Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway
Abstract
1. Introduction
2. Results
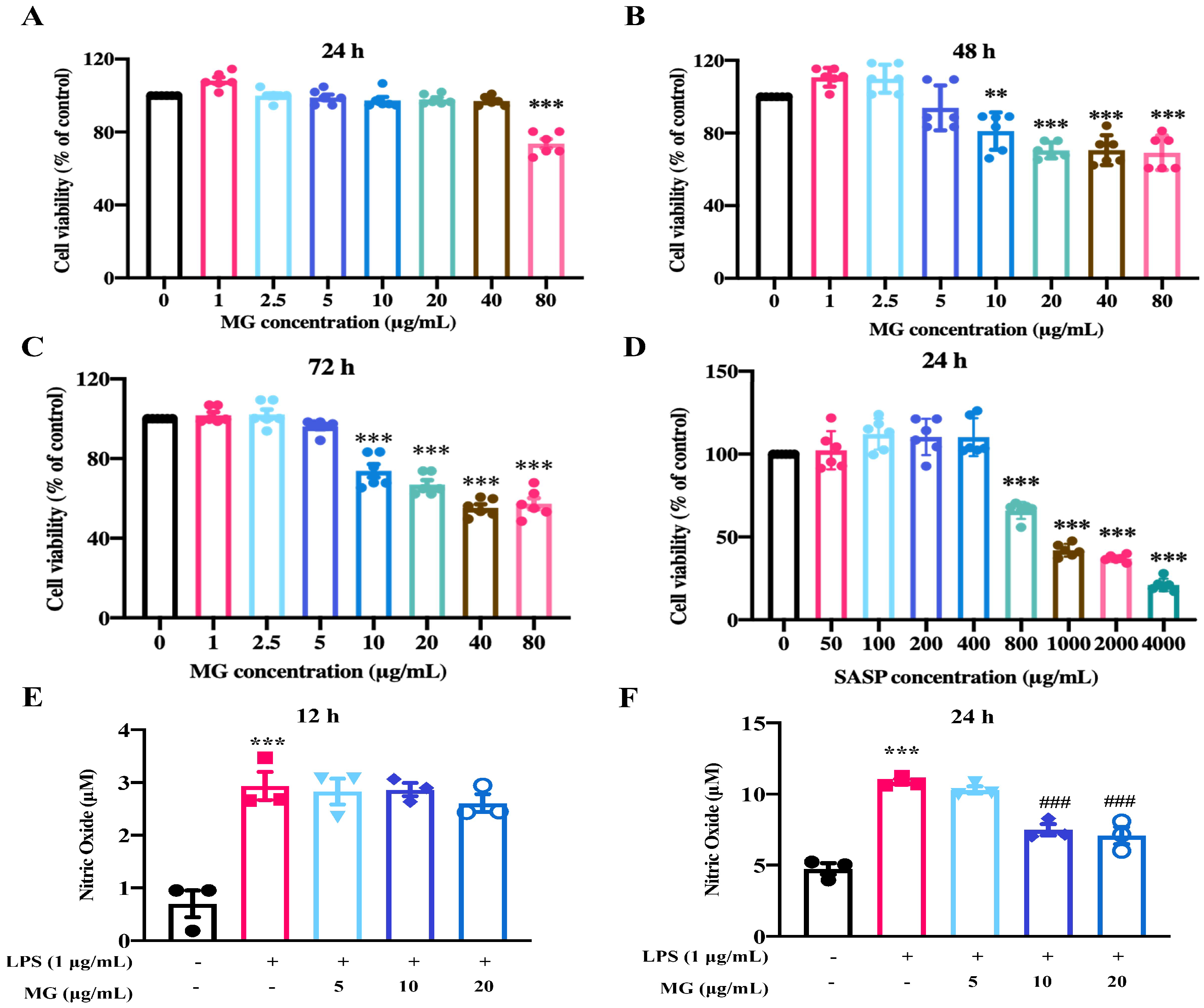
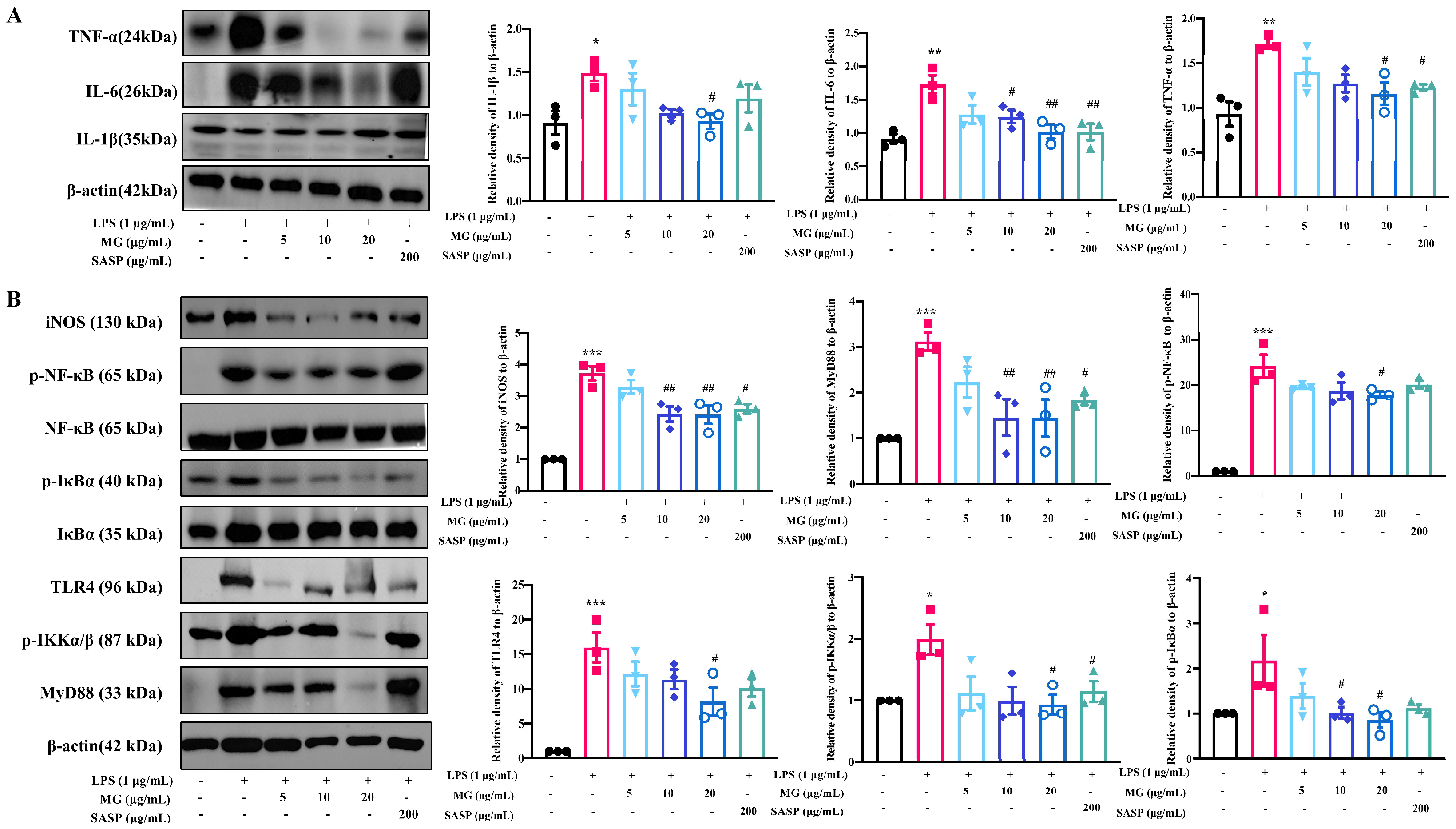
2.1. MG Alleviated Inflammatory Response in the Lipopolysaccharide (LPS)-Induced UC Model In Vitro
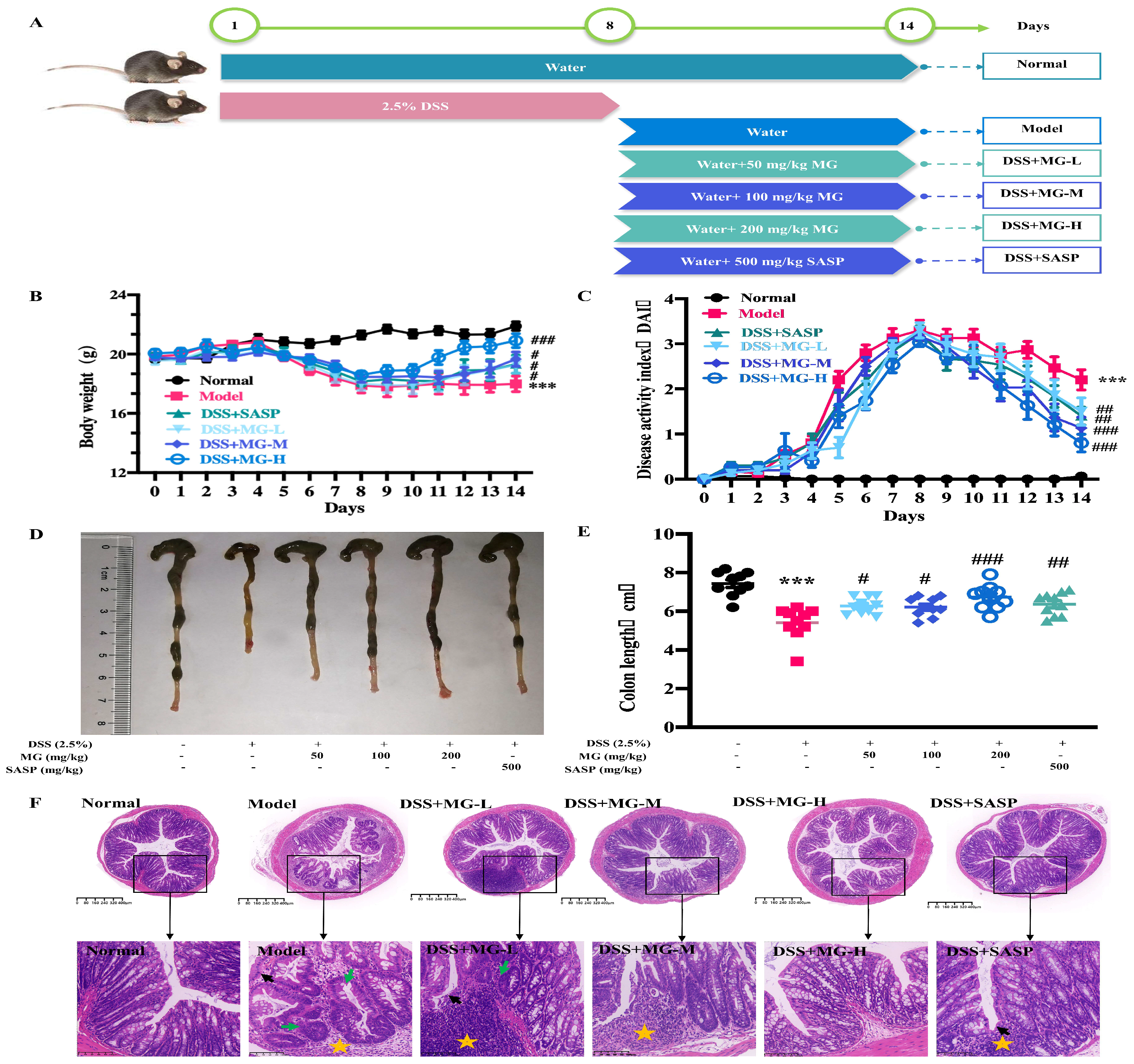
2.2. MG Alleviated the Inflammatory Response of Dextran Sulphate Sodium (DSS)-Induced UC Model Mice
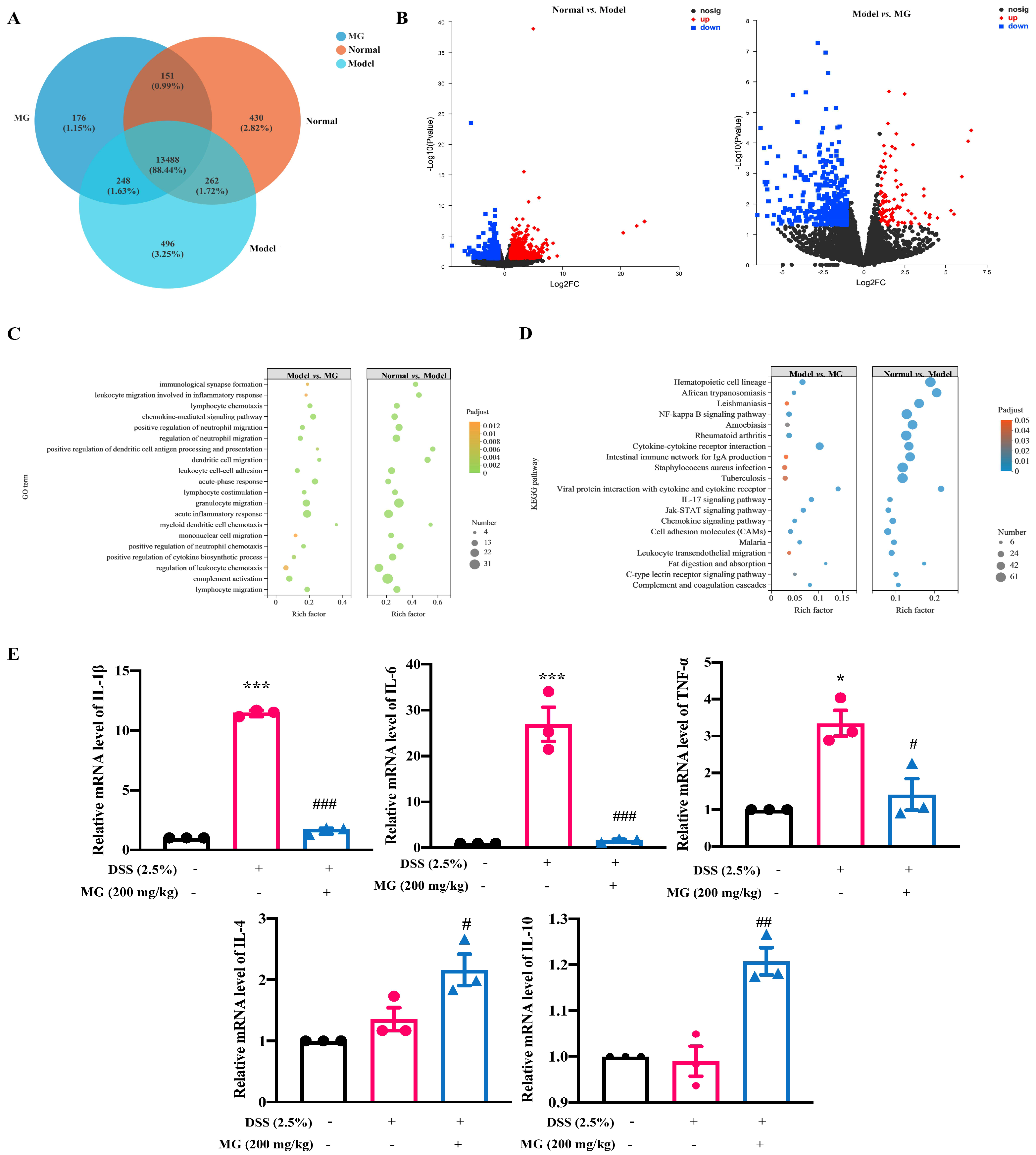
2.3. Bioinformatics Analysis Indicated the Underlying Biological Function of MG in DSS-Induced Ulcerative Colitis
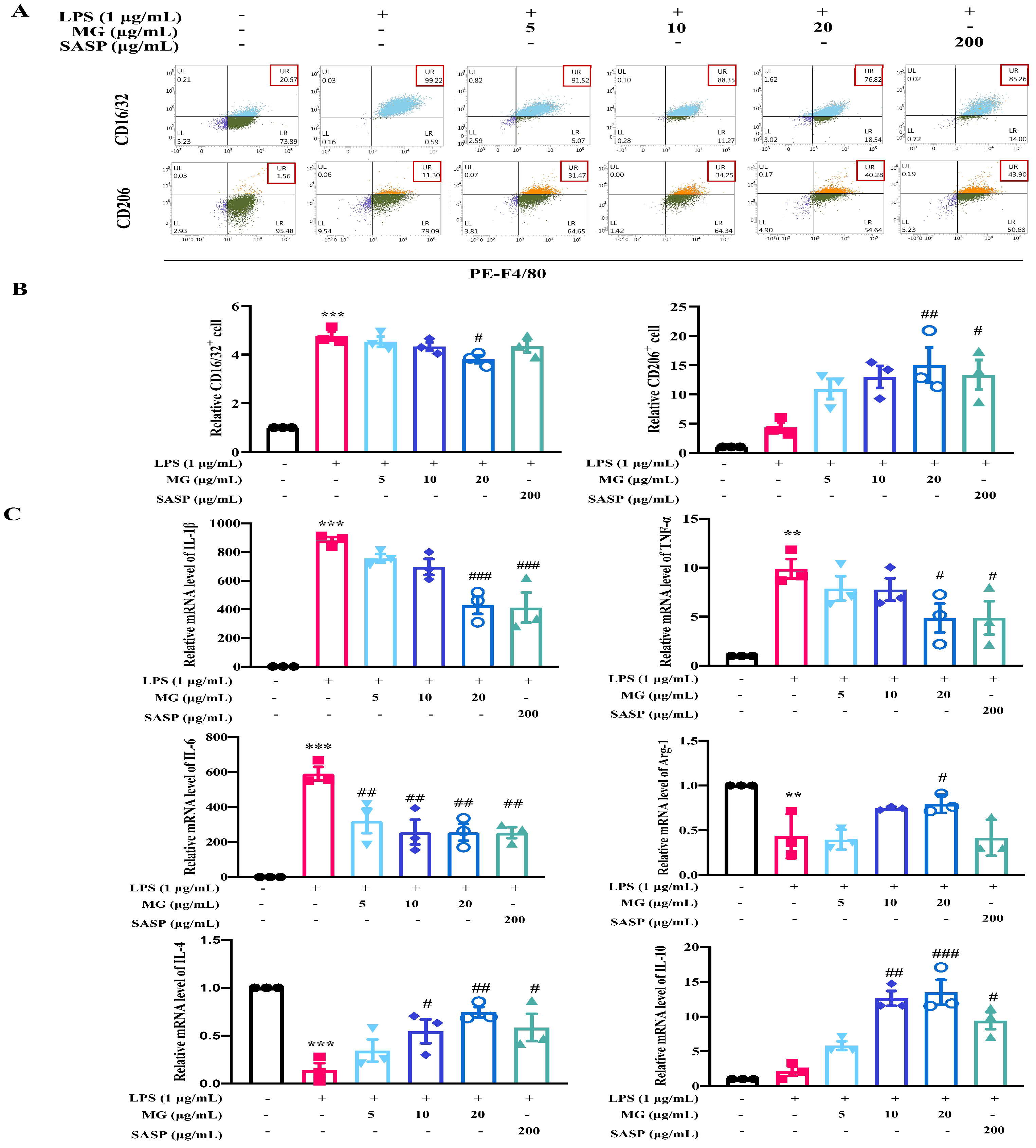
2.4. The Mechanisms of MG Inhibiting Gut Immunity, Macrophage Infiltration and Inflammatory Cytokines
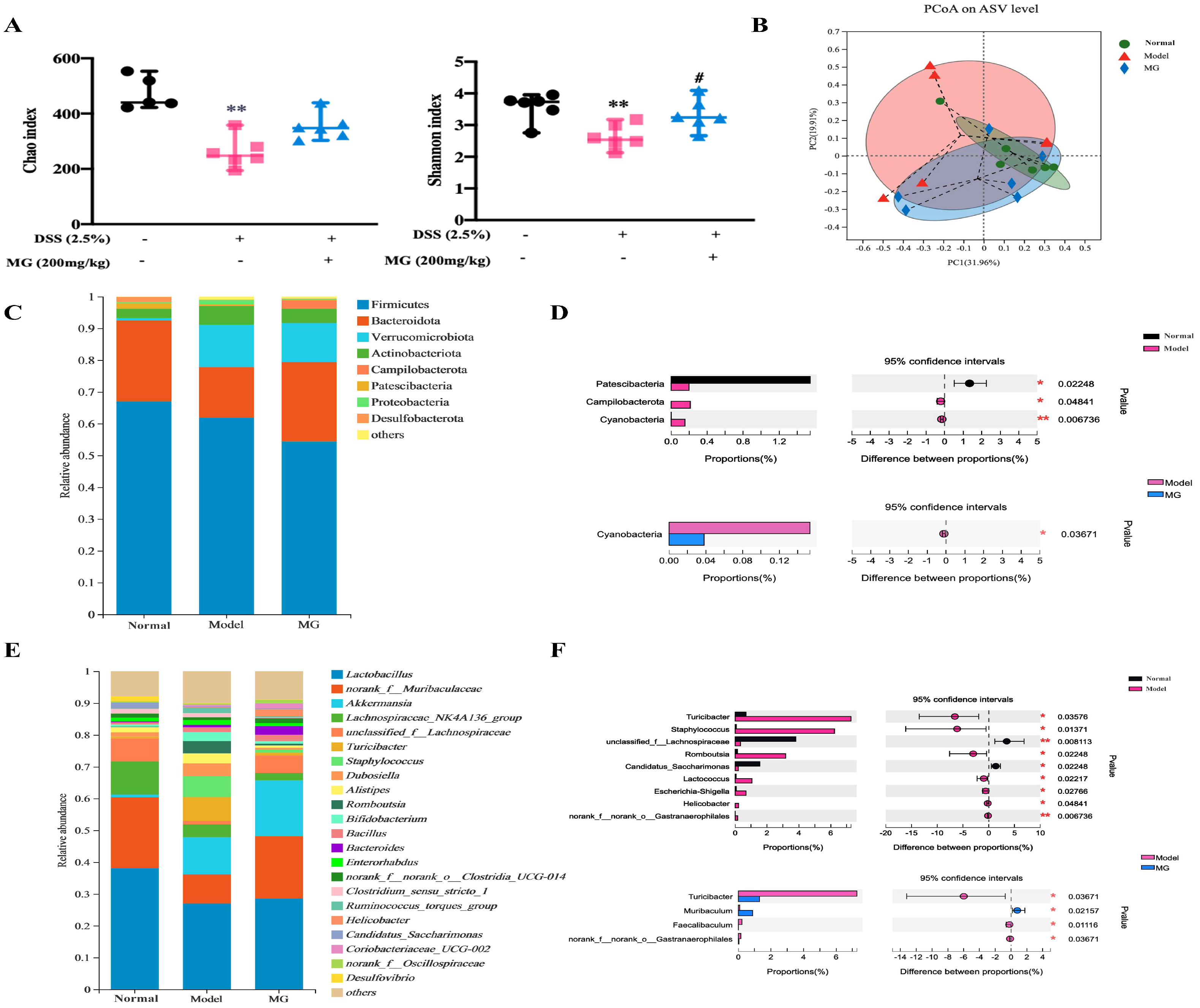
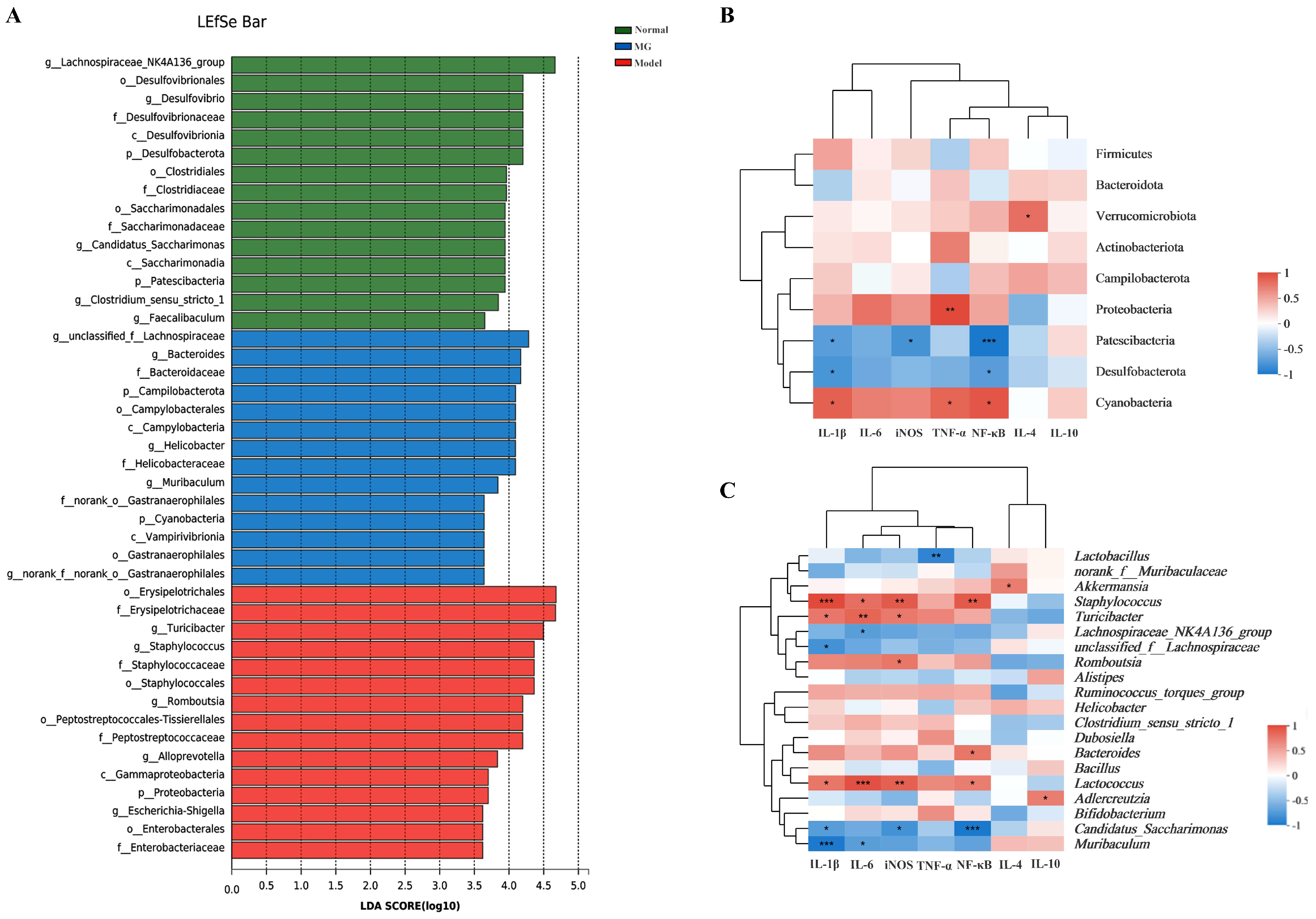
2.5. MG Rectified Gut Microbiota Imbalance in Ulcerative Colitis
2.6. MG May Restore Gut Immunity by Altering Gut Microbiota in Ulcerative Colitis
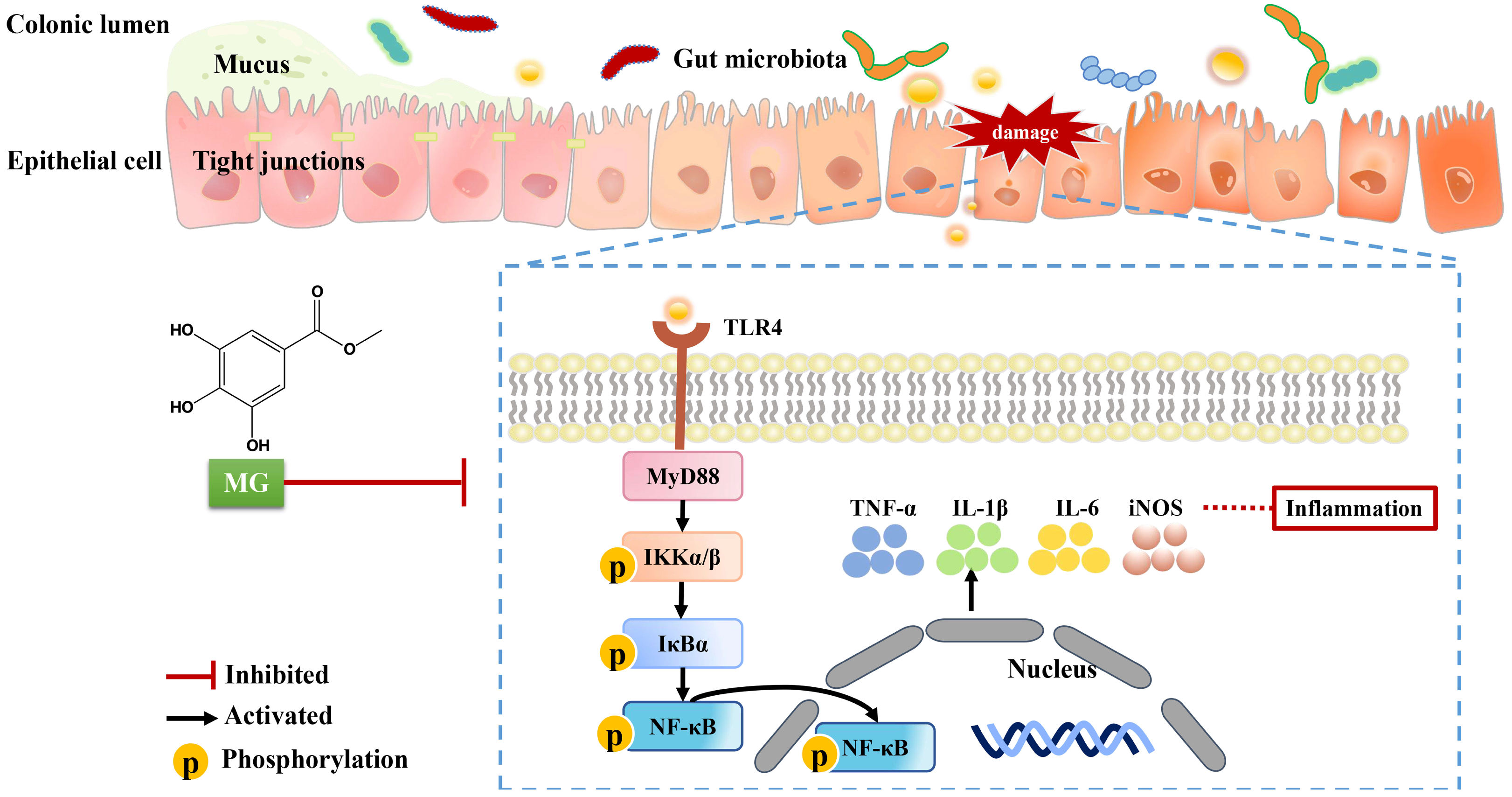
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Animals
4.3. Induction of DSS Colitis in Mice and Drug Treatment
4.4. Disease Activity Index (DAI)
4.5. Histological Analysis
4.6. Cell Culture and Treatment
4.7. Cell Viability
4.8. Nitric Oxide (NO) Production and Quantification
4.9. Flow Cytometry Analysis
4.10. RNA Preparation and qPCR Assay
4.11. Transcriptome Analysis
4.12. 16S rRNA Microbiota Analysis
4.13. Western Blot Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Eisenstein, M. Ulcerative colitis: Towards remission. Nature 2018, 563, S33. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.T.; Zhang, J.X.; Fang, Y.F.; Wang, R.; Tang, X.P.; Zhao, P.F.; Zhao, Y.G.; Zhang, M.; Huang, Y.Z. