An Overview of the Current Known and Unknown Roles of Vitamin D3 in the Female Reproductive System: Lessons from Farm Animals, Birds, and Fish
Abstract
1. Introduction
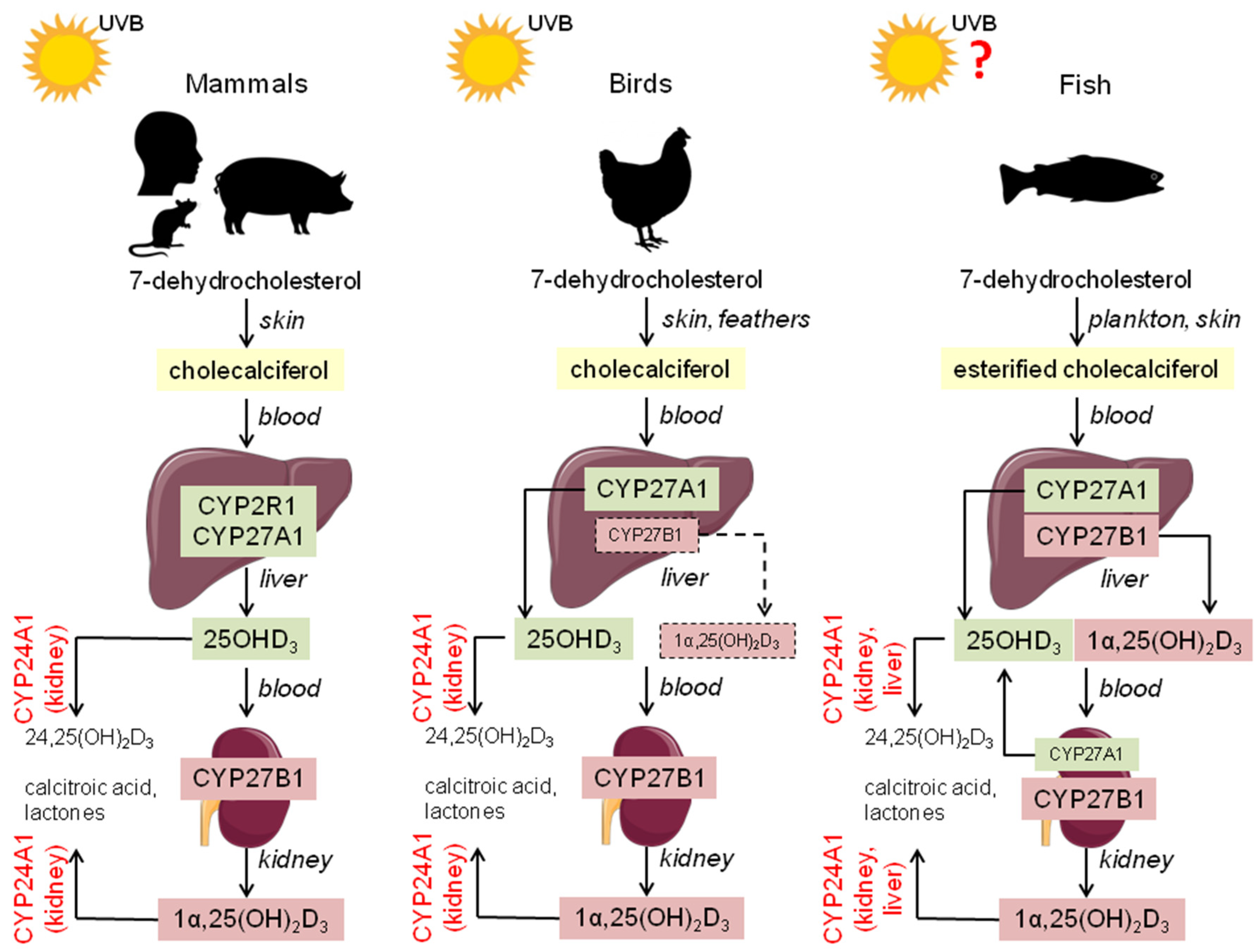
2. Vitamin D3 Metabolism—Comparative Aspects
3. Genomic and Non-Genomic Action of Vitamin D3
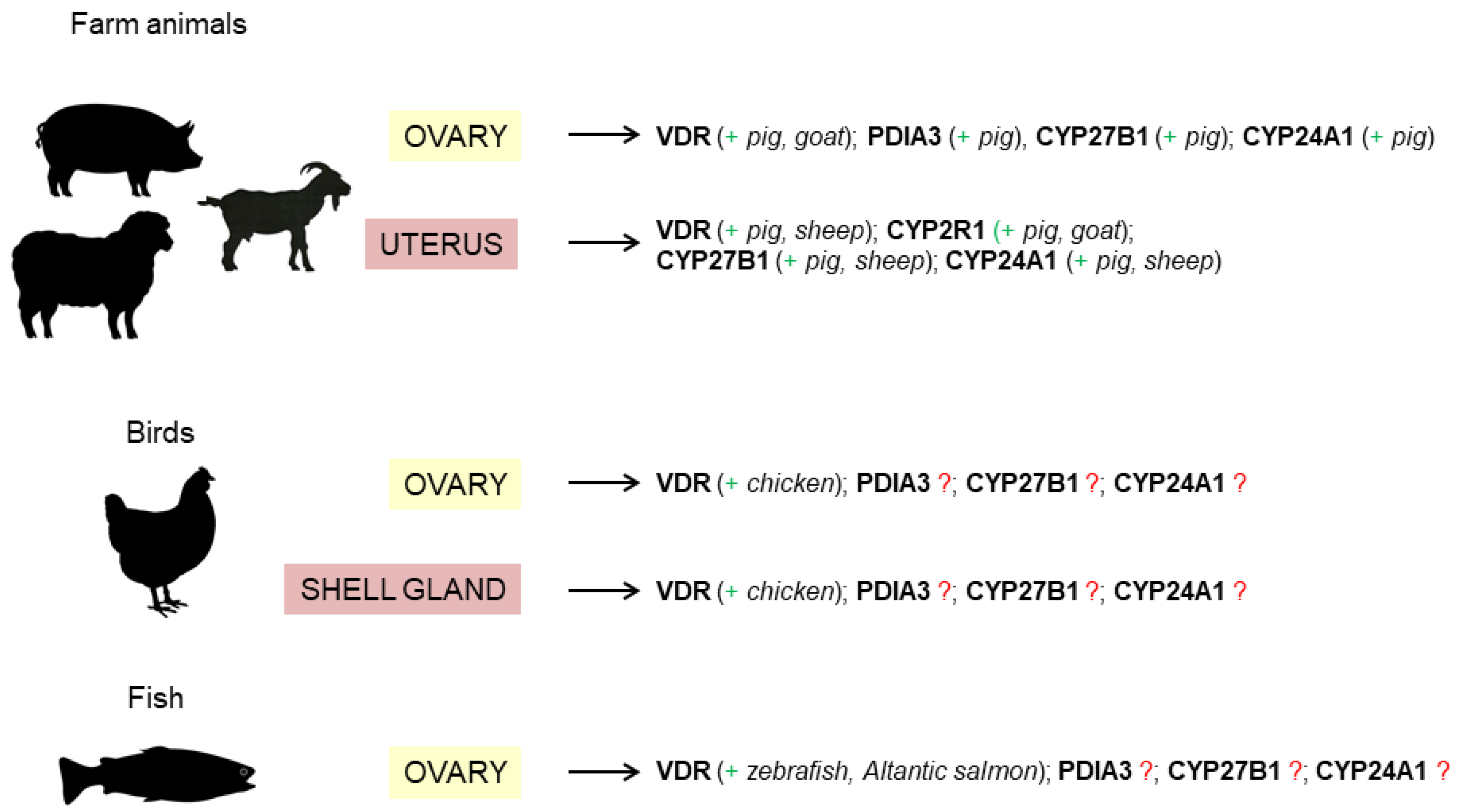
4. Vitamin D3 Action in the Female Reproductive System of Farm Animals
4.1. Ovary
4.2. Uterus
5. Vitamin D3 Action in the Female Reproductive System of Birds
5.1. Ovary
5.2. Oviduct
| Tissue | Species | Cell Type | Treatment | Effect | References |
|---|---|---|---|---|---|
| Ovary | Goat | Gc | 1, 10, 100 nM | ↑ proliferation | [27,70] |
| Goat | Gc | 10 nM | ↓ reactive oxygen species | [70] | |
| Goat | Gc | VDR silencing | ↑ apoptosis | [70] | |
| Goat | Gc | 10 nM | ↑ P4 and E2 | [27] | |
| Pig | SF, MF | 10, 50 ng/mL | ↑ P4 | [29] | |
| Pig | Gc | 100 nM | ↓ P4 | [74] | |
| Pig | Gc | 100 ng/mL | → P4 | [73] | |
| Pig | Gc | 100 ng/mL | ↑ E2 | [73] | |
| Pig | Gc | 100 ng/mL | ↑ E2 | [74] | |
| Pig | SF | 100 ng/mL | ↑ E2 | [29] | |
| Pig | MF | 1–100 ng/mL | ↑ E2 | [29] | |
| Pig | SF, MF, LF | 1–100 ng/mL | → T | [29] | |
| Chicken | Gc | 10, 100 nM | ↑ proliferation | [30] | |
| Chicken | Gc | 10, 100 nM | ↓ AMH mRNA | [30] | |
| Chicken | Gc | 100 nM | ↑ FSHR mRNA | [30] | |
| Chicken | Gc | 100 nM | ↑ Kit ligand mRNA | [87] | |
| Chicken | - | vitamin D3-deficient diet | ↓ E2 and P4 | [86] | |
| Uterus | Pig | M | 10, 50 ng/mL | ↑ E2 | [33] |
| Pig | E | 2–200 µM | ↑ implantation-related genes | [34] |
6. Vitamin D3 Action in the Female Reproductive System of Fish
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, A.; Bentley, G.E.; Cabrera, R.; Ortega, H.H.; Perfito, N.; Wu, T.J.; Micevych, P. Hormonal regulation of female reproduction. Horm. Metab. Res. 2012, 44, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.L. Ovarian follicle selection and granulosa cell differentiation. Poult. Sci. 2015, 94, 781–785. [Google Scholar] [CrossRef] [PubMed]
- Lubzens, E.; Bobe, J.; Young, G.; Sullivan, C.V. Maternal investment in fish oocytes and eggs: The molecular cargo and its contributions to fertility and early development. Aquaculture 2017, 472, 107–143. [Google Scholar] [CrossRef]
- Biran, J.; Levavi-Sivan, B. Endocrine Control of Reproduction, Fish. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 362–368. [Google Scholar] [CrossRef]
- Gershon, E.; Dekel, N. Newly identified regulators of ovarian folliculogenesis and ovulation. Int. J. Mol. Sci. 2020, 21, 4565. [Google Scholar] [CrossRef] [PubMed]
- van der Klein, S.A.; Zuidhof, M.J.; Bédécarrats, G.Y. Diurnal and seasonal dynamics affecting egg production in meat chickens: A review of mechanisms associated with reproductive dysregulation. Anim. Reprod. Sci. 2020, 213, 106257. [Google Scholar] [CrossRef]
- Hrabia, A. Reproduction in the Female. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Dridi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 941–986. [Google Scholar] [CrossRef]
