Bamboo Salt and Triple Therapy Synergistically Inhibit Helicobacter pylori-Induced Gastritis In Vivo: A Preliminary Study
Abstract
1. Introduction
2. Results
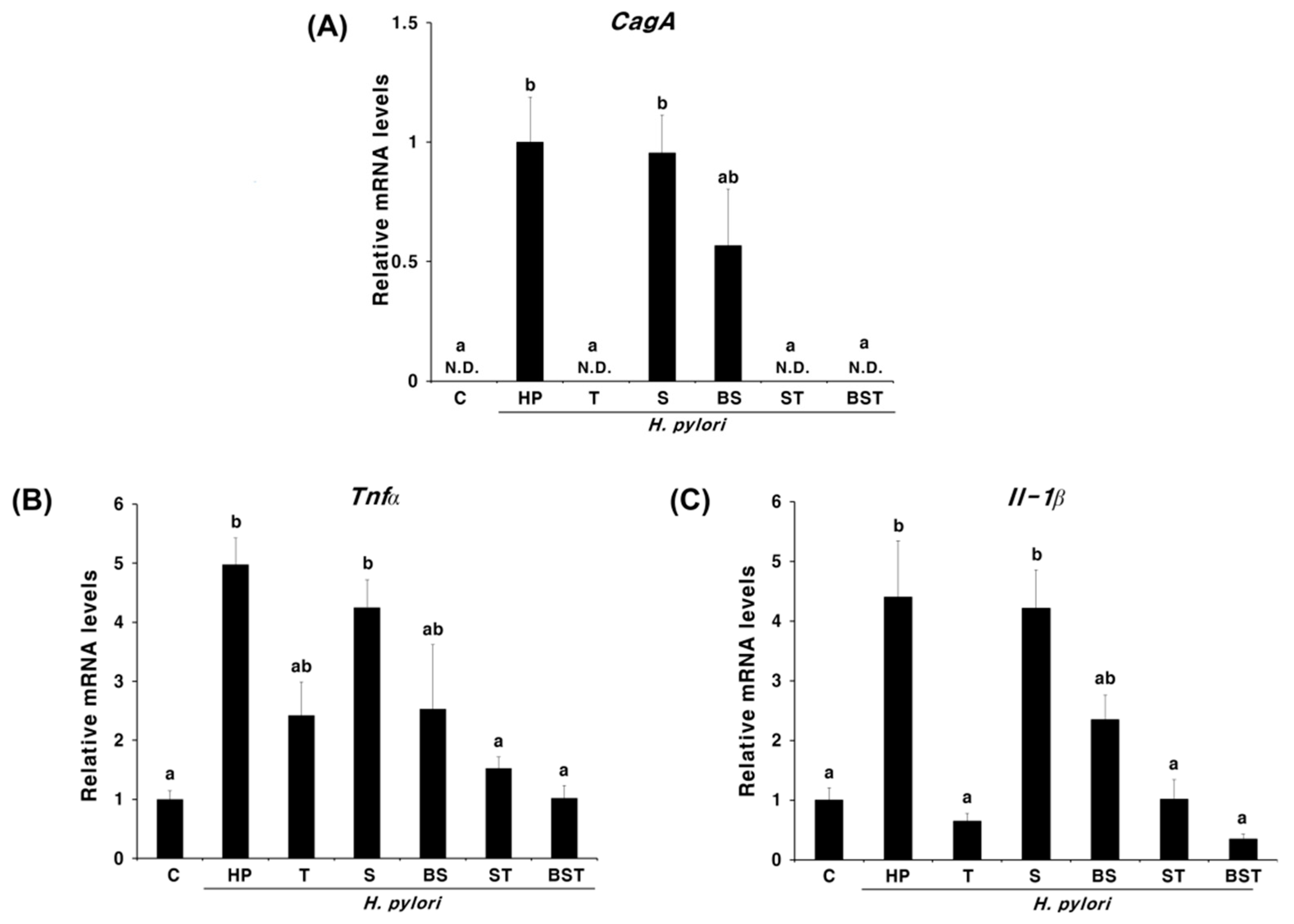
2.1. Effect of Bamboo Salt on the Eradication of H. pylori Infection and Cytokine Expression
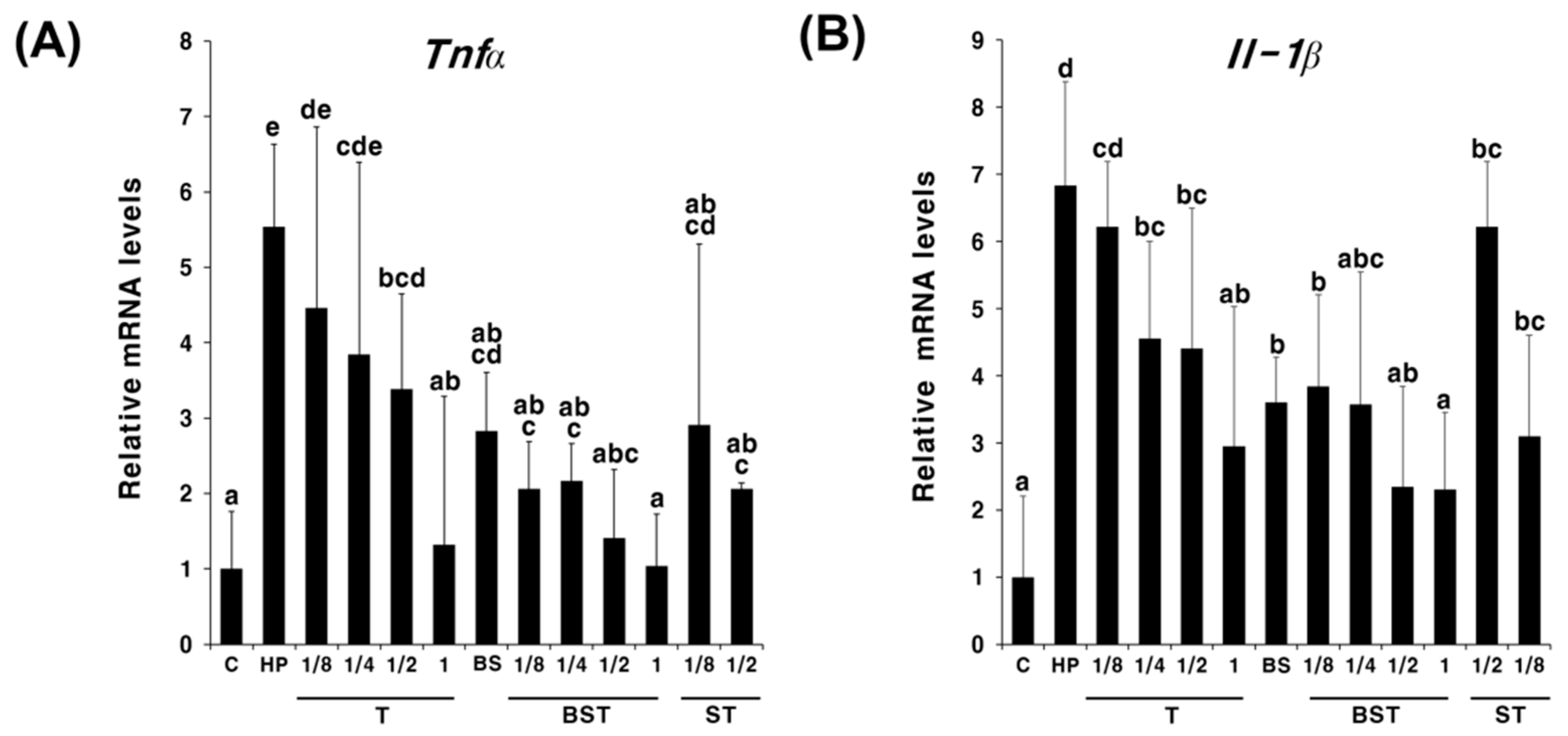
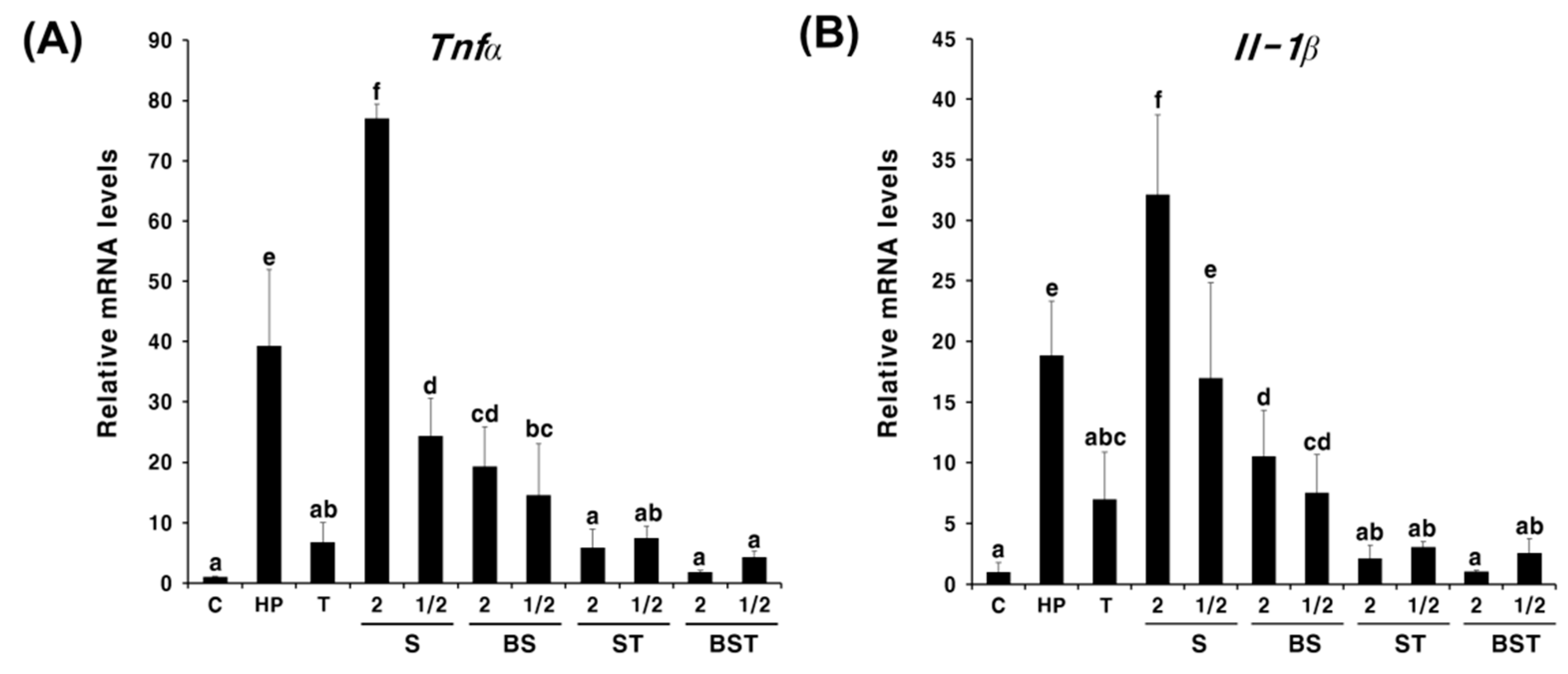
2.2. Synergistic Effects of Bamboo Salt in Combination with Triple Therapy
2.3. Effect of Bamboo Salt on the Gastric Tissue Histology of H. pylori-Infected Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. H. pylori Strain and Culture Conditions
4.3. H. pylori Infection of Animals
4.4. Treatment of Animals with Bamboo Salt and Triple Therapy Drugs after H. pylori Infection
4.5. Gene Expression Analysis
4.6. Histological Examination
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kusters, J.G.; van Vliet, A.H.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.S. Helicobacter pylori gastritis, peptic ulcer, and gastric cancer: Clinical and molecular aspects. Clin. Infect. Dis. 1997, 25, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Charitos, I.A.; D’Agostino, D.; Topi, S.; Bottalico, L. 40 Years of Helicobacter pylori: A Revolution in Biomedical Thought. Gastroenterol. Insights 2021, 12, 111–135. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Pellicano, R.; Ianiro, G.; Fagoonee, S.; Settanni, C.R.; Gasbarrini, A. Review: Extragastric diseases and Helicobacter pylori. Helicobacter 2020, 25, e12741. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Delchier, J.C.; Malfertheiner, P.; Thieroff-Ekerdt, R. Use of a combination formulation of bismuth, metronidazole and tetracycline with omeprazole as a rescue therapy for eradication of Helicobacter pylori. Aliment. Pharmacol. Ther. 2014, 40, 171–177. [Google Scholar] [CrossRef]
- O’Connor, A.; Furuta, T.; Gisbert, J.P.; O’Morain, C. Review—Treatment of Helicobacter pylori infection 2020. Helicobacter 2020, 25, e12743. [Google Scholar] [CrossRef]