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol. Sin. 2021, 42, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Lok, K.H.; Hung, H.G.; Ng, C.H.; Kwong, K.C.; Yip, W.M.; Lau, S.F.; Li, K.K.; Li, K.F.; Szeto, M.L. Epidemiology and clinical characteristics of ulcerative colitis in Chinese population: Experience from a single center in Hong Kong. J. Gastroenterol. Hepatol. 2008, 23, 406–410. [Google Scholar] [CrossRef]
- Meli, V.S.; Veerasubramanian, P.K.; Atcha, H.; Reitz, Z.; Downing, T.L.; Liu, W.F. Biophysical regulation of macrophages in health and disease. J. Leukoc. Biol. 2019, 106, 283–299. [Google Scholar] [CrossRef]
- Azad, S.; Sood, N.; Sood, A. Biological and histological parameters as predictors of relapse in ulcerative colitis: A prospective study. Saudi J. Gastroenterol. 2011, 17, 194–198. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef]
- Aximujiang, K.; Kaheman, K.; Wushouer, X.; Wu, G.; Ahemaiti, A.; Yunusi, K. Lactobacillus acidophilus and HKL Suspension Alleviates Ulcerative Colitis in Rats by Regulating Gut Microbiota, Suppressing TLR9, and Promoting Metabolism. Front. Pharmacol. 2022, 13, 859628. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, K.; Li, G. Protective Effect of Prim-O-Glucosylcimifugin on Ulcerative Colitis and Its Mechanism. Front. Pharmacol. 2022, 13, 882924. [Google Scholar] [CrossRef]
- Hirten, R.P.; Sands, B.E. New therapeutics for ulcerative colitis. Annu. Rev. Med. 2021, 72, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Su, Y.; An, Z.; Zhang, P.; Yue, Q.; Zhao, C.; Sun, X.; Zhang, S.; Liu, X.; Li, K.; et al. Fermentation products of Danshen relieved dextran sulfate sodium-induced experimental ulcerative colitis in mice. Sci. Rep. 2021, 11, 16210. [Google Scholar] [CrossRef] [PubMed]
- Jie, F.; Xiao, S.; Qiao, Y.; You, Y.; Feng, Y.; Long, Y.; Li, S.; Wu, Y.; Li, Y.; Du, Q. Kuijieling decoction suppresses NLRP3-Mediated pyroptosis to alleviate inflammation and experimental colitis in vivo and in vitro. J. Ethnopharmacol. 2021, 264, 113243. [Google Scholar] [CrossRef]
