Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes—A Narrative Review
Abstract
1. Introduction
- The latest research on the neurobiology of maternal anxiety, stress, and depression and the transmission mechanisms at the molecular level to the fetus and child;
- The longitudinal studies in which early child development is monitored to the presence of maternal distress during pregnancy and the postpartum period.
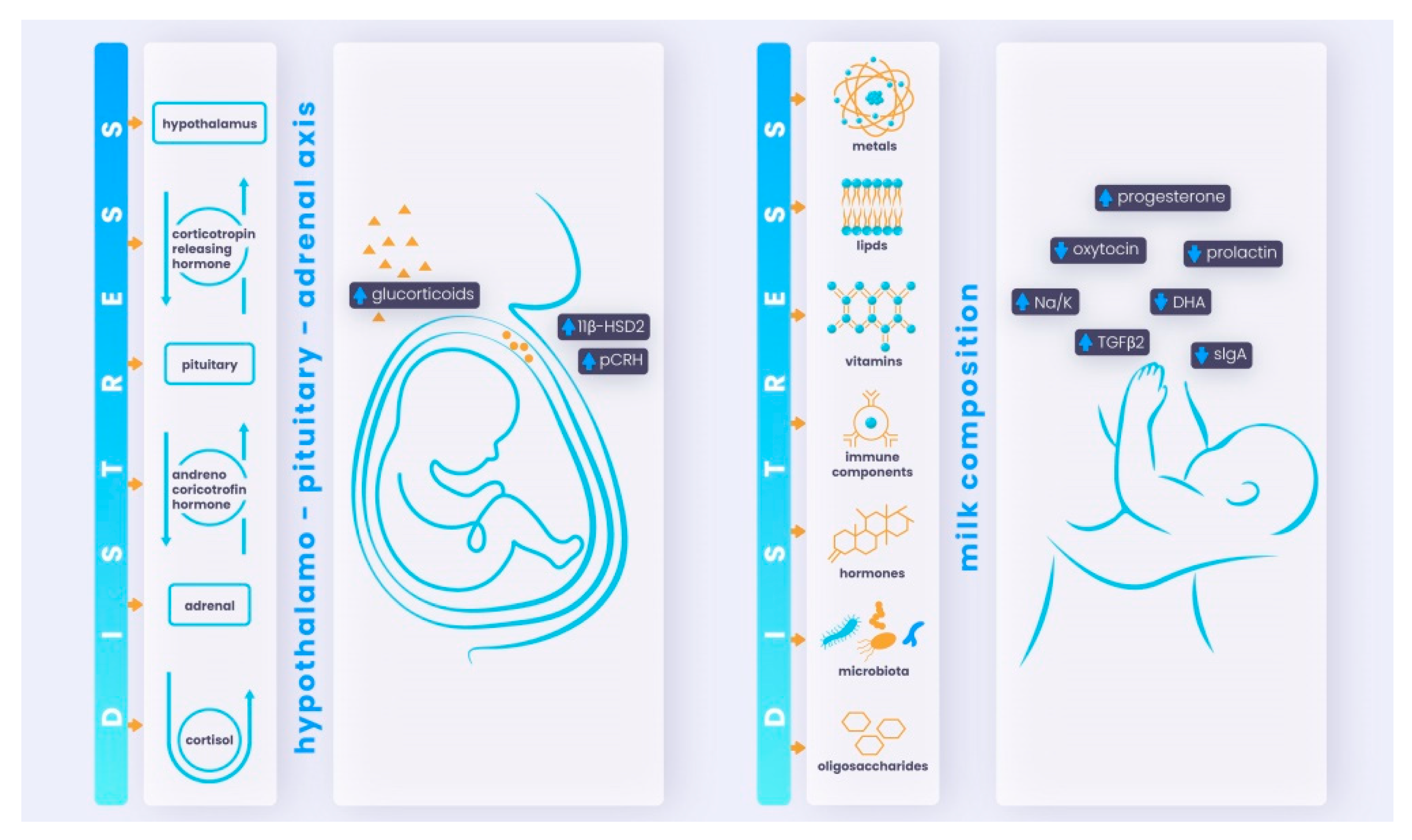
2. Factors and Biological Mechanisms Underlying the Transmission of Maternal Distress to the (Unborn) Child
2.1. Mechanisms Involved in Stress Responses
2.2. Biological Mechanisms and Factors Mediating Maternal Distress in the Prenatal Period
2.2.1. HPA Axis Dysfunction
2.2.2. Placental Mechanism
- Prenatally, the fetus could be affected while still in the uterus via the placenta and dysregulated maternal HPA axis;
- Postnatally, the newborn infant could be affected via breastfeeding and changed milk composition (disrupted concentrations of hormones, immune cells, and other components).
2.2.3. Catecholamine, Uteroplacental, and Fetal Hemodynamics
2.2.4. Immune System and Inflammation as Maternal Mediators of Stress
2.2.5. Serotonin and Tryptophan
2.2.6. Oxidative Stress: Interaction between Maternal and Fetal Oxidative Systems
2.2.7. Neuroactive Steroids
2.2.8. Maternal Microbiota as a Potential Stress-Transfer Mechanism
2.2.9. Autonomic Nervous System
2.2.10. Gene–Environment Interactions, Epigenetics, and Prenatal Stressors
DNA Methylation
Histone Modification
Changes in Non-Coding RNAs
2.2.11. Neurodevelopmental Mechanisms
2.3. Biological Mechanisms Underlying the Maternal Distress Transmission to the Child in the Postpartum Period
2.3.1. Effects of Maternal Distress on Milk Composition
2.3.2. Mother-Child Bonding and Caregiving Mechanism
3. Maternal Distress as a Risk Factor for Adverse Fetal and Child Developmental Outcomes
3.1. Cognition
3.2. Socio-Emotional Development
3.3. Fine and Gross Motor Development
3.4. Neurodevelopmental Disorders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DOHaD | Developmental Origin of Health and Disease |
| DOBHaD | Developmental Origins of Behavior, Health and Disease |
| COVID-19 | Coronavirus Disease 2019 caused by SARS-CoV-2 virus |
| HPA | Hypothalamic–pituitary–adrenal |
| CRH | Corticotropin releasing hormone |
| ACTH | Adrenocorticotropic hormone |
| 11β-HSD2 | 11β-hydroxysteroid Dehydrogenase type 2 |
| 11β-HSD1 | 11β-hydroxysteroid Dehydrogenase type 1 |
| NAD+ | Nicotinamide adenine dinucleotide |
| IGF2 | Insulin-Like Growth Factor 2 |
| H19 | H19 Imprinted Maternally Expressed Transcript |
| HSD11B2 | Hydroxysteroid 11-Beta Dehydrogenase 2 gene |
| NR3C1 | Nuclear receptor subfamily 3 group C member 1 |
| LINE 1 | The long interspersed nucleotide elements 1 |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| ANS | Autonomic nervous system |
| SAM axis | Sympathetic-adrenal-medullary axis |
| HPT axis | Hypothalamic-pituitary-thyroid axis |
| THs | Thyroid Hormones |
| GCs | Glucocorticoids |
| SNS | Sympathetic nervous system |
| PNS | Parasympathetic nervous system |
| AVP | Arginine-vasopressin |
| POMC | Pro-opiomelanocortin |
| NE | Norepinephrine |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
| TNF-α | Tumour necrosis factor |
| 5-HT | 5-hydroxytryptamine or serotonin |
| 5HIAA | 5-hydroxyindoleacetic acid |
| TPH | Tryptophan hydroxylase |
| 5-HT1A | Serotonin 1A receptor |
| SERT | Serotonin transporter or 5HTT |
| MAO A | Monoamine oxidase A |
| ROS | Reactive oxygen species |
| THDOC | Tetrahydrodeoxycorticosterone |
| HR | Heart rate |
| HRV | Heart rate variability |
| DNAm | DNA methylation |
| FKBP5 | FK506 binding protein 5 gene |
| SLC6A4 | Solute carrier family 6 member 4 gene |
| 5-HTT gene | Serotonim transporter gene (or SLC6A4 or SERT) |
| FKBP51 | FK506-binding protein 51 |
| OXTR | Oxytocin receptor gene |
| BDNF | Brain-derived neurotrophic factor |
| ICR | Imprinted control region |
| MEG3 gene | Maternally expressed gene 3 |
| PLAGL1 gene | PLAG1-Like Zinc Finger 1 gene |
| PEG3 gene | Paternally expressed gene 3 |
| ncRNAs | Non-coding RNAs |
| snRNAs | Small nuclear RNAs |
| snoRNAs | Small nucleolar RNAs |
| rRNAs | Ribosomal RNAs |
| tRNAs | Transfer RNAs |
| cRNAs | Circular RNAs |
| piRNAs | Piwi-interacting RNAs |
| Na | Sodium |
| Na/K | Sodium-Potassium |
| TGFβ2 | Transforming growth beta factor-2 |
| sIgA | Secretory IgA |
| DHA | Docosahexaenoic acid |
| ADHD | Attention deficit hyperactivity disorder |
| ASD | Autism spectrum disorder |
References
- Newman, L.; Judd, F.; Olsson, C.A.; Castle, D.; Bousman, C.; Sheehan, P.; Pantelis, C.; Craig, J.M.; Komiti, A.; Everall, I. Early origins of mental disorder-risk factors in the perinatal and infant period. BMC Psychiatry 2016, 16, 270. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Walker, S.P.; Fernald, L.C.; Andersen, C.T.; DiGirolamo, A.M.; Lu, C.; McCoy, D.C.; Fink, G.; Shawar, Y.R.; Shiffman, J. Early childhood development coming of age: Science through the life course. Lancet 2017, 389, 77–90. [Google Scholar] [CrossRef]
- Britto, P.R.; Lye, S.J.; Proulx, K.; Yousafzai, A.K.; Matthews, S.G.; Vaivada, T.; Perez-Escamilla, R.; Rao, N.; Ip, P.; Fernald, L.C. Nurturing care: Promoting early childhood development. Lancet 2017, 389, 91–102. [Google Scholar] [CrossRef]