- Jung, H.K.; Kang, S.J.; Lee, Y.C.; Yang, H.J.; Park, S.Y.; Shin, C.M.; Kim, S.E.; Lim, H.C.; Kim, J.H.; Nam, S.Y.; et al. Evidence-Based Guidelines for the Treatment of Helicobacter pylori Infection in Korea 2020. Gut Liver 2021, 15, 168–195. [Google Scholar] [CrossRef]
- Ariño Pérez, I.; Martínez-Domínguez, S.J.; Alfaro Almajano, E.; Carrera-Lasfuentes, P.; Lanas, Á. Management of Helicobacter pylori Infection and Effectiveness Rates in Daily Clinical Practice in Spain: 2010–2019. Antibiotics 2022, 11, 698. [Google Scholar] [CrossRef]
- Roszczenko-Jasinska, P.; Wojtys, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef] [PubMed]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Perez-Aisa, A.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp--EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21 533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Wasielica-Berger, J.; Gugnacki, P.; Mlynarczyk, M.; Rogalski, P.; Swidnicka-Siergiejko, A.; Antonowicz, S.; Krzyzak, M.; Maslach, D.; Dabrowski, A.; Daniluk, J. Comparative Effectiveness of Various Eradication Regimens for Helicobacter pylori Infection in the Northeastern Region of Poland. Int. J. Environ. Res. Public Health 2022, 19, 6921. [Google Scholar] [CrossRef]
- Hutchinson, C.; Geissler, C.A.; Powell, J.J.; Bomford, A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut 2007, 56, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Jalving, M.; Koornstra, J.J.; Wesseling, J.; Boezen, H.M.; De Jong, S.; Kleibeuker, J.H. Increased risk of fundic gland polyps during long-term proton pump inhibitor therapy. Aliment. Pharmacol. Ther. 2006, 24, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Driman, D.K.; Wright, C.; Tougas, G.; Riddell, R.H. Omeprazole produces parietal cell hypertrophy and hyperplasia in humans. Dig. Dis. Sci. 1996, 41, 2039–2047. [Google Scholar] [CrossRef]
