Encapsulation Reduces the Deleterious Effects of Salicylic Acid Treatments on Root Growth and Gravitropic Response
Abstract
1. Introduction
2. Results and Discussion
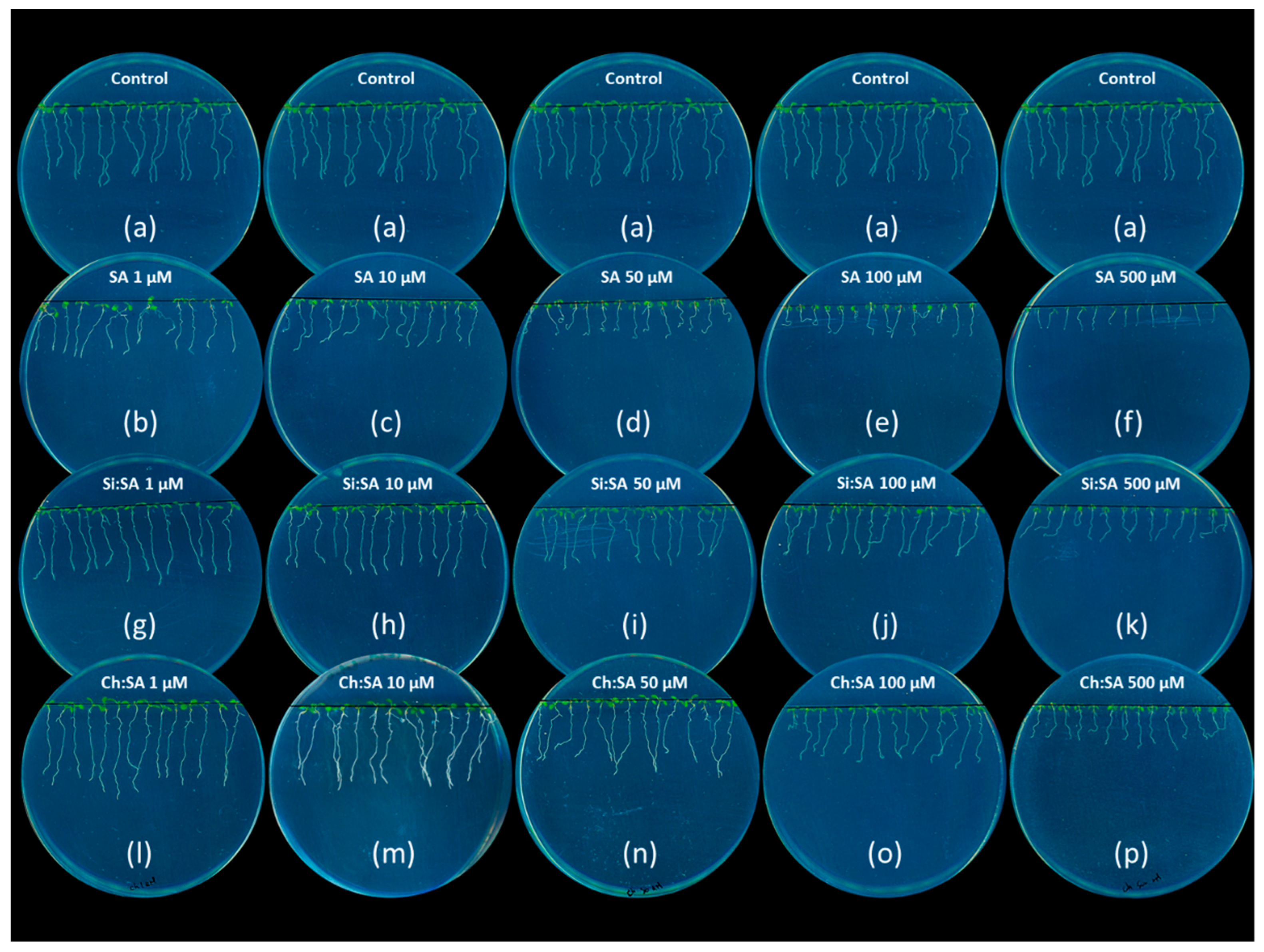
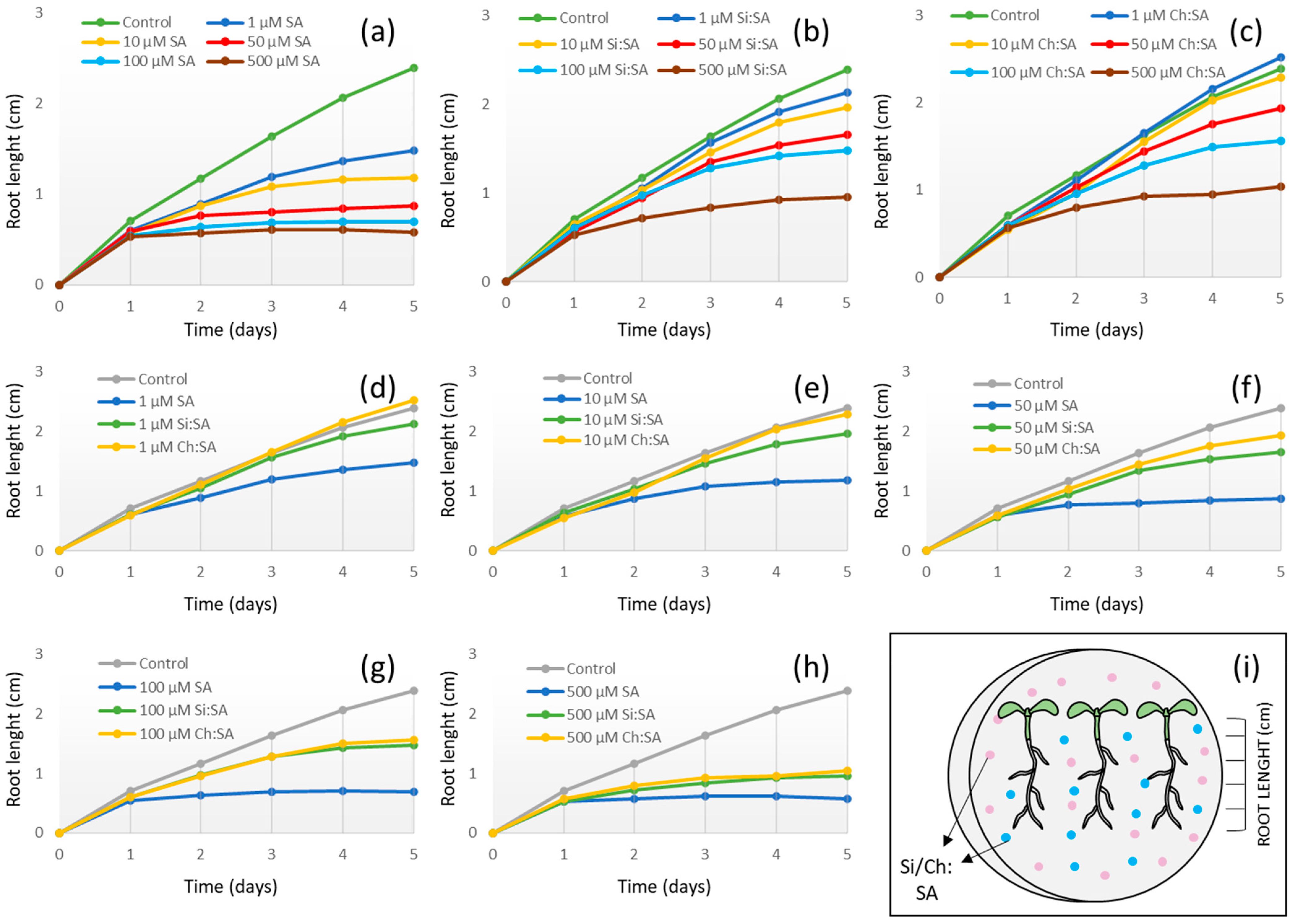
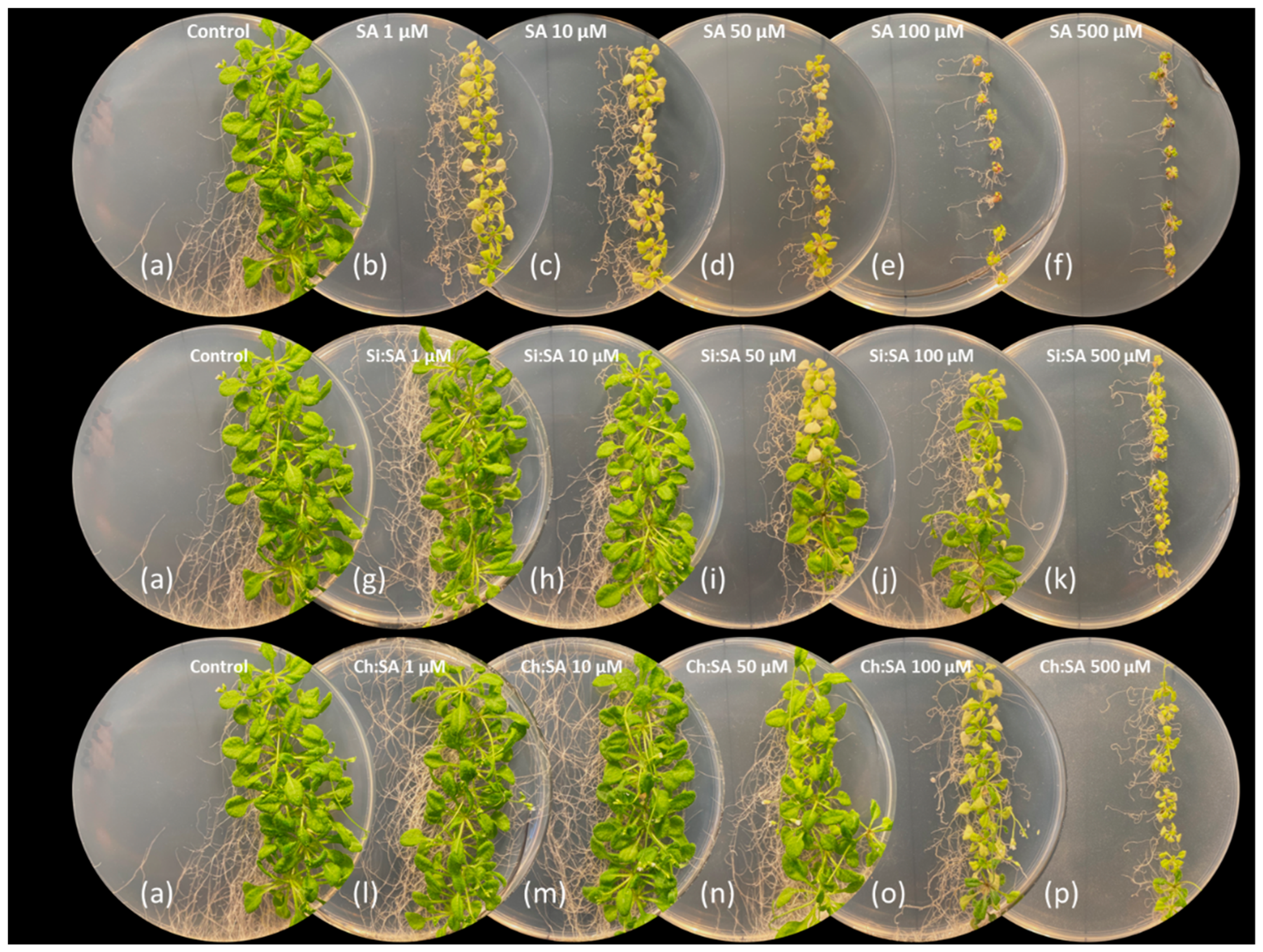
2.1. Encapsulation Decreases the SA Negative Effect on Primary Root Growth
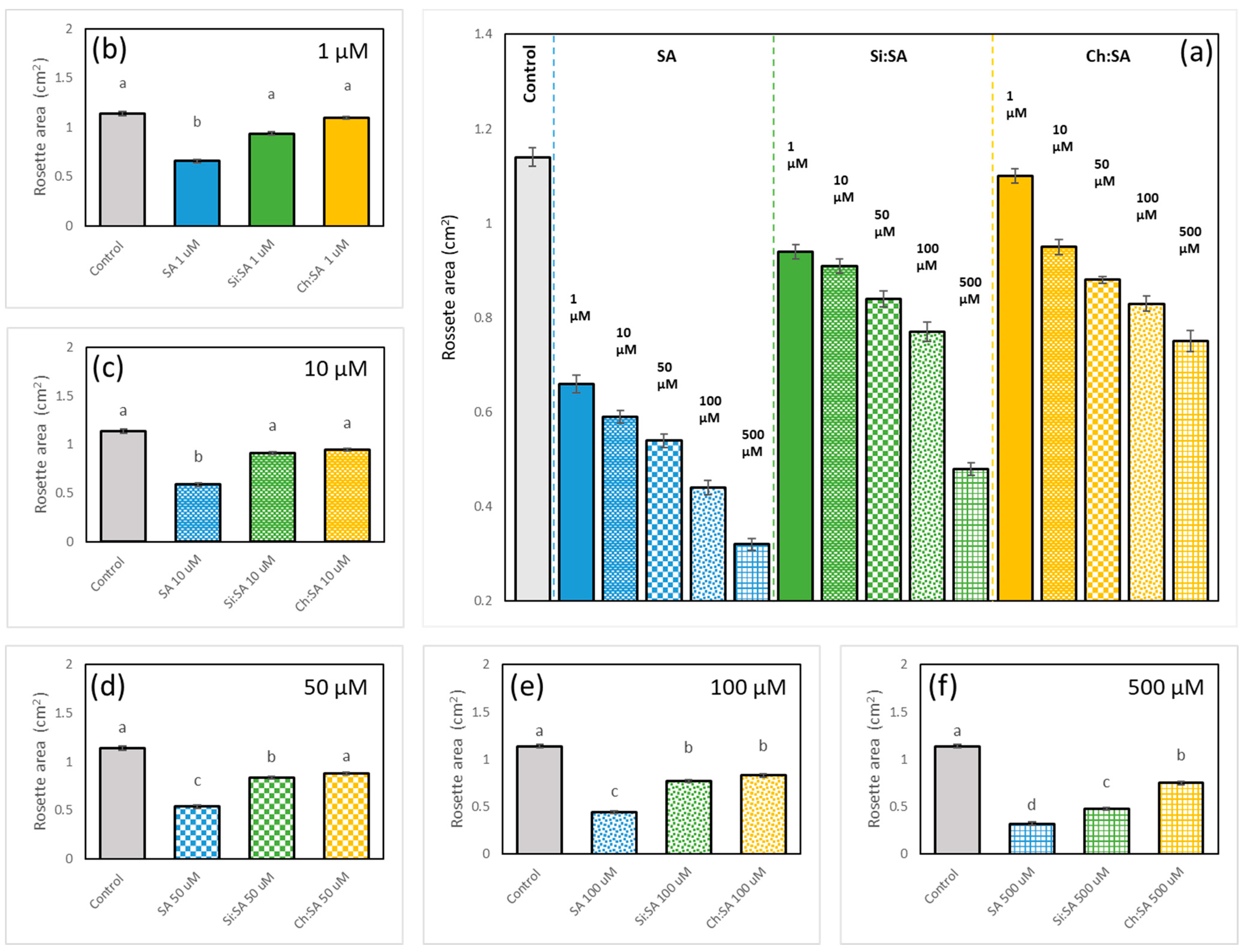
2.2. The Encapsulation of SA Allows for the Correct Development of the Rosette
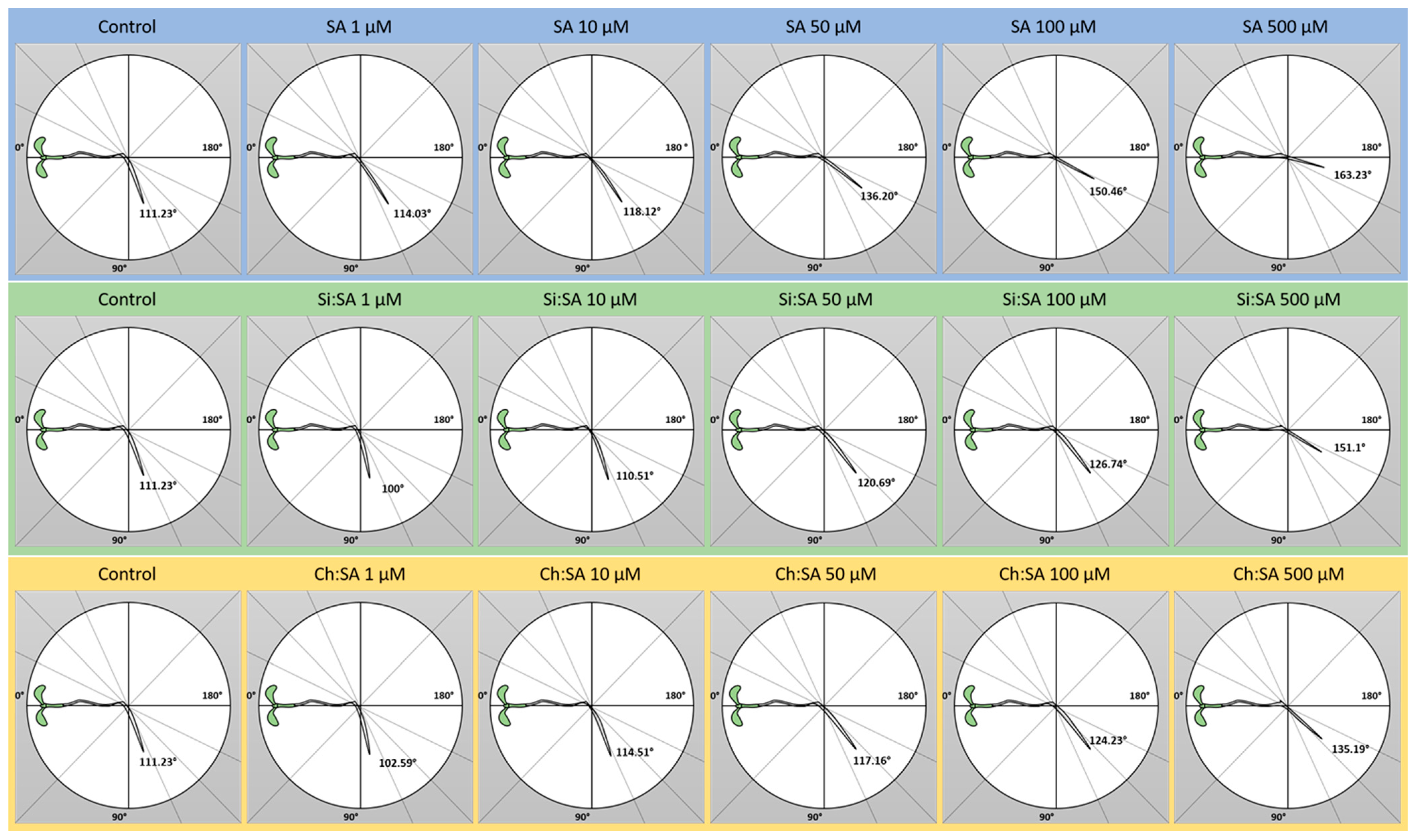
2.3. The Encapsulation of SA Prevents Alteration of Root Gravitropism
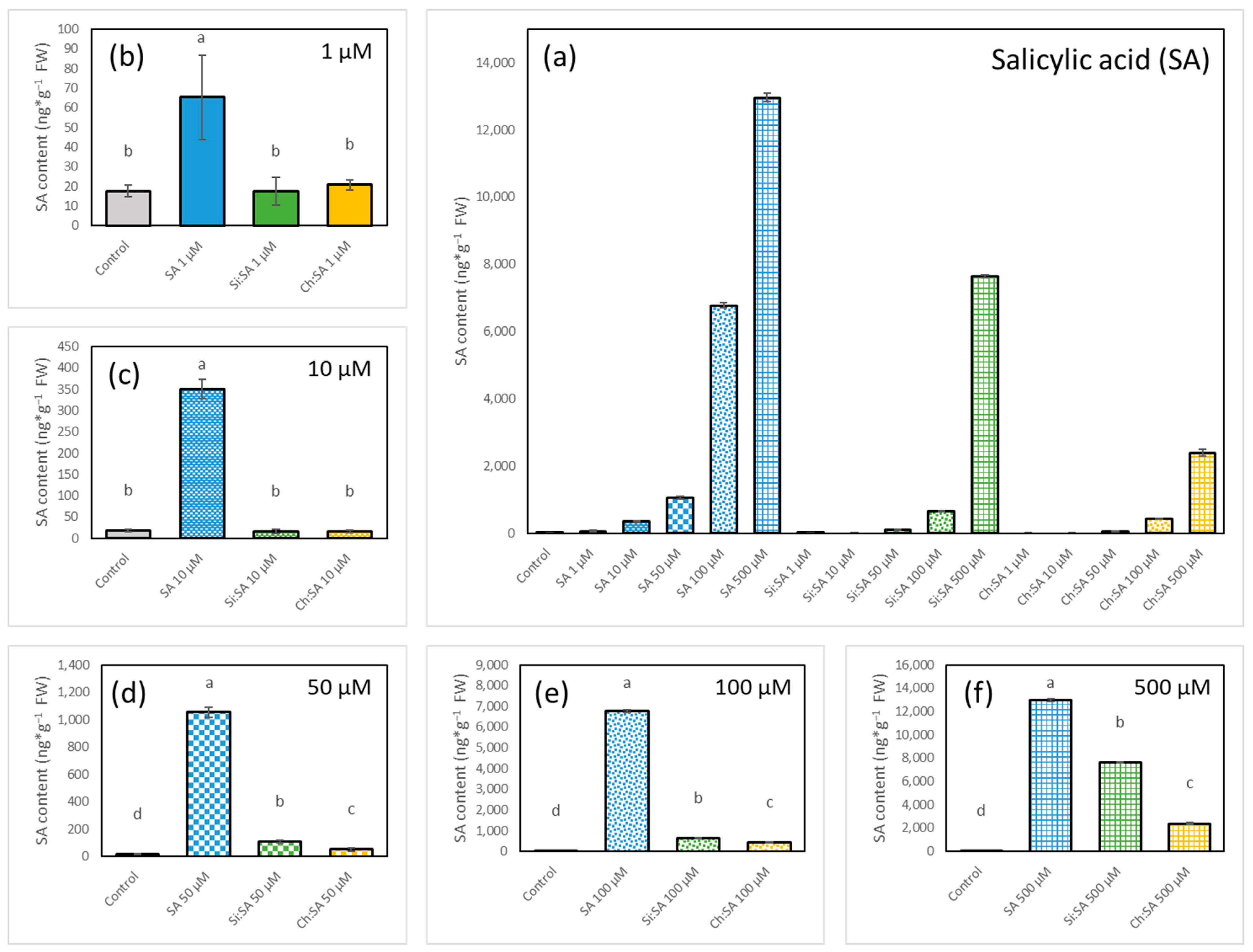
2.4. The Encapsulation of SA Modulates Endogenous SA Accumulation in Plants
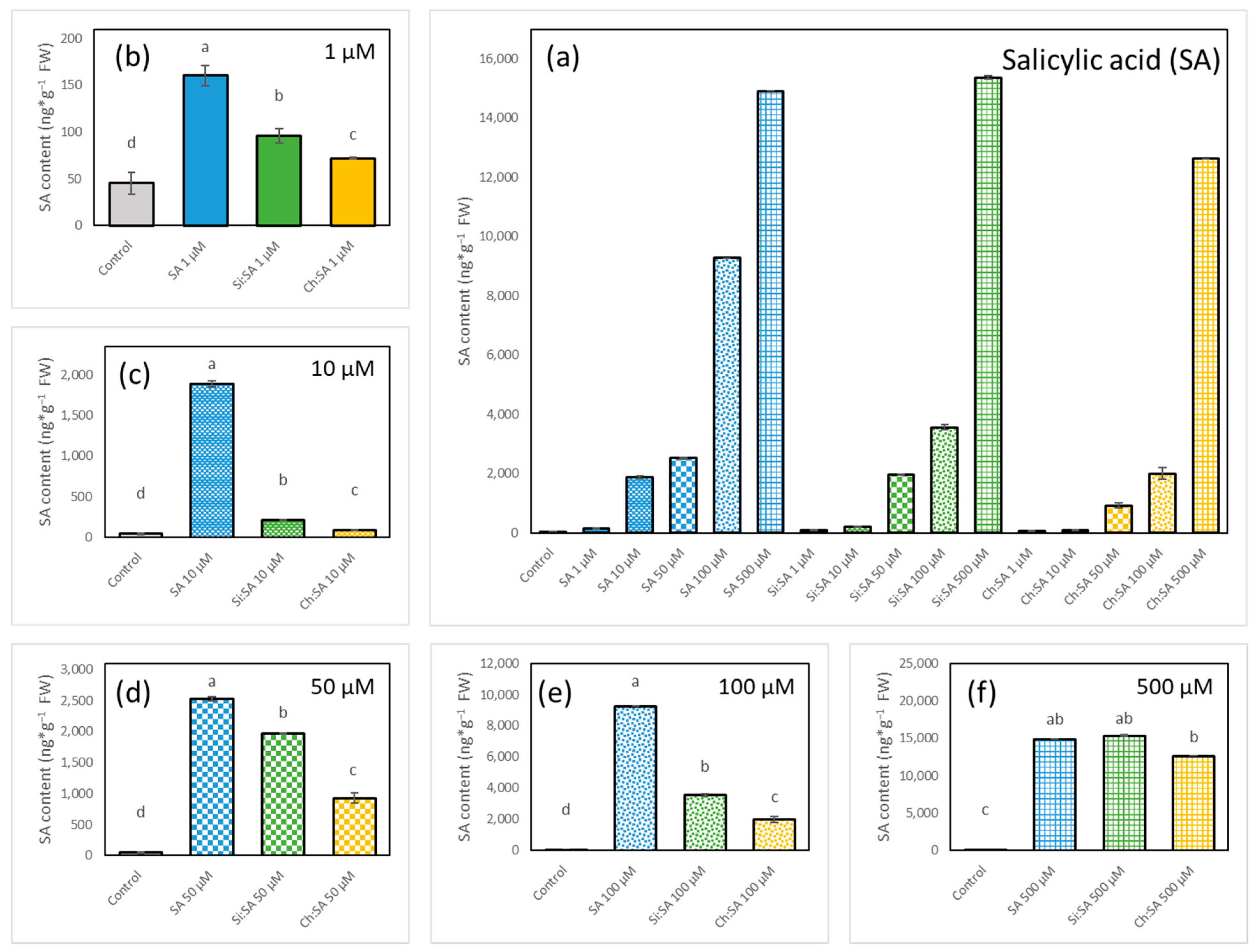
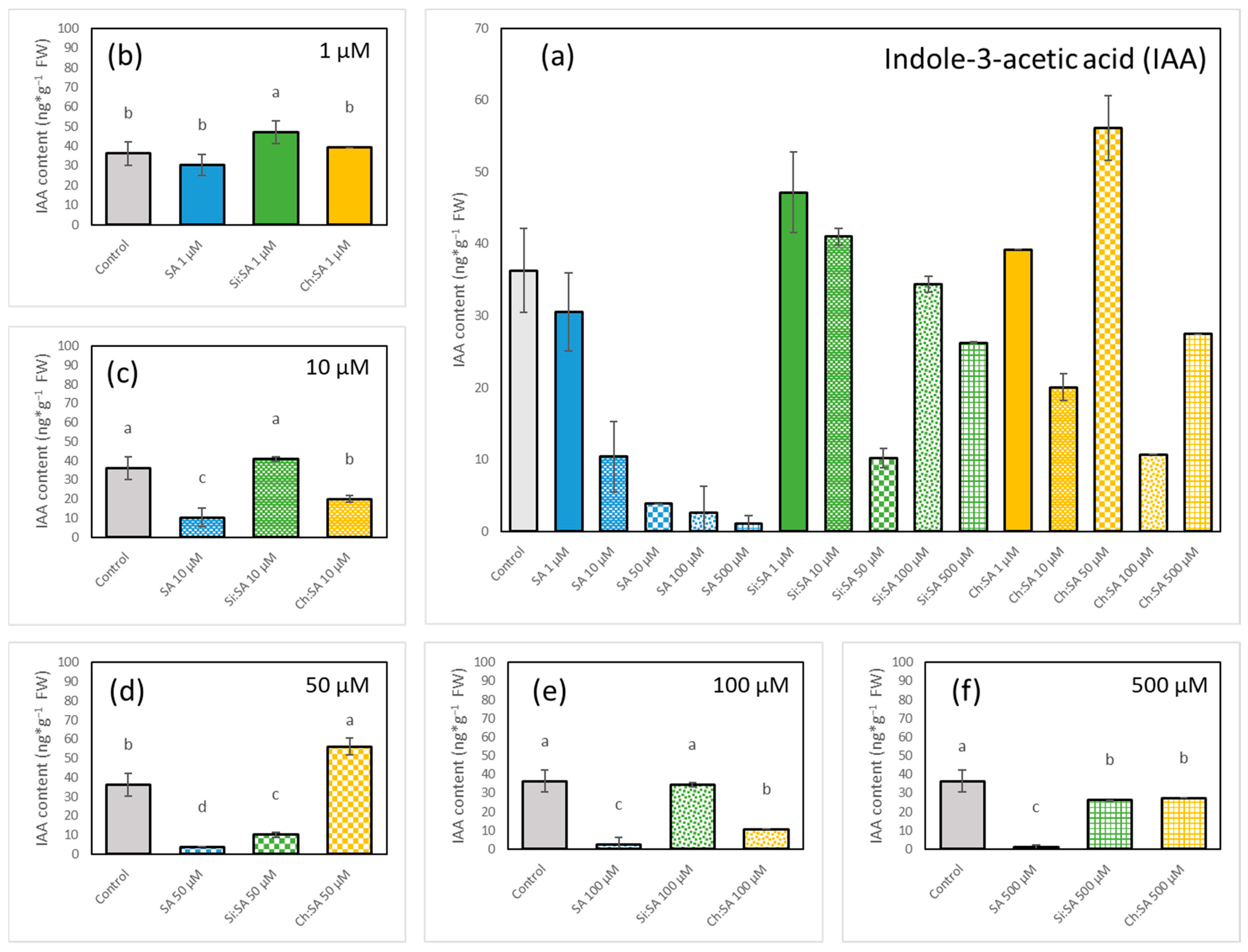
2.5. The Encapsulation of SA Modulates Endogenous IAA Accumulation in Roots
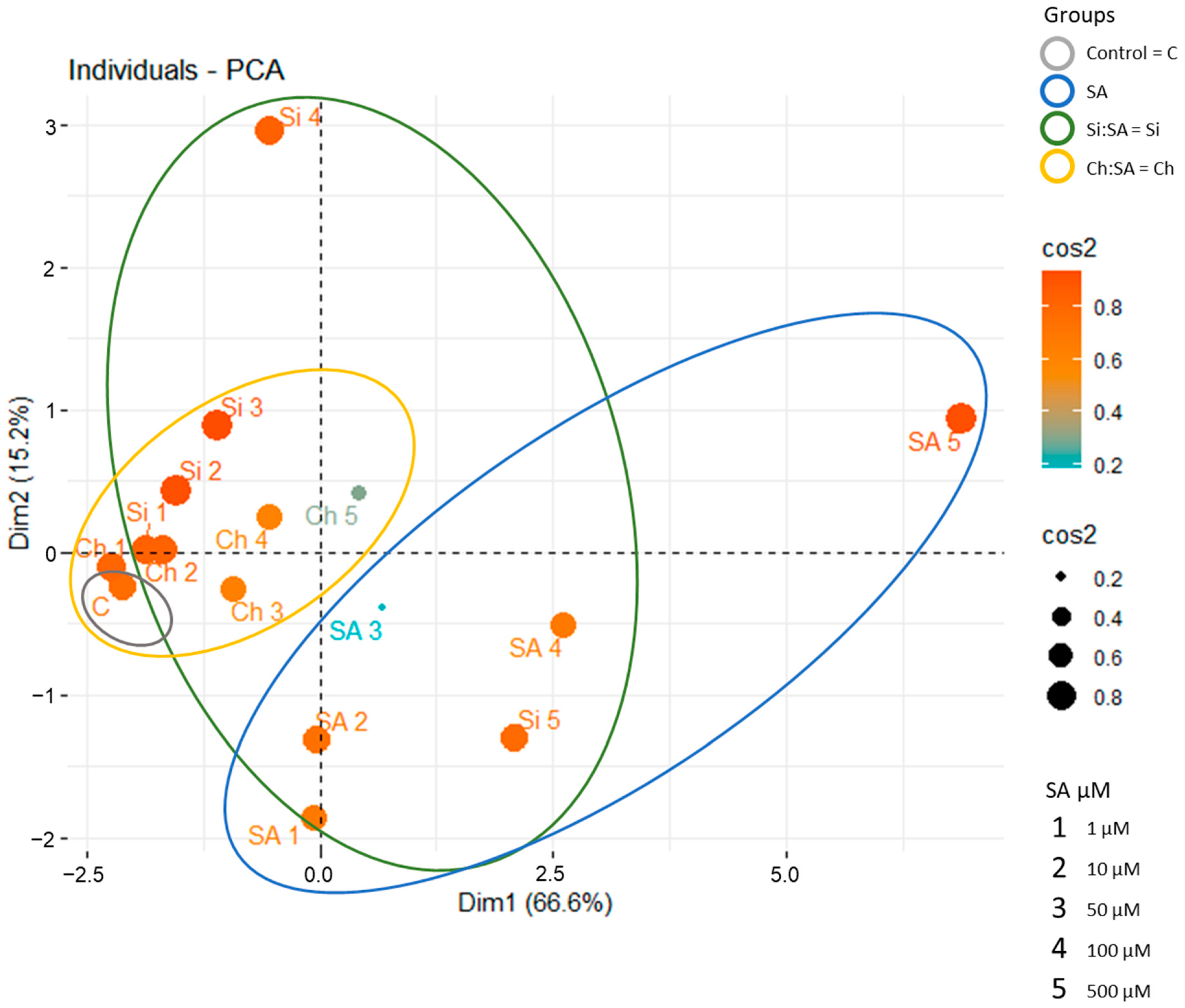
2.6. General Comparison of Free SA vs. Encapsulated SA Treatments
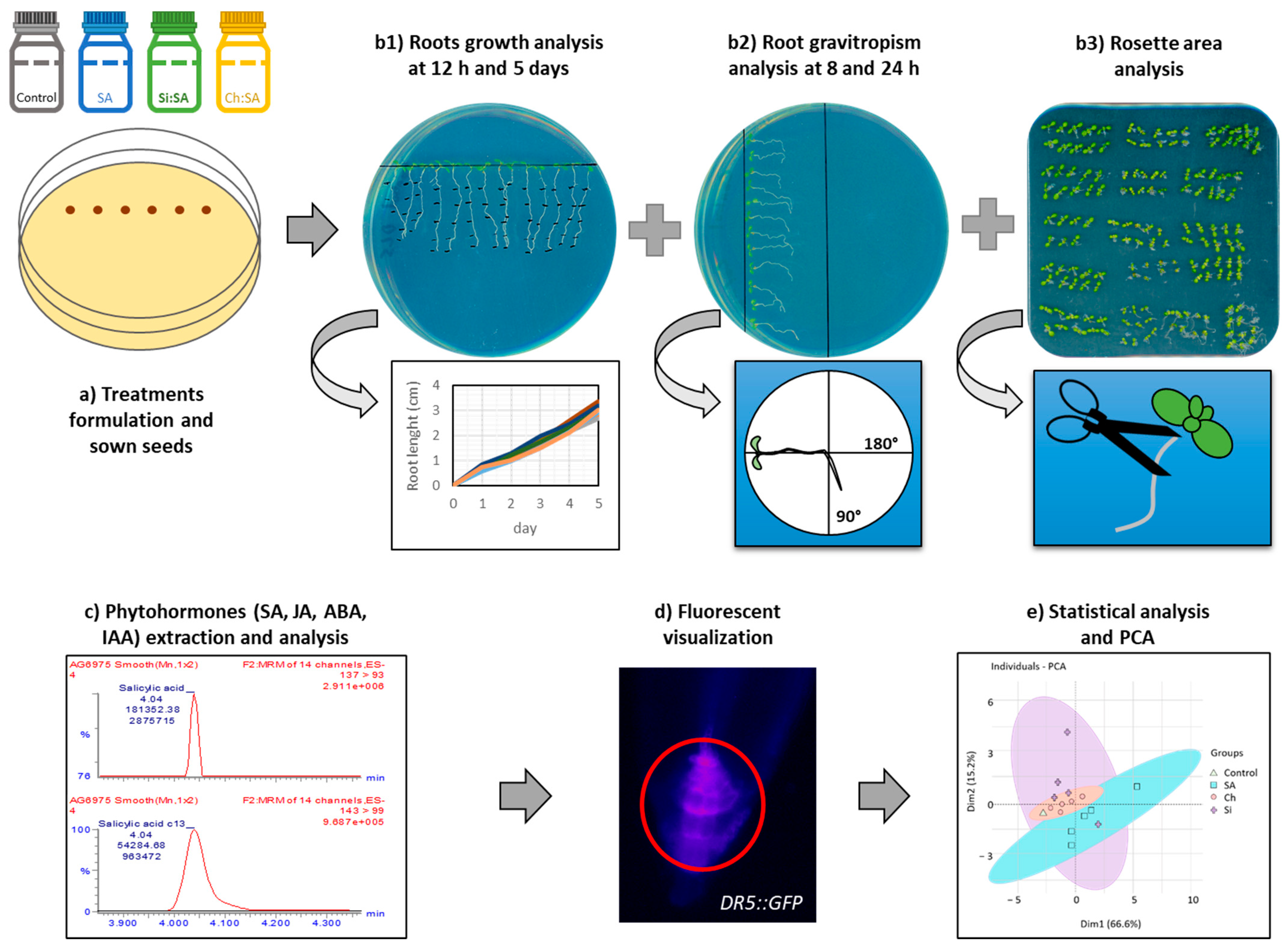
3. Materials and Methods
3.1. Materials and Plant Growth Conditions
3.2. SA Treatment Conditions
3.3. Determination of Root Growth, Rosette Area and Root Gravitropism
3.4. Extraction and Phytohormones Analysis
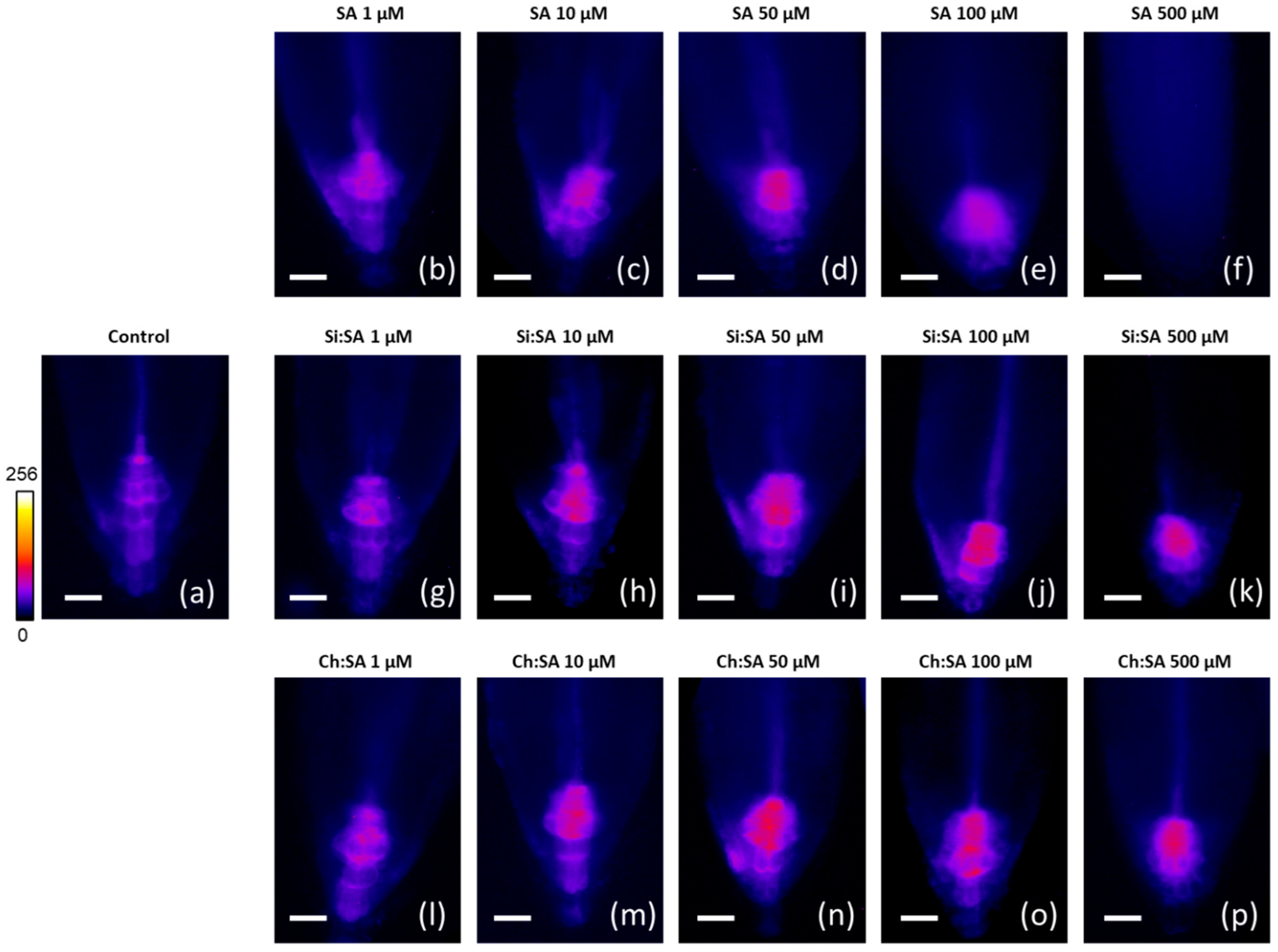
3.5. Fluorescence Analysis
3.6. Statistical Analysis and Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabagh, A.E.L.; Mbarki, S.; Hossain, A.; Iqbal, M.A.; Islam, M.S.; Raza, A.; Llanes, A.; Reginato, M.; Rahman, M.A.; Mahboob, W.; et al. Potential role of plant growth regulators in administering crucial processes against abiotic stresses. Front. Agron. 2021, 3. [Google Scholar] [CrossRef]