- Van den Bergh, B.R. Developmental programming of early brain and behaviour development and mental health: A conceptual framework. Dev. Med. Child Neurol. 2011, 53, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Pinal, C. The developmental origins of adult disease. Matern. Child Nutr. 2005, 1, 130–141. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A. Developmental origins of disease paradigm: A mechanistic and evolutionary perspective. Pediatric Res. 2004, 56, 311–317. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.; Mulder, E.J.; Mennes, M.; Glover, V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci. Biobehav. Rev. 2005, 29, 237–258. [Google Scholar] [CrossRef]
- Wadhwa, P.D.; Buss, C.; Entringer, S.; Swanson, J.M. Developmental origins of health and disease: Brief history of the approach and current focus on epigenetic mechanisms. In Seminars in Reproductive Medicine; © Thieme Medical Publishers: New York, NY, USA, 2009; pp. 358–368. [Google Scholar]
- Ibanez, G.; Bernard, J.Y.; Rondet, C.; Peyre, H.; Forhan, A.; Kaminski, M.; Saurel-Cubizolles, M.-J.; Group, E.M.-C.C.S. Effects of antenatal maternal depression and anxiety on children’s early cognitive development: A prospective cohort study. PLoS ONE 2015, 10, e0135849. [Google Scholar] [CrossRef]
- Heindel, J.J.; Vandenberg, L.N. Developmental origins of health and disease: A paradigm for understanding disease etiology and prevention. Curr. Opin. Pediatrics 2015, 27, 248. [Google Scholar] [CrossRef]
- Painter, R.C.; Roseboom, T.J.; Van Montfrans, G.A.; Bossuyt, P.M.; Krediet, R.T.; Osmond, C.; Barker, D.J.; Bleker, O.P. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J. Am. Soc. Nephrol. 2005, 16, 189–194. [Google Scholar] [CrossRef]
- Lopuhaä, C.; Roseboom, T.; Osmond, C.; Barker, D.; Ravelli, A.; Bleker, O.; Van Der Zee, J.; Van Der Meulen, J. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax 2000, 55, 555–561. [Google Scholar] [CrossRef]
- Kim, D.R.; Bale, T.L.; Epperson, C.N. Prenatal programming of mental illness: Current understanding of relationship and mechanisms. Curr. Psychiatry Rep. 2015, 17, 5. [Google Scholar] [CrossRef]
- Roseboom, T.J.; van der Meulen, J.H.; Osmond, C.; Barker, D.J.; Ravelli, A.C.; Bleker, O.P. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am. J. Clin. Nutr. 2000, 72, 1101–1106. [Google Scholar] [CrossRef]
- Kyle, U.G.; Pichard, C. The Dutch Famine of 1944–1945: A pathophysiological model of long-term consequences of wasting disease. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 388–394. [Google Scholar] [CrossRef]
- Schmutte, C.; Jones, P.A. Involvement of DNA methylation in human carcinogenesis. Biol. Chem. 1998, 379, 377–388. [Google Scholar]
- Williams, K.T.; Garrow, T.A.; Schalinske, K.L. Type I diabetes leads to tissue-specific DNA hypomethylation in male rats. J. Nutr. 2008, 138, 2064–2069. [Google Scholar] [CrossRef]
- Bogdarina, I.; Welham, S.; King, P.J.; Burns, S.P.; Clark, A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007, 100, 520–526. [Google Scholar] [CrossRef]
- Goyal, R.; Galffy, A.; Field, S.A.; Gheorghe, C.P.; Mittal, A.; Longo, L.D. Maternal protein deprivation: Changes in systemic renin-angiotensin system of the mouse fetus. Reprod. Sci. 2009, 16, 894–904. [Google Scholar] [CrossRef]
- Goyal, R.; Longo, L.D. Maternal protein deprivation: Sexually dimorphic programming of hypertension in the mouse. Hypertens. Res. 2013, 36, 29–35. [Google Scholar] [CrossRef]
- Obeid, R.; Schadt, A.; Dillmann, U.; Kostopoulos, P.; Fassbender, K.; Herrmann, W. Methylation status and neurodegenerative markers in Parkinson disease. Clin. Chem. 2009, 55, 1852–1860. [Google Scholar] [CrossRef]
- Goyal, R.; Goyal, D.; Leitzke, A.; Gheorghe, C.P.; Longo, L.D. Brain renin-angiotensin system: Fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod. Sci. 2010, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Goyal, D.; Chu, N.; Van Wickle, J.; Longo, L.D. Cerebral artery alpha-1 AR subtypes: High altitude long-term acclimatization responses. PLoS ONE 2014, 9, e112784. [Google Scholar] [CrossRef] [PubMed]
- Ducsay, C.A.; Goyal, R.; Pearce, W.J.; Wilson, S.; Hu, X.-Q.; Zhang, L. Gestational hypoxia and developmental plasticity. Physiol. Rev. 2018, 98, 1241–1334. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.; Stroud, L.; Buka, S. Maternal stress and child outcomes: Evidence from siblings. J. Hum. Resour. 2016, 51, 523–555. [Google Scholar] [CrossRef]
- Christian, L.M. Stress and immune function during pregnancy: An emerging focus in mind-body medicine. Curr. Dir. Psychol. Sci. 2015, 24, 3–9. [Google Scholar] [CrossRef]
- Huizink, A.; Menting, B.; Oosterman, M.; Verhage, M.; Kunseler, F.; Schuengel, C. The interrelationship between pregnancy-specific anxiety and general anxiety across pregnancy: A longitudinal study. J. Psychosom. Obstet. Gynecol. 2014, 35, 92–100. [Google Scholar] [CrossRef]
- Kinsella, M.T.; Monk, C. Impact of maternal stress, depression & anxiety on fetal neurobehavioral development. Clin. Obstet. Gynecol. 2009, 52, 425. [Google Scholar]
- Mulder, E.J.; De Medina, P.R.; Huizink, A.C.; Van den Bergh, B.R.; Buitelaar, J.K.; Visser, G.H. Prenatal maternal stress: Effects on pregnancy and the (unborn) child. Early Hum. Dev. 2002, 70, 3–14. [Google Scholar] [CrossRef]
- Vehmeijer, F.O.; Guxens, M.; Duijts, L.; El Marroun, H. Maternal psychological distress during pregnancy and childhood health outcomes: A narrative review. J. Dev. Orig. Health Dis. 2019, 10, 274–285. [Google Scholar] [CrossRef]