- Chonan, O.; Takahashi, R.; Yasui, H.; Watanuki, M. Effect of L-lactic acid on calcium absorption in rats fed omeprazole. J. Nutr. Sci. Vitaminol. 1998, 44, 473–481. [Google Scholar] [CrossRef]
- O’Connell, M.B.; Madden, D.M.; Murray, A.M.; Heaney, R.P.; Kerzner, L.J. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. Am. J. Med. 2005, 118, 778–781. [Google Scholar] [CrossRef]
- Ha, J.O.; Park, K.Y. Comparison of Mineral Content and External Structure of Various Salts. J. Korean Soc. Food Sci. Nutr. 1998, 27, 413–418. [Google Scholar]
- Jin, Y.X.; Kim, H.R.; Kim, S. Analysis of components according to different collecting time and production method in sun-dried salt. Korean J. Food Preserv. 2013, 20, 791–797. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.H.; Ryu, H.I. Elements in a Bamboo Salt and Comparison of Its Elemental Contents with Those in Other Salts. Pharm. Soc. Korea 2003, 47, 135–141. [Google Scholar]
- Han, Y.Y.; Shang, Q.W.; Yao, J.; Ji, Y. Hydrogen sulfide: A gaseous signaling molecule modulates tissue homeostasis: Implications in ophthalmic diseases. Cell Death Dis. 2019, 10, 293. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.C.; da Silva, E.F.; Silveira, L.L.; de Paiva, Y.B.; de Castro, C.H.; Freiria-Oliveira, A.H.; Rosa, D.A.; Ferreira, P.M.; Xavier, C.H.; Colombari, E.; et al. High sodium intake during postnatal phases induces an increase in arterial blood pressure in adult rats. Br. J. Nutr. 2014, 112, 1923–1932. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fox, J.G.; Dangler, C.A.; Taylor, N.S.; King, A.; Koh, T.J.; Wang, T.C. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999, 59, 4823–4828. [Google Scholar]
- Joossens, J.V.; Hill, M.J.; Elliott, P.; Stamler, R.; Lesaffre, E.; Dyer, A.; Nichols, R.; Kesteloot, H. Dietary salt, nitrate and stomach cancer mortality in 24 countries. European Cancer Prevention (ECP) and the INTERSALT Cooperative Research Group. Int. J. Epidemiol. 1996, 25, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.Y.; Lee, E.H.; Kim, C.Y.; Shin, T.Y.; Kim, S.D.; Song, Y.S.; Lee, K.N.; Hong, S.H.; Kim, H.M. Anti-inflammatory activity of Korean folk medicine purple bamboo salt. Immunopharmacol. Immunotoxicol. 2003, 25, 377–384. [Google Scholar] [CrossRef]
