Abstract
Previous studies have indicated that Brca1 (Breast cancer suppressor gene 1) plays an important role in neural development and degenerative diseases. However, the bioactivity and regulatory mechanism of Brca1 expression in retinal neurocytes remain unclear. In the present study, our data indicated that Brca1 maintains the state of neuronal precursor cells. Brca1 silencing induces differentiation in 661W cells. Nestin, a marker of precursor cells, was significantly decreased in parallel with Brca1 silencing in 661W cells, whereas Map2 (Microtubule associated protein 2), a marker of differentiated neurons, was significantly increased. Neurite outgrowth was increased by ~4.0-fold in Brca1-silenced cells. Moreover, DNA affinity purification assays and ChIP assays demonstrated that Gata3 (GATA binding protein 3) regulates Brca1 transcription in 661W cells. Silencing or overexpressing Gata3 could significantly regulate the expression of Brca1 and affect its promoter inducibility. Furthermore, the expression of Gata3 generally occurred in parallel with that of Brca1 in developing mouse retinas. Both Gata3 and Brca1 are expressed in the neonatal mouse retina but are developmentally silenced with age. Exogenous Gata3 significantly inhibited neural activity by decreasing synaptophysin and neurite outgrowth. Thus, this study demonstrated that Brca1 is transcriptionally regulated by Gata3. Brca1/Gata3 silencing is involved in neuronal differentiation and maturation.
1. Introduction
Brca1, the breast cancer susceptibility gene, contains an N-terminal RING domain, nuclear localization signals (NLS) and two C-terminal BRCT domains [1]. A growing body of literature has demonstrated that Brca1 functions in tumor suppression via DNA repair, genomic stability, cell cycle and transcriptional regulation during tumorigenesis and development [2,3,4]. Patients with mutations in Brca1 are prone to developing breast and ovarian cancer [5,6]. Thus, Brca1 has been identified as a target gene in precision medicine [7]. Moreover, the transcriptional mechanism of Brca1 is well defined in tumor cells. However, the bioactivity and regulatory mechanism of Brca1 expression in the retina remain unclear.
Although studies of Brca1 in neural science are limited, much evidence indicates that Brca1 may play an important role in neural development. Based on an analysis of in silico network construction in 2008, Bromberg et al. indicated that Brca1 functions as a “hanger” in neural development [8]. The expression of Brca1 declines with developmental stage progression [9]. Moreover, conditional knockout of Brca1 with a neural progenitor-specific driver could induce early apoptosis and reduce brain volume [10]. However, Brca1 bioactivity is self-contradictory in neurodegenerative diseases. For example, Brca1 can rescue neurons from cerebral ischemia/reperfusion injury [11]. In contrast, under pathological conditions, Brca1 is abnormally colocalized with Tau in neurofibrillary tangles, a hallmark lesion of Alzheimer’s disease. Thus, these studies suggested that overexpression of Brca1 was associated with proapoptotic phenotypes and ultimately resulted in neuron death [12,13]. Moreover, Evans et al. suggested that Brca1 might initiate re-entry into the cell cycle of neurons [12]. Thus, Brca1 may play various roles in different physiological conditions and stages. Its exact role in the retina still requires investigation.
The transcriptional mechanism of Brca1 has also been well documented in tumor tissues. Brca1 is regulated by methylation (DNA, histone), sumoylation or hypoxic conditions. The methylation status of the white blood cell Brca1 promoter could be a predictive biomarker of ovarian cancer [14]. Sumoylation by SUMO1 negatively regulates Brca1 transcription via modulation of promoter occupancy [15]. Conditions mimicking the tumor microenvironment, such as hypoxia, also silenced Brca1 expression [16,17]. However, the mechanism of Brca1 transcription in retinal neurocytes is not well defined. Our previous studies have demonstrated that the DNA demethylation agent 5-Aza could upregulate Brca1 expression in postnatal rat retinal neurons [18]. However, we did not find a methylated site in the Brca1 promoter through bisulfite sequencing. Thus, more investigation is required to reveal the transcriptional mechanism of Brca1 expression in retinal neurocytes.
To address these questions, we investigated Brca1 bioactivity during proliferation, differentiation and neurite outgrowth using cell lines and primary retinal cells coupled with gene interference. Transcription factors regulating Brca1 expression were identified using DNA affinity purification, ChIP and promoter inducibility assays [19]. Thus, this study reveals the bioactivity and transcriptional mechanism of Brca1 expression in retinal neurocytes.
2. Results
2.1. Downregulation of Brca1 Promotes Differentiation of Retinal Precursor Cells
The 661W cell, a cone-photoreceptor-specific expressing precursor-like cell line, can be used as an ideal tool for investigating the differentiation of retinal neurocytes [20,21]. As shown in Figure 1A, 661W cells were characterized as having characteristics of stem cells. Nestin (red), a marker of precursor cells, was expressed in the cytoplasm, and Brca1 was expressed in the nuclei of 661W cells; there was no obvious staining for Map2, a marker of neurons.
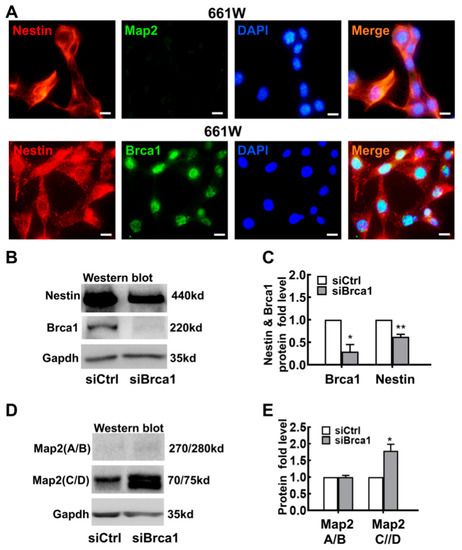
Figure 1.
Brca1 silencing induces differentiation of 661W cells. (A) Strong Nestin expression (red) and faint Map2 expression were observed in the cell cytoplasm of 661W cells. Brca1 was expressed at high levels in the cellular nuclei of 661W cells (green). The nuclei were stained with DAPI (blue). (B) Nestin expression was decreased in Brca1-silenced 661W cells, as evidenced by Western blot. (C) The relative expression levels of Nestin and Brca1 in 661W cells are presented as a histogram. (D) Brca1 silencing significantly increased the expression of Map2 (C/D) in 661W cells. (E) The relative protein expression levels of Map2 (A/B) and Map2 (C/D) are presented as histograms. Scale bars represent 10 μm. The asterisks indicate statistically significant differences (* p < 0.05, ** p < 0.01).