- Vlahoviček-Kahlina, K.; Jurić, S.; Marijan, M.; Mutaliyeva, B.; Khalus, S.V.; Prosyanik, A.V.; Vinceković, M. Synthesis, characterization, and encapsulation of novel plant growth regulators (PGRs) in biopolymer matrices. Int. J. Mol. Sci. 2021, 22, 1847. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Farooq, T. Chapter 7—Triazole-Based Plant Growth-Regulating Agents: A Recent Update; Farooq, T.B.T.-A., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 169–185. ISBN 978-0-12-817113-4. [Google Scholar]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, W. Plant Growth Regulators: Backgrounds and Uses in Plant Production. J. Plant Growth Regul. 2015, 34, 845–872. [Google Scholar] [CrossRef]
- Feller, U.; Kopriva, S.; Vassileva, V. Plant nutrient dynamics in stressful environments: Needs interfere with burdens. Agriculture 2018, 8, 97. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Gómez-Cadenas, A.; Mittler, R. Plant responses to climate change: Metabolic changes under combined abiotic stresses. J. Exp. Bot. 2022, 73, 3339–3354. [Google Scholar] [CrossRef]
- Neill, E.M.; Byrd, M.C.R.; Billman, T.; Brandizzi, F.; Stapleton, A.E. Plant growth regulators interact with elevated temperature to alter heat stress signaling via the unfolded protein response in maize. Sci. Rep. 2019, 9, 10392. [Google Scholar] [CrossRef]
- Raskin, I. Salicylate, A New Plant Hormone 1. Plant Physiol. 1992, 99, 799–803. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Pérez-Clemente, R.M.; Montoliu, A.; Vives-Peris, V.; Arbona, V.; Gómez-Cadenas, A. Hormonal and metabolic responses of Mexican lime plants to CTV infection. J. Plant Physiol. 2019, 238, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef] [PubMed]
- Nunes da Silva, M.; Carvalho, S.M.P.; Rodrigues, A.M.; Gómez-Cadenas, A.; António, C.; Vasconcelos, M.W. Defence-related pathways, phytohormones and primary metabolism are key players in kiwifruit plant tolerance to Pseudomonas syringae pv. actinidiae. Plant. Cell Environ. 2022, 45, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Espinoza, V.A.; López-Climent, M.F.; Casaretto, J.A.; Gómez-Cadenas, A. Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Front. Plant Sci. 2015, 6, 997. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Horváth, E.; Pál, M.; Szalai, G.; Páldi, E.; Janda, T. Exogenous 4-hydroxybenzoic acid and salicylic acid modulate the effect of short-term drought and freezing stress on wheat plants. Biol. Plant. 2007, 51, 480–487. [Google Scholar] [CrossRef]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Salicylic acid improves the salinity tolerance of medicago sativa in symbiosis with sinorhizobium meliloti by preventing nitrogen fixation inhibition. Plant Sci. 2013, 208, 75–82. [Google Scholar] [CrossRef]
- Jayakannan, M.; Bose, J.; Babourina, O.; Rengel, Z.; Shabala, S. Salicylic acid improves salinity tolerance in Arabidopsis by restoring membrane potential and preventing salt-induced K+ loss via a GORK channel. J. Exp. Bot. 2013, 64, 2255–2268. [Google Scholar] [CrossRef]
- Ma, X.; Zheng, J.; Zhang, X.; Hu, Q.; Qian, R. Salicylic acid alleviates the adverse effects of salt stress on Dianthus superbus (caryophyllaceae) by activating photosynthesis, protecting morphological structure, and enhancing the antioxidant system. Front. Plant Sci. 2017, 8, 600. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Rashighi, M.; Harris, J.E. 乳鼠心肌提取 HHS Public Access. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef] [PubMed]
- De Alcantara Lemos, J.; Oliveira, A.E.M.F.M.; Araujo, R.S.; Townsend, D.M.; Ferreira, L.A.M.; de Barros, A.L.B. Recent progress in micro and nano-encapsulation of bioactive derivatives of the Brazilian genus Pterodon. Biomed. Pharmacother. 2021, 143, 112137. [Google Scholar] [CrossRef] [PubMed]
- Atienza, M.T.J.A.; Magpantay, M.D.A.; Santos, K.L.T.; Mora, N.B.; Balaraman, R.P.; Gemeinhardt, M.E.; Dela Cueva, F.M.; Paterno, E.S.; Fernando, L.M.; Kohli, P. Encapsulation of plant growth-promoting bacterial crude extract in nanoliposome and its antifungal property against Fusarium oxysporum. ACS Agric. Sci. Technol. 2021, 1, 691–701. [Google Scholar] [CrossRef]