- Zhao, X.; Song, J.L.; Jung, O.S.; Lim, Y.I.; Park, K.Y. Chemical Properties and In vivo Gastric Protective Effects of Bamboo Salt. Food Sci. Biotechnol. 2014, 23, 895–902. [Google Scholar] [CrossRef]
- Zhao, X.; Deng, X.; Park, K.Y.; Qiu, L.; Pang, L. Purple bamboo salt has anticancer activity in TCA8113 cells in vitro and preventive effects on buccal mucosa cancer in mice in vivo. Exp. Ther. Med. 2013, 5, 549–554. [Google Scholar] [CrossRef]
- Shin, H.Y.; Na, H.J.; Moon, P.D.; Shin, T.; Shin, T.Y.; Kim, S.H.; Hong, S.H.; Kim, H.M. Inhibition of mast cell-dependent immediate-type hypersensitivity reactions by purple bamboo salt. J. Ethnopharmacol. 2004, 91, 153–157. [Google Scholar] [CrossRef]
- You, Y.O.; Shin, H.Y.; Yu, H.H.; Yoo, S.J.; Kim, S.H.; Kim, Y.K.; Hong, S.H.; Shin, T.Y.; Kim, H.M. Effect of Powerdental on caries-inducing properties of Streptococcus mutans and TNF-alpha secretion from HMC-1 cells. J. Ethnopharmacol. 2004, 92, 331–335. [Google Scholar] [CrossRef]
- Gao, T.C.; Cho, J.Y.; Feng, L.Y.; Chanmuang, S.; Park, S.Y.; Auh, C.K.; Pai, T.K.; Ham, K.S. Mineral-rich Solar Sea Salt Generates Less Oxidative Stress in Rats than Mineral-deficient Salt. Food Sci. Biotechnol. 2014, 23, 951–956. [Google Scholar] [CrossRef]
- Gao, T.C.; Cho, J.Y.; Feng, L.Y.; Chanmuang, S.; Pongworn, S.; Jaiswal, L.; Auh, C.K.; Pai, T.K.; Ham, K.S. Heat-Treated Solar Sea Salt Has Antioxidant Activity in Vitro and Produces Less Oxidative Stress in Rats Compared with Untreated Solar Sea Salt. J. Food Biochem. 2015, 39, 631–641. [Google Scholar] [CrossRef]
- Zhao, X.; Jung, O.S.; Park, K.Y. Alkaline and Antioxidant Effects of Bamboo Salt. J. Korean Soc. Food Sci. Nutr. 2012, 41, 1301–1304. [Google Scholar] [CrossRef]