- Jiang, N.; Li, H.; Sun, Y.; Zeng, J.; Yang, F.; Kantawong, F.; Wu, J. Sanguisorba OfficinalisNetwork Pharmacology and Pharmacological Evaluation Reveals the Mechanism of the in Suppressing Hepatocellular Carcinoma. Front. Pharmacol. 2021, 12, 618522. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, K.; Jiyan, L. Clinical Study on Treatment of Ulcerative Colitis (Left Half Colon) with Bingji Diyu Decoction for Enema. J. Liaoning Univ. Tradit. Chin. Med. 2019, 21, 25–28. [Google Scholar]
- Wang, Z.J.; Li, H.X.; Liang, J.; Hou, C.Y. Influence of Xuejie(Risina Draconis)combining Diyu(Radix Sanguisorbae)on mucosal healing of ulcerative colitis. J. Beijing Univ. Tradit. Chin. Med. 2013, 36, 426–428. [Google Scholar]
- Prihantini, A.I.; Tachibana, S.; Itoh, K. Evaluation of antioxidant and α-glucosidase inhibitory activities of some subtropical plants. Pak. J. Biol. Sci. 2014, 17, 1106–1114. [Google Scholar] [CrossRef][Green Version]
- Qiu, Y.; Xiao, Z.; Wang, Y.; Zhang, D.; Zhang, W.; Wang, G.; Chen, W.; Liang, G.; Li, X.; Zhang, Y.; et al. Optimization and anti-inflammatory evaluation of methyl gallate derivatives as a myeloid differentiation protein 2 inhibitor. Bioorg. Med. Chem. 2019, 27, 115049. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, Y.J.; Wei, P.L.; Hung, C.S.; Wang, W. Methyl gallate, gallic acid-derived compound, inhibit cell proliferation through increasing ROS production and apoptosis in hepatocellular carcinoma cells. PLoS ONE 2021, 16, e0248521. [Google Scholar] [CrossRef]