To reveal its bioactivity in retinal progenitor cells, Brca1 was silenced by siRNA interference. After 48 h of treatment with siBrca1 or siControl, total protein was extracted from 661W cells and measured by Western blot. As shown in Figure 1B,C, Brca1 silencing (Brca1: siControl, 1; siBrca1 0.29 ± 0.15; * p < 0.05) significantly decreased the expression of Nestin in 661W cells (Nestin: siControl, 1; siBrca1 0.627 ± 0.05; ** p < 0.01), which implies that Brca1 is upstream of Nestin. Map2 can be alternatively spliced into multiple isoforms in neural cells. The high-molecular-weight isoform Map2 A/B functions in neuronal maturation, and the low-molecular-weight isoform Map2 C/D promotes neurogenesis and neurite initiation. As shown in Figure 1D,E, the expression of Map2 (C/D) was significantly increased in Brca1-silenced 661W cells compared with control cells (C/D: siControl, 1; siBrca1, 1.79 ± 0.19; * p < 0.05), which indicates downregulation of Brca1 mainly promotes neural differentiation but not maturation.
To observe changes in cell shape following treatment with siBrca1, the cells were double stained with anti-Map2 and anti-Nestin antibodies. As shown in Figure 2A, Map2 (green) was expressed in 661W cells treated with siBrca1. Fully Map2-positive cells had a smaller soma and longer dendrites (green, white arrow). In contrast, little Nestin expression was observed in differentiated cells with developed neurites (red, white arrowhead). Further experiments confirmed these results via double staining with anti-Brca1 and anti-Map2/anti-Nestin in Brca1-silenced cells. Brca1 silencing significantly promoted retinal neurite outgrowth in 661W cells (siControl, 1.16 ± 0.11; siBrca1, 4.31 ± 0.14; ** p < 0.01) (Figure 2B). Accordingly, Brca1 silencing significantly inhibited cell viability (siControl, 1; siBrca1, 0.65 ± 0.06; ** p < 0.01) (Figure 2C). Collectively, these data indicate that Brca1 is involved in maintaining neural precursor status and that its silencing promotes neuronal differentiation.
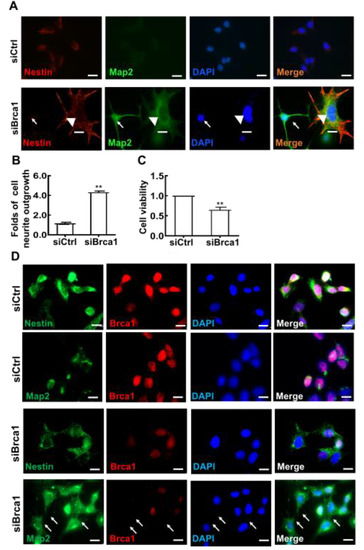
Figure 2.
Brca1 is involved in the differentiation of 661W cells. (A,D), Brca1 interference decreased Nestin expression (red, cell cytoplasm) while increasing Map2 expression in 661W cells (green, cell cytoplasm). The cell nuclei were stained with DAPI (blue). The white arrows indicate dendrites or neurites stained by Map2, the white arrowhead indicates neurite stained by Nestin. (B) Brca1 silencing promoted retinal neurite outgrowth. Scale bars represent 20 μm. (C) Brca1 interference by siRNA inhibited the viability of 661W cells. The asterisks indicate statistically significant differences (** p < 0.01).
2.2. Brca1 Is Transcriptionally Regulated by Gata3 In Vitro
To reveal the transcriptional mechanisms involved in regulating Brca1 expression in retinal neurons, DNA affinity purification assays were performed to investigate the proteins that bind to the Brca1 promoter. The experimental procedure is summarized in Figure 3A. Several bands (asterisks) appeared only in the presence of the Brca1 promoter (+) and disappeared in the absence of the Brca1 promoter (−) due to the higher NaCl elutions (Figure 3B). These bands containing proteins that bound the Brca1 promoter with high affinity and specificity were digested and identified by mass spectrometry. The following proteins were identified: Gata3, Twist 1, ZFP521 and SFPQ, among others (Figure 3C). As reported previously, these proteins are known to be transcription regulators that bind to other promoters [22,23,24,25]. Among them, Gata3 is characterized as an important transcription factor involved in central nervous system development [26]. Moreover, Gata3 was strongly expressed and colocalized with Brca1 in the nucleus of 661W cells (white arrows), as evidenced by immunofluorescence assays (Figure 3D).
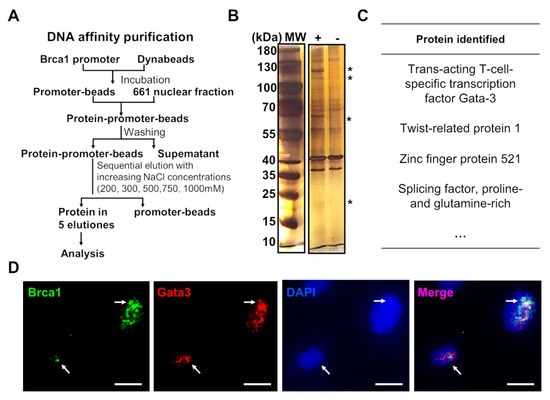
Figure 3.
The transcription mechanisms of Brca1 expression. (A) A summary of the experimental procedures used for DNA affinity purification assays. (B) Several bands specifically appeared (asterisk) in the presence of the Brca1 promoter (+), but disappeared in the absence of the Brca1 promoter (-). (C) Proteins that might be related to transcriptional mechanisms were identified by mass spectrometry. (D) Gata3 (red) is strongly expressed and colocalized with Brca1 (green) in the cellular nuclei of 661W cells. Scale bars represent 10 μm. The asterisks indicate statistically significant differences (*p < 0.05, ** p < 0.01). The white aroows indicate colocalization of Brca1 and Gata3 in the nucleus.
To further explore the potential relationship between Gata3 and Brca1, 661W cells were transfected with either Gata3 siRNA (siGata3) or the plasmid pFLAG-Gata3; control cells were transfected with either scramble siRNA (siControl) or the empty vector, pcDNA3.1. At 24 h after transfection, total protein was extracted and measured by Western blot assay. As shown in Figure 4A,B, Gata3 expression was significantly downregulated by Gata3 siRNA transfection compared with that of the control siRNA (siControl, 1; siGata3, 0.33 ± 0.10; ** p < 0.01) (Figure 4B). Consistently, the expression of Brca1 was also significantly decreased in Gata3-silenced 661W cells (siControl, 1; siGata3, 0.57 ± 0.21; * p < 0.05) (Figure 4A,B). In contrast, the expression of Brca1 was increased in parallel with the upregulation of Gata3 in the 661W cells transfected with the pFLAG-Gata3 plasmid (Figure 4C). The relative protein expression levels of Gata3 and Brca1 in 661W cells are presented as a histogram, demonstrating that Gata3 expression was upregulated by 10.8-fold (10.80 ± 3.50, * p < 0.05) after pFLAG-Gata3 plasmid transfection and, in parallel, Brca1 expression was also increased by 1.57-fold (1.57 ± 0.14, ** p < 0.01) (Figure 4D). The inconsistency in the ratio of their increased expression implies that Gata3 is a transcription factor that regulates Brca1.