- Lorenzen, M.; Boisen, I.M.; Mortensen, L.J.; Lanske, B.; Juul, A.; Blomberg Jensen, M. Reproductive endocrinology of vitamin D. Mol. Cell. Endocrinol. 2017, 453, 103–112. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Altieri, B.; de Angelis, C.; Palomba, S.; Pivonello, R.; Colao, A.; Orio, F. Shedding new light on female fertility: The role of vitamin D. Rev. Endocr. Metab. Disord. 2017, 18, 273–283. [Google Scholar] [CrossRef]
- Fichera, M.; Török, P.; Tesarik, J.; Della Corte, L.; Rizzo, G.; Garzon, S.; Carlea, A.; Di Angelo Antonio, S.; Zito, G.; Panella, M.M. Vitamin D, reproductive disorders and assisted reproduction: Evidences and perspectives. Int. J. Food. Sci. Nutr. 2020, 71, 276–285. [Google Scholar] [CrossRef]
- Dabrowski, F.A.; Grzechocinska, B.; Wielgos, M. The role of vitamin D in reproductive health—A trojan horse or the golden fleece? Nutrients 2015, 7, 4139–4153. [Google Scholar] [CrossRef]
- Grzesiak, M. Vitamin D3 action within the ovary–an updated review. Physiol. Res. 2020, 69, 371–378. [Google Scholar] [CrossRef]
- Kaminska, K.; Grzesiak, M. The relationship between vitamin D3 and insulin in polycystic ovary syndrome-a critical review. J. Physiol. Pharmacol. 2021, 72, 13–22. [Google Scholar] [CrossRef]
- Handel, I.; Watt, K.A.; Pilkington, J.G.; Pemberton, J.M.; Macrae, A.; Scott, P.; McNeilly, T.N.; Berry, J.L.; Clements, D.N.; Nussey, D.H.; et al. Vitamin D status predicts reproductive fitness in a wild sheep population. Sci. Rep. 2016, 6, 18986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; McEvoy, T.G.; Gill, A.C.; Lambe, N.R.; Morgan-Davies, C.R.; Hurst, E.; Sargison, N.D.; Mellanby, R.J. Investigation of relationship between vitamin D status and reproductive fitness in Scottish hill sheep. Sci. Rep. 2019, 9, 1162. [Google Scholar] [CrossRef]
- Hurst, E.A.; Homer, N.Z.; Mellanby, R.J. Vitamin D metabolism and profiling in veterinary species. Metabolites 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.M.; Ingold, B.C.; Young, J.L.; Fensterseifer, S.R.; Wechsler, P.J.; Austin, K.J.; Larson-Meyer, D.E. Sunlight exposure increases vitamin D sufficiency in growing pigs fed a diet formulated to exceed requirements. Domest. Anim. Endocrinol. 2017, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Coffey, J.D.; Hines, E.A.; Starkey, J.D.; Starkey, C.W.; Chung, T.K. Feeding 25-hydroxycholecalciferol improves gilt reproductive performance and fetal vitamin D status. J. Anim. Sci. 2012, 90, 3783–3788. [Google Scholar] [CrossRef]
- Narbaitz, R.; Tsang, C.P.W.; Grunder, A.A.; Soares, J.H., Jr. Scanning electron microscopy of thin and soft shells induced by feeding calcium-deficient or vitamin D-deficient diets to laying hens. Poult. Sci. 1987, 66, 341–347. [Google Scholar] [CrossRef]
- Luk, J.; Torrealday, S.; Neal Perry, G.; Pal, L. Relevance of vitamin D in reproduction. Hum. Reprod. 2012, 27, 3015–3027. [Google Scholar] [CrossRef]
- Anagnostis, P.; Karras, S.; Goulis, D.G. Vitamin D in human reproduction: A narrative review. Int. J. Clin. Pract. 2013, 67, 225–235. [Google Scholar] [CrossRef]
- Irani, M.; Merhi, Z. Role of vitamin D in ovarian physiology and its implication in reproduction: A systematic review. Fertil. Steril. 2014, 102, 460–468. [Google Scholar] [CrossRef]