- Correa, L.B.; Seito, L.N.; Manchope, M.F.; Verri, W.A.; Cunha, T.M.; Henriques, M.G.; Rosas, E.C. Methyl gallate attenuates inflammation induced by Toll-like receptor ligands by inhibiting MAPK and NF-κb signaling pathways. Inflamm. Res. 2020, 69, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.; Cho, C.-Y.; Ho, A.; Huang, J.-Y.; Martin, B.; Gilbert, E.S. 4-Ethoxybenzoic acid inhibits Staphylococcus aureus biofilm formation and potentiates biofilm sensitivity to vancomycin. Int. J. Antimicrob. Agents. 2020, 56, 106086. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Xu, Y.; Yuan, G.; Wu, X.; Li, Q. Bacterial ClpP protease is a potential target for methyl gallate. Front. Microbiol. 2020, 11, 598692. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.J.; Li, J.H.; Zhang, Z.; Zhang, J.Y.; Liu, S.Q.; Yang, J. Suppression of MIF-induced neuronal apoptosis may underlie the therapeutic effects of effective components of Fufang Danshen in the treatment of Alzheimer’s disease. Acta Pharmacol. Sin. 2018, 39, 1421–1438. [Google Scholar] [CrossRef]
- Argollo, M.; Fiorino, G.; Hindryckx, P.; Peyrin-Biroulet, L.; Danese, S. Novel therapeutic targets for inflammatory bowel disease. J. Autoimmun. 2017, 85, 103–116. [Google Scholar] [CrossRef]
- Liu, W.; Wang, C.; Tang, L.; Yang, H. Associations between gene polymorphisms in pro-inflammatory cytokines and the risk of inflammatory bowel disease: A meta-analysis. Immunol. Investig. 2021, 50, 869–883. [Google Scholar] [CrossRef]
- Ge, C.Y.; Wei, L.Y.; Tian, Y.; Wang, H.H. A seven-NF-kapaB-related gene signature may distinguish patients with ulcerative colitis-associated colorectal carcinoma. Pharmgenomics Pers. Med. 2020, 13, 707–718. [Google Scholar] [CrossRef]
- Papoutsopoulou, S.; Burkitt, M.D.; Bergey, F.; England, H.; Hough, R.; Schmidt, L.; Spiller, D.G.; White, M.H.R.; Paszek, P.; Jackson, D.A.; et al. Macrophage-Specific NF-κB activation dynamics can segregate inflammatory bowel disease patients. Front. Immunol. 2019, 10, 2168. [Google Scholar] [CrossRef]
