regulated by microRNA-107. Exp. Ther. Med. 2016, 11, 503–509. [Google Scholar] [CrossRef][Green Version]
- Velasco-Velázquez, M.; Jiao, X.; De La Fuente, M.; Pestell, T.G.; Ertel, A.; Lisanti, M.P.; Pestell, R.G. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012, 72, 3839–3850. [Google Scholar] [CrossRef]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor microenvironment as a “game changer” in cancer radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the tumor microenvironment in breast cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Arneth, B. Tumor microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Brett, E.; Sauter, M.; Timmins, É.; Azimzadeh, O.; Rosemann, M.; Merl-Pham, J.; Hauck, S.M.; Nelson, P.J.; Becker, K.F.; Schunn, I.; et al. Oncogenic Linear Collagen VI of Invasive Breast Cancer Is Induced by CCL5. J. Clin. Med. 2020, 9, 991. [Google Scholar] [CrossRef]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor microenvironment as a regulator of radiation therapy: New insights into stromal-mediated radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Brett, E.; Rosemann, M.; Azimzadeh, O.; Pagani, A.; Prahm, C.; Daigeler, A.; Duscher, D.; Kolbenschlag, J. Irradiated Triple-Negative Breast Cancer Co-Culture Produces a Less Oncogenic Extracellular Matrix. Int. J. Mol. Sci. 2022, 23, 8265. [Google Scholar] [CrossRef] [PubMed]
- Grayson, M.H.; Holtzman, M.J. Chemokine complexity: The case for CCL5. Am. J. Respir. Cell Mol. Biol. 2006, 35, 143–146. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 axis in cancer progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Kufel, W.D. Antibody-based strategies in HIV therapy. Int. J. Antimicrob. Agents 2020, 56, 106186. [Google Scholar] [CrossRef]
- Ni, J.; Wang, D.; Wang, S. The CCR5-Delta32 Genetic Polymorphism and HIV-1 Infection Susceptibility: A Meta-analysis. Open Med. 2018, 13, 467–474. [Google Scholar] [CrossRef]
- Novembre, J.; Galvani, A.P.; Slatkin, M. The geographic spread of the CCR5 Delta32 HIV-resistance allele. PLoS Biol. 2005, 3, e339. [Google Scholar] [CrossRef]
- Jiao, X.; Nawab, O.; Patel, T.; Kossenkov, A.V.; Halama, N.; Jaeger, D.; Pestell, R.G. Recent Advances Targeting CCR5 for Cancer and Its Role in Immuno-OncologyTargeting CCR5 for Cancer. Cancer Res. 2019, 79, 4801–4807. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Lan, T.; Wei, Y.; Wei, X. CCL5/CCR5 axis in human diseases and related treatments. Genes Dis. 2021, 9, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Schödel, J.; Grampp, S.; Maher, E.R.; Moch, H.; Ratcliffe, P.J.; Russo, P.; Mole, D.R. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur. Urol. 2016, 69, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Aitzetmüller, M.M.; Brett, E.A.; König, V.; Wenny, R.; Thor, D.; Radtke, C.; Huemer, G.M.; Machens, H.G.; Duscher, D.; et al. Skin rejuvenation through HIF-1α modulation. Plast. Reconstr. Surg. 2018, 141, 600e–607e. [Google Scholar] [CrossRef] [PubMed]
- Pagani, A.; Kirsch, B.M.; Hopfner, U.; Aitzetmueller, M.M.; Brett, E.A.; Thor, D.; Mela, P.; Machens, H.-G.; Duscher, D. Deferiprone Stimulates Aged Dermal Fibroblasts via HIF-1α Modulation. Aesthetic Surg. J. 2020, 41, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Fong, Y.-C.; Lee, C.-Y.; Chen, M.-Y.; Tsai, H.-C.; Hsu, H.-C.; Tang, C.-H. CCL5 increases lung cancer migration via PI3K, Akt and NF-κB pathways. Biochem. Pharmacol. 2009, 77, 794–803. [Google Scholar] [CrossRef]
- Gils, A.; Declerck, P.J. Three Decades of Research on Plasminogen Activator Inhibitor-1: A Multifaceted Serpin. Semin. Thromb. Hemost. 2013, 39, 356–364. [Google Scholar] [CrossRef]
- Mi, Z.; Bhattacharya, S.D.; Kim, V.M.; Guo, H.; Talbot, L.J.; Kuo, P.C. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 2011, 32, 477–487. [Google Scholar] [CrossRef]