- Sousa, F.L.; Santos, M.; Rocha, S.M.; Trindade, T. Encapsulation of essential oils in SiO2 microcapsules and release behaviour of volatile compounds. J. Microencapsul. 2014, 31, 627–635. [Google Scholar] [CrossRef]
- Wu, B.; Yang, C.; Li, B.; Feng, L.; Hai, M.; Zhao, C.X.; Chen, D.; Liu, K.; Weitz, D.A. Active encapsulation in biocompatible nanocapsules. Small 2020, 16, 2002716. [Google Scholar] [CrossRef]
- Sampedro-Guerrero, J.; Vives-Peris, V.; Gomez-Cadenas, A.; Clausell-Terol, C. Improvement of salicylic acid biological effect through its encapsulation with silica or chitosan. Int. J. Biol. Macromol. 2022, 199, 108–120. [Google Scholar] [CrossRef]
- Marques Mandaji, C.; da Silva Pena, R.; Campos Chisté, R. Encapsulation of bioactive compounds extracted from plants of genus Hibiscus: A review of selected techniques and applications. Food Res. Int. 2022, 151, 110820. [Google Scholar] [CrossRef]
- Xu, D.; Watahiki, M.K. Phytohormone-Mediated Homeostasis of Root System Architecture; Gonzalez, A., Rodriguez, M., Sağlam, N.G., Eds.; IntechOpen: Rijeka, Croatia, 2020; p. Ch. 2. ISBN 978-1-78984-747-5. [Google Scholar]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef]
- Yazdanbakhsh, N.; Fisahn, J. Analysis of Arabidopsis thaliana root growth kinetics with high temporal and spatial resolution. Ann. Bot. 2010, 105, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Bouallègue, A.; Souissi, F.; Nouairi, I.; Souibgui, M.; Abbes, Z.; Mhadhbi, H. Salicylic acid and hydrogen peroxide pretreatments alleviate salt stress in faba bean (Vicia faba) seeds during germination. Seed Sci. Technol. 2017, 45, 675–690. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, A.; Chaturvedi, V.; Kumar, A.; Bose, B.B. Effects of salicylic acid on seedling growth and nitrogen metabolism in cucumber (Cucumis sativus L.). Orig. Text J. Stress Physiol. Biochem. 2010, 6, 102–113. [Google Scholar]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Salicylic acid functionalized chitosan nanoparticle: A sustainable biostimulant for plant. Int. J. Biol. Macromol. 2019, 123, 59–69. [Google Scholar] [CrossRef]
- Mur, L.A.J.; Kenton, P.; Atzorn, R.; Miersch, O.; Wasternack, C. The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol. 2006, 140, 249–262. [Google Scholar] [CrossRef]
- Faragó, D.; Sass, L.; Valkai, I.; Andrási, N.; Szabados, L. PlantSize offers an affordable, non-destructive method to measure plant size and color in vitro. Front. Plant Sci. 2018, 9, 219. [Google Scholar] [CrossRef]
- Nishimura, N.; Okamoto, M.; Narusaka, M.; Yasuda, M.; Nakashita, H.; Shinozaki, K.; Narusaka, Y.; Hirayama, T. ABA hypersensitive germination2-1 causes the activation of both abscisic acid and salicylic acid responses in Arabidopsis. Plant Cell Physiol. 2009, 50, 2112–2122. [Google Scholar] [CrossRef]
- Bagautdinova, Z.Z.; Omelyanchuk, N.; Tyapkin, A.V.; Kovrizhnykh, V.V.; Lavrekha, V.V.; Zemlyanskaya, E.V. Salicylic acid in root growth and development. Int. J. Mol. Sci. 2022, 23, 2228. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Yang, K.-Z.; Zou, J.-J.; Zhu, L.-L.; Xie, Z.D.; Morita, M.T.; Tasaka, M.; Friml, J.; Grotewold, E.; Beeckman, T.; et al. Transcriptional regulation of PIN genes by FOUR LIPS and MYB88 during Arabidopsis root gravitropism. Nat. Commun. 2015, 6, 8822. [Google Scholar] [CrossRef]
- Watanabe, S.; Takahashi, N.; Kanno, Y.; Suzuki, H.; Aoi, Y.; Takeda-Kamiya, N.; Toyooka, K.; Kasahara, H.; Hayashi, K.-I.; Umeda, M.; et al. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. BioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Konstantinova, N.; Korbei, B.; Luschnig, C. Auxin and root gravitropism: Addressing basic cellular processes by exploiting a defined growth response. Int. J. Mol. Sci. 2021, 22, 2749. [Google Scholar] [CrossRef] [PubMed]