- Keane, K.N.; Cruzat, V.F.; Calton, E.K.; Hart, P.H.; Soares, M.J.; Newsholme, P.; Yovich, J.L. Molecular actions of Vitamin D in reproductive cell biology. Reproduction 2017, 153, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Cermisoni, G.C.; Alteri, A.; Corti, L.; Rabellotti, E.; Papaleo, E.; Viganò, P.; Sanchez, A.M. Vitamin D and endometrium: A systematic review of a neglected area of research. Int. J. Mol. Sci. 2018, 19, 2320. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Tamblyn, J.A.; Finn-Sell, S.; Chan, S.Y.; Westwood, M.; Gupta, J.; Kilby, M.D.; Gross, S.R.; Hewison, M. Vitamin D, the placenta and early pregnancy: Effects on trophoblast function. J. Endocrinol. 2018, 236, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wolf, S.; Green, O.; Xu, J. Vitamin D in follicular development and oocyte maturation. Reproduction 2021, 161, 129–137. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, G.; Guo, Y.; EI-Samahy, M.; Wang, S.; Wan, Y.; Han, L.; Liu, Z.; Wang, F.; Zhang, Y. Vitamin D receptor expression and potential role of vitamin D on cell proliferation and steroidogenesis in goat ovarian granulosa cells. Theriogenology 2017, 102, 162–173. [Google Scholar] [CrossRef]
- Herian, M.; Luck, M.R.; Grzesiak, M. The influence of testosterone on the expression and function of vitamin D3 receptor (VDR) protein in the porcine ovarian follicle. Physiol. Res. 2018, 67, 515–519. [Google Scholar] [CrossRef]
- Grzesiak, M.; Knapczyk-Stwora, K.; Slomczynska, M. Vitamin D3 in ovarian antral follicles of mature gilts: Expression of its receptors and metabolic enzymes, concentration in follicular fluid and effect on steroid secretion in vitro. Theriogenology 2021, 160, 151–160. [Google Scholar] [CrossRef]
- Wojtusik, J.; Johnson, P.A. Vitamin D regulates anti-Mullerian hormone expression in granulosa cells of the hen. Biol. Reprod. 2012, 86, 91. [Google Scholar] [CrossRef]
- Craig, T.A.; Sommer, S.; Sussman, C.R.; Grande, J.P.; Kumar, R. Expression and regulation of the vitamin D receptor in the zebrafish. Danio rerio. J. Bone. Miner. Res. 2008, 23, 1486–1496. [Google Scholar] [CrossRef]
- Emam, M.A.; Abouelroos, M.E.; Gad, F.A. Expression of calbindin-D9k and vitamin D receptor in the uterus of Egyptian buffalo during follicular and luteal phases. Acta Histochem. 2016, 118, 471–477. [Google Scholar] [CrossRef]
- Grzesiak, M.; Waszkiewicz, E.; Wojtas, M.; Kowalik, K.; Franczak, A. Expression of vitamin D receptor in the porcine uterus and effect of 1,25(OH)2D3 on progesterone and estradiol-17β secretion by uterine tissues in vitro. Theriogenology 2019, 125, 102–108. [Google Scholar] [CrossRef]