- Skodol, A.E.; Shrout, P.E. Use of DSM-III axis IV in clinical practice: Rating etiologically significant stressors. Am. J. Psychiatry 1989, 146, 61–66. [Google Scholar]
- Leach, L.S.; Poyser, C.; Fairweather-Schmidt, K. Maternal perinatal anxiety: A review of prevalence and correlates. Clin. Psychol. 2017, 21, 4–19. [Google Scholar] [CrossRef]
- Melville, J.L.; Gavin, A.; Guo, Y.; Fan, M.-Y.; Katon, W.J. Depressive disorders during pregnancy: Prevalence and risk factors in a large urban sample. Obstet. Gynecol. 2010, 116, 1064. [Google Scholar] [CrossRef]
- Woods, S.M.; Melville, J.L.; Guo, Y.; Fan, M.-Y.; Gavin, A. Psychosocial stress during pregnancy. Am. J. Obstet. Gynecol. 2010, 202, 61.e1-61.e7. [Google Scholar] [CrossRef]
- Rees, S.; Channon, S.; Waters, C.S. The impact of maternal prenatal and postnatal anxiety on children’s emotional problems: A systematic review. Eur. Child Adolesc. Psychiatry 2019, 28, 257–280. [Google Scholar] [CrossRef]
- Quinlivan, J.; Lambregtse-van den Berg, M. Will COVID-19 Impact upon Pregnancy, Childhood and Adult Outcomes? A Call to Establish National Longitudinal Datasets; Taylor & Francis: Abingdon, UK, 2020; Volume 41, pp. 165–166. [Google Scholar]
- Berthelot, N.; Lemieux, R.; Garon-Bissonnette, J.; Drouin-Maziade, C.; Martel, É.; Maziade, M. Uptrend in distress and psychiatric symptomatology in pregnant women during the coronavirus disease 2019 pandemic. Acta Obstet. Et Gynecol. Scand. 2020, 99, 848–855. [Google Scholar] [CrossRef]
- Lebel, C.; MacKinnon, A.; Bagshawe, M.; Tomfohr-Madsen, L.; Giesbrecht, G. Elevated depression and anxiety symptoms among pregnant individuals during the COVID-19 pandemic. J. Affect. Disord. 2020, 277, 5–13. [Google Scholar] [CrossRef]
- Preis, H.; Mahaffey, B.; Heiselman, C.; Lobel, M. Pandemic-related pregnancy stress and anxiety among women pregnant during the coronavirus disease 2019 pandemic. Am. J. Obstet. Gynecol. MFM 2020, 2, 100155. [Google Scholar] [CrossRef]
- Yue, C.; Liu, C.; Wang, J.; Zhang, M.; Wu, H.; Li, C.; Yang, X. Association between social support and anxiety among pregnant women in the third trimester during the coronavirus disease 2019 (COVID-19) epidemic in Qingdao, China: The mediating effect of risk perception. Int. J. Soc. Psychiatry 2021, 67, 120–127. [Google Scholar] [CrossRef]
- Omowale, S.S.; Casas, A.; Lai, Y.-H.; Sanders, S.A.; Hill, A.V.; Wallace, M.L.; Rathbun, S.L.; Gary-Webb, T.L.; Burke, L.E.; Davis, E.M. Trends in stress throughout pregnancy and postpartum period during the COVID-19 pandemic: Longitudinal study using ecological momentary assessment and data from the Postpartum Mothers Mobile Study. JMIR Ment. Health 2021, 8, e30422. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of adult disease. Eur. J. Epidemiol. 2003, 23, 733–736. [Google Scholar] [CrossRef]
- Nugent, B.M.; Bale, T.L. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front. Neuroendocrinol. 2015, 39, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Lehrner, A. Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Rakers, F.; Rupprecht, S.; Dreiling, M.; Bergmeier, C.; Witte, O.W.; Schwab, M. Transfer of maternal psychosocial stress to the fetus. Neurosci. Biobehav. Rev. 2020, 117, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Matas-Blanco, C.; Caparros-Gonzalez, R.A. Influence of maternal stress during pregnancy on child’s neurodevelopment. Psych 2020, 2, 16. [Google Scholar] [CrossRef]
- Cao-Lei, L.; van den Heuvel, M.I.; Huse, K.; Platzer, M.; Elgbeili, G.; Braeken, M.A.; Otte, R.A.; Witte, O.W.; Schwab, M.; Van den Bergh, B.R. Epigenetic modifications associated with maternal anxiety during pregnancy and children’s behavioral measures. Cells 2021, 10, 2421. [Google Scholar] [CrossRef]
- Veru, F.; Laplante, D.P.; Luheshi, G.; King, S. Prenatal maternal stress exposure and immune function in the offspring. Stress 2014, 17, 133–148. [Google Scholar] [CrossRef]
- Graignic-Philippe, R.; Dayan, J.; Chokron, S.; Jacquet, A.; Tordjman, S. Effects of prenatal stress on fetal and child development: A critical literature review. Neurosci. Biobehav. Rev. 2014, 43, 137–162. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef]
- Van den Bergh, B.R.; van den Heuvel, M.I.; Lahti, M.; Braeken, M.; de Rooij, S.R.; Entringer, S.; Hoyer, D.; Roseboom, T.; Räikkönen, K.; King, S. Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci. Biobehav. Rev. 2020, 117, 26–64. [Google Scholar] [CrossRef]
- Mueller, B.R.; Bale, T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef]
- Carpenter, T.; Grecian, S.; Reynolds, R. Sex differences in early-life programming of the hypothalamic–Pituitary–Adrenal axis in humans suggest increased vulnerability in females: A systematic review. J. Dev. Orig. Health Dis. 2017, 8, 244–255. [Google Scholar] [CrossRef]
- Mansell, T.; Novakovic, B.; Meyer, B.; Rzehak, P.; Vuillermin, P.; Ponsonby, A.; Collier, F.; Burgner, D.; Saffery, R.; Ryan, J. The effects of maternal anxiety during pregnancy on IGF2/H19 methylation in cord blood. Transl. Psychiatry 2016, 6, e765. [Google Scholar] [CrossRef]
- Appleton, A.A.; Armstrong, D.A.; Lesseur, C.; Lee, J.; Padbury, J.F.; Lester, B.M.; Marsit, C.J. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS ONE 2013, 8, e74691. [Google Scholar] [CrossRef]
- Stroud, L.R.; Papandonatos, G.D.; Parade, S.H.; Salisbury, A.L.; Phipps, M.G.; Lester, B.; Padbury, J.F.; Marsit, C.J. Prenatal major depressive disorder, placenta glucocorticoid and serotonergic signaling, and infant cortisol response. Psychosom. Med. 2016, 78, 979. [Google Scholar] [CrossRef]
