New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis
Abstract
1. Introduction
2. Results and Discussion
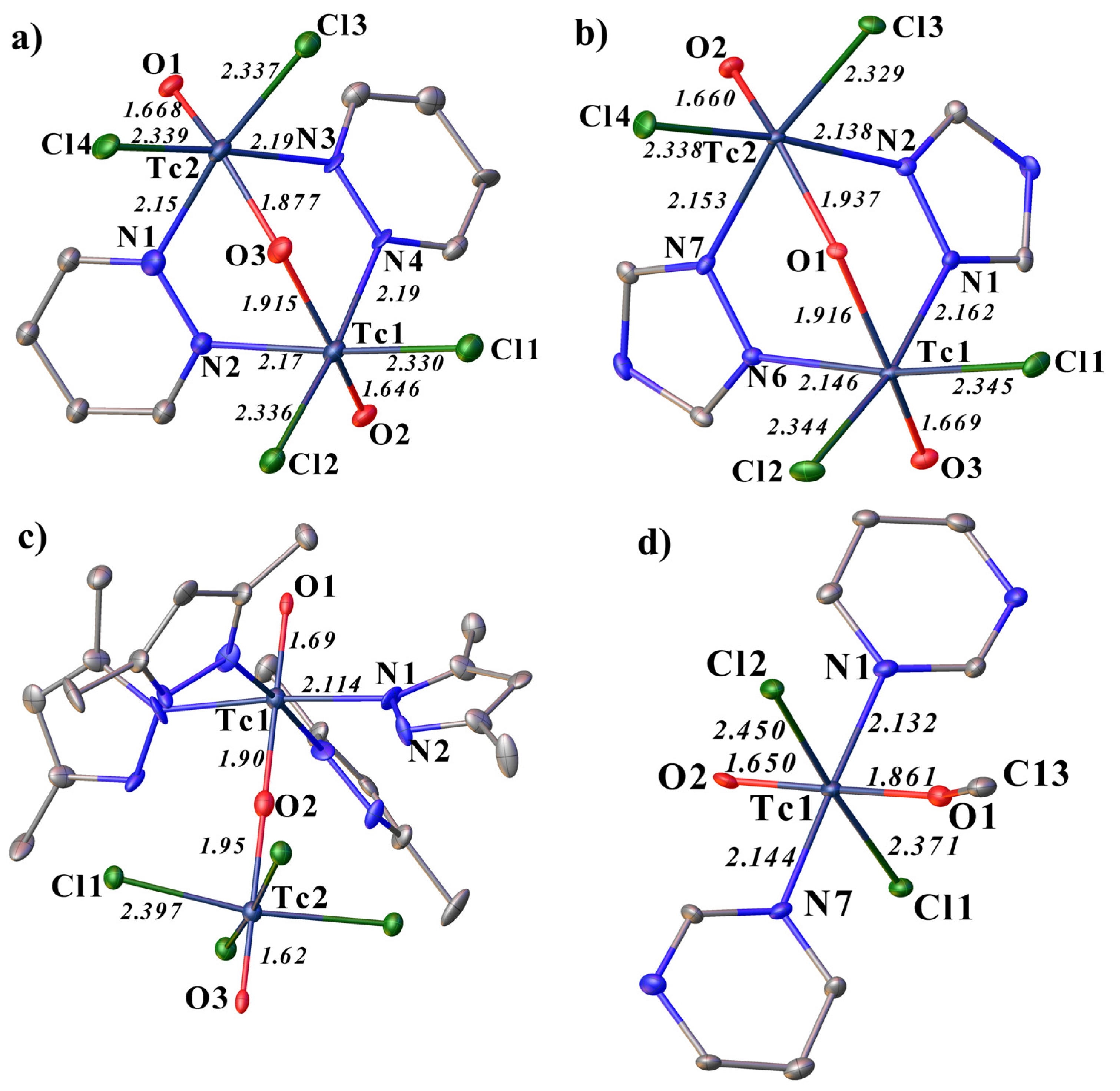
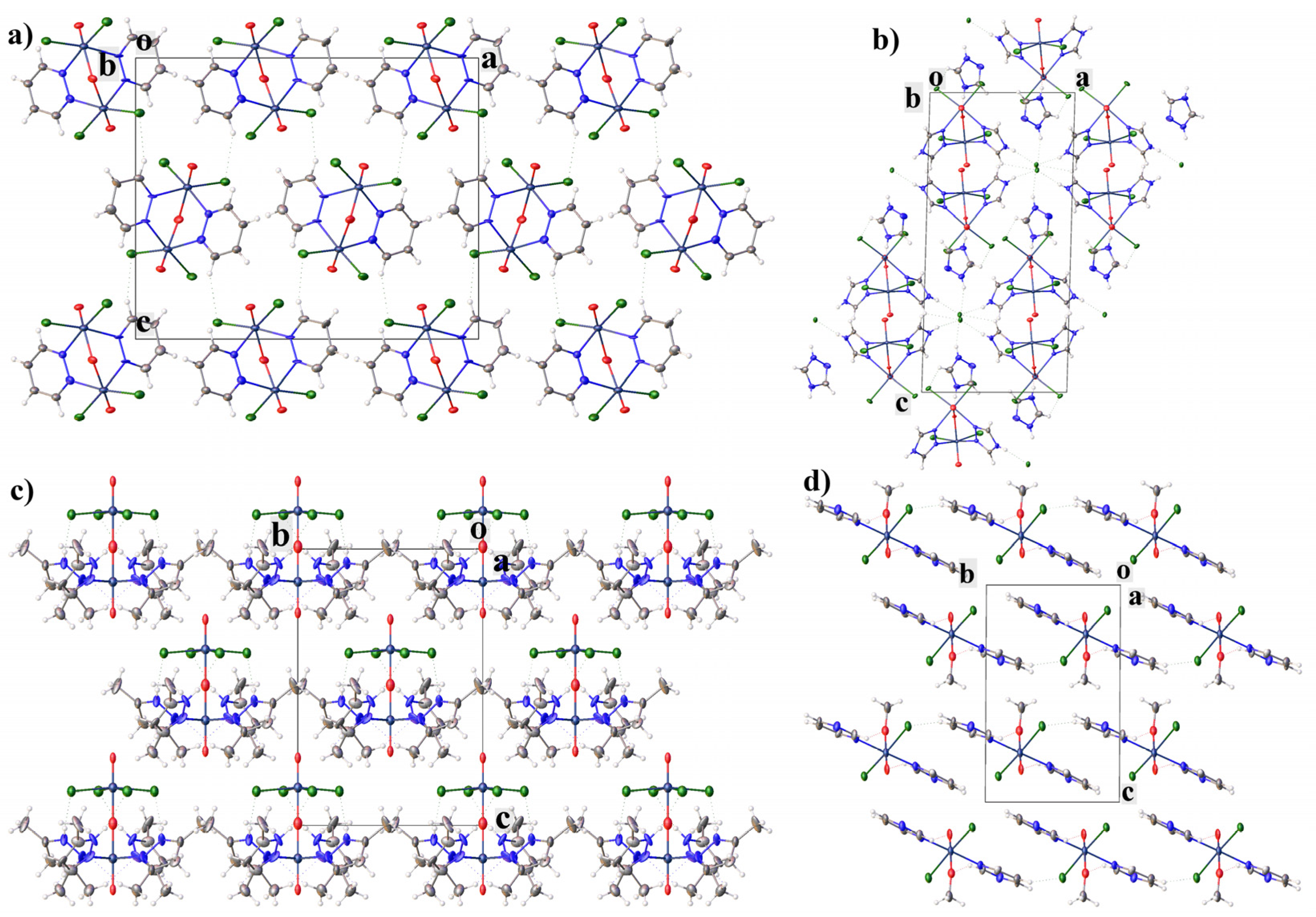
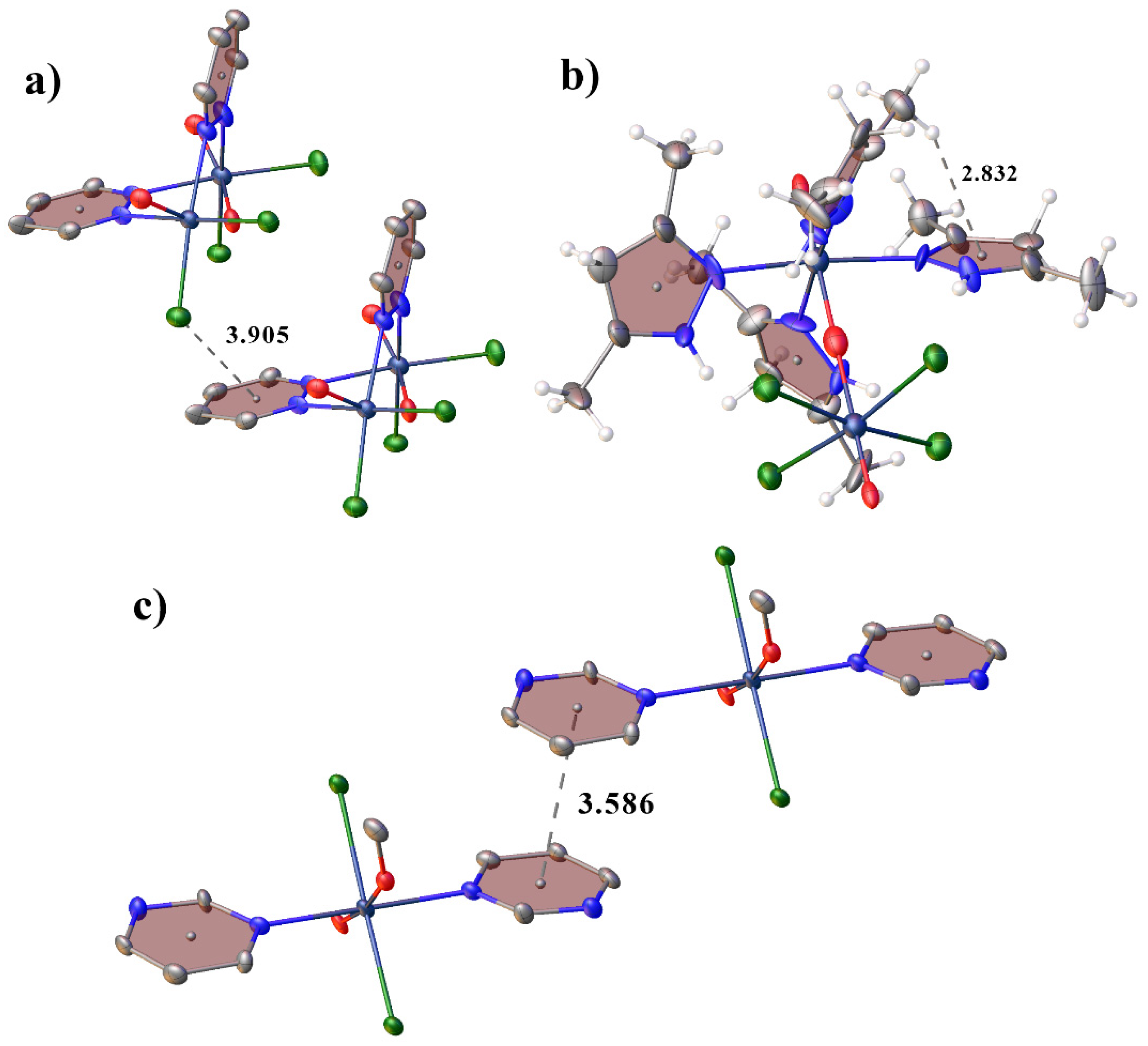
2.1. Structural Description of Tc(V) Complexes
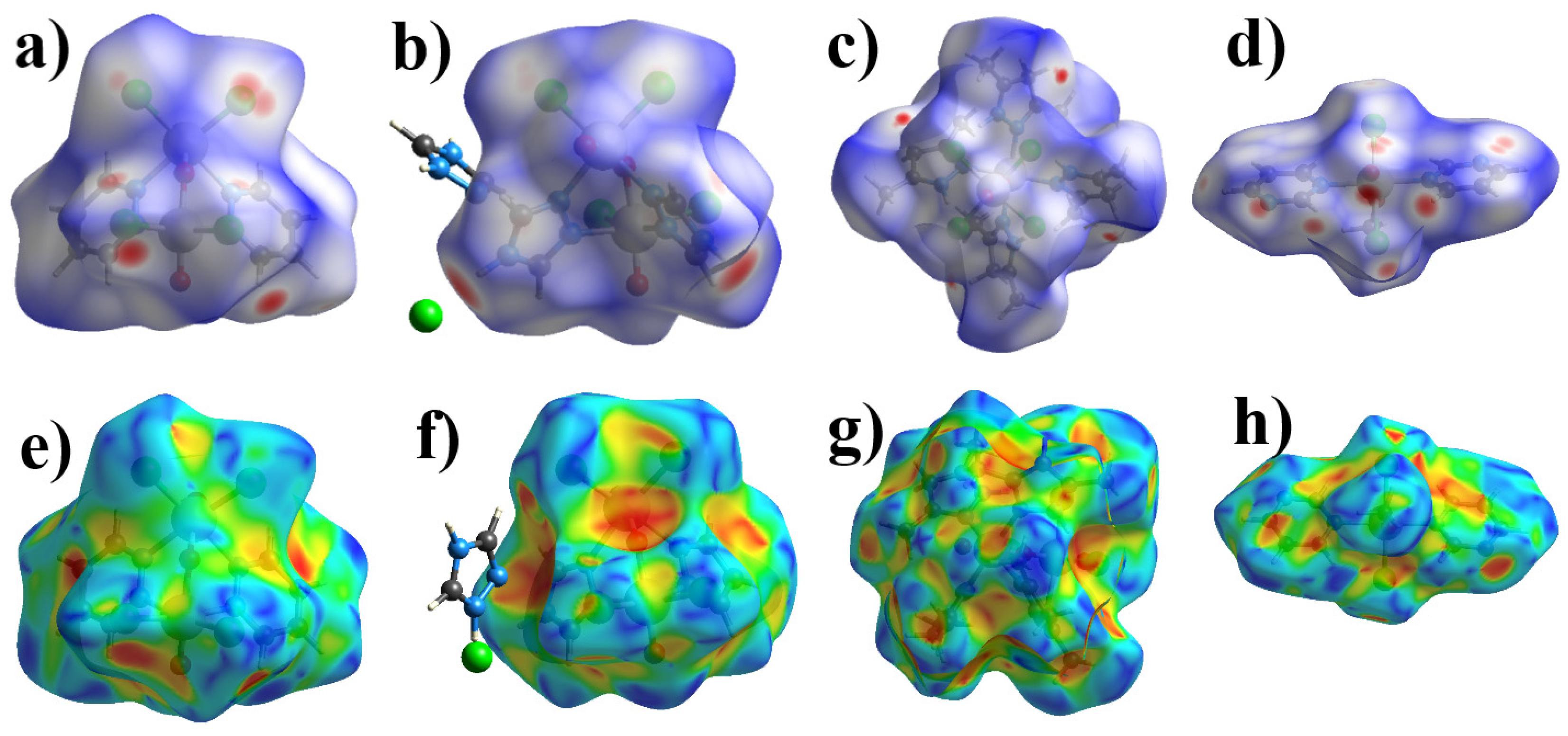
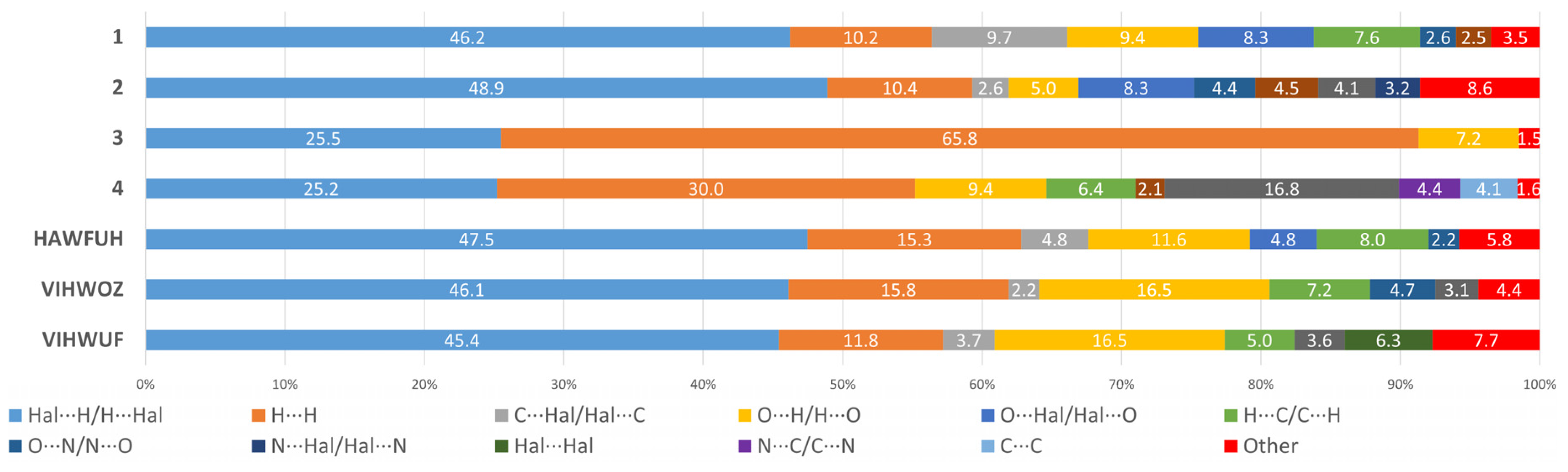
2.2. Hirshfeld Surface Analysis
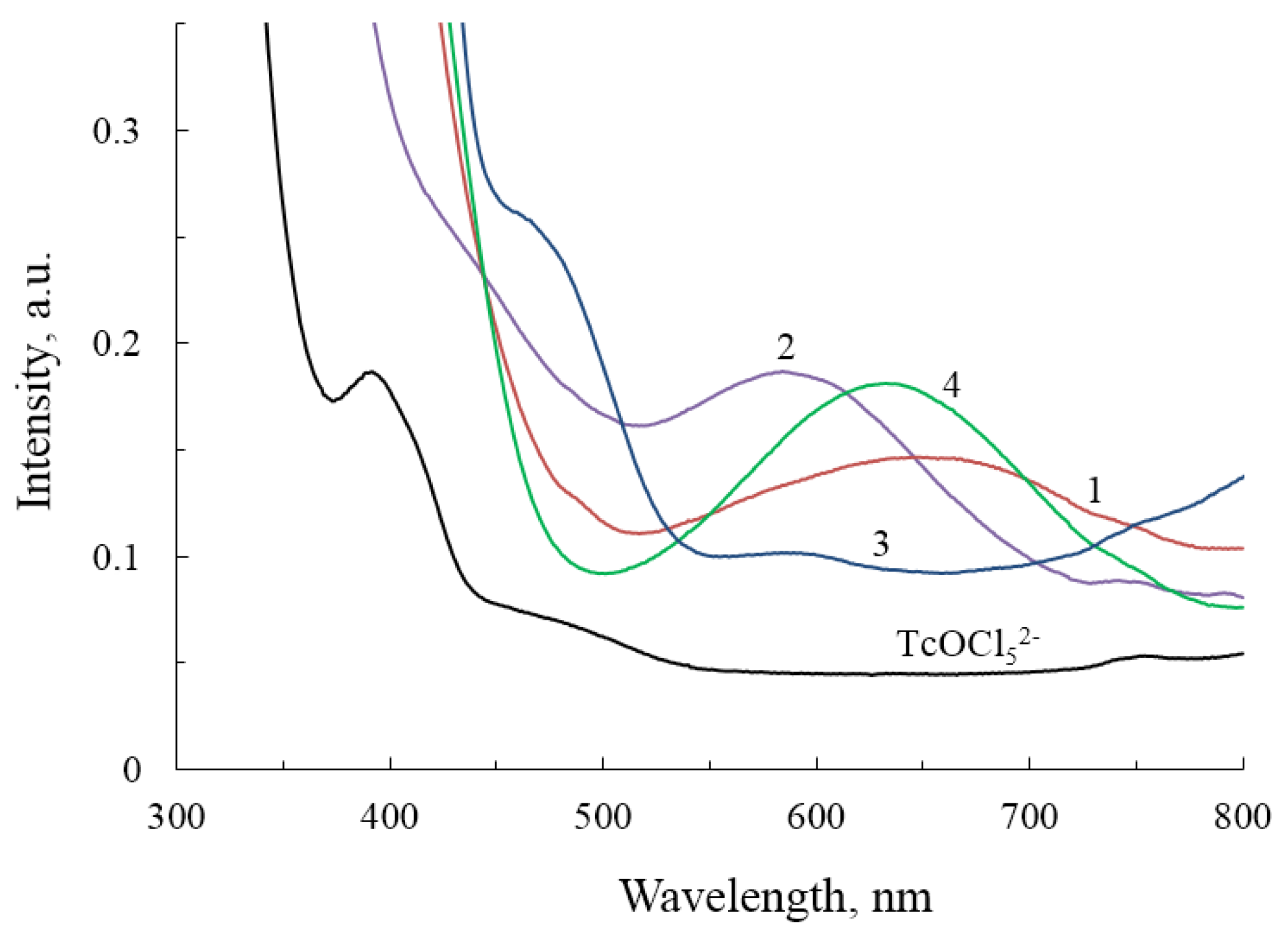
2.3. Spectroscopic Studies
2.4. MALDI-ToF Analysis of the Mother Liquor of 1
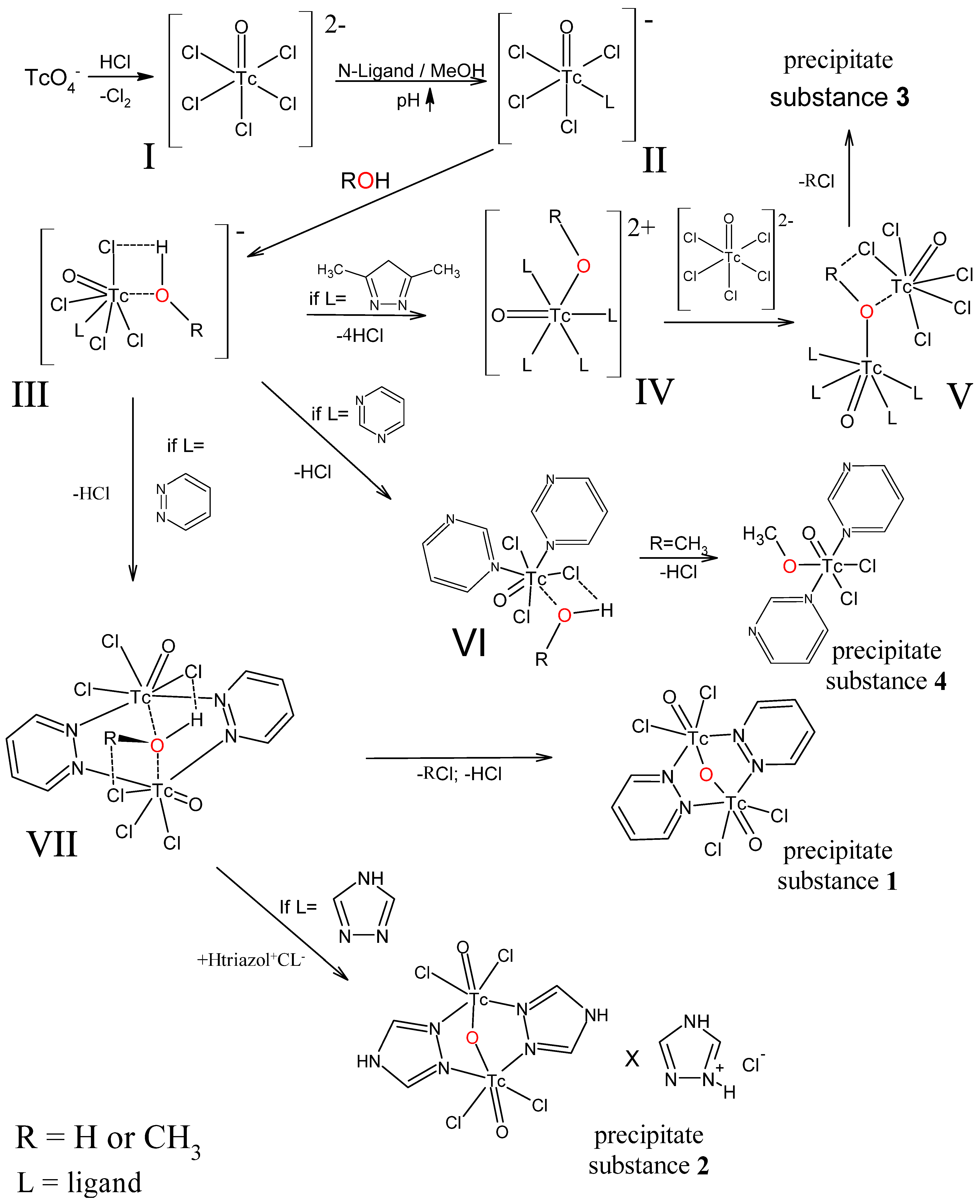
2.5. Proposed Mechanism for the Formation of Complexes
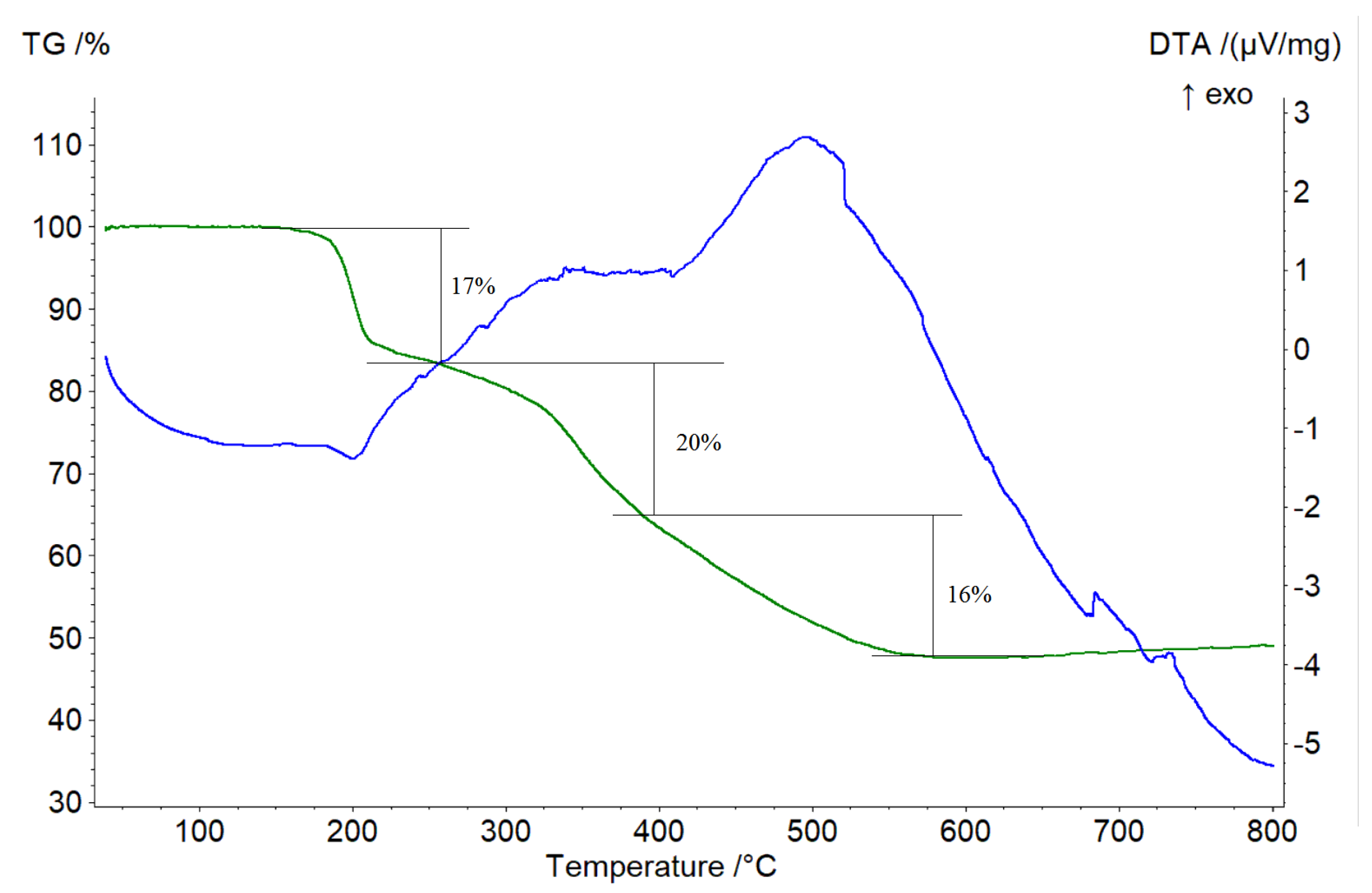
2.6. DTA-Analysis
3. Materials and Methods
3.1. Synthesis of Complexes
3.2. Single-Crystal XRD Analysis
3.3. Spectroscopic Analysis
3.4. MALDI-ToF—Analysis
3.5. Thermal Analysis
3.6. Elemental Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volkov, M.A.; Fedoseev, A.M.; Krivoborodov, E.G.; Toropygin, I.Y.; German, K.E.; Grigoriev, M.S.; Kuznetsov, V.V.; Budantseva, N.A.; Novikov, A.P.; Mezhuev, Y.O. A new method for the synthesis of polynuclear carboxylate complexes of technetium (II, III). J. Organomet. Chem. 2022, 957, 122146. [Google Scholar] [CrossRef]
- Alberto, R. From oxo to carbonyl and arene complexes; A journey through technetium chemistry. J. Organomet. Chem. 2018, 869, 264–269. [Google Scholar] [CrossRef]
- Sidorenko, G.V.; Miroslavov, A.E. Higher Technetium(I) Carbonyls and Possibility of Using Them in Nuclear Medicine: Problems and Prospects. Radiochemistry 2021, 63, 253–262. [Google Scholar] [CrossRef]
- Zegke, M.; Grödler, D.; Roca Jungfer, M.; Haseloer, A.; Kreuter, M.; Neudörfl, J.M.; Sittel, T.; James, C.M.; Rothe, J.; Altmaier, M.; et al. Ammonium Pertechnetate in Mixtures of Trifluoromethanesulfonic Acid and Trifluoromethanesulfonic Anhydride. Angew. Chem. Int. Ed. 2022, 61, e202113777. [Google Scholar] [CrossRef] [PubMed]
- German, K.E.; Fedoseev, A.M.; Grigoriev, M.S.; Kirakosyan, G.A.; Dumas, T.; Den Auwer, C.; Moisy, P.; Lawler, K.V.; Forster, P.M.; Poineau, F. A 70-Year-Old Mystery in Technetium Chemistry Explained by the New Technetium Polyoxometalate [H7O3]4[Tc20O68]⋅4H2O. Chem. Eur. J. 2021, 27, 13624–13631. [Google Scholar] [CrossRef] [PubMed]
- Abram, U.; Beyer, R.; Münze, R.; Stach, J.; Kaden, L.; Lorenz, B.; Findeisen, M. Mixed-ligand complexes of technetium-III. Synthesis and characterization of [bis(diphenylphosphino)ethane]tetrakis(trimethylphosphite)technetium(I) hexafluorophosphate, [Tc(DPPE)(TMP)4]PF6. Polyhedron 1989, 8, 1201–1204. [Google Scholar] [CrossRef]
