3. Discussion
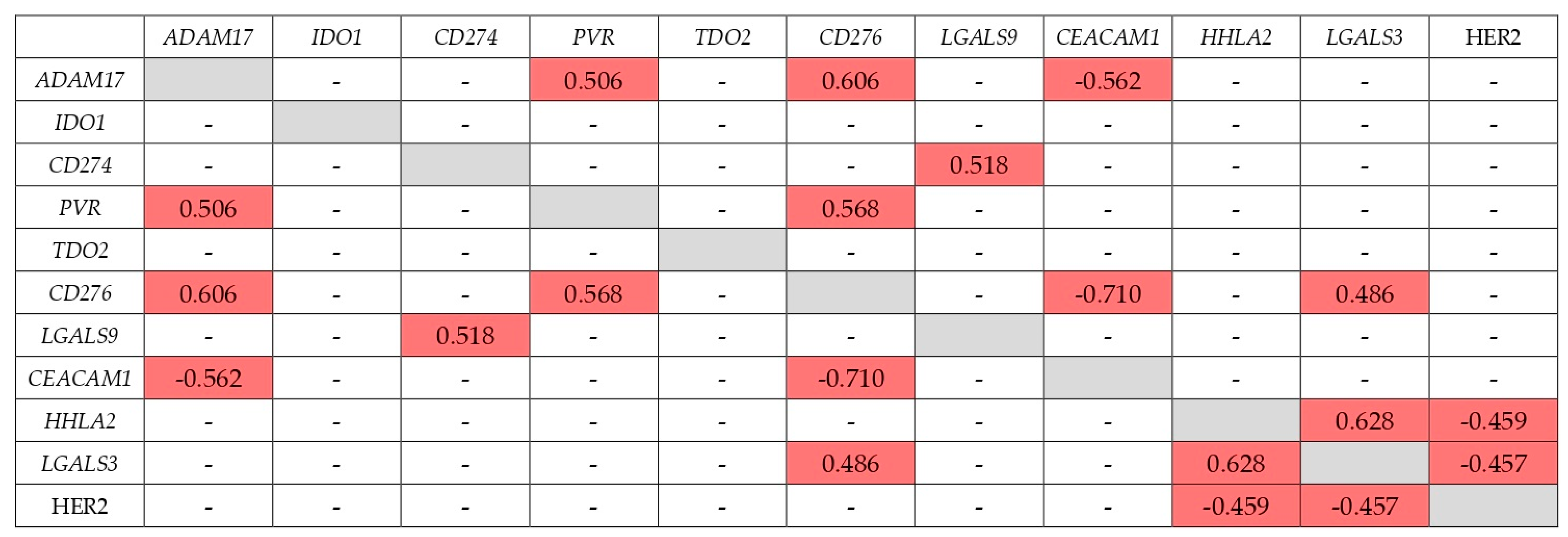
A very significant factor influencing the therapeutic effect of IC inhibition may be the co-expression of another IC with respect to the inhibited one. Such co-expression may also be able to block the antitumor effect of the immune system, which would lead to the lack of a therapeutic effect of immunotherapy based on the inhibition of IC. In this work, for the first time, we studied the co-expression of a set of IC genes—ADAM17, PVR, TDO2, CD274, CD276, CEACAM1, IDO1, LGALS3, LGALS9, and HHLA2—in GC tumors at the early stages of its development and during metastasis.
As was found, 9 out of 10 genes studied have at some stage other IC genes with expression coordinated with them. The exception is the
TDO2 gene, whose expression does not correlate with the expression of other genes studied at all stages of GC. However, Cui et al. found a correlation between
TDO2 expression level and a number of IC genes and, including the
CD274,
CD276, and
IDO1 genes in a sample of GC from the TCGA database, that may be due to the difference in samples [
8]. In metastatic GC, the expression of the
IDO1 gene does not correlate with any other gene. Except these genes, for the remaining eight there is a significant probability of co-expression of other IC genes. This is especially important to keep in mind for the
CD274 gene, encoding PD-L1, which is co-expressed with
IDO1 at early stage of GC and with
LGALS9 at late stage. Although the inhibition of these ICs has not yet been used in practice, they are being actively studied and have the prospect of entering the arsenal of immunotherapy tools [
9,
10].