- Sun, Y.L.; Zheng, J.Y.; Yi, J.J.; Cai, S.B. Investigation on the Effects and Mechanisms of Alkaline Natural Mineral Water and Distilled Water on Ethanol-Induced Gastric Ulcers In Vivo and In Vitro. Processes 2022, 10, 498. [Google Scholar] [CrossRef]
- Kim, N.R.; Nam, S.Y.; Ryu, K.J.; Kim, H.M.; Jeong, H.J. Effects of bamboo salt and its component, hydrogen sulfide, on enhancing immunity. Mol. Med. Rep. 2016, 14, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Kim, H.S.; Kang, S.Y.; Jung, K.K.; Kim, T.G.; Han, H.M.; Rheu, H.M.; Moon, A.R. Characterization and anti-gastric ulcer activity of bamboo salt. J. Food Hyg. Saf. 1998, 13, 252–257. [Google Scholar]
- Linden, D.R. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid. Redox Signal. 2014, 20, 818–830. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, K.Y.; Kim, S.J.; Oh, S.; Moon, J.H. Antimicrobial activity of the synthesized non-allergenic urushiol derivatives. Biosci. Biotechnol. Biochem. 2015, 79, 1915–1918. [Google Scholar] [CrossRef]
- Jeong, H.Y.; Lee, T.H.; Kim, J.G.; Lee, S.; Moon, C.; Truong, X.T.; Jeon, T.I.; Moon, J.H. 3-Pentylcatechol, a Non-Allergenic Urushiol Derivative, Displays Anti-Helicobacter pylori Activity In Vivo. Pharmaceuticals 2020, 13, 384. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; O’Rourke, J.; De Ungria, M.C.; Robertson, B.; Daskalopoulos, G.; Dixon, M.F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology 1997, 112, 1386–1397. [Google Scholar] [CrossRef]
- Suk, K.T.; Baik, S.K.; Kim, H.S.; Park, S.M.; Paeng, K.J.; Uh, Y.; Jang, I.H.; Cho, M.Y.; Choi, E.H.; Kim, M.J.; et al. Antibacterial effects of the urushiol component in the sap of the lacquer tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter 2011, 16, 434–443. [Google Scholar] [CrossRef]