- Ostlund, B.D.; Conradt, E.; Crowell, S.E.; Tyrka, A.R.; Marsit, C.J.; Lester, B.M. Prenatal stress, fearfulness, and the epigenome: Exploratory analysis of sex differences in DNA methylation of the glucocorticoid receptor gene. Front. Behav. Neurosci. 2016, 10, 147. [Google Scholar] [CrossRef]
- Braithwaite, E.; Kundakovic, M.; Ramchandani, P.; Murphy, S.; Champagne, F. Maternal prenatal depressive symptoms predict infant NR3C1 1F and BDNF IV DNA methylation. Epigenetics 2015, 10, 408–417. [Google Scholar] [CrossRef]
- Kemeny, M.E. The psychobiology of stress. Curr. Dir. Psychol. Sci. 2003, 12, 124–129. [Google Scholar] [CrossRef]
- Najafzadeh, A. Stress and preterm birth: Biological and vascular mechanisms affecting the feto-placental circulation and the length of gestation. Sonography 2016, 3, 95–102. [Google Scholar] [CrossRef]
- Sheng, J.A.; Bales, N.J.; Myers, S.A.; Bautista, A.I.; Roueinfar, M.; Hale, T.M.; Handa, R.J. The hypothalamic-pituitary-adrenal axis: Development, programming actions of hormones, and maternal-fetal interactions. Front. Behav. Neurosci. 2021, 14, 601939. [Google Scholar] [CrossRef]
- Hobel, C.J.; Goldstein, A.; Barrett, E.S. Psychosocial stress and pregnancy outcome. Clin. Obstet. Gynecol. 2008, 51, 333–348. [Google Scholar] [CrossRef]
- Benfield, R.D.; Newton, E.R.; Tanner, C.J.; Heitkemper, M.M. Cortisol as a biomarker of stress in term human labor: Physiological and methodological issues. Biol. Res. Nurs. 2014, 16, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.E.; Keller-Wood, M. The critical importance of the fetal hypothalamus-pituitary-adrenal axis. F1000Research 2016, 5, F1000. [Google Scholar] [CrossRef] [PubMed]
- Dusek, J.A.; Benson, H. Mind-body medicine: A model of the comparative clinical impact of the acute stress and relaxation responses. Minn. Med. 2009, 92, 47. [Google Scholar] [PubMed]
- Anifantaki, F.; Pervanidou, P.; Lambrinoudaki, I.; Panoulis, K.; Vlahos, N.; Eleftheriades, M. Maternal prenatal stress, thyroid function and neurodevelopment of the offspring: A mini review of the literature. Front. Neurosci. 2021, 15, 692446. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, F.; Rodríguez, J.S. Thyroid hormone therapy modulates hypothalamo-pituitary-adrenal axis. Endocr. J. 2011, 58, 137–142. [Google Scholar] [CrossRef]
- Lautarescu, A.; Craig, M.C.; Glover, V. Prenatal stress: Effects on fetal and child brain development. Int. Rev. Neurobiol. 2020, 150, 17–40. [Google Scholar]
- Glover, V.; O’Donnell, K.J.; O’Connor, T.G.; Fisher, J. Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—A global perspective. Dev. Psychopathol. 2018, 30, 843–854. [Google Scholar] [CrossRef]
- Martinez, C.A.; Marteinsdottir, I.; Josefsson, A.; Sydsjö, G.; Theodorsson, E.; Rodriguez-Martinez, H. Expression of stress-mediating genes is increased in term placentas of women with chronic self-perceived anxiety and depression. Genes 2020, 11, 869. [Google Scholar] [CrossRef]
- Charil, A.; Laplante, D.P.; Vaillancourt, C.; King, S. Prenatal stress and brain development. Brain Res. Rev. 2010, 65, 56–79. [Google Scholar] [CrossRef]
- Seckl, J.R.; Holmes, M.C. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal ’programming’ of adult pathophysiology. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 479–488. [Google Scholar] [CrossRef]
- Ryan, J.; Mansell, T.; Fransquet, P.; Saffery, R. Does maternal mental well-being in pregnancy impact the early human epigenome? Epigenomics 2017, 9, 313–332. [Google Scholar] [CrossRef]
- Shallie, P.D.; Naicker, T. The placenta as a window to the brain: A review on the role of placental markers in prenatal programming of neurodevelopment. Int. J. Dev. Neurosci. 2019, 73, 41–49. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, J.; Huang, J.; Jia, Y.; Zhang, J.; Yan, C.; Zhang, J. Effects of prenatal and postnatal maternal emotional stress on toddlers’ cognitive and temperamental development. J. Affect. Disord. 2017, 207, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, P.D.; Entringer, S.; Buss, C.; Lu, M.C. The contribution of maternal stress to preterm birth: Issues and considerations. Clin. Perinatol. 2011, 38, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Q.; Deyssenroth, M.; Lambertini, L.; Finik, J.; Ham, J.; Huang, Y.; Tsuchiya, K.J.; Pehme, P.; Buthmann, J. Timing of prenatal exposure to trauma and altered placental expressions of hypothalamic-pituitary-adrenal axis genes and genes driving neurodevelopment. J. Neuroendocrinol. 2018, 30, e12581. [Google Scholar] [CrossRef]
- Ruffaner-Hanson, C.; Noor, S.; Sun, M.S.; Solomon, E.; Marquez, L.E.; Rodriguez, D.E.; Allan, A.M.; Caldwell, K.K.; Bakhireva, L.N.; Milligan, E.D. The maternal-placental-fetal interface: Adaptations of the HPA axis and immune mediators following maternal stress and prenatal alcohol exposure. Exp. Neurol. 2022, 355, 114121. [Google Scholar] [CrossRef] [PubMed]
- Kosicka, K.; Siemiątkowska, A.; Główka, F.K. 11β-Hydroxysteroid dehydrogenase 2 in preeclampsia. Int. J. Endocrinol. 2016, 2016, 5279462. [Google Scholar] [CrossRef]
- O’Donnell, K.J.; Jensen, A.B.; Freeman, L.; Khalife, N.; O’Connor, T.G.; Glover, V. Maternal prenatal anxiety and downregulation of placental 11β-HSD2. Psychoneuroendocrinology 2012, 37, 818–826. [Google Scholar] [CrossRef]