- Mitra, A.K.; Zillhardt, M.; Hua, Y.; Tiwari, P.; Murmann, A.E.; Peter, M.E.; Lengyel, E. MicroRNAs Reprogram Normal Fibroblasts into Cancer-Associated Fibroblasts in Ovarian CancermiRNAs Regulate CAFs. Cancer Discov. 2012, 2, 1100–1108. [Google Scholar] [CrossRef]
- Xia, L.; Zhu, X.; Zhang, L.; Xu, Y.; Chen, G.; Luo, J. EZH2 enhances expression of CCL5 to promote recruitment of macrophages and invasion in lung cancer. Biotechnol. Appl. Biochem. 2019, 67, 1011–1019. [Google Scholar] [CrossRef]
- Jin, K.; Pandey, N.B.; Popel, A.S. Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res. 2018, 20, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Korkaya, H.; Kim, G.-I.; Davis, A.; Malik, F.; Henry, N.L.; Ithimakin, S.; Quraishi, A.A.; Tawakkol, N.; D’Angelo, R.; Paulson, A.K.; et al. Activation of an IL6 Inflammatory Loop Mediates Trastuzumab Resistance in HER2+ Breast Cancer by Expanding the Cancer Stem Cell Population. Mol. Cell 2012, 47, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Nosalski, R.; Szczepaniak, P.; Budzyn, K.; Osmenda, G.; Skiba, D.; Sagan, A.; Wu, J.; Vinh, A.; Marvar, P.J.; et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. FASEB J. 2016, 30, 1987–1999. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Velasco-Velázquez, M.A.; Wang, M.; Li, Z.; Rui, H.; Peck, A.R.; Korkola, J.E.; Chen, X.; Xu, S.; DuHadaway, J.B.; et al. CCR5 Governs DNA Damage Repair and Breast Cancer Stem Cell Expansion. Cancer Res. 2018, 78, 1657–1671. [Google Scholar] [CrossRef]
- Schlecker, E.; Stojanovic, A.; Eisen, C.; Quack, C.; Falk, C.S.; Umansky, V.; Cerwenka, A.; Xu, M.; Hadinoto, V.; Appanna, R.; et al. Tumor-Infiltrating Monocytic Myeloid-Derived Suppressor Cells Mediate CCR5-Dependent Recruitment of Regulatory T Cells Favoring Tumor Growth. J. Immunol. 2012, 189, 5602–5611. [Google Scholar] [CrossRef]
- Sleeman, J.P. The lymph node pre-metastatic niche. J. Mol. Med. 2015, 93, 1173–1184. [Google Scholar] [CrossRef]
- Tan, M.C.B.; Goedegebuure, P.S.; Belt, B.A.; Flaherty, B.; Sankpal, N.; Gillanders, W.E.; Eberlein, T.J.; Hsieh, C.-S.; Linehan, D.C. Disruption of CCR5-Dependent Homing of Regulatory T Cells Inhibits Tumor Growth in a Murine Model of Pancreatic Cancer. J. Immunol. 2009, 182, 1746–1755. [Google Scholar] [CrossRef]
- Halvorsen, E.C.; Hamilton, M.J.; Young, A.; Wadsworth, B.J.; LePard, N.E.; Lee, H.N.; Firmino, N.; Collier, J.L.; Bennewith, K.L. Maraviroc decreases CCL8-mediated migration of CCR5+ regulatory T cells and reduces metastatic tumor growth in the lungs. OncoImmunology 2016, 5, e1150398. [Google Scholar] [CrossRef]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Arwert, E.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018, 23, 1239–1248. [Google Scholar] [CrossRef]
- Nishikawa, G.; Kawada, K.; Nakagawa, J.; Toda, K.; Ogawa, R.; Inamoto, S.; Mizuno, R.; Itatani, Y.; Sakai, Y. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Luboshits, G.; Shina, S.; Kaplan, O.; Engelberg, S.; Nass, D.; Lifshitz-Mercer, B.; Chaitchik, S.; Keydar, I.; Ben-Baruch, A. Elevated expression of the CC chemokine regulated on activation, normal T cell expressed and secreted (RANTES) in advanced breast carcinoma. Cancer Res. 1999, 59, 4681–4687. [Google Scholar] [PubMed]
- Fujimoto, Y.; Inoue, N.; Morimoto, K.; Watanabe, T.; Hirota, S.; Imamura, M.; Matsushita, Y.; Katagiri, T.; Okamura, H.; Miyoshi, Y. Significant association between high serum CCL5 levels and better disease-free survival of patients with early breast cancer. Cancer Sci. 2019, 111, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Back, D.J.; Vourvahis, M. Maraviroc: Pharmacokinetics and drug interactions. Antivir. Ther. 2009, 14, 607–618. [Google Scholar] [CrossRef]
- Swaisland, H.C.; Ranson, M.; Smith, R.P.; Leadbetter, J.; Laight, A.; McKillop, D.; Wild, M.J. Pharmacokinetic Drug Interactions of Gefitinib with Rifampicin, Itraconazole and Metoprolol. Clin. Pharmacokinet. 2005, 44, 1067–1081. [Google Scholar] [CrossRef]