- Duatti, A. Review on 99mTc radiopharmaceuticals with emphasis on new advancements. Nucl. Med. Biol. 2021, 92, 202–216. [Google Scholar] [CrossRef]
- Jürgens, S.; Herrmann, W.A.; Kühn, F.E. Rhenium and technetium based radiopharmaceuticals: Development and recent advances. J. Organomet. Chem. 2014, 751, 83–89. [Google Scholar] [CrossRef]
- Melent’ev, A.B.; Mashkin, A.N.; German, K.E. The influence of deviations in process parameters on the purification of uranium from different radionuclides. Theor. Found. Chem. Eng. 2016, 50, 554–561. [Google Scholar] [CrossRef]
- Fackler, P.H.; Lindsay, M.J.; Clarke, M.J.; Kastner, M.E. Synthesis and structure of trans-[O2(Im)4Tc]Cl·2H2O, trans-[O2(1-meIm)4Tc]Cl·3H2O and related compounds. Inorg. Chim. Acta 1985, 109, 39–49. [Google Scholar] [CrossRef]
- Welch, M.J.; Redvanly, C.S. Handbook of Radiopharmaceuticals: Radiochemistry and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003; ISBN 978-0-470-84638-4. [Google Scholar]
- Abram, U.; Alberto, R. Technetium and rhenium: Coordination chemistry and nuclear medical applications. J. Braz. Chem. Soc. 2006, 17, 1486–1500. [Google Scholar] [CrossRef]
- Liu, S. The role of coordination chemistry in the development of target-specific radiopharmaceuticals. Chem. Soc. Rev. 2004, 33, 445–461. [Google Scholar] [CrossRef]
- Maruk, A.Y.; Bruskin, A.B.; Kodina, G.E. Novel 99m Tc radiopharmaceuticals with bifunctional chelating agents. Radiochemistry 2011, 53, 341–353. [Google Scholar] [CrossRef]
- Günther, T.; Konrad, M.; Stopper, L.; Kunert, J.P.; Fischer, S.; Beck, R.; Casini, A.; Wester, H.J. Optimization of the Pharmacokinetic Profile of [99mTc]Tc-N4-Bombesin Derivatives by Modification of the Pharmacophoric Gln-Trp Sequence. Pharmaceuticals 2022, 15, 1133. [Google Scholar] [CrossRef]
- Volkov, M.A.; Novikov, A.P.; Grigoriev, M.S.; Fedoseev, A.M.; German, K.E. Novel Synthesis Methods of New Imidazole-Containing Coordination Compounds Tc(IV, V, VII)—Reaction Mechanism, Xrd and Hirshfeld Surface Analysis. Int. J. Mol. Sci. 2022, 23, 9461. [Google Scholar] [CrossRef]
- Sergienko, V.S.; Churakov, A.V. Specific features of technetium mononuclear octahedral oxo complexes: A review. Crystallogr. Rep. 2013, 58, 1–25. [Google Scholar] [CrossRef]