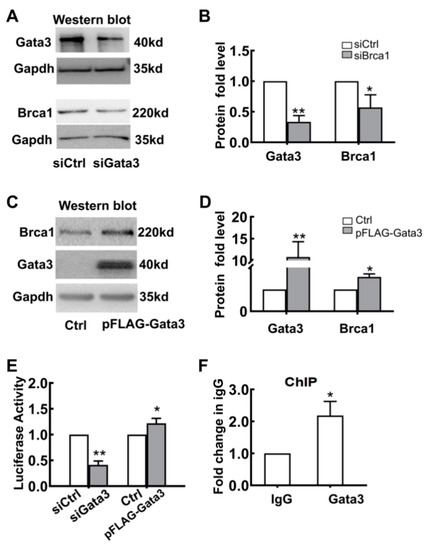
Figure 4.
Brca1 is transcriptionally regulated by Gata3. (A) Brca1 expression decreased in the Gata3-silenced 661W cells, as evidenced by Western blot. (B) The relative protein expression levels of Gata3 and Brca1 are presented as histograms. (C) Exogenous Gata3 significantly increased Brca1 expression in 661W cells. (D) The relative protein expression levels of Gata3 and Brca1 are presented as histograms. (E) The luciferase activity of the Brca1 promoter decreased with the downregulation of Gata3 but increased with the upregulation of Gata3 in 661W cells. (F) Gata3 might bind to the Brca1 promoter and regulate its activity, as evidenced by ChIP assays. The asterisks indicate statistically significant differences (* p < 0.05, ** p < 0.01).
To confirm whether Gata3 directly regulates the transcription of Brca1, luciferase reporter and ChIP assays were used. Gata3-silenced or Gata3-overexpressing 661W cells were cotransfected with pCMV-RL-pFLAG-Gata3 or pcDNA (control). The levels of luciferase activity were measured and normalized to Renilla luciferase. As shown in Figure 4E, the luciferase activity of the Brca1 promoter decreased by approximately 60% with the downregulation of Gata3 expression (siControl, 1; siGata3, 0.41 ± 0.08; ** p < 0.01) and increased by approximately 22% with the upregulation of Gata3 expression in 661W cells (Control, 1; pFLAG-Gata3, 1.22 ± 0.1; * p < 0.05). Moreover, the binding status of Gata3 to the Brca1 promoter in 661W cells was detected by ChIP assays using an antibody against Gata3 and a control normal rabbit IgG antibody. Our data showed that, compared with mock precipitation, there was an approximately 2.2-fold greater enrichment for the Brca1 promoter with the Gata3 antibody (IgG, 1; anti-Gata3, 2.18 ± 0.44; * p < 0.05), suggesting that Gata3 binds to the Brca1 promoter in vitro (Figure 4F). Thus, this evidence strongly suggests that Gata3 may bind to the Brca1 promoter and regulate its activity, eventually altering the expression of the Brca1 protein.
2.3. Gata3 Silencing Promoted Cell Differentiation Similar to Brca1 Silencing in 661W Cells
Since Gata3 positively regulated the expression of Brca1, Gata3 may also be involved in neuron differentiation. To verify this hypothesis, control and Gata3-silenced 661w cells were double stained with anti-Nestin and anti-Map2. As shown in Figure 5A, consistent with our previous study, Nestin was strongly expressed in control 661W cells (red), whereas the Map2 signal was very weak (green) [27]. By contrast, weak Nestin and strong Map2 staining were observed in Gata3-silenced 661W cells. In addition, unlike the control cells that appeared ovular and had elongated central axes with no distinct neuritis, the Gata3-silenced 661W cells presented neurite outgrowth and displayed primary and secondary branching (white arrowheads). Nestin was weakly expressed in these neurite outgrowths (white arrows). Similar to Brca1 silencing, silencing Gata3 significantly reduced cell viability (siControl, 1; siGata3, 0.51 ± 0.05; ** p < 0.01) (Figure 5B). Silencing Brca1 or Gata3 induced synaptophysin expression in 661W cells (Figure 5C). This evidence indicates that the level of cell differentiation is correlated with the level of Gata3 expression in 661W cells, further confirming the role of Gata3 in cell differentiation.
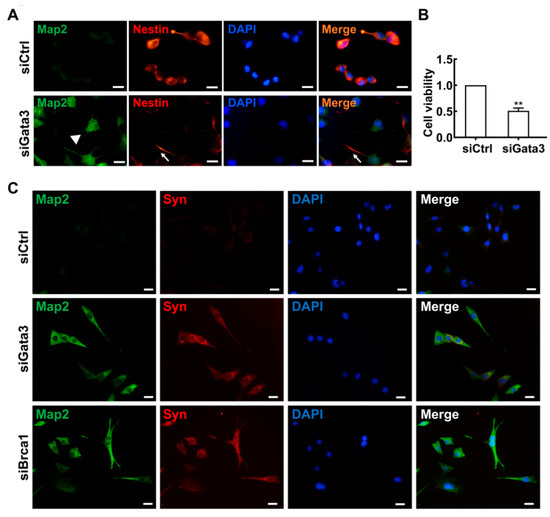
Figure 5.
Gata3 inhibition induces cell differentiation in 661W cells. (A) Strong Nestin (red) and weak Map2 (green) expression were observed in the 661W cells. In Gata3-silenced 661W cells, Nestin expression was decreased, and Map2 expression was observed. The white arrow indicates neurite outgrowth stained by Nestin, the white arrowhead indicates neurite outgrowth stained by Map2. (B) Gata3 inhibition decreased the viability of 661W cells. (C), Both Gata3 silencing and Brca1 silencing induce synaptophysin (red, cell cytoplasm) expression in 661W cells. Scale bars represent 20 μm. The asterisks indicate statistically significant differences (** p < 0.01).