- Togher, K.L.; Treacy, E.; O’Keeffe, G.W.; Kenny, L.C. Maternal distress in late pregnancy alters obstetric outcomes and the expression of genes important for placental glucocorticoid signalling. Psychiatry Res. 2017, 255, 17–26. [Google Scholar] [CrossRef]
- Coussons-Read, M.E.; Okun, M.L.; Nettles, C.D. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav. Immun. 2007, 21, 343–350. [Google Scholar] [CrossRef]
- Karlsson, L.; Nousiainen, N.; Scheinin, N.M.; Maksimow, M.; Salmi, M.; Lehto, S.M.; Tolvanen, M.; Lukkarinen, H.; Karlsson, H. Cytokine profile and maternal depression and anxiety symptoms in mid-pregnancy—The FinnBrain Birth Cohort Study. Arch. Women’s Ment. Health 2017, 20, 39–48. [Google Scholar] [CrossRef]
- Glover, V. Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. In Perinatal Programming of Neurodevelopment; Springer: Berlin/Heidelberg, Germany, 2015; pp. 269–283. [Google Scholar]
- Raghupathy, R.; Kalinka, J. Cytokine imbalance in pregnancy complications and its modulation. Front. Biosci. -Landmark 2008, 13, 985–994. [Google Scholar] [CrossRef]
- Aaltonen, R.; Heikkinen, T.; Hakala, K.; Laine, K.; Alanen, A. Transfer of proinflammatory cytokines across term placenta. Obstet. Gynecol. 2005, 106, 802–807. [Google Scholar] [CrossRef]
- Izvolskaia, M.; Sharova, V.; Zakharova, L. Prenatal programming of neuroendocrine system development by lipopolysaccharide: Long-term effects. Int. J. Mol. Sci. 2018, 19, 3695. [Google Scholar] [CrossRef]
- Cory-Slechta, D.A. Enduring Behavioral and Brain Impacts of Prenatal Stress and Childhood Adversity and Their Potential Multigenerational Consequences. In Advances in Neurotoxicology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2, pp. 265–300. [Google Scholar]
- St-Pierre, J.; Laurent, L.; King, S.; Vaillancourt, C. Effects of prenatal maternal stress on serotonin and fetal development. Placenta 2016, 48, S66–S71. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Rasmussen, J.M.; Lindsay, K.; Gillen, D.L.; Cooper, D.M.; Wadhwa, P.D. Maternal cortisol during pregnancy and infant adiposity: A prospective investigation. J. Clin. Endocrinol. Metab. 2017, 102, 1366–1374. [Google Scholar] [CrossRef]
- Blakeley, P.M.; Capron, L.E.; Jensen, A.B.; O’Donnell, K.J.; Glover, V. Maternal prenatal symptoms of depression and down regulation of placental monoamine oxidase A expression. J. Psychosom. Res. 2013, 75, 341–345. [Google Scholar] [CrossRef]
- Bonnin, A.; Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef]
- Szumiel, I. Ionizing radiation-induced oxidative stress, epigenetic changes and genomic instability: The pivotal role of mitochondria. Int. J. Radiat. Biol. 2015, 91, 1–12. [Google Scholar] [CrossRef]
- Niu, Y.; DesMarais, T.L.; Tong, Z.; Yao, Y.; Costa, M. Oxidative stress alters global histone modification and DNA methylation. Free. Radic. Biol. Med. 2015, 82, 22–28. [Google Scholar] [CrossRef]
- Jašarević, E.; Rodgers, A.B.; Bale, T.L. A novel role for maternal stress and microbial transmission in early life programming and neurodevelopment. Neurobiol. Stress 2015, 1, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Talarowska, M. Epigenetic mechanisms in the neurodevelopmental theory of depression. Depress. Res. Treat. 2020, 2020, 6357873. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.; Spengler, D. Environmental factors and epigenetics of neuropsychiatric disorders. In Neuropsychiatric Disorders and Epigenetics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 9–33. [Google Scholar]
- Cao-Lei, L.; De Rooij, S.; King, S.; Matthews, S.; Metz, G.; Roseboom, T.; Szyf, M. Prenatal stress and epigenetics. Neurosci. Biobehav. Rev. 2020, 117, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Šerman, A.; Vlahović, M.; Šerman, L.; Bulić-Jakuš, F. DNA methylation as a regulatory mechanism for gene expression in mammals. Coll. Antropol. 2006, 30, 665–671. [Google Scholar] [PubMed]
- Shimada-Sugimoto, M.; Otowa, T.; Hettema, J.M. Genetics of anxiety disorders: Genetic epidemiological and molecular studies in humans. Psychiatry Clin. Neurosci. 2015, 69, 388–401. [Google Scholar] [CrossRef]
- Conradt, E.; Lester, B.M.; Appleton, A.A.; Armstrong, D.A.; Marsit, C.J. The roles of DNA methylation of NR3C1 and 11β-HSD2 and exposure to maternal mood disorder in utero on newborn neurobehavior. Epigenetics 2013, 8, 1321–1329. [Google Scholar] [CrossRef]
- Hompes, T.; Izzi, B.; Gellens, E.; Morreels, M.; Fieuws, S.; Pexsters, A.; Schops, G.; Dom, M.; Van Bree, R.; Freson, K. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. J. Psychiatr. Res. 2013, 47, 880–891. [Google Scholar] [CrossRef]
- Mansell, T.; Vuillermin, P.; Ponsonby, A.-L.; Collier, F.; Saffery, R.; Ryan, J. Maternal mental well-being during pregnancy and glucocorticoid receptor gene promoter methylation in the neonate. Dev. Psychopathol. 2016, 28, 1421–1430. [Google Scholar] [CrossRef]
- Monk, C.; Feng, T.; Lee, S.; Krupska, I.; Champagne, F.A.; Tycko, B. Distress during pregnancy: Epigenetic regulation of placenta glucocorticoid-related genes and fetal neurobehavior. Am. J. Psychiatry 2016, 173, 705–713. [Google Scholar] [CrossRef]
- Marsit, C.J.; Maccani, M.A.; Padbury, J.F.; Lester, B.M. Placental 11-beta hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS ONE 2012, 7, e33794. [Google Scholar] [CrossRef]
- Devlin, A.M.; Brain, U.; Austin, J.; Oberlander, T.F. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS ONE 2010, 5, e12201. [Google Scholar] [CrossRef]