- Kastner, M.E.; Lindsay, M.J.; Clarke, M.J. Synthesis and Structure of trans-[O2(en)2Tcv]+. Inorg. Chem. 1982, 21, 2037–2040. [Google Scholar] [CrossRef]
- Zuckman, S.A.; Freeman, G.M.; Troutner, D.E.; Volkert, W.A.; Holmes, R.A.; Van Derveer, D.G.; Barefield, E.K. Preparation and X-ray Structure of trans-Dioxo(1,4,8,11-tetraazacyclotetradecane)technetium(V) Perchlorate Hydrate. Inorg. Chem. 1981, 20, 2386–2389. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Trieu, T.N.; Abram, U. Syntheses and Structures of Nitridorhenium(V) and Nitridotechnetium(V) Complexes with N,N-[(Dialkylamino)(thiocarbonyl)]-N′-(2-hydroxyphenyl)benzamidines. Z. Anorg. Allg. Chem. 2011, 637, 1330–1333. [Google Scholar] [CrossRef]
- Clarke, M.J.; Lu, J. Synthesis and Spectra of Cis-[NCl(phen)2Tc]Cl-H2O and c/s-[NCl(phen)2Tc]PF6 and Considerations of their Structural Distortions. Inorg. Chem. 1992, 31, 2476–2480. [Google Scholar] [CrossRef]
- Rue, K.L.; McLachlan, J.R.; Cazzaniga, J.A.; Chakraborty, I.; Dares, C.J.; Raptis, R.G. Redox-active dinuclear oxorhenium(V) pyrazolate complexes. Inorg. Chim. Acta 2021, 516, 120126. [Google Scholar] [CrossRef]
- MacHura, B.; Dziȩgielewski, J.O.; Kruszynski, R.; Bartczak, T.J.; Kusz, J. Linear and bent oxo-bridged dinuclear rhenium(V) complexes with terminal and bridging pyrazole ligands. Inorg. Chim. Acta 2004, 357, 1011–1022. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Jaworska, M.; Lodowski, P. Synthesis, spectroscopic characterization, crystal, molecular and electronic structure of the [{ReOBr2(pyz)2}2(μ-O)] and [{ReOBr2}2(μ-O)(μ-pyd)2] complexes. Polyhedron 2005, 24, 1893–1906. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Kusz, J. Reactivity of [ReOX3(PPh3)2] complexes towards pyridazine. X-ray structures of [{ReOCl2}2(μ-O)(μ-pyd)2]·C6H6 and [{ReOBr2}2(μ-O)(μ-pyd)2]·CH3CN, and DFT calculations for [{ReOCl2}2(μ-O)(μ-pyd)2]. Polyhedron 2007, 26, 3054–3062. [Google Scholar] [CrossRef]
- Machura, B.; Wolff, M.; Palion, J.; Benoist, E. Synthesis, spectroscopic characterization and X-ray crystal structures of mononuclear and binuclear oxidorhenium(V) complexes containing indazolyl moieties. Inorg. Chim. Acta 2013, 404, 144–154. [Google Scholar] [CrossRef]
- Fackler, P.H.; Kastner, M.E.; Clarke, M.J. Synthesis, spectra, and structure of af-dibromo-b-ethoxo-d-oxo-ce-bis(4-nitropyridine)technetium(V) and related. Inorg. Chem. 1984, 23, 3968–3972. [Google Scholar] [CrossRef]