- Jang, H.; Choi, Y.; Yoo, I.; Han, J.; Hong, J.S.; Kim, Y.Y.; Ka, H. Vitamin D-metabolic enzymes and related molecules: Expression at the maternal-conceptus interface and the role of vitamin D in endometrial gene expression in pigs. PLoS ONE 2017, 12, e0187221. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, C.; Halloran, K.M.; Newton, M.G.; Gaddy, D.; Suva, L.J.; Bazer, F.W. Novel mineral regulatory pathways in ovine pregnancy: II. Calcium-binding proteins, calcium transporters, and vitamin D signaling. Biol. Reprod. 2021, 105, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Ieda, T.; Saito, N.; Ono, T.; Shimada, K. Effects of presence of an egg and calcium deposition in the shell gland on levels of messenger ribonucleic acid of CaBP-D28K and of vitamin D3 receptor in the shell gland of the laying hen. Gen. Comp. Endocrinol. 1995, 99, 145–151. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Ohira, H.; Tamura, T. Immunocytochemical localization of vitamin D receptors in the shell gland of immature, laying, and molting hens. Gen. Comp. Endocrinol. 1997, 108, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Stenhouse, C.; Halloran, K.M.; Moses, R.M.; Seo, H.; Gaddy, D.; Johnson, G.A.; Wu, G.; Suva, L.J.; Bazer, F.W. Effects of progesterone and interferon tau on ovine endometrial phosphate, calcium, and vitamin D signaling. Biol. Reprod. 2022, 106, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Grzesiak, M.; Kaminska, K.; Bodzioch, A.; Drzewiecka, E.M.; Franczak, A.; Knapczyk-Stwora, K. Vitamin D3 metabolic enzymes in the porcine uterus: Expression, localization and sutoregulation by 1,25(OH)2D3 in vitro. Int. J. Mol. Sci. 2022, 23, 3972. [Google Scholar] [CrossRef]
- Hanel, A.; Carlberg, C. Vitamin D and evolution: Pharmacologic implications. Biochem. Pharmacol. 2020, 173, 113595. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: An ancient hormone. Exp. Dermatol. 2011, 20, 7–13. [Google Scholar] [CrossRef]
- Hernigou, P.; Auregan, J.C.; Dubory, A. Vitamin D: Part I; from plankton and calcified skeletons (500 million years ago) to rickets. Int. Orthop. 2018, 42, 2273–2285. [Google Scholar] [CrossRef]
- Hamre, K.; Krossøy, C.; Lock, E.J.; Moren, M. Roles of lipid-soluble vitamins during ontogeny of marine fish larvae. Aquac. Res. 2010, 41, 745–750. [Google Scholar] [CrossRef]
- Lock, E.J.; Ornsrud, R.; Aksnes, L.; Spanings, F.A.; Waagbø, R.; Flik, G. The vitamin D receptor and its ligand 1alpha,25-dihydroxyvitamin D3 in Atlantic salmon (Salmo salar). J. Endocrinol. 2007, 193, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Pierens, S.L.; Fraser, D.R. The origin and metabolism of vitamin D in rainbow trout. J. Steroid. Biochem. Mol. Biol. 2015, 145, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Donati, S.; Palmini, G.; Aurilia, C.; Falsetti, I.; Miglietta, F.; Iantomasi, T.; Brandi, M.L. Rapid nontranscriptional effects of calcifediol and calcitriol. Nutrients 2022, 14, 1291. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Bevelander, G.S.; Pinto, E.S.; Canario, A.V.; Spanings, T.; Flik, G. CYP27A1 expression in gilthead sea bream (Sparus auratus, L.): Effects of calcitriol and parathyroid hormone-related protein. J. Endocrinol. 2008, 196, 625–635. [Google Scholar] [CrossRef]