- Feng, J.; Zhu, Y.; Chen, L.; Wang, M. Clinical significance of microRNA-146a in patients with ulcerative colitis. Ann. Clin. Lab. Sci. 2020, 50, 463–467. [Google Scholar]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Immune response and inflammatory pathway of ulcerative colitis. J. Basic Clin. Physiol. Pharmacol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Yan, Y.X.; Shao, M.J.; Qi, Q.; Xu, Y.S.; Yang, X.Q.; Zhu, F.H.; He, S.J.; He, P.L.; Feng, C.L.; Wu, Y.W.; et al. Artemisinin analogue SM934 ameliorates DSS-induced mouse ulcerative colitis via suppressing neutrophils and macrophages. Acta Pharmacol. Sin. 2018, 39, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, B.; Duan, Z.; Xia, Z.; Ding, Y.; Chen, T.; Liu, H.; Wang, B.; Yang, B.; Wang, X.; et al. Depression and anxiety in patients with active ulcerative colitis: Crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779. [Google Scholar] [CrossRef] [PubMed]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Larabi, A.; Barnich, N.; Nguyen, H.T.T. New insights into the interplay between autophagy, gut microbiota and inflammatory responses in IBD. Autophagy 2020, 16, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.G.; Zhang, M.N.; Wen, X.; He, L.; Zhang, M.H.; Zhang, J.L.; Yang, X.Z. Cepharanthine ameliorates dextran sulphate sodium-induced colitis through modulating gut microbiota. Microb. Biotechnol. 2022, 15, 2208–2222. [Google Scholar] [CrossRef]
- Shang, L.; Liu, H.; Yu, H.; Chen, M.; Yang, T.; Zeng, X.; Qiao, S. Core Altered microorganisms in colitis mouse model: A comprehensive time-point and fecal microbiota transplantation analysis. Antibiotics 2021, 10, 643. [Google Scholar] [CrossRef]