- Dorr, P.; Westby, M.; Dobbs, S.; Griffin, P.; Irvine, B.; Macartney, M.; Mori, J.; Rickett, G.; Smith-Burchnell, C.; Napier, C.; et al. Maraviroc (UK-427,857), a Potent, Orally Bioavailable, and Selective Small-Molecule Inhibitor of Chemokine Receptor CCR5 with Broad-Spectrum Anti-Human Immunodeficiency Virus Type 1 Activity. Antimicrob. Agents Chemother. 2005, 49, 4721–4732. [Google Scholar] [CrossRef]
- Perry, C.M. Maraviroc. Drugs 2010, 70, 1189–1213. [Google Scholar] [CrossRef]
- Westby, M.; Smith-Burchnell, C.; Mori, J.; Lewis, M.; Mosley, M.; Stockdale, M.; Dorr, P.; Ciaramella, G.; Perros, M. Reduced Maximal Inhibition in Phenotypic Susceptibility Assays Indicates that Viral Strains Resistant to the CCR5 Antagonist Maraviroc Utilize Inhibitor-Bound Receptor for Entry. J. Virol. 2007, 81, 2359–2371. [Google Scholar] [CrossRef]
- Casagrande, N.; Borghese, C.P.; Visser, L.; Mongiat, M.; Colombatti, A.; Aldinucci, D. CCR5 antagonism by maraviroc inhibits Hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica 2018, 104, 564–575. [Google Scholar] [CrossRef]
- Pervaiz, A.; Zepp, M.; Mahmood, S.; Ali, D.M.; Berger, M.R.; Adwan, H. CCR5 blockage by maraviroc: A potential therapeutic option for metastatic breast cancer. Cell. Oncol. 2018, 42, 93–106. [Google Scholar] [CrossRef]
- Lalezari, J.; Thompson, M.; Kumar, P.; Piliero, P.; Davey, R.; Patterson, K.; Shachoy-Clark, A.; Adkison, K.; Demarest, J.; Lou, Y.; et al. Antiviral activity and safety of 873140, a novel CCR5 antagonist, during short-term monotherapy in HIV-infected adults. AIDS 2005, 19, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Nichols, W.G.; Steel, H.M.; Bonny, T.; Adkison, K.; Curtis, L.; Millard, J.; Kabeya, K.; Clumeck, N. Hepatotoxicity Observed in Clinical Trials of Aplaviroc (GW873140). Antimicrob. Agents Chemother. 2008, 52, 858–865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qi, B.; Fang, Q.; Liu, S.; Hou, W.; Li, J.; Huang, Y.; Shi, J. Advances of CCR5 antagonists: From small molecules to macromolecules. Eur. J. Med. Chem. 2020, 208, 112819. [Google Scholar] [CrossRef]
- Friedman, S.L.; Ratziu, V.; Harrison, S.A.; Abdelmalek, M.F.; Aithal, G.P.; Caballeria, J.; Francque, S.; Farrell, G.; Kowdley, K.V.; Craxi, A.; et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018, 67, 1754–1767. [Google Scholar] [CrossRef]
- Files, D.C.; Tacke, F.; O’Sullivan, A.; Dorr, P.; Ferguson, W.G.; Powderly, W.G. Rationale of using the dual chemokine receptor CCR2/CCR5 inhibitor cenicriviroc for the treatment of COVID-19. PLoS Pathog. 2022, 18, e1010547. [Google Scholar] [CrossRef]
- Kanmogne, G.; Woollard, S. Maraviroc: A review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015, 9, 5447–5468. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.P.D.; Lim, J.K. Evaluating the Therapeutic Potential of Cenicriviroc in the Treatment of Nonalcoholic Steatohepatitis with Fibrosis: A Brief Report on Emerging Data. Hepatic Med. Évid. Res. 2020, 12, 115–123. [Google Scholar] [CrossRef]
- Monickaraj, F.; Oruganti, S.R.; McGuire, P.; Das, A. A potential novel therapeutic target in diabetic retinopathy: A chemokine receptor (CCR2/CCR5) inhibitor reduces retinal vascular leakage in an animal model. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 93–100. [Google Scholar] [CrossRef]