- Philosoph-Hadas, S.; Friedman, H.; Meir, S. Gravitropic bending and plant hormones. Vitam. Horm. 2005, 72, 31–78. [Google Scholar] [PubMed]
- Tan, S.; Abas, M.; Verstraeten, I.; Glanc, M.; Molnár, G.; Hajný, J.; Lasák, P.; Petřík, I.; Russinova, E.; Petrášek, J. Salicylic acid targets protein phosphatase 2A to attenuate growth in plants. Curr. Biol. 2020, 30, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Monshausen, G.B.; Ding, L.; Assmann, S.M. Regulation of root-wave response by extra large and conventional G proteins in Arabidopsis thaliana. Plant J. 2008, 55, 311–322. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Mahata, M.K.; Kim, K.-T. Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles. Adv. Sci. 2022, 9, 2105373. [Google Scholar] [CrossRef]
- Ozga, J.A.; Van Huizen, R.; Reinecke, D.M. Hormone and seed-specific regulation of pea fruit growth. Plant Physiol. 2002, 128, 1379–1389. [Google Scholar] [CrossRef]
- Czesnick, H.; Lenhard, M. Size Control in Plants—Lessons from Leaves. Cold Spring Harb. Perspect. Biol. 2015, 7, a019190. [Google Scholar] [CrossRef]
- Machado, C.A.; Robbins, N.; Gilbert, M.T.P.; Herre, E.A. Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism. Proc. Natl. Acad. Sci. USA 2005, 102, 6558–6565. [Google Scholar] [CrossRef]
- Tian, H.; De Smet, I.; Ding, Z. Shaping a root system: Regulating lateral versus primary root growth. Trends Plant Sci. 2014, 19, 426–431. [Google Scholar] [CrossRef]