- Takeuchi, A.; Okano, T.; Kobayashi, T. The existence of 25-hydroxyvitamin D3-1α-hydroxylase in the liver of carp and bastard halibut. Life Sci. 1991, 48, 275–282. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Breck, O.; Sandvik, R.; Waagbø, R.; Berg, A.; Ørnsrud, R. Supplementation of dietary minerals during the early seawater phase increase vertebral strength and reduce the prevalence of vertebral deformities in fast growing under-yearling Atlantic salmon (Salmo salar L.) smolt. Aquac. Nutr. 2009, 15, 366–378. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Shehabi, H.Z.; Semak, I.; Tang, E.K.; Nguyen, M.N.; Benson, H.A.; Korik, E.; Janjetovic, Z.; Chen, J.; et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012, 26, 3901–3915. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Li, W.; Postlethwaite, A.; Tieu, E.W.; Tang, E.K.Y.; Tuckey, R.C. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Horst, R.L.; Reinhardt, T.A.; Reddy, G.S. Vitamin D metabolism. In Vitamin D, 2nd ed.; Feldman, D., Wesley Pike, J., Glorieux, F., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 1, pp. 15–36. [Google Scholar]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Nykjaer, A.; Fyfe, J.C.; Kozyraki, R.; Leheste, J.R.; Jacobsen, C.; Nielsen, M.S.; Verroust, P.J.; Aminoff, M.; De La Chapelle, A.; Moestrup, S.K.; et al. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D3. Proc. Natl. Acad. Sci. USA 2001, 98, 13895–13900. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhou, S.; Luu, S.; Glowacki, J. Megalin mediates 25-hydroxyvitamin D3 actions in human mesenchymal stem cells. FASEB J. 2019, 33, 7684–7693. [Google Scholar] [CrossRef]
- DeLuca, H.F. Evolution of our understanding of vitamin D. Nutr. Rev. 2008, 66, 537–587. [Google Scholar] [CrossRef]
- Mizwicki, M.T.; Keidel, D.; Bula, C.M.; Bishop, J.E.; Zanello, L.P.; Wurtz, J.M.; Moras, D.; Norman, A.W. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1α,25(OH) 2-vitamin D3 signaling. Proc. Natl. Acad. Sci. USA 2004, 101, 12876–12881. [Google Scholar] [CrossRef]
- Haussler, M.R.; Jurutka, P.W.; Mizwicki, M.; Norman, A.W. Vitamin D receptor (VDR)-mediated actions of 1α,25(OH)2vitamin D3: Genomic and non-genomic mechanisms. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 543–559. [Google Scholar] [CrossRef]
- Ricca, C.; Aillon, A.; Bergandi, L.; Alotto, D.; Castagnoli, C.; Silvagno, F. Vitamin D receptor is necessary for mitochondrial function and cell health. Int. J. Mol. Sci. 2018, 19, 1672. [Google Scholar] [CrossRef]
- Filipović, N.; Bočina, I.; Restović, I.; Grobe, M.; Kretzschmar, G.; Kević, N.; Mašek, T.; Vitlov Uljević, M.; Jurić, M.; Vukojević, K.; et al. Ultrastructural characterization of vitamin D receptors and metabolizing enzymes in the lipid droplets of the fatty liver in rat. Acta Histochem. 2020, 122, 151502. [Google Scholar] [CrossRef]
- Hii, C.S.; Ferrante, A. The non-genomic actions of vitamin D. Nutrients 2016, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Ma, L. Development of the mammalian female reproductive tract. J. Biochem. 2005, 137, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Handa, Y.; Uematsu, Y.; Takeda, S.; Sekine, K.; Yoshihara, Y.; Kawakami, T.; Arioka, K.; Sato, H.; Uchiyama, Y.; et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat. Genet. 