2.4. The Expression of Gata3 in Developing Mouse Retinas Generally Occurs in Parallel with That of Brca1 in Precursor-like Cells
To further verify our in vitro results, we quantitatively examined the protein expression patterns of Brca1 and Gata3 in the mouse retina by Western blot. The relative intensities of the bands obtained by Western blot were quantified by densitometry and normalized to Tubulin levels. As shown in Figure 6A,B, the expression of both Brca1 and Gata3 was significantly induced after birth, with intense bands detected in the lysates of P1 mouse retinas; however, very weak expression was observed in adult mouse retinas (Brca1: P1, 0.316 ± 0.187; adult: 0.041 ± 0.007; * p < 0.05; Gata3: P1, 0.848 ± 0.272; adult, 0.293 ± 0.02; ** p < 0.070) (Figure 6A,B).
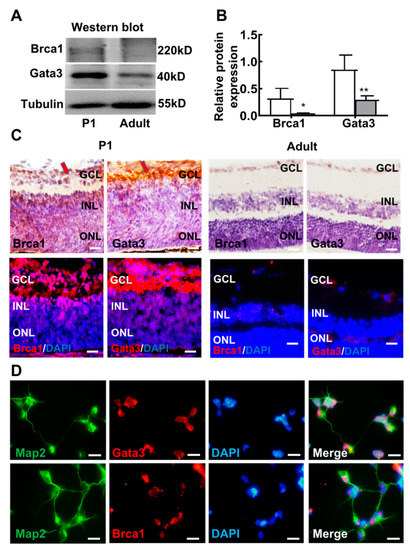
Figure 6.
The expression profile of Gata3 generally parallels that of Brca1 in the retina. (A) Both Gata3 and Brca1 are developmentally downregulated in the mouse retina in vivo, as evidenced by Western blot. (B) The relative protein expression levels of Gata3 and Brca1 are presented as histograms. (C) The expression and localization profiles of Gata3 are coincident with that of Brca1 in the mouse retina, as evidenced by immunohistochemistry and immunofluorescence assays, Gata3 and Brca1 were stained as red in immunofluorescence. (D) Map2 (green, cell cytoplasm) marks neurons, both Brca1 (above, red, cell nuclei) and Gata3 (below, red, cell nuclei) were expressed in P1 retinal neurons. Scale bars represent 20 μm. The asterisks indicate statistically significant differences (* p < 0.05, ** p < 0.01).
Moreover, the localization profiles of Brca1 and Gata3 in the mouse retina were analyzed by immunohistochemistry and immunofluorescence (Figure 6C. Brca1 was predominantly distributed in the ganglion layer (GCL) and inner nuclear layer (INL) of the immature P1 neonatal mouse retina. Slight immunoreactive cells were observed in the GCL and INL of the differentiated retina neurons of adult mice. The expression and localization profiles of Gata3 were coincident with those of Brca1 in the mouse retina. Taken together, this evidence reveals similar expression patterns between Nestin, Brca1 and Gata3 in the mouse retina, further suggesting a regulatory relationship in the differentiation and maturation of retinal neurons.
Primary retinal neurons (P1) were double stained with anti-Map2 and anti-Gata3/anti-Brca1. Figure 6D shows that Gata3 and Brca1 were expressed in P1 retinal neurons. Map2 (green) was located in neural outgrowths and the cytoplasm, whereas Gata3 and Brca1 (red) were located in the nuclei of retinal neurons.
2.5. Brca1 Might Be Transcriptionally Regulated by Gata3 in Primary Retinal Neurocytes In Vitro
Since the Brca1/Gata3 pathway is involved in the differentiation of retinal cell lines, we further confirmed its role in primary retinal neurocytes. Primary retinal neurocytes (P1) were infected with AAV-Re-Gata3-GFP or AAV-Re-GFP adenovirus. At 5 days after infection, GFP was highly expressed in the cells (Figure 7A). Western blot assays indicated that a more than 60-fold increase in Gata3 expression was observed in primary retinal cells infected with AAV-Re-Gata3-GFP compared with that of cells infected with AAV-Re-GFP (AAV-Re-GFP, 1; AAV-Re-Gata3-GFP, 61.76 ± 10.65; ** p = 0.01) (Figure 7B,C). In parallel, Brca1 expression was mildly increased (AAV-Re-GFP, 1; AAV-Re-Gata3-eGFP, 1.35 ± 0.10; * p < 0.05) (Figure 7C). Thus, these data further indicate that Gata3 might be a transcription factor that regulates the expression of Brca1.
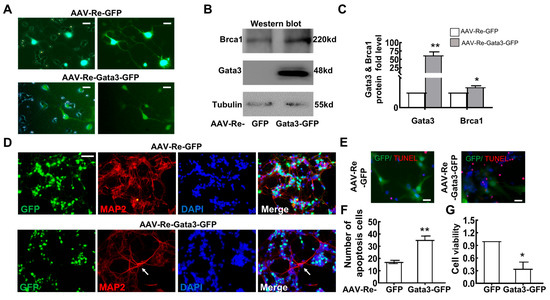
Figure 7.
Brca1 might be transcriptionally regulated by Gata3 in vitro. (A) Primary retinal neurocytes (P1) were infected with AAV-Re-Gata3-GFP (green) or AAV-Re-GFP (green) adenoviruses. (B) AAV-Re-Gata3-GFP transfection upregulated the expression of not only Gata3 but also Brca1 in primary retinal neurocytes. (C) The relative protein expression levels of Gata3 and Brca1 are presented as histograms. (D) Gata3 overexpression impeded differentiation in primary retinal neurocytes, as evidenced by staining with anti-Map2 (red) in primary retinal neurocytes treated as above (green). (E) The number of apoptotic cells is presented as a histogram. (F) Exogenous Gata3 significantly reduced the viability of primary retinal neurocytes. The asterisks indicate statistically significant differences (* p < 0.05, ** p < 0.01). Scale bars represent 20 μm.
To confirm the impact of Gata3 overexpressing on retinal neurocytes, staining with anti-Map2 in primary retinal neurocytes exposed to AAV infection was performed. As shown in Figure 7C, primary retinal neurocytes infected with AAV-Re-GFP exhibit strong Map2 expression and normal neurite growth. In contrast, AAV-Re-Gata3-GFP infection induces weak Map2 expression and limited neurite growth in GFP-positive neurocytes, while the GFP-negative neurons show strong Map2 staining and distinct neuritis (White arrow). Our data indicate that Gata3-overexpressing impedes differentiation of retinal neurocytes.
Moreover, we found that overexpressing Gata3 also induced cell death of primary retinal neurons. The cells were analyzed at 5 days after transfection by TUNEL assays. As shown in Figure 7D,E, overexpressing Gata3 induced a significant increase in death (AAV-Re-GFP, 17.11 ± 1.26%; AAV-Re-Gata3-GFP, 35.10 ± 3.22%; ** p < 0.01). The CCK-8 assay also indicated that exogenous Gata3 decreased cell viability (AAV-Re-GFP, 1; AAV-Re-Gata3-GFP, 0.344 ± 0.16; * p < 0.05) (Figure 7F).