- Lock, C.J.L.; Turner, G. Studies of the rhenium-oxygen bond. III. The crystal and molecular structure of 1-oxo-6-ethoxo-2,4-dichloro-3,5-dipyridinerhenium(V). Can. J. Chem. 1972, 55, 333–339. [Google Scholar] [CrossRef]
- Fortin, S.; Beauchamp, A.L. Synthesis and crystal structure of a new isomer of the μ-oxo-bis[dichlorooxobis(pyridine)rhenium(V)] complex {OReCl2py2}2O. Inorg. Chim. Acta 1998, 279, 159–164. [Google Scholar] [CrossRef]
- Iengo, E.; Zangrando, E.; Mestroni, S.; Fronzoni, G.; Stener, M.; Alessio, E. Complexed bridging ligands: Oxorhenium(V) compounds with mono-coordinated pyrazine or pyrimidine as possible building blocks for the construction of polynuclear architectures. J. Chem. Soc. Dalton Trans. 2001, 1338–1346. [Google Scholar] [CrossRef]
- Daolio, A.; Pizzi, A.; Terraneo, G.; Frontera, A.; Resnati, G. Anion⋅Anion Interactions Involving σ-Holes of Perrhenate, Pertechnetate and Permanganate Anions. ChemPhysChem 2021, 22, 2281–2285. [Google Scholar] [CrossRef]
- Novikov, A.P.; German, K.E.; Safonov, A.V.; Grigoriev, M.S. Cation Protonation Degree Influence on the Formation of Anion⋅⋅⋅Anion and Other Non-Valent Interactions in Guaninium Perrhenates and Pertechnetate. ChemistrySelect 2022, 7, e202202814. [Google Scholar] [CrossRef]
- Xie, R.; Shen, N.; Chen, X.; Li, J.; Wang, Y.; Zhang, C.; Xiao, C.; Chai, Z.; Wang, S. 99TcO4-Separation through Selective Crystallization Assisted by Polydentate Benzene-Aminoguanidinium Ligands. Inorg. Chem. 2021, 60, 6463–6471. [Google Scholar] [CrossRef]
- Zhu, L.; Sheng, D.; Xu, C.; Dai, X.; Silver, M.A.; Li, J.; Li, P.; Wang, Y.; Wang, Y.; Chen, L.; et al. Identifying the Recognition Site for Selective Trapping of 99TcO4- in a Hydrolytically Stable and Radiation Resistant Cationic Metal-Organic Framework. J. Am. Chem. Soc. 2017, 139, 14873–14876. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xiao, C.; Dai, X.; Li, J.; Gui, D.; Sheng, D.; Chen, L.; Zhou, R.; Chai, Z.; Albrecht-Schmitt, T.E.; et al. Exceptional perrhenate/pertechnetate uptake and subsequent immobilization by a low-dimensional cationic coordination polymer: Overcoming the hofmeister bias selectivity. Environ. Sci. Technol. Lett. 2017, 4, 316–322. [Google Scholar] [CrossRef]
- Sheng, D.; Zhu, L.; Dai, X.; Xu, C.; Li, P.; Pearce, C.I.; Xiao, C.; Chen, J.; Zhou, R.; Duan, T.; et al. Successful Decontamination of 99TcO4− in Groundwater at Legacy Nuclear Sites by a Cationic Metal-Organic Framework with Hydrophobic Pockets. Angew. Chem. 2019, 131, 5022–5026. [Google Scholar] [CrossRef]