Basically, the correlation of the expression of the genes studied is positive. An exception is the negative correlation between
CD276 and
CEACAM1 gene expression both at the early and metastatic stages. So far, the therapeutic efficacy of CEACAM1 inhibition has not been determined, but the sum of available results does not exclude such a prospect [
1]. In this case, inhibition of CEACAM1 may be particularly beneficial in the absence of expression of B7-H3, encoded by the
CD276 gene. It should be noted that this gene has the highest number of expression correlations with other ICs among the ones studied. This feature may reduce the therapeutic efficacy of B7-H3 inhibition.
An essential characteristic of IC is the association of expression with tumor metastasis, which may be due to the blocking of the action of the immune system on cells detached from the tumor. On the other hand, differences in expression at different stages of tumor development may serve as an indication of the stage at which inhibition of this IC may be the most effective therapeutically. In our study, the expression levels of two genes were associated with GC metastasis—
TDO2 and
LGALS3. TDO2 has one of the key roles in the catabolism of the Tryptophan to Kynurenine, along with IDO1 and IDO2. It is known that IDO1 catabolizes most of the tryptophan in various organs, while TDO2 is mainly expressed in the liver [
11]. Increased TDO2 expression accelerates this process, leading to a decrease in the concentration of Tryptophan and an increase in the concentration of Kynurenine. This reduces the proliferation and activity of CD8+ T-cells and the strengthening of their apoptosis, contributing to the evasion of the tumor from the immune response [
12]. This mechanism corresponds to our experimental results, in which increased TDO2 mRNA expression was first associated with distant metastasis in GC. In metastatic tumors TDO2 was expressed four times higher than in non-metastatic. In Pham et al. study, TDO2 expression was correlated with progression and outcome in GC [
13]. Meta-analysis on prognosis and clinical features of TDO2 expression in various malignancies revealed that TDO2 overexpression has been associated with poor survival, TNM stage, and regional lymph node metastasis [
14]. Moreover, bioinformatic analysis showed a correlation of high TDO2 expression with a poor prognosis in many cancer types [
8]. The revealed features of TDO2—a significant increase in expression at the metastatic stage, the absence of other IC genes with correlated expression—indicate the prospect of inhibiting this gene in a metastatic GC.
Galectin-3 (Gal-3) is a member of the galectin family that is widespread in mammalian tissues and is determined by its carbohydrate recognition domains with a specific binding affinity for β-galactosides [
15]. Gal-3, encoded by the
LGALS3 gene, is involved in the regulation of tumor cell growth, transformation, apoptosis, immunosuppression, angiogenesis, adhesion, invasion, and metastasis [
16]. A decrease in Gal-3 expression reduces adhesion between tumor cells and facilitates the invasion of cancer cells [
17]. Earlier, in the meta-analysis of Gal-3 expression in GC, measured by immunohistochemistry (IHC) method, the association of reduced Gal-3 expression with poor prognosis and high TNM stage was shown [
18]. In our work, for the first time we have shown the association of reduced
LGALS3 gene expression in the tumor with a distant metastasis in GC. In metastatic tumors,
LGALS3 was expressed two-fold lower than in non-metastatic one. This suggests a greater therapeutic efficacy of inhibition of
LGALS3 in the early stages of GC in relation to metastatic tumors, if any.
IDO1 and TDO2 are intracellular metalloproteins that catalyze the first step of the kynurenine pathway that converts the essential amino acid tryptophan to kynurenine. IDO1 expression may be induced by IFN-γ. Overactivation of the kynurenine pathway results in a decrease in tryptophan and an increase in the kynurenine level. Accumulation of kynurenine is toxic to immune cells and can lead to arrest of cell cycle in CD8+ T-cells, NK-cells, and NKT-cells through the GCN and mTOR signaling pathways [
19]. High expression of IDO1 in patients with GC is positively associated with tumor invasion and metastasis. In addition, increased IDO1 expression is associated with fewer number of CD4+ and CD8+ T-cells and higher number of Treg cells in tumors [
20,
21]. In GC, increased expression of IDO1 is associated with poor OS [
20,
22,
23]. We found the association of increased expression of the
IDO1 gene with a poor differentiation in GC for the first time. The IDO1 may be considered for inhibition in tumors with a low differentiation degree, as connected with GC differentiation.
CD274 gene encodes the PD-L1 IC, which is essential in the immune response. Interaction of PD-L1 with PD-1 receptor leads to inhibition of T-cell activation, CD8+ cytotoxic T-cells apoptosis, and increase of Foxp3+ Tregs number, which contributes the tumor to evade the immunity [
24]. PD-L1 expression is regulated by several signaling pathways such as PI3K/AKT, MAPK, JAK-STAT, WNT, NF-κB, and Hedgehog [
25]. PD-L1 is expressed in tumor cells of 30% of GC cases, but not in non-neoplastic gastric epithelium [
26]. The expression of PD-L1 is being studied as a marker of poor prognosis in various types of malignancies [
27,
28,
29]. Increased PD-L1 expression is also associated with a poor prognosis in GC [
30,
31,
32]. We found an association of increased CD274 mRNA expression with a diffuse/mixed type according to the Lauren classification. Chen et al. study also showed that the expression of PD-L1, measured by the IHC method, correlated with the Lauren type [
33].