- Kaplan, G.G.; Ng, S.C. Globalisation of inflammatory bowel disease: Perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 2016, 1, 307–316. [Google Scholar] [CrossRef]
- Li, H.; Jiang, X.; Shen, X.; Sun, Y.; Jiang, N.; Zeng, J.; Lin, J.; Yue, L.; Lai, J.; Li, Y.; et al. TMEA, a Polyphenol in Sanguisorba officinal, Promotes Thrombocytopoiesis by Upregulating PI3K/Akt Signaling. Front. Cell Dev. Biol. 2021, 9, 708331. [Google Scholar] [CrossRef]
- Dharmasiri, S.; Garrido-Martin, E.M.; Harris, R.J.; Bateman, A.C.; Collins, J.E.; Cummings, J.R.F.; Sanchez-Elsner, T. Human intestinal macrophages are involved in the pathology of both ulcerative colitis and crohn disease. Inflamm. Bowel Dis. 2021, 27, 1641–1652. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Lau, J.T.; Whelan, F.J.; Herath, I.; Lee, C.H.; Collins, S.M.; Bercik, P.; Surette, M.G. Capturing the diversity of the human gut microbiota through culture-enriched molecular profiling. Genome Med. 2016, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, J.; Chen, L.; Wu, X.; Gan, Y.; Xu, N.; Li, M.; Luo, H.; Guan, F.; Su, Z.; et al. β-patchoulene simultaneously ameliorated dextran sulfate sodium-induced colitis and secondary liver injury in mice via suppressing colonic leakage and flora imbalance. Biochem. Pharmacol. 2020, 182, 114260. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Y.; Ma, L.L.; Zhang, C.; Lin, J.Z.; Han, L.; He, Y.N.; Xie, C.G. Exploring the mechanism of indigo naturalis in the treatment of ulcerative colitis based on tlr4/myd88/nf-κb signaling pathway and gut microbiota. Front. Pharmacol. 2021, 12, 674416. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wu, M.; Xiong, W.; Li, J.; An, Y.; Ren, J.; Xie, Y.; Xue, H.; Yan, D.; Li, M.; et al. Saikosaponin-d ameliorates dextran sulfate sodium-induced colitis by suppressing NF-κB activation and modulating the gut microbiota in mice. Int. Immunopharmacol. 