- Go, D.H.; Lee, Y.G.; Lee, D.H.; Kim, J.A.; Jo, I.H.; Han, Y.S.; Jo, Y.H.; Kim, K.Y.; Seo, Y.K.; Moon, J.H.; et al. 3-Decylcatechol induces autophagy-mediated cell death through the IRE1alpha/JNK/p62 in hepatocellular carcinoma cells. Oncotarget 2017, 8, 58790–58800. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Son, Y.; Kim, J.; Kang, S.; Ahn, W.; Kim, S.H.; Kim, J.C.; Shin, T.; et al. Involvement of BDNF/ERK signaling in spontaneous recovery from trimethyltin-induced hippocampal neurotoxicity in mice. Brain Res. Bull. 2016, 121, 48–58. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence | |
|---|---|---|
| Forward | Reverse | |
| Rplp0 | GTGCTGATGGGCAAGAAC | AGGTCCTCCTTGGTGAAC |
| Tnfα | CGAGTGACAAGCCTGTAGCC | AGCTGCTCCTCCACTTGGT |
| Il-1β | ATGAGAGCATCCAGCTTCAA | TGAAGGAAAAGAAGGTGCTC |
| CagA | CCGATCGATCCGAAATTTTA | CGTTCGGATTTGATTCCCTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.; Jeong, H.Y.; An, D.Y.; Kim, H.; Cho, J.-Y.; Hwang, D.Y.; Lee, H.J.; Ham, K.-S.; Moon, J.-H. Bamboo Salt and Triple Therapy Synergistically Inhibit Helicobacter pylori-Induced Gastritis In Vivo: A Preliminary Study. Int. J. Mol. Sci. 2022, 23, 13997. https://doi.org/10.3390/ijms232213997
Lee TH, Jeong HY, An DY, Kim H, Cho J-Y, Hwang DY, Lee HJ, Ham K-S, Moon J-H. Bamboo Salt and Triple Therapy Synergistically Inhibit Helicobacter pylori-Induced Gastritis In Vivo: A Preliminary Study. International Journal of Molecular Sciences. 2022; 23(22):13997. https://doi.org/10.3390/ijms232213997
Chicago/Turabian StyleLee, Tae Ho, Hang Yeon Jeong, Do Yeon An, Haesung Kim, Jeong-Yong Cho, Do Young Hwang, Hyoung Jae Lee, Kyung-Sik Ham, and Jae-Hak Moon. 2022. "Bamboo Salt and Triple Therapy Synergistically Inhibit Helicobacter pylori-Induced Gastritis In Vivo: A Preliminary Study" International Journal of Molecular Sciences 23, no. 22: 13997. https://doi.org/10.3390/ijms232213997
APA StyleLee, T. H., Jeong, H. Y., An, D. Y., Kim, H., Cho, J.-Y., Hwang, D. Y., Lee, H. J., Ham, K.-S., & Moon, J.-H. (2022). Bamboo Salt and Triple Therapy Synergistically Inhibit Helicobacter pylori-Induced Gastritis In Vivo: A Preliminary Study. International Journal of Molecular Sciences, 23(22), 13997. https://doi.org/10.3390/ijms232213997