LGALS9 gene encodes Galectin-9 (Gal-9), another member of the galectin family. Gal-9 triggers the signaling pathways required for stimulation of innate immunity, recruits eosinophils and neutrophils to the site of infection, and facilitates the maturation of dendritic cells. Interaction of Gal-9 with receptors on the cell surface leads to the production of pro-inflammatory cytokines and chemokines by activated T-cells. Changes in intra- and extracellular Gal-9 concentration lead to physiological changes [
34]. One of the Gal-9 receptors is Tim-3, an exhaustion marker that is expressed by activated T-cells. Binding of Gal-9 to Tim-3 leads to apoptosis of peripheral T-cells through the calcium-calpain-caspase-1 pathway. Most TIM-3+ T-cells in tumors co-express PD-1, another receptor of Gal-9. Gal-9–PD-1 binding contributes to the persistence of PD-1 + TIM-3+ T cells and attenuates Gal-9/Tim-3-induced cell death [
9]. Gal-9 expression is significantly altered in most malignant tumors [
35]. In solid tumors, increased expression of Gal-9 was significantly correlated with a lower depth of invasion, an earlier histopathological stage, the absence of lymph node metastases and the absence of distant metastases [
36]. In GC some studies find better survival with increased expression of Gal-9, and there are also conflicting results in different studies [
35,
37,
38].
In our study, elevated LGALS9 expression was associated with poor differentiation and diffuse/mixed Lauren type. Furthermore, according to the results of multiple logistic regression, the Lauren type is an independent feature, while tumor differentiation degree is associated with the expression of LGALS9 as a secondary characteristic. The feature found should be taken into account in further studies of this gene as a therapeutic target for immunotherapy. The expression of the IC genes studied does not correlate positively with the expression of HER2. This result indicates in favor of the possibility of independent use of inhibitors of the studied ICs and HER2.
5. Conclusions
For the first time, data were obtained on the correlation of the expression of 10 IC genes in GC at early stages of development and during metastasis. Almost all genes studied, except TDO2, have at some stage other IC genes with expression coordinated with them. This should be taken into account in the study and in the possible use of IC inhibition. Of particular interest in this regard is the co-expression of the CD274 gene, which encodes PD-L1, at early stage of GC with IDO1 and with LGALS9 at late stage. It is not excluded, that correlation of PD-L1 expression with another ICs may be a cause of resistance to PD-L1 inhibition. At stage IV, the expression of the IDO1 gene does not correlate with any other gene. This suggests that IDO1 inhibition at stage IV may be the most effective. As a rule, the correlation is positive, although there are exceptions. The expression of the CD276 and CEACAM1 genes negatively correlates both at the early and metastatic stages. The inhibition of CEACAM1 may be used in absence of B7-H3 expression, encoded by the CD276—gene with the highest number of expression correlations with other ICs. These data and their possible practical consequence accented the importance of parallel investigation of IC expression association with cancer development and IC expression coordination.
In particular, the expression of the TDO2 and LGALS3 genes had a statistically significant relationship with GC metastasis. In metastatic tumors TDO2 was expressed four-fold higher than in non-metastatic tumors, but LGALS3 was two-fold lower. The differentiation was associated with another IC gene, IDO1. The expression of CD274 (PD-L1) and LGALS9 was associated with the type of GC by Lauren classification that should be considered in future studies and practical PD-L1 inhibition.
The revealed features of TDO2—a significant increase in expression at the metastatic stage, the absence of other IC genes with correlated expression—indicate the prospect of inhibiting this gene in a metastatic GC tumor. The IDO1, as connected with GC differentiation, may be considered for inhibition in tumors with a low differentiation degree.

 significant correlation;
significant correlation;  there is no sig-nificant correlation.
there is no sig-nificant correlation.
 significant correlation;
significant correlation;  there is no sig-nificant correlation.
there is no sig-nificant correlation.
 significant correlation;
significant correlation;  there is no significant correlation.
there is no significant correlation.
 significant correlation;
significant correlation;  there is no significant correlation.
there is no significant correlation.