Alternate-