1997, 16, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kinuta, K.; Tanaka, H.; Moriwake, T.; Aya, K.; Kato, S.; Seino, Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000, 141, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.K.; Miao, D.; Tremblay, M.L.; Sirois, J.; Farookhi, R.; Hendy, G.N.; Goltzman, D. Targeted ablation of the 25-hydroxyvitamin D 1α-hydroxylase enzyme: Evidence for skeletal, reproductive, and immune dysfunction. Proc. Natl. Acad. Sci. USA 2001, 98, 7498–7503. [Google Scholar] [CrossRef]
- Drummond, A.E. The role of steroids in follicular growth. Reprod. Biol. Endocrinol. 2006, 4, 1–11. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; El-Samahy, M.A.; Ren, C.; Liu, Z.; Wang, F.; You, P. Roles of vitamin D and its receptor in the proliferation and apoptosis of luteinised granulosa cells in the goat. Reprod. Fertil. Dev. 2020, 32, 335–348. [Google Scholar] [CrossRef]
- Grzesiak, M.; Socha, M.; Hrabia, A. Altered vitamin D metabolic system in follicular cysts of sows. Reprod. Domest. Anim. 2021, 56, 193–196. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, Y.; Li, Y.Y.; Liu, X.M.; Wang, X.X.; Zhang, C.L.; Hao, C.F.; Deng, S.L. Regulation of follicular development and differentiation by intra-ovarian factors and endocrine hormones. Front. Biosci. 2019, 24, 983–993. [Google Scholar] [CrossRef]
- Smolikova, K.; Mlynarcikova, A.; Scsukova, S. Effect of 1 α,25-dihydroxyvitamin D3 on progesterone secretion by porcine ovarian granulosa cells. Endocr. Regul. 2013, 47, 123–131. [Google Scholar] [CrossRef]
- Hong, S.H.; Lee, J.E.; Kim, H.S.; Jung, Y.J.; Hwang, D.; Lee, J.H.; Yang, S.Y.; Kim, S.C.; Cho, S.K.; An, B.S. Effect of vitamin D3 on production of progesterone in porcine granulosa cells by regulation of steroidogenic enzymes. J. Biomed. Res. 2016, 30, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Lee, J.E.; An, S.M.; Shin, Y.Y.; Hwang, D.Y.; Yang, S.Y.; Cho, S.K.; An, B.S. Effect of vitamin D3 on biosynthesis of estrogen in porcine granulosa cells via modulation of steroidogenic enzymes. Toxicol. Res. 2017, 33, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhao, Y.; Mangelsdorf, D.J.; Simpson, E.R. Characterization of a region upstream of exon I.1 of the human CYP19 (aromatase) gene that mediates regulation by retinoids in human choriocarcinoma cells. Endocrinology 1998, 139, 1684–1691. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spencer, T.E.; Johnson, G.A.; Burghard, R.C.; Bazer, F.W. Progesterone and placental hormone actions on the uterus: Insights from domestic animals. Biol. Reprod. 2004, 71, 2–10. [Google Scholar] [CrossRef]
- Kovacs, C.S. Bone development and mineral homeostasis in the fetus and neonate: Roles of the calciotropic and phosphotropic hormones. Physiol. Rev. 2014, 94, 1143–1218. [Google Scholar] [CrossRef]
- Choi, Y.; Jang, H.; Seo, H.; Yoo, I.; Han, J.; Kim, M.; Lee, S.; Ka, H. Changes in calcium levels in the endometrium throughout pregnancy and the role of calcium on endometrial gene expression at the time of conceptus implantation in pigs. Mol. Reprod. Dev. 2019, 86, 883–895. [Google Scholar] [CrossRef]