- Sosnowski, D.W.; Booth, C.; York, T.P.; Amstadter, A.B.; Kliewer, W. Maternal prenatal stress and infant DNA methylation: A systematic review. Dev. Psychobiol. 2018, 60, 127–139. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Rialdi, A.; Mystal, E.; Ly, J.; Finik, J.; Davey, T.; Lambertini, L.; Nomura, Y. Influences of maternal stress during pregnancy on the epi/genome: Comparison of placenta and umbilical cord blood. J. Depress. Anxiety 2014, 3, 152. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Seo, M.K.; Kim, S.-g.; Seog, D.-H.; Bahk, W.-M.; Kim, S.-H.; Park, S.W.; Lee, J.G. Effects of early life stress on epigenetic changes of the glucocorticoid receptor 17 promoter during adulthood. Int. J. Mol. Sci. 2020, 21, 6331. [Google Scholar] [CrossRef]
- Deussing, J.M.; Jakovcevski, M. Histone modifications in major depressive disorder and related rodent models. Neuroepigenomics Aging Dis. 2017, 978, 169–183. [Google Scholar]
- Kumar, S.; Gonzalez, E.A.; Rameshwar, P.; Etchegaray, J.-P. Non-coding RNAs as mediators of epigenetic changes in malignancies. Cancers 2020, 12, 3657. [Google Scholar] [CrossRef]
- Miguel, V.; Lamas, S.; Espinosa-Diez, C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox Biol. 2020, 37, 101580. [Google Scholar] [CrossRef]
- Ding, X.-X.; Wu, Y.-L.; Xu, S.-J.; Zhu, R.-P.; Jia, X.-M.; Zhang, S.-F.; Huang, K.; Zhu, P.; Hao, J.-H.; Tao, F.-B. Maternal anxiety during pregnancy and adverse birth outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Affect. Disord. 2014, 159, 103–110. [Google Scholar] [CrossRef]
- Troller-Renfree, S.V.; Brito, N.H.; Desai, P.M.; Leon-Santos, A.G.; Wiltshire, C.A.; Motton, S.N.; Meyer, J.S.; Isler, J.; Fifer, W.P.; Noble, K.G. Infants of mothers with higher physiological stress show alterations in brain function. Dev. Sci. 2020, 23, e12976. [Google Scholar] [CrossRef]
- Buss, C.; Davis, E.P.; Muftuler, L.T.; Head, K.; Sandman, C.A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 2010, 35, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lu, Y.-C.; Jacobs, M.; Pradhan, S.; Kapse, K.; Zhao, L.; Niforatos-Andescavage, N.; Vezina, G.; du Plessis, A.J.; Limperopoulos, C. Association of prenatal maternal psychological distress with fetal brain growth, metabolism, and cortical maturation. JAMA Netw. Open 2020, 3, e1919940. [Google Scholar] [CrossRef] [PubMed]
- Mikšić, Š.; Uglešić, B.; Jakab, J.; Holik, D.; Milostić Srb, A.; Degmečić, D. Positive effect of breastfeeding on child development, anxiety, and postpartum depression. Int. J. Environ. Res. Public Health 2020, 17, 2725. [Google Scholar] [CrossRef] [PubMed]
- Di Benedetto, M.G.; Bottanelli, C.; Cattaneo, A.; Pariante, C.M.; Borsini, A. Nutritional and immunological factors in breast milk: A role in the intergenerational transmission from maternal psychopathology to child development. Brain Behav. Immun. 2020, 85, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Serim Demirgoren, B.; Ozbek, A.; Ormen, M.; Kavurma, C.; Ozer, E.; Aydın, A. Do mothers with high sodium levels in their breast milk have high depression and anxiety scores? J. Int. Med. Res. 2017, 45, 843–848. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shin, H.-S.; Lee, W.-H. Impact of endocrine-disrupting chemicals in breast milk on postpartum depression in Korean mothers. Int. J. Environ. Res. Public Health 2021, 18, 4444. [Google Scholar] [CrossRef]
- Shariat, M.; Abedinia, N.; Rezaei, N.; Farrokhzad, N. Increase concentration of transforming growth factor beta (TGF-β) in breast milk of mothers with psychological disorders. Acta Med. Iran. 2017, 55, 429–436. [Google Scholar]
- Kang, L.J.; Koleva, P.T.; Field, C.J.; Giesbrecht, G.F.; Wine, E.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Subbarao, P.; Sears, M.R. Maternal depressive symptoms linked to reduced fecal Immunoglobulin A concentrations in infants. Brain Behav. Immun. 2018, 68, 123–131. [Google Scholar] [CrossRef]
- Kawano, A.; Emori, Y. The relationship between maternal postpartum psychological state and breast milk secretory immunoglobulin A level. J. Am. Psychiatr. Nurses Assoc. 2015, 21, 23–30. [Google Scholar] [CrossRef]
- Moirasgenti, M.; Doulougeri, K.; Panagopoulou, E.; Theodoridis, T. Psychological stress reduces the immunological benefits of breast milk. Stress Health 2019, 35, 681–685. [Google Scholar] [CrossRef]
- Aparicio, M.; Browne, P.D.; Hechler, C.; Beijers, R.; Rodríguez, J.M.; de Weerth, C.; Fernández, L. Human milk cortisol and immune factors over the first three postnatal months: Relations to maternal psychosocial distress. PLoS ONE 2020, 15, e0233554. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Apanasewicz, A.; Danel, D.P.; Babiszewska, M.; Piosek, M.; Orczyk-Pawiłowicz, M. Maternal distress and social support are linked to human milk immune properties. Nutrients 2021, 13, 1857. [Google Scholar] [CrossRef]
- Lauritzen, L.a. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog. Lipid Res. 2001, 40, 1–94. [Google Scholar] [CrossRef]
- Keim, S.A.; Daniels, J.L.; Siega-Riz, A.M.; Dole, N.; Herring, A.H.; Scheidt, P.C. Depressive symptoms during pregnancy and the concentration of fatty acids in breast milk. J. Hum. Lact. 2012, 28, 189–195. [Google Scholar] [CrossRef]
- Ziomkiewicz, A.; Babiszewska, M.; Apanasewicz, A.; Piosek, M.; Wychowaniec, P.; Cierniak, A.; Barbarska, O.; Szołtysik, M.; Danel, D.; Wichary, S. Psychosocial stress and cortisol stress reactivity predict breast milk composition. Sci. Rep. 2021, 11, 11576. [Google Scholar] [CrossRef]