- Ke, M.; Ma, Z.; Wang, D.; Sun, Y.; Wen, C.; Huang, D.; Chen, Z.; Yang, L.; Tan, S.; Li, R.; et al. Salicylic acid regulates PIN2 auxin transporter hyperclustering and root gravitropic growth via Remorin-dependent lipid nanodomain organisation in Arabidopsis thaliana. New Phytol. 2021, 229, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Jin, G.; Li, X.; Tang, C.; Zhang, Y.; Liang, Y.; Yu, J. Phosphorus and magnesium interactively modulate the elongation and directional growth of primary roots in Arabidopsis thaliana (L.) Heynh. J. Exp. Bot. 2015, 66, 3841–3854. [Google Scholar] [CrossRef] [PubMed]
- Ken-ichiro, H.; Shouichi, N.; Shiho, F.; Takeshi, N.; Jenness, M.K.; Murphy, A.S.; Hiroyasu, M.; Hiroshi, N.; Masahiko, F.; Takashi, A. Auxin transport sites are visualized in planta using fluorescent auxin analogs. Proc. Natl. Acad. Sci. USA 2014, 111, 11557–11562. [Google Scholar] [CrossRef]
- Chen, Y.; Yordanov, Y.S.; Ma, C.; Strauss, S.; Busov, V.B. DR5 as a reporter system to study auxin response in Populus. Plant Cell Rep. 2013, 32, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Ivanchenko, M.G.; Napsucialy-Mendivil, S.; Dubrovsky, J.G. Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. Plant J. 2010, 64, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Zhaojun, D.; Jiří, F. Auxin regulates distal stem cell differentiation in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 2010, 107, 12046–12051. [Google Scholar] [CrossRef]
- Manacorda, C.A.; Asurmendi, S. Arabidopsis phenotyping through geometric morphometrics. Gigascience 2018, 7, giy073. [Google Scholar] [CrossRef]
- Lever, J.; Krzywinski, M.; Altman, N. Principal component analysis. Nat. Methods 2017, 14, 641–642. [Google Scholar] [CrossRef]
- Miranda-Sánchez, F.; Rivera, J.; Vinuesa, P. Diversity patterns of Rhizobiaceae communities inhabiting soils, root surfaces and nodules reveal a strong selection of rhizobial partners by legumes. Environ. Microbiol. 2016, 18, 2375–2391. [Google Scholar] [CrossRef]