- Bahr, J.M.; Johnson, A.L. Regulation of the follicular hierarchy and ovulation. J. Exp. Zool. Part A Ecol. Integr. Physiol. 1984, 232, 495–500. [Google Scholar] [CrossRef]
- Hrabia, A.; Socha, J.K.; Sechman, A. Involvement of matrix metalloproteinases (MMP-2,-7,-9) and their tissue inhibitors (TIMP-2,-3) in the regression of chicken postovulatory follicles. Gen. Comp. Endocrinol. 2018, 260, 32–40. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J.; Rodriguez-Navarro, A.B.; Hincke, M. Mechanisms and Hormonal Regulation of Shell Formation: Supply of Ionic and Organic Precursors, Shell Mineralization. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Dridi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 833–879. [Google Scholar] [CrossRef]
- Dokoh, S.; Donaldson, C.A.; Marion, S.L.; Pike, J.W.; Haussler, M.R. The ovary: A target organ for 1, 25-dihydroxyvitamin D3. Endocrinology 1983, 112, 200–206. [Google Scholar] [CrossRef]
- Pawłowska, K.; Sechman, A.; Suchanek, I.; Grzegorzewska, A.; Rząsa, J. Effect of 9-cis retinoic acid (RA) on progesterone and estradiol secretion and RA receptor expression in the chicken ovarian follicles. Folia Biol. 2008, 56, 65–72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruschkowski, S.R.; Hart, L.E. Ionic and endocrine characteristics of reproductive failure in calcium-deficient and vitamin D-deficient laying hens. Poult. Sci. 1992, 71, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Kundu, M.C.; Wojtusik, J.; Johnson, P.A. Expression and regulation of Kit ligand in the ovary of the hen. Gen. Comp. Endocrinol. 2012, 179, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Coty, W.A. A specific, high affinity binding protein for 1α, 25-dihydroxy vitamin D in the chick oviduct shell gland. Biochem. Biophys. Res. Commun. 1980, 93, 285–292. [Google Scholar] [CrossRef]
- Johnson, A.L. Reproduction in the Female. In Sturkie’s Avian Physiology, 6th ed.; Scanes, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 635–665. [Google Scholar] [CrossRef]
- Sechman, A.; Shimada, K.; Saito, N.; Ieda, T.; Ono, T. Tissue-specific expression of calbindin-D28K gene during ontogeny of the chicken. J. Exp. Zool. 1994, 269, 450–457. [Google Scholar] [CrossRef]
- Jonchère, V.; Brionne, A.; Gautron, J.; Nys, Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol. 2012, 12, 1–17. [Google Scholar] [CrossRef]
- Corradino, R.A. Calbindin D28K regulation in precociously matured chick egg shell gland in vitro. Gen. Comp. Endocrinol. 1993, 91, 158–166. [Google Scholar] [CrossRef]
- Christakos, S.; Lieben, L.; Masuyama, R.; Carmeliet, G. Vitamin D endocrine system and the intestine. BoneKEy Rep. 2014, 3, 496. [Google Scholar] [CrossRef]
- Nys, Y.; Le Roy, N. Calcium Homeostasis and Eggshell Biomineralization in Female Chicken. In Vitamin D, 4th ed.; Feldman, D., Wesley Pike, M., Bouillon, R., Giovannucci, E., Goltzman, D., Hewison, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 1, pp. 361–382. [Google Scholar] [CrossRef]
- Fernandez, M.S.; Escobar, C.; Lavelin, I.; Pines, M.; Arias, J.L. Localization of osteopontin in oviduct tissue and eggshell during different stages of the avian egg laying cycle. J. Struct. Biol. 2003, 143, 171–180. [Google Scholar] [CrossRef]