- Artemjev, A.A.; Novikov, A.P.; Burkin, G.M.; Sapronov, A.A.; Kubasov, A.S.; Nenajdenko, V.G.; Khrustalev, V.N.; Borisov, A.V.; Kirichuk, A.A.; Kritchenkov, A.S.; et al. Towards Anion Recognition and Precipitation with Water-Soluble 1,2,4-Selenodiazolium Salts: Combined Structural and Theoretical Study. Int. J. Mol. Sci. 2022, 23, 6372. [Google Scholar] [CrossRef] [PubMed]
- Chotkowski, M.; Wrzosek, B.; Grdeń, M. Intermediate oxidation states of technetium in concentrated sulfuric acid solutions. J. Electroanal. Chem. 2018, 814, 83–90. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shen, N.; Chen, L.; Guo, Q.; Chen, L.; He, L.; Dai, X.; Chai, Z.; Wang, S. Task-Specific Tailored Cationic Polymeric Network with High Base-Resistance for Unprecedented 99TcO4-Cleanup from Alkaline Nuclear Waste. ACS Cent. Sci. 2021, 7, 1441–1450. [Google Scholar] [CrossRef]
- Machura, B.; Kruszynski, R.; Kusz, J. A novel oxo-bridged rhenium complex with Re centers in different coordination environments–Synthesis, spectroscopic characterization and X-ray structure. Inorg. Chem. Commun. 2007, 10, 494–497. [Google Scholar] [CrossRef]
- Pervukhina, N.V.; Sokolov, M.N.; Fedorova, N.; Fedorov, V.E. Crystal Structures of Two Re(V) Complexes of 3,5-Dimethylpyrazole with Linear and Bent [OReOReO]4+ Fragments. J. Struct. Chem. 2001, 42, 833–837. [Google Scholar] [CrossRef]
- Kückmann, T.I.; Abram, U. Oxorhenium(V)- and Tricarbonylrhenium(I) Complexes with Substituted Pyrazoles as Products of the Degradation of Hydrotrispyrazolylborates. Z. Anorg. Allg. Chem. 2004, 630, 783–785. [Google Scholar] [CrossRef]
- Sokolov, M.N.; Fedorova, N.E.; Pervukhina, N.V.; Peresypkina, E.V.; Virovets, A.V.; Pätow, R.; Fedorov, V.E.; Fenske, D. Rhenium(IV) and rhenium(V) complexes with 3,5-dimethylpyrazole. Russ. Chem. Bull. 2006, 55, 53–61. [Google Scholar] [CrossRef]
- Novikov, A.P.; Volkov, M.A.; Safonov, A.V.; Grigoriev, M.S. Synthesis, Crystal Structure, and Hirshfeld Surface Analysis of Hexachloroplatinate and Tetraclorouranylate of 3-Carboxypyridinium—Halogen Bonds and π-Interactions vs. Hydrogen Bonds. Crystals 2022, 12, 271. [Google Scholar] [CrossRef]
- Alvarez, S. A cartography of the van der Waals territories. Dalt. Trans. 2013, 42, 8617–8636. [Google Scholar] [CrossRef] [PubMed]