3. Discussion
In this study, we first demonstrated that Brca1 is involved in maintaining the state of retinal precursor cells. Silencing Brca1 by siRNA interference significantly downregulated Nestin, a marker of stem cells, increased the expression of Map2, and promoted neurite outgrowth in 661W cells (Figure 1 and Figure 2). Moreover, the expression pattern of Brca1 in the retina in vivo was similar to that in vitro (Figure 6). Nestin was highly expressed in the retina of P1 (Figure 6), which indicated that the cells of this stage have characteristics of neural precursor cells. Brca1 was expressed in postnatal mouse retinas but not adult retinas. Thus, these data indicated that Brca1 silencing is involved in the differentiation of retinal precursor cells. This conclusion is partially consistent with previous studies. For example, Brca1 was expressed at leading edges in migrating cells, particularly in proliferative zones, during brain development [28,29]. Brca1 plays a role as a centrosomal factor in maintaining neural progenitors [13]. Oligodendrocyte precursor cells (OPCs) were also maintained by Brca1 and Brm [30]. Inactivation of Brca1 by cisplatin could increase growth cones in embryonic mouse brain precursor cells [31].
However, why does silencing Brca1 cause such typical neural differentiation? We speculate that Brca1 might function as a transcription factor in precursor cells. A previous study demonstrated that Sox2 expression depended on Brca1 via chromatin remodeling and histone modification in neural stem-like cells. Brca1 contributes to the conversion of oligodendrocyte precursors into neural stem cells [30]. Moreover, Brca1 might be a candidate ubiquitin ligase to degrade proteins in cholinergic neuritis [28]. However, for retinal precursor cells, more investigation is required to reveal the exact mechanism of Brca1-mediated differentiation in the future.
Second, a strength of our study was that Gata3 transcriptionally regulated Brca1 in retinal cells. Analysis of DNA affinity purification and ChIP assays indicated that Gata3 binds to the Brca1 promoter in 661W cells (Figure 3). Silencing Gata3 also decreased promoter inducibility and the expression level of Brca1 in vitro. Previous studies partially support our discovery. Celikkaya et al. reported that Gata3 promoted the neural progenitor state [26]. Gata3 is required for reactive proliferation of progenitors [32]. However, the decreased expression of Brca1 was not consistent with that of Gata3 (Figure 4A,B). Accordingly, exogenous Gata3 expression was also up to ~10-fold higher compared to the control, while Brca1 was only increased by ~50% (Figure 4D). Moreover, exogenous Gata3 notably induced an upregulation of Brca1 in primary postnatal retinal neurons (Figure 7A,B,D). However, the increased Brca1 expression was notably lower than that of Gata3. Therefore, we speculated that Gata3 might act as one of the transcription factors promoting Brca1 promoter activity along with other factors in postnatal retinal cells.
In addition, our data indicated that the effect of Brca1 on cell viability depends on cell type. Currently, there are different viewpoints about Brca1 in the central nervous system. Some studies suggested that the function of Brca1 was unique in neurons and increasing Brca1 could promote cell cycle re-entry and induce neuronal death in Alzheimer’s diseases [12,13]. However, Xu et al. indicated that Brca1 protected neurons from cerebral ischemia/reperfusion injury through the NRF2-mediated antioxidant pathway [11]. Our previous study also indicated that Brca1 contributed to neural viability by enhancing DNA stability in RGC5, an immortal neural cell line [33]. Why did these studies suggest different conclusions? The data presented here can account for this discrepancy. We found that Brca1 and Gata3 promoted the viability of precursor cells (Figure 1F and Figure 5D), whereas cell viability was decreased in differentiated P1 retinal neurons that re-expressed both genes (Figure 7). Thus, we speculate that Brca1 rescues neurons by affecting precursor cells in cerebral tissue in cerebral ischemia/reperfusion injury. Brca1 and Gata3 play different roles in neural progenitor cells and fully differentiated neurons.
4. Materials and Methods
661W cell culture. The mouse retinal cell line, 661W, purchased from ATCC (Manassas, VA, USA), was cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, Carlsbad, CA, USA) and 1% penicillin/streptomycin (Gibco, Carlsbad, CA, USA) in a humidified 5% CO2 incubator.
Primary mouse retinal neuron culture and treatment. Primary mouse retinal neurons were cultured as described previously. Mice were provided by the animal center of Zhongshan Ophthalmic Center, Sun Yat-sen University, China. The culturing protocol followed Reference [34]. The cells were maintained in complete medium (DMEM supplemented with 10% FBS) and characterized by Map2 staining (Boster, Wuhan, China, BM1243, 1:100). Three days after culture, the cells were used in the experiments described below.
Reporter and plasmid construction. The PGL3-WT plasmid carrying the mouse Brca1 promoter region (−1012 to +208) was generated as follows. The required Brca1 promoter region was amplified by PCR using the primer pair 5′-GGGGTACCCCCTTCCTTACCAGCTTTCCGC-3′ and 5′-CCCAAGCTTGGGCTGTTCCTCAGGGCTGTCTC-3′ (Kpn I and Hind III sites are underlined, respectively). The PCR products and the PGL3-WT vector were digested with Kpn I and Hind III, and the Brca1 promoter fragment (1220 bp) was inserted into PGL3-WT at identical sites following the manufacturer’s protocol, yielding the vector PGL3-Brca1-promoter. The integrity of the fragment was verified by DNA sequencing.
Virus preparation. The recombinant adeno-associated viruses AAV-Re-GFP and AAV-Re-Gata3-GFP were produced and packaged into recombinant adeno-associated virus 2 retro [35] by Cyagen Biosciences using the mouse Gata binding protein 3 (Gata3) cDNA sequence (accession number NM_001002295.2). AAVs were stored in 150 mM NaCl, 2 mM MgCl2 and 50 mM Tris (pH 8.0) at −80 °C for less than 1 year and thawed on ice on the day of use.
Brca1 promoter-reporter assay. Mouse retinal 661W cells were seeded in 24-well plates and cultured overnight. Then, the luciferase reporter pGL3-Brca1-luc was cotransfected with different plasmids or siRNAs, including pcDNA, a Gata3 overexpression vector (pFLAG-Gata3; Addgene, Cambridge, MA, USA), and Gata3 siRNA and scramble control siRNA. The pCMV-RL plasmid encoding Renilla luciferase was included in all the samples to monitor transfection efficiency. Twenty-four hours after transfection, the cells were harvested, and luciferase activity was measured using the Dual-Glo Luciferase Assay (Promega, Madison, WI, USA). The levels of firefly luciferase activity were normalized to Renilla luciferase activity.