- Adams, D.L.; Raghavakaimal, A.; Tang, C.-M.; Gardner, K.P.; Kelly, S.; Pourhassan, N.; Ray, N. Safety, efficacy, and clinical outcomes of the anti-CCR5 inhibitor (Leronlimab): A pooled analysis of three clinical trials in patients with mTNBC. J. Clin. Oncol. 2022, 40, e13062. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, F.; Yao, X.; Yi, C.; Tan, C.; Wei, L.; Sun, S. Role of CCL5 in invasion, proliferation and proportion of CD44+/CD24− phenotype of MCF-7 cells and correlation of CCL5 and CCR5 expression with breast cancer progression. Oncol. Rep. 2009, 21, 1113–1121. [Google Scholar]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Qi, Y.; Wang, Z.; Zeng, H.; Zhang, H.; Liu, Z.; Huang, Q.; Xiong, Y.; Wang, J.; Chang, Y.; et al. CCR5 blockade inflames antitumor immunity in BAP1-mutant clear cell renal cell carcinoma. J. Immunother. Cancer 2019, 8, e000228. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; Zepp, M.; Georges, R.; Bergmann, F.; Mahmood, S.; Faiza, S.; Berger, M.R.; Adwan, H. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J. Cancer Res. Clin. Oncol. 2020, 147, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Mañes, S.; Mira, E.; Colomer, R.; Montero, S.; Real, L.M.; Gómez-Moutón, C.; Jiménez-Baranda, S.; Garzón, A.; LaCalle, R.A.; Harshman, K.; et al. CCR5 Expression Influences the Progression of Human Breast Cancer in a p53-dependent Manner. J. Exp. Med. 2003, 198, 1381–1389. [Google Scholar] [CrossRef]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned Collagen Is a Prognostic Signature for Survival in Human Breast Carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef]
- Brett, E.A.; Sauter, M.A.; Machens, H.-G.; Duscher, D. Tumor-associated collagen signatures: Pushing tumor boundaries. Cancer Metab. 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Vangelista, L.; Vento, S. The expanding therapeutic perspective of CCR5 blockade. Front. Immunol. 2018, 8, 1981. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, C.E.; Oda, J.M.; Losi Guembarovski, R.; de Oliveira, K.B.; Ariza, C.B.; Neto, J.S.; Banin Hirata, B.K.; Watanabe, M.A. CC chemokine receptor 5: The interface of host immunity and cancer. Dis. Markers 2014, 2014, 126954. [Google Scholar] [CrossRef]
- Lapteva, N.; Huang, X.F. CCL5 as an adjuvant for cancer immunotherapy. Expert Opin. Biol. Ther. 2010, 10, 725–733. [Google Scholar] [CrossRef]
- Abayev, M.; Rodrigues, J.P.G.L.M.; Srivastava, G.; Arshava, B.; Jaremko, Ł.; Jaremko, M.; Naider, F.; Levitt, M.; Anglister, J. The solution structure of monomeric CCL 5 in complex with a doubly sulfated N-terminal segment of CCR 5. FEBS J. 2018, 285, 1988–2003. [Google Scholar] [CrossRef]
- Anderson, J.; Akkina, R. Complete knockdown of CCR5 by lentiviral vector-expressed siRNAs and protection of transgenic macrophages against HIV-1 infection. Gene Ther. 2007, 14, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.S.; Su, J.L.; Cha, S.T.; Tarn, W.Y.; Wang, M.Y.; Hsu, H.C.; Lin, M.T.; Chu, C.Y.; Hua, K.T.; Chen, C.N.; et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J. Clin. Investig. 2011, 121, 3442–3455. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.M.; Kuenzi, G.; Gaertner, H.; Foti, M.; Hartley, O. CC chemokine receptor 5 (CCR5) desensitization: Cycling receptors accumulate in the trans-Golgi network. J. Biol. Chem. 2010, 285, 41772–41780. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brett, E.; Duscher, D.; Pagani, A.; Daigeler, A.; Kolbenschlag, J.; Hahn, M. Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment. Int. J. Mol. Sci. 2022, 23, 14159. https://doi.org/10.3390/ijms232214159
Brett E, Duscher D, Pagani A, Daigeler A, Kolbenschlag J, Hahn M. Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment. International Journal of Molecular Sciences. 2022; 23(22):14159. https://doi.org/10.3390/ijms232214159
Chicago/Turabian StyleBrett, Elizabeth, Dominik Duscher, Andrea Pagani, Adrien Daigeler, Jonas Kolbenschlag, and Markus Hahn. 2022. "Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment" International Journal of Molecular Sciences 23, no. 22: 14159. https://doi.org/10.3390/ijms232214159
APA StyleBrett, E., Duscher, D., Pagani, A., Daigeler, A., Kolbenschlag, J., & Hahn, M. (2022). Naming the Barriers between Anti-CCR5 Therapy, Breast Cancer and Its Microenvironment. International Journal of Molecular Sciences, 23(22), 14159. https://doi.org/10.3390/ijms232214159