- Spiwok, V. CH/π Interactions in Carbohydrate Recognition. Molecules 2017, 22, 1038. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hao, J.; Zhai, L.X.; Zhang, Y.; Dong, W.K. Synthesis, Crystal Structure, Luminescence, Electrochemical and Antimicrobial Properties of Bis(salamo)-Based Co(II) Complex. Crystals 2017, 7, 277. [Google Scholar] [CrossRef]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in organic reactions. Tetrahedron 2005, 61, 6923–6950. [Google Scholar] [CrossRef]
- Zhuo, H.; Li, Q.; Li, W.; Cheng, J. Is π halogen bonding or lone pair⋯π interaction formed between borazine and some halogenated compounds? Phys. Chem. Chem. Phys. 2013, 16, 159–165. [Google Scholar] [CrossRef]
- Shah, M.B.; Liu, J.; Zhang, Q.; Stout, C.D.; Halpert, J.R. Halogen-π Interactions in the Cytochrome P450 Active Site: Structural Insights into Human CYP2B6 Substrate Selectivity. ACS Chem. Biol. 2017, 12, 1210. [Google Scholar] [CrossRef]
- Mitra, D.; Bankoti, N.; Michael, D.; Sekar, K.; Row, T.N.G. C-halogen…pi interactions in nucleic acids: A database study. J. Chem. Sci. 2020, 132, 93. [Google Scholar] [CrossRef]
- Riley, K.E.; Tran, K.A. Strength and Character of R–X···π Interactions Involving Aromatic Amino Acid Sidechains in Protein-Ligand Complexes Derived from Crystal Structures in the Protein Data Bank. Crystals 2017, 7, 273. [Google Scholar] [CrossRef]
- Dumitrescu, D.; Shova, S.; Man, I.C.; Caira, M.R.; Popa, M.M.; Dumitrascu, F. 5-Iodo-1-Arylpyrazoles as Potential Benchmarks for Investigating the Tuning of the Halogen Bonding. Crystals 2020, 10, 1149. [Google Scholar] [CrossRef]
- Novikov, A.P.; Volkov, M.A.; Safonov, A.V.; Grigoriev, M.S.; Abkhalimov, E.V. Synthesis and Characterization of New Guanine Complexes of Pt(IV) and Pd(II) by X-Ray Diffraction and Hirshfeld Surface Analysis. Crystals 2021, 11, 1417. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Scheiner, S. Noncovalent Forces; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; ISBN 9783319141633. [Google Scholar]
- Psycharis, V.; Dermitzaki, D.; Raptopoulou, C.P. The Use of Hirshfeld Surface Analysis Tools to Study the Intermolecular Interactions in Single Molecule Magnets. Crystals 2021, 11, 1246. [Google Scholar] [CrossRef]
- Liu, M.; Yin, C.; Chen, P.; Zhang, M.; Parkin, S.; Zhou, P.; Li, T.; Yu, F.; Long, S. sp2CH⋯Cl hydrogen bond in the conformational polymorphism of 4-chloro-phenylanthranilic acid. CrystEngComm 2017, 19, 4345–4354. [Google Scholar] [CrossRef]