- Bar, A. Calcium transport in strongly calcifying laying birds: Mechanisms and regulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 447–469. [Google Scholar] [CrossRef]
- Zohar, Y. Fish reproductive biology-reflecting on five decades of fundamental and translational research. Gen. Comp. Endocrinol. 2021, 300, 113544. [Google Scholar] [CrossRef] [PubMed]
| Molecule | Tissue | Species | Compartment | mRNA | Protein (IHC/WB) | References |
|---|---|---|---|---|---|---|
| VDR | Ovary | Goat | Gc, Tc, O | + | + | [27] |
| Pig | Gc, Tc | na | + | [28] | ||
| Pig | Gc, Tc | + | + | [29] | ||
| Chicken | Gc | + | + | [30] | ||
| Fish | - | na | + | [31] | ||
| Uterus | Buffalo cow | E | na | + | [32] | |
| Pig | E, M | + | + | [33] | ||
| Pig | Gravid E | + | + | [34] | ||
| Sheep | Gravid E, M | + | + | [35] | ||
| Shell gland | Chicken | - | + | na | [36] | |
| Chicken | E, TG | na | + | [37] | ||
| Placenta | Pig | - | + | + | [34] | |
| Sheep | - | + | + | [35] | ||
| PDIA3 | Ovary | Pig | Gc, Tc | + | + | [29] |
| CYP2R1 | Uterus | Pig | Gravid E | + | na | [34] |
| Sheep | Gravid E, M | + | + | [35,38] | ||
| Placenta | Pig | - | + | na | [34] | |
| Sheep | - | + | na | [35] | ||
| CYP27B1 | Ovary | Pig | Gc, Tc | + | + | [29] |
| Uterus | Pig | E, M | + | + | [39] | |
| Pig | Gravid E | + | na | [34] | ||
| Sheep | Gravid E, M | + | + | [35,38] | ||
| Placenta | Pig | - | + | na | [34] | |
| Sheep | - | + | na | [35] | ||
| CYP24A1 | Ovary | Pig | Gc, Tc | + | + | [29] |
| Uterus | Pig | E | + | + | [39] | |
| Pig | Gravid E | + | na | [34] | ||
| Sheep | Gravid E | + | na | [35] | ||
| Placenta | Pig | - | + | na | [34] | |
| Sheep | - | + | na | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzesiak, M.; Tchurzyk, M.; Socha, M.; Sechman, A.; Hrabia, A. An Overview of the Current Known and Unknown Roles of Vitamin D3 in the Female Reproductive System: Lessons from Farm Animals, Birds, and Fish. Int. J. Mol. Sci. 2022, 23, 14137. https://doi.org/10.3390/ijms232214137
Grzesiak M, Tchurzyk M, Socha M, Sechman A, Hrabia A. An Overview of the Current Known and Unknown Roles of Vitamin D3 in the Female Reproductive System: Lessons from Farm Animals, Birds, and Fish. International Journal of Molecular Sciences. 2022; 23(22):14137. https://doi.org/10.3390/ijms232214137
Chicago/Turabian StyleGrzesiak, Malgorzata, Marcelina Tchurzyk, Magdalena Socha, Andrzej Sechman, and Anna Hrabia. 2022. "An Overview of the Current Known and Unknown Roles of Vitamin D3 in the Female Reproductive System: Lessons from Farm Animals, Birds, and Fish" International Journal of Molecular Sciences 23, no. 22: 14137. https://doi.org/10.3390/ijms232214137
APA StyleGrzesiak, M., Tchurzyk, M., Socha, M., Sechman, A., & Hrabia, A. (2022). An Overview of the Current Known and Unknown Roles of Vitamin D3 in the Female Reproductive System: Lessons from Farm Animals, Birds, and Fish. International Journal of Molecular Sciences, 23(22), 14137. https://doi.org/10.3390/ijms232214137