Real-time RT–PCR. Total RNA was isolated with TRIzol Reagent (Invitrogen, Carlsbad, California, USA). The following primer pairs were used: mouse Gata3, 5′-CGAGATGGTACCGGGCACTA-3′ (sense) and 5′-GACAGTTCGCGCAGGATGT-3′ (antisense); mouse Brca1, 5′-TCTGGCAGCATGTTCTCTTC-3′ (sense) and 5′-CTCATTCCCACACTGGTGAC-3′ (antisense); mouse β-actin, 5′-AGGTCATCACTATTGGCAACG-3′ (sense) and 5′-ACGGATGTCAACGTCACACTT-3′ (antisense); mouse Map2, 5′-AAGTCACTGATGGAATAAGC-3′ (sense) and 5′-CTCTGCGAATTGGTTCTG-3′ (antisense); mouse Nestin, 5′-TCAAGATGTCCCTTAGTCTGGA-3′ (sense) and 5′-TGGTCCTCTGGTATCCCAAGG-3′ (antisense).
Western blotting. Total protein was extracted from cells using RIPA buffer supplemented with PMSF. The following primary antibodies were used: Gata3 (Abcam, Cambridge, MA, USA, ab199428, 1:500), Brca1 (Santa Cruz, Dallas, TX, USA, ab199428, 1:500), Map2 (Boster, Wuhan, China, BM1243, 1:1000), Nestin (Millipore, Burlington, MA, USA, mab353, 1:1000) and synaptophysin (Abcam, USA, mab353, 1:1000). The membrane was incubated with horseradish peroxidase-conjugated secondary anti-rabbit (CST, USA, 7074s, 1:10,000) or anti-mouse antibody (CST, USA, 7076s, 1:10,000). Gadph (Protein Tech Group, Wuhan, China, 10494-1-AP, 1:2000) served as a loading control. Protein bands were detected using an enhanced chemiluminescence detection system (Millipore, USA).
Immunofluorescence analysis. Immunofluorescence assays were performed according to the standard protocol. In brief, cells were fixed with paraformaldehyde after the treatments mentioned above. The primary antibodies included mouse anti-Map2 (Boster, China, BM1243, 1:100), rabbit anti-Map2 (Abcam, USA, ab254264, 1:200), rabbit anti-Nestin (Millipore, USA, mab353, 1:100), mouse anti-synaptophysin (Abcam, USA, ab8049, 1:500), rabbit anti-Gata3 (Abcam, USA, ab199428, 1:100), rabbit anti-Brca1 (Proteintech Group, Wuhan, China, 20649-1-AP) and mouse anti-Flag (Sigma, St. Louis, MO, USA, ab199428, 1:100). Cell death was detected by the TUNEL staining (kit from Elabscience, China, E-CK-A320) according to the manufacturer’s manual, the GFP+TUNEL+ cells were calculated as dead cells.
DNA affinity purification. DNA affinity purification was performed following previously described procedures with some modifications [36]. The nuclear extract was isolated from 661W cells using a nuclear and cytoplasmic protein extraction kit (Beyotime, China, P0027). The Brca1 promoter corresponding to positions −28 to −407 relative to the transcription initiation site (+1) was amplified by PCR using 5′-biotin-labeled primers (up: 5′-biotin-CAGGAAGGCTGAGGGAGGGA-3′; down: 5′-CTAAAATTCCCGCGCTCTCC-3′) and bound to streptavidin Dynabeads (Invitrogen, Carlsbad, CA, USA, 65305). Nuclear extracts were incubated with the DNA beads and washed extensively with BS/THES buffer, taking advantage of the magnetic properties of the Dynabeads when changing buffers. As a negative control, similar incubation was also carried out with beads lacking DNA bait. Bound proteins were eluted by increasing NaCl concentrations to 200, 300, 500, 750 and 1000 mM. Lower NaCl concentrations (100–300 mM) eluted proteins that bound to the promoter through weak interactions, while elution with higher NaCl concentrations (500–1000 mM) yielded proteins that have high affinity for the Brca1 promoter. Eluted samples were analyzed by SDS–PAGE followed by silver staining. Protein bands were excised, digested with trypsin and analyzed by liquid chromatography (LC)-MS/MS spectrometry on a Q Exactive (Thermo Fisher Scientific, San Jose, CA, USA) coupled online to high-performance liquid chromatography (HPLC). Proteins were identified by database searches using the Mascot search engine (Matrix Science, London, UK), and the data were collated into a list of proteins containing annotated transcription factors exclusively.
Immunohistochemical assay. Immunohistochemical assays were performed on retinal slides of postnatal day 1 and adult mice according to the manufacturer’s protocols of the SABC-POD (F) rabbit IgG kit (Boster, China, SA1028). Rabbit anti-Gata3 (1:100, Abcam, USA, ab199428, 1:100) and rabbit anti-Brca1 (Santa Cruz, USA, sc-135732, 1:100) were used as primary antibodies, and biotinylated anti-rabbit IgG antibodies were used as secondary antibodies. Following washing, the sections were developed with 3′-diaminobenzidine tetrahydrochloride (DAB) peroxidase substrate (Boster, China, AR1000) and counterstained with hematoxylin.
Cell viability assayed by CCK-8. Cell viability was determined by a Cell Counting Kit-8 (CCK-8) assay (Dojindo, Japan). Cells were incubated with CCK-8 reagent for 2 h at 37 °C followed by measuring the optical density at 450 nm. Cell viability was normalized to the untreated control.
ChIP assay. ChIP was carried out using a ChIP Assay Kit (Upstate Cell Signaling Solutions, Lake Placid, NY, USA) following the manufacturer’s instructions. Briefly, the chromatin from cells crosslinked with 1% formaldehyde was sheared by sonication. Some of the sheared chromatin was kept as an input control, and the rest was incubated with either Gata3 antibody (Abcam, Cambridge, MA, USA, ab199428, 1:100) or normal IgG as a negative control. The precipitated DNA was subjected to real-time PCR using the following primers that span the Gata3 binding site on the Brca1 promoter: forward, 5′-ACCTCGTTTTGCAACTGCTT-3′; reverse, 5′-GGTTTGCGATTGGCTACCTA-3′.