- Koij, F.S.; Saba, J. Using cluster analysis and principal component analysis to group lines and determine important traits in white bean. Procedia Environ. Sci. 2015, 29, 38–40. [Google Scholar] [CrossRef]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Durgbanshi, A.; Arbona, V.; Pozo, O.; Miersch, O.; Sancho, J.V.; Gómez-Cadenas, A. Simultaneous determination of multiple phytohormones in plant extracts by liquid chromatography−electrospray tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 8437–8442. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84); R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses [R Package Factoextra Version 1.0.7]. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 6 November 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampedro-Guerrero, J.; Vives-Peris, V.; Gomez-Cadenas, A.; Clausell-Terol, C. Encapsulation Reduces the Deleterious Effects of Salicylic Acid Treatments on Root Growth and Gravitropic Response. Int. J. Mol. Sci. 2022, 23, 14019. https://doi.org/10.3390/ijms232214019
Sampedro-Guerrero J, Vives-Peris V, Gomez-Cadenas A, Clausell-Terol C. Encapsulation Reduces the Deleterious Effects of Salicylic Acid Treatments on Root Growth and Gravitropic Response. International Journal of Molecular Sciences. 2022; 23(22):14019. https://doi.org/10.3390/ijms232214019
Chicago/Turabian StyleSampedro-Guerrero, Jimmy, Vicente Vives-Peris, Aurelio Gomez-Cadenas, and Carolina Clausell-Terol. 2022. "Encapsulation Reduces the Deleterious Effects of Salicylic Acid Treatments on Root Growth and Gravitropic Response" International Journal of Molecular Sciences 23, no. 22: 14019. https://doi.org/10.3390/ijms232214019
APA StyleSampedro-Guerrero, J., Vives-Peris, V., Gomez-Cadenas, A., & Clausell-Terol, C. (2022). Encapsulation Reduces the Deleterious Effects of Salicylic Acid Treatments on Root Growth and Gravitropic Response. International Journal of Molecular Sciences, 23(22), 14019. https://doi.org/10.3390/ijms232214019