2020, 81, 106288. [Google Scholar] [CrossRef]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Dai, T.; Shi, C.; Tao, L.; Wang, Y.; Tian, Y.; Sheng, J. Astragalin attenuates dextran sulfate sodium (dss)-induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting nf-κb activation in mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [CrossRef]
- Fenton, C.G.; Taman, H.; Florholmen, J.; Sørbye, S.W.; Paulssen, R.H. Transcriptional signatures that define ulcerative colitis in remission. Inflamm. Bowel Dis. 2021, 27, 94–105. [Google Scholar] [CrossRef]
- Wang, R.; Tang, R.; Li, B.; Ma, X.; Schnabl, B.; Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell Mol. Immunol. 2021, 18, 4–17. [Google Scholar] [CrossRef]
- Pei, L.Y.; Ke, Y.S.; Zhao, H.H.; Wang, L.; Jia, C.; Liu, W.Z.; Fu, Q.H.; Shi, M.N.; Cui, J.; Li, S.C. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 2019, 19, 10. [Google Scholar] [CrossRef]
- Ryan, F.J.; Ahern, A.M.; Fitzgerald, R.S.; Laserna-Mendieta, E.J.; Power, E.M.; Clooney, A.G.; O’Donoghue, K.W.; McMurdie, P.J.; Iwai, S.; Crits-Christoph, A.; et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat. Commun. 2020, 11, 1512. [Google Scholar] [CrossRef]
- Tang, X.; Wang, W.; Hong, G.; Duan, C.; Zhu, S.; Tian, Y.; Han, C.; Qian, W.; Lin, R.; Hou, X. Gut microbiota-mediated lysophosphatidylcholine generation promotes colitis in intestinal epithelium-specific Fut2 deficiency. J. Biomed. Sci. 2021, 28, 20. [Google Scholar] [CrossRef]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, S.; Schulte, B.; Al-Massad, N.; Thieme, F.; Schulte, D.M.; Bethge, J.; Rehman, A.; Tran, F.; Aden, K.; Häsler, R.; et al. Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology 2017, 153, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Cooper, H.S.; Murthy, S.N.; Shah, R.S.; Sedergran, D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Investig. 1993, 69, 238–249. [Google Scholar] [PubMed]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kuri-García, A.; Godínez-Santillán, R.I.; Mejía, C.; Ferriz-Martínez, R.A.; García-Solís, P.; Enríquez-Vázquez, A.; García-Gasca, T.; Guzmán-Maldonado, S.H.; Chávez-Servín, J.L. Preventive effect of an infusion of the aqueous extract of chaya leaves (Cnidoscolus aconitifolius) in an aberrant crypt foci rat model induced by azoxymethane and dextran sulfate sodium. J. Med. Food 2019, 22, 851–860. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, P.; Lai, J.; Li, Y.; Deng, J.; Zhao, C.; Huang, Q.; Yang, F.; Yang, S.; Wu, Y.; Tang, X.; et al. Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 14024. https://doi.org/10.3390/ijms232214024
Zhou P, Lai J, Li Y, Deng J, Zhao C, Huang Q, Yang F, Yang S, Wu Y, Tang X, et al. Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway. International Journal of Molecular Sciences. 2022; 23(22):14024. https://doi.org/10.3390/ijms232214024
Chicago/Turabian StyleZhou, Ping, Jia Lai, Yueyue Li, Junzhu Deng, Chunling Zhao, Qianqian Huang, Fei Yang, Shuo Yang, Yuesong Wu, Xiaoqin Tang, and et al. 2022. "Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway" International Journal of Molecular Sciences 23, no. 22: 14024. https://doi.org/10.3390/ijms232214024
APA StyleZhou, P., Lai, J., Li, Y., Deng, J., Zhao, C., Huang, Q., Yang, F., Yang, S., Wu, Y., Tang, X., Huang, F., Wang, L., Huang, X., Zou, W., & Wu, J. (2022). Methyl Gallate Alleviates Acute Ulcerative Colitis by Modulating Gut Microbiota and Inhibiting TLR4/NF-κB Pathway. International Journal of Molecular Sciences, 23(22), 14024. https://doi.org/10.3390/ijms232214024