RNA interference. The sequences of Brca1 siRNA and control siRNA were as follows: Brca1-siRNA, 5′-GCAGGAGCCAAAUCUAUAA(dTdT)-3′ and control siRNA, 5′-CCUACGCCACCAAUUUCGU(dTdT)-3′. The siRNA targeting Gata3 is a pool of three different sequences: Gata3-siRNA-1, 5′-GACGGAAGAGGUGGACGUA(dTdT)-3′; Gata3-siRNA-2, 5′-UCGUACAUGGAAGCUCAGU(dTdT)-3′; Gata3-siRNA-3, 5′-GAUUUCAGAUCUGGGCAAU(dTdT)-3′. The oligos were synthesized by RiboBio (Guangzhou, China). Transfections were performed with Lipofectamine RNAiMAX (Invitrogen Waltham, MA, USA), and the expression levels of Gata3 and Brca1 were measured by Western blot at 24 h post-transfection.
Statistical analysis. All in vitro experiments were performed at least 3 times. Data are expressed as the means ± SEMs. The differences between mean values were evaluated with the two-tailed Student’s t-test (for two groups), analysis of variance (ANOVA, for 2 groups) and analysis of variance (for >2 groups). All calculations and statistical tests were performed using the computer programs Microsoft Excel 2003 (Microsoft, Redmond, WA, USA) and SPSS 11.5 (SPSS, Chicago, IL, USA). p < 0.05 was considered significant for all analyses.
5. Conclusions
In conclusion, the study presented here sheds light on the underlying transcriptional mechanism and bioactivity of Brca1 expression in retinal neurons. Brca1 and the upstream Gata3 are likely to be relevant for neuronal differentiation.
Author Contributions
Conceptualization, Z.Y. and J.Z. (Jing Zhuang); data curation: J.Z. (Jiejie Zhuang), Y.W., Q.W. and Z.J. (Jing Zhuang); formal analysis, P.C. and J.Z. (Jiejie Zhuang); funding acquisition, P.C., Z.Y. and J.Z. (Jing Zhuang); investigation, P.C., J.Z. (Jiejie Zhuang) and Y.W.; methodology, P.C., Y.L. and J.Z. (Jing Zhuang); project administration, Z.Y. and J.Z. (Jing Zhuang); resources, J.Z. (Jiejie Zhuang), Y.W., Q.L. and Q.W.; supervision, Z.Y. and J.Z. (Jing Zhuang); validation, Z.Y. and J.Z. (Jing Zhuang); visualization, S.C., X.C. and J.Q.; writing—original draft, P.C., J.Z. (Jiejie Zhuang) and J.Z. (Jing Zhuang); writing—review and editing and J.Z. (Jing Zhuang), P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (Project: 81470626 (J.Z.), 81670848 (J.Z.), and 82101134 (P.C.)) and the Science and Technology Project of Guangzhou Municipal (202102080039, Z.Y).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Zhongshan Ophthalmic Center (Permit Number: SYXK (YUE) 2010-0058).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to following study request, but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clark, S.L.; Rodriguez, A.M.; Snyder, R.R.; Hankins, G.D.; Boehning, D. Structure-Function of The Tumor Suppressor Brca1. Comput. Struct. Biotechnol. J. 2012, 1, e201204005. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Zhang, J.; Willers, H.; Wang, H.; Chung, J.H.; van Gent, D.C.; Hallahan, D.E.; Powell, S.N.; Xia, F. Checkpoint kinase 2-mediated phosphorylation of Brca1 regulates the fidelity of nonhomologous end-joining. Cancer Res. 2006, 66, 1401–1408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chiang, H.C.; Zhang, X.; Li, J.; Zhao, X.; Chen, J.; Wang, H.T.; Jatoi, I.; Brenner, A.; Hu, Y.; Li, R. Brca1-associated R-loop affects transcription and differentiation in breast luminal epithelial cells. Nucleic Acids Res. 2019, 47, 5086–5099. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.D.; Park, M.A.; Lee, J.S. Suppression and recovery of Brca1-mediated transcription by HP1γ via modulation of promoter occupancy. Nucleic Acids Res. 2012, 40, 11321–11338. [Google Scholar] [CrossRef] [PubMed]
- Rousset-Jablonski, C.; Gompel, A. Screening for familial cancer risk: Focus on breast cancer. Maturitas 2017, 105, 69–77. [Google Scholar] [CrossRef]
- Tangutoori, S.; Baldwin, P.; Sridhar, S. PARP inhibitors: A new era of targeted therapy. Maturitas 2015, 81, 5–9. [Google Scholar] [CrossRef]
- Levy-Lahad, E.; Lahad, A.; King, M.C. Precision medicine meets public health: Population screening for Brca1 and BRCA2. J. Natl. Cancer Inst. 2015, 107, 420. [Google Scholar] [CrossRef]
- Bromberg, K.D.; Ma’ayan, A.; Neves, S.R.; Iyengar, R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science 2008, 320, 903–909. [Google Scholar] [CrossRef]
- Korhonen, L.; Brannvall, K.; Skoglosa, Y.; Lindholm, D. Tumor suppressor gene BRCA-1 is expressed by embryonic and adult neural stem cells and involved in cell proliferation. J. Neurosci. Res. 2003, 71, 769–776. [Google Scholar] [CrossRef]
- Pao, G.M.; Zhu, Q.; Perez-Garcia, C.G.; Chou, S.J.; Suh, H.; Gage, F.H.; O’Leary, D.D.; Verma, I.M. Role of Brca1 in brain development. Proc. Natl. Acad. Sci. USA 2014, 111, E1240–E1248. [Google Scholar] [CrossRef]
- Xu, P.; Liu, Q.; Xie, Y.; Shi, X.; Li, Y.; Peng, M.; Guo, H.; Sun, R.; Li, J.; Hong, Y.; et al. Breast cancer susceptibility protein 1 (Brca1) rescues neurons from cerebral ischemia/reperfusion injury through NRF2-mediated antioxidant pathway. Redox Biol. 2018, 18, 158–172. [Google Scholar] [CrossRef]
- Evans, T.A.; Raina, A.K.; Delacourte, A.; Aprelikova, O.; Lee, H.G.; Zhu, X.; Perry, G.; Smith, M.A. Brca1 may modulate neuronal cell cycle re-entry in Alzheimer disease. Int. J. Med. Sci. 2007, 4, 140–145. [Google Scholar] [CrossRef]
- Wezyk, M.; Zekanowski, C. Role of Brca1 in Neuronal Death in Alzheimer’s Disease. ACS Chem. Neurosci. 2018, 9, 870–872. [Google Scholar] [CrossRef]
- Lonning, P.E.; Berge, E.O.; Bjornslett, M.; Minsaas, L.; Chrisanthar, R.; Hoberg-Vetti, H.; Dulary, C.; Busato, F.; Bjorneklett, S.; Eriksen, C.; et al. White Blood Cell Brca1 Promoter Methylation Status and Ovarian Cancer Risk. Ann. Intern. Med. 2018, 168, 326–334. [Google Scholar] [CrossRef]