- Abou-Saleh, M.T.; Ghubash, R.; Karim, L.; Krymski, M.; Bhai, I. Hormonal aspects of postpartum depression. Psychoneuroendocrinology 1998, 23, 465–475. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Grewen, K.; Meltzer-Brody, S. Association between maternal mood and oxytocin response to breastfeeding. J. Women’s Health 2013, 22, 352–361. [Google Scholar] [CrossRef]
- Vass, R.A.; Kiss, G.; Bell, E.F.; Roghair, R.D.; Miseta, A.; Bódis, J.; Funke, S.; Ertl, T. Breast Milk for Term and Preterm Infants—Own Mother’s Milk or Donor Milk? Nutrients 2021, 13, 424. [Google Scholar] [CrossRef]
- De Weerth, C.; Aatsinki, A.-K.; Azad, M.B.; Bartol, F.F.; Bode, L.; Collado, M.C.; Dettmer, A.M.; Field, C.J.; Guilfoyle, M.; Hinde, K. Human milk: From complex tailored nutrition to bioactive impact on child cognition and behavior. Crit. Rev. Food Sci. Nutr. 2022, 1–38. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Slupsky, C.M.; Aatsinki, A.-K.; Sinkkonen, J.; Karlsson, L.; Linderborg, K.M.; Yang, B.; Karlsson, H.; Kailanto, H.-M. Human milk metabolome is associated with symptoms of maternal psychological distress and milk cortisol. Food Chem. 2021, 356, 129628. [Google Scholar] [CrossRef]
- Newman, L.; Sivaratnam, C.; Komiti, A. Attachment and early brain development–neuroprotective interventions in infant–Caregiver therapy. Transl. Dev. Psychiatry 2015, 3, 28647. [Google Scholar] [CrossRef]
- Cooke, J.E.; Racine, N.; Plamondon, A.; Tough, S.; Madigan, S. Maternal adverse childhood experiences, attachment style, and mental health: Pathways of transmission to child behavior problems. Child Abus. Negl. 2019, 93, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Provençal, N.; Binder, E.B. The effects of early life stress on the epigenome: From the womb to adulthood and even before. Exp. Neurol. 2015, 268, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Blackmore, E.R.; Gustafsson, H.; Gilchrist, M.; Wyman, C.; O’Connor, T.G. Pregnancy-related anxiety: Evidence of distinct clinical significance from a prospective longitudinal study. J. Affect. Disord. 2016, 197, 251–258. [Google Scholar] [CrossRef]
- Ibanez, G.; Charles, M.-A.; Forhan, A.; Magnin, G.; Thiebaugeorges, O.; Kaminski, M.; Saurel-Cubizolles, M.-J.; Group, E.M.C.C.S. Depression and anxiety in women during pregnancy and neonatal outcome: Data from the EDEN mother–child cohort. Early Hum. Dev. 2012, 88, 643–649. [Google Scholar] [CrossRef]
- Lobel, M.; Cannella, D.L.; Graham, J.E.; DeVincent, C.; Schneider, J.; Meyer, B.A. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008, 27, 604. [Google Scholar] [CrossRef]
- Rifkin-Graboi, A.; Meaney, M.J.; Chen, H.; Bai, J.; Hameed, W.B.r.; Tint, M.T.; Broekman, B.F.; Chong, Y.-S.; Gluckman, P.D.; Fortier, M.V. Antenatal maternal anxiety predicts variations in neural structures implicated in anxiety disorders in newborns. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 313–321 e2. [Google Scholar] [CrossRef]
- King, S.; Dancause, K.; Turcotte-Tremblay, A.M.; Veru, F.; Laplante, D.P. Using natural disasters to study the effects of prenatal maternal stress on child health and development. Birth Defects Res. Part C Embryo Today Rev. 2012, 96, 273–288. [Google Scholar] [CrossRef]
- Manzari, N.; Matvienko-Sikar, K.; Baldoni, F.; O’Keeffe, G.W.; Khashan, A.S. Prenatal maternal stress and risk of neurodevelopmental disorders in the offspring: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2019, 54, 1299–1309. [Google Scholar] [CrossRef]
- Simcock, G.; Laplante, D.P.; Elgbeili, G.; Kildea, S.; Cobham, V.; Stapleton, H.; King, S. Infant neurodevelopment is affected by prenatal maternal stress: The QF 2011 Queensland Flood Study. Infancy 2017, 22, 282–302. [Google Scholar] [CrossRef]
- Brouwers, E.P.; van Baar, A.L.; Pop, V.J. Maternal anxiety during pregnancy and subsequent infant development. Infant Behav. Dev. 2001, 24, 95–106. [Google Scholar] [CrossRef]
- Buitelaar, J.K.; Huizink, A.C.; Mulder, E.J.; De Medina, P.G.R.; Visser, G.H. Prenatal stress and cognitive development and temperament in infants. Neurobiol. Aging 2003, 24, S53–S60. [Google Scholar] [CrossRef]
- Laplante, D.P.; Hart, K.J.; O’Hara, M.W.; Brunet, A.; King, S. Prenatal maternal stress is associated with toddler cognitive functioning: The Iowa Flood Study. Early Hum. Dev. 2018, 116, 84–92. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, M.S.; Hao, J.H.; Chen, Y.J.; Jiang, X.M.; Tao, R.X.; Huang, K.; Tao, F.B. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev. Med. Child Neurol. 2014, 56, 283–289. [Google Scholar] [CrossRef]
- Keim, S.A.; Daniels, J.L.; Dole, N.; Herring, A.H.; Siega-Riz, A.M.; Scheidt, P.C. A prospective study of maternal anxiety, perceived stress, and depressive symptoms in relation to infant cognitive development. Early Hum. Dev. 2011, 87, 373–380. [Google Scholar] [CrossRef]
- Li, J.; Robinson, M.; Malacova, E.; Jacoby, P.; Foster, J.; Van Eekelen, A. Maternal life stress events in pregnancy link to children’s school achievement at age 10 years. J. Pediatrics 2013, 162, 483–489. [Google Scholar] [CrossRef]
- Porter, E.; Lewis, A.J.; Watson, S.J.; Galbally, M. Perinatal maternal mental health and infant socio-emotional development: A growth curve analysis using the MPEWS cohort. Infant Behav. Dev. 2019, 57, 101336. [Google Scholar] [CrossRef]