- Marek, P.H.; Urban, M.; Madura, I.D. The study of interactions with a halogen atom: Influence of NH2 group insertion on the crystal structures of meta-bromonitrobenzene derivatives. Acta Crystallogr. Sect. C Struct. Chem. 2018, 74, 1509–1517. [Google Scholar] [CrossRef]
- Novikov, A.P.; Bezdomnikov, A.A.; Grigoriev, M.S.; German, K.E. Synthesis, crystal structure and Hirshfeld surface analysis of 2-(perfluorophenyl)acetamide in comparison with some related compounds. Acta Crystallogr. Sect. E Crystallogr. Commun. 2022, 78, 80–83. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Dirghangi, B.K.; Menon, M.; Pramanik, A.; Chakravorty, A. A Triad of Variable-Valent Rhenium Aldimine and Amide Systems Interrelated by Successive Oxygen Atom Transfer. Inorg. Chem. 1997, 36, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, G.K.; Goswami, S.; Falvello, L.R.; Chakravorty, A. A New Family of Semibent Rhenium(V) Arylimides Formed by Azo Splitting: Structure, Bonding, and Electrooxidation to Rhenium(VI) Congeners. Inorg. Chem. 1987, 26, 3365–3370. [Google Scholar] [CrossRef]
- Du Preez, J.G.H.; Gerber, T.I.A.; Fourie, P.J.; Van Wyk, A.J. The Chemistry of rhenium and technetium. Part 1. Syntheses and characterisation of new dioxo technetium(V) complexes with schiff base type ligands. Inorg. Chim. Acta 1984, 82, 201–205. [Google Scholar] [CrossRef]
- L’Annunziata, M.F. (Ed.) Handbook of Radioactivity Analysis: Volume 2. Radioanalytical Applications; Academic Press: Cambridge, MA, USA, 2020; ISBN 978-0-12-814395-7. [Google Scholar]
- Bruker AXS Inc.: Madison, WI, USA. SAINT, V8.40B, Elsevier: Edinburgh, UK, 2020.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]

| Identification Code | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Empirical formula | C8H8Cl4N4O3Tc2 | C6H10Cl5N9O3Tc2 | C20H36Cl4N8O3Tc2 | C9H11Cl2N4O2Tc |
| Formula weight | 545.98 | 631.30 | 774.37 | 376.12 |
| Temperature/K | 100(2) | |||
| Crystal system | orthorhombic | monoclinic | tetragonal | triclinic |
| Space group | Pna21 | P21/n | I4 | P-1 |
| a/Å | 17.929(2) | 10.4766(9) | 10.059(6) | 6.168(3) |
| b/Å | 6.1567(9) | 8.4060(7) | 10.059(6) | 8.617(4) |
| c/Å | 14.726(2) | 21.6695(18) | 15.055(13) | 13.054(6) |
| α/° | 90 | 90 | 90 | 89.553(17) |
| β/° | 90 | 91.572(3) | 90 | 89.505(19) |
| γ/° | 90 | 90 | 90 | 69.338(16) |