- Park, M.A.; Seok, Y.J.; Jeong, G.; Lee, J.S. SUMO1 negatively regulates Brca1-mediated transcription, via modulation of promoter occupancy. Nucleic Acids Res. 2008, 36, 263–283. [Google Scholar] [CrossRef]
- Wiedmeier, J.E.; Ohlrich, A.; Chu, A.; Rountree, M.R.; Turker, M.S. Induction of the long noncoding RNA NBR2 from the bidirectional Brca1 promoter under hypoxic conditions. Mutat. Res. 2017, 796, 13–19. [Google Scholar] [CrossRef]
- Lu, Y.; Chu, A.; Turker, M.S.; Glazer, P.M. Hypoxia-induced epigenetic regulation and silencing of the Brca1 promoter. Mol. Cell. Biol. 2011, 31, 3339–3350. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Chen, P.; Xu, Z.; Qiu, J.; Ge, J.; Yu, K.; Zhuang, J. Brca1 Is Upregulated by 5-Aza-CdR and Promotes DNA Repair and Cell Survival, and Inhibits Neurite Outgrowth in Rat Retinal Neurons. Int. J. Mol. Sci. 2018, 19, 1214. [Google Scholar] [CrossRef]
- Kadonaga, J.T.; Tjian, R. Affinity purification of sequence-specific DNA binding proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 5889–5893. [Google Scholar] [CrossRef]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Invest. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef]
- Sayyad, Z.; Sirohi, K.; Radha, V.; Swarup, G. 661W is a retinal ganglion precursor-like cell line in which glaucoma-associated optineurin mutants induce cell death selectively. Sci. Rep. 2017, 7, 16855. [Google Scholar] [CrossRef] [PubMed]
- Kulkeaw, K.; Inoue, T.; Iino, T.; Tani, K.; Akashi, K.; Speck, N.A.; Nakanishi, Y.; Sugiyama, D. Twist1 regulates embryonic hematopoietic differentiation through binding to Myb and Gata2 promoter regions. Blood Adv. 2017, 1, 1672–1681. [Google Scholar] [CrossRef] [PubMed]
- Garrison, B.S.; Rybak, A.P.; Beerman, I.; Heesters, B.; Mercier, F.E.; Scadden, D.T.; Bryder, D.; Baron, R.; Rossi, D.J. ZFP521 regulates murine hematopoietic stem cell function and facilitates MLL-AF9 leukemogenesis in mouse and human cells. Blood 2017, 130, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Zarei-Kheirabadi, M.; Hesaraki, M.; Kiani, S.; Baharvand, H. In vivo conversion of rat astrocytes into neuronal cells through neural stem cells in injured spinal cord with a single zinc-finger transcription factor. Stem Cell Res. Ther. 2019, 10, 380. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Jinu, S.; Gordon, P.M.; Fielding, T.; Taylor, R.; Smith, B.N.; Snowden, V.; Blanc, E.; Vance, C.; Topp, S.; Wong, C.H.; et al. Non-nuclear Pool of Splicing Factor SFPQ Regulates Axonal Transcripts Required for Normal Motor Development. Neuron 2017, 94, 322–336.e5. [Google Scholar] [CrossRef]
- Celikkaya, H.; Cosacak, M.I.; Papadimitriou, C.; Popova, S.; Bhattarai, P.; Biswas, S.N.; Siddiqui, T.; Wistorf, S.; Nevado-Alcalde, I.; Naumann, L.; et al. Gata3 Promotes the Neural Progenitor State but Not Neurogenesis in 3D Traumatic Injury Model of Primary Human Cortical Astrocytes. Front. Cell Neurosci. 2019, 13, 23. [Google Scholar] [CrossRef]
- Chen, P.; Wu, Y.; Zhuang, J.; Liu, X.; Luo, Q.; Wang, Q.; Jiang, Z.; He, A.; Chen, S.; Chen, X.; et al. Gata3 Silencing Is Involved in Neuronal Differentiation and Its Abnormal Expression Impedes Neural Activity in Adult Retinal Neurocytes. Int. J. Mol. Sci. 2022, 23, 2495. [Google Scholar] [CrossRef]
- Coene, E.D.; Gadelha, C.; White, N.; Malhas, A.; Thomas, B.; Shaw, M.; Vaux, D.J. A novel role for Brca1 in regulating breast cancer cell spreading and motility. J. Cell Biol. 2011, 192, 497–512. [Google Scholar] [CrossRef]
- Pulvers, J.N.; Huttner, W.B. Brca1 is required for embryonic development of the mouse cerebral cortex to normal size by preventing apoptosis of early neural progenitors. Development 2009, 136, 1859–1868. [Google Scholar] [CrossRef]
- Kondo, T.; Raff, M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes Dev. 2004, 18, 2963–2972. [Google Scholar] [CrossRef]
- Keimpema, E.; Tortoriello, G.; Alpar, A.; Capsoni, S.; Arisi, I.; Calvigioni, D.; Hu, S.S.; Cattaneo, A.; Doherty, P.; Mackie, K.; et al. Nerve growth factor scales endocannabinoid signaling by regulating monoacylglycerol lipase turnover in developing cholinergic neurons. Proc. Natl. Acad. Sci. USA 2013, 110, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Kizil, C.; Kyritsis, N.; Dudczig, S.; Kroehne, V.; Freudenreich, D.; Kaslin, J.; Brand, M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev. Cell 2012, 23, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hu, H.; Chen, Z.; Cai, X.; Zhang, Z.; Yang, Y.; Yu, N.; Zhang, J.; Xia, L.; Ge, J.; et al. Brca1 silencing is associated with failure of DNA repairing in retinal neurocytes. PLoS ONE 2014, 9, e99371. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Tian, S.; Li, F.; Hu, H.; Chen, P.; Cai, X.; Xu, L.; Zhang, J.; Chen, Z.; et al. Lithium promotes DNA stability and survival of ischemic retinal neurocytes by upregulating DNA ligase IV. Cell Death Dis. 2016, 7, e2473. [Google Scholar] [CrossRef]
- Tervo, D.G.; Hwang, B.Y.; Viswanathan, S.; Gaj, T.; Lavzin, M.; Ritola, K.D.; Lindo, S.; Michael, S.; Kuleshova, E.; Ojala, D.; et al. A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 2016, 92, 372–382. [Google Scholar] [CrossRef]
- Jutras, B.L.; Verma, A.; Stevenson, B. Identification of novel DNA-binding proteins using DNA-affinity chromatography/pull down. Curr. Protoc. Microbiol. 2012, 24, 1F.1.1–1F.1.13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).