- Van den Heuvel, M.; Johannes, M.; Henrichs, J.; Van den Bergh, B. Maternal mindfulness during pregnancy and infant socio-emotional development and temperament: The mediating role of maternal anxiety. Early Hum. Dev. 2015, 91, 103–108. [Google Scholar] [CrossRef]
- Nolvi, S.; Karlsson, L.; Bridgett, D.J.; Korja, R.; Huizink, A.C.; Kataja, E.-L.; Karlsson, H. Maternal prenatal stress and infant emotional reactivity six months postpartum. J. Affect. Disord. 2016, 199, 163–170. [Google Scholar] [CrossRef]
- Olulade, O.A.; Seydell-Greenwald, A.; Chambers, C.E.; Turkeltaub, P.E.; Dromerick, A.W.; Berl, M.M.; Gaillard, W.D.; Newport, E.L. The neural basis of language development: Changes in lateralization over age. Proc. Natl. Acad. Sci. USA 2020, 117, 23477–23483. [Google Scholar] [CrossRef] [PubMed]
- Laplante, D.P.; Brunet, A.; King, S. The effects of maternal stress and illness during pregnancy on infant temperament: Project Ice Storm. Pediatric Res. 2016, 79, 107–113. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.; Boivin, J.; Gibson, F.; Hammarberg, K.; Wynter, K.; Saunders, D.; Fisher, J. Pregnancy-specific anxiety, ART conception and infant temperament at 4 months post-partum. Hum. Reprod. 2013, 28, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Dubber, S.; Reck, C.; Müller, M.; Gawlik, S. Postpartum bonding: The role of perinatal depression, anxiety and maternal–Fetal bonding during pregnancy. Arch. Women’s Ment. Health 2015, 18, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Otte, R.; Donkers, F.; Braeken, M.; Van den Bergh, B. Multimodal processing of emotional information in 9-month-old infants II: Prenatal exposure to maternal anxiety. Brain Cogn. 2015, 95, 107–117. [Google Scholar] [CrossRef]
- Mughal, M.K.; Giallo, R.; Arnold, P.; Benzies, K.; Kehler, H.; Bright, K.; Kingston, D. Trajectories of maternal stress and anxiety from pregnancy to three years and child development at 3 years of age: Findings from the All Our Families (AOF) pregnancy cohort. J. Affect. Disord. 2018, 234, 318–326. [Google Scholar] [CrossRef]
- Kikkert, H.K.; Middelburg, K.J.; Hadders-Algra, M. Maternal anxiety is related to infant neurological condition, paternal anxiety is not. Early Hum. Dev. 2010, 86, 171–177. [Google Scholar] [CrossRef]
- Cao, X.; Laplante, D.P.; Brunet, A.; Ciampi, A.; King, S. Prenatal maternal stress affects motor function in 5½-year-old children: Project Ice Storm. Dev. Psychobiol. 2014, 56, 117–125. [Google Scholar] [CrossRef]
- Grace, T.; Bulsara, M.; Robinson, M.; Hands, B. The impact of maternal gestational stress on motor development in late childhood and adolescence: A longitudinal study. Child Dev. 2016, 87, 211–220. [Google Scholar] [CrossRef]
- Hansen, B.H.; Oerbeck, B.; Skirbekk, B.; Petrovski, B.É.; Kristensen, H. Neurodevelopmental disorders: Prevalence and comorbidity in children referred to mental health services. Nord. J. Psychiatry 2018, 72, 285–291. [Google Scholar] [CrossRef]
- Say, G.N.; Karabekiroğlu, K.; Babadağı, Z.; Yüce, M. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatrics Int. 2016, 58, 265–269. [Google Scholar] [CrossRef]
- Class, Q.A.; Abel, K.M.; Khashan, A.S.; Rickert, M.E.; Dalman, C.; Larsson, H.; Hultman, C.M.; Långström, N.; Lichtenstein, P.; D‘Onofrio, B.M. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychol. Med. 2014, 44, 71–84. [Google Scholar] [CrossRef]
- Li, J.; Olsen, J.; Vestergaard, M.; Obel, C. Attention-deficit/hyperactivity disorder in the offspring following prenatal maternal bereavement: A nationwide follow-up study in Denmark. Eur. Child Adolesc. Psychiatry 2010, 19, 747–753. [Google Scholar] [CrossRef]
- Walder, D.J.; Laplante, D.P.; Sousa-Pires, A.; Veru, F.; Brunet, A.; King, S. Prenatal maternal stress predicts autism traits in 6½ year-old children: Project Ice Storm. Psychiatry Res. 2014, 219, 353–360. [Google Scholar] [CrossRef]
- Zhu, P.; Hao, J.-H.; Tao, R.-X.; Huang, K.; Jiang, X.-M.; Zhu, Y.-D.; Tao, F.-B. Sex-specific and time-dependent effects of prenatal stress on the early behavioral symptoms of ADHD: A longitudinal study in China. Eur. Child Adolesc. Psychiatry 2015, 24, 1139–1147. [Google Scholar] [CrossRef]
- Varcin, K.J.; Alvares, G.A.; Uljarević, M.; Whitehouse, A.J. Prenatal maternal stress events and phenotypic outcomes in Autism Spectrum Disorder. Autism Res. 2017, 10, 1866–1877. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeličić, L.; Veselinović, A.; Ćirović, M.; Jakovljević, V.; Raičević, S.; Subotić, M. Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 13932. https://doi.org/10.3390/ijms232213932
Jeličić L, Veselinović A, Ćirović M, Jakovljević V, Raičević S, Subotić M. Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes—A Narrative Review. International Journal of Molecular Sciences. 2022; 23(22):13932. https://doi.org/10.3390/ijms232213932
Chicago/Turabian StyleJeličić, Ljiljana, Aleksandra Veselinović, Milica Ćirović, Vladimir Jakovljević, Saša Raičević, and Miško Subotić. 2022. "Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes—A Narrative Review" International Journal of Molecular Sciences 23, no. 22: 13932. https://doi.org/10.3390/ijms232213932
APA StyleJeličić, L., Veselinović, A., Ćirović, M., Jakovljević, V., Raičević, S., & Subotić, M. (2022). Maternal Distress during Pregnancy and the Postpartum Period: Underlying Mechanisms and Child’s Developmental Outcomes—A Narrative Review. International Journal of Molecular Sciences, 23(22), 13932. https://doi.org/10.3390/ijms232213932