| Volume/Å3 | 1625.5(4) | 1907.6(3) | 1523(2) | 649.2(5) |
| Z | 4 | 4 | 2 | 2 |
| ρcalcg/cm3 | 2.231 | 2.198 | 1.688 | 1.924 |
| μ/mm−1 | 2.368 | 2.176 | 1.294 | 1.519 |
| F(000) | 1048.0 | 1216.0 | 780.0 | 372.0 |
| Crystal size/mm3 | 0.3 × 0.12 × 0.05 | 0.22 × 0.09 × 0.06 | 0.06 × 0.05 × 0.04 | 0.39 × 0.12 × 0.04 |
| Radiation | MoKα (λ = 0.71073) | |||
| 2Θ range for data collection/° | 8.494 to 54.986 | 8.326 to 59.998 | 9.078 to 59.994 | 9.37 to 59.982 |
| Index ranges | −23 ≤ h ≤ 23, −7 ≤ k ≤ 7, −19 ≤ l ≤ 18 | −14 ≤ h ≤ 14, −11 ≤ k ≤ 9, −30 ≤ l ≤ 30 | −14 ≤ h ≤ 13, −13 ≤ k ≤ 13, −21 ≤ l ≤ 20 | −7 ≤ h ≤ 8, −12 ≤ k ≤ 11, −18 ≤ l ≤ 18 |
| Reflections collected | 18191 | 32465 | 3234 | 10884 |
| Independent reflections | 3664 [Rint = 0.1657, Rsigma = 0.1277] | 5544 [Rint = 0.0925, Rsigma = 0.0779] | 1764 [Rint = 0.1930, Rsigma = 0.3190] | 3691 [Rint = 0.1534, Rsigma = 0.2125] |
| Data/restraints/parameters | 3664/1/162 | 5544/0/239 | 1764/1/83 | 3691/0/165 |
| Goodness-of-fit on F2 | 1.025 | 1.023 | 0.941 | 0.986 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0691, wR2 = 0.1416 | R1 = 0.0426, wR2 = 0.0734 | R1 = 0.0971, wR2 = 0.1829 | R1 = 0.1046, wR2 = 0.2348 |
| Final R indexes [all data] | R1 = 0.1262, wR2 = 0.1680 | R1 = 0.0659, wR2 = 0.0805 | R1 = 0.2371, wR2 = 0.2429 | R1 = 0.1907, wR2 = 0.2894 |
| Largest diff. peak/hole/e Å−3 | 2.42/−1.45 | 0.97/−0.88 | 1.39/−1.28 | 3.04/−2.23 |
| Flack parameter | 0.5(2) | 0.06(19) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novikov, A.P.; Volkov, M.A. New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis. Int. J. Mol. Sci. 2022, 23, 14034. https://doi.org/10.3390/ijms232214034
Novikov AP, Volkov MA. New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis. International Journal of Molecular Sciences. 2022; 23(22):14034. https://doi.org/10.3390/ijms232214034
Chicago/Turabian StyleNovikov, Anton Petrovich, and Mikhail Alexandrovich Volkov. 2022. "New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis" International Journal of Molecular Sciences 23, no. 22: 14034. https://doi.org/10.3390/ijms232214034
APA StyleNovikov, A. P., & Volkov, M. A. (2022). New O- and N-N-Bridging Complexes of Tc(V), the Role of the Nitrogen Atom Position in Aromatic Rings: Reaction Mechanism, Spectroscopy, DTA, XRD and Hirshfeld Surface Analysis. International Journal of Molecular Sciences, 23(22), 14034. https://doi.org/10.3390/ijms232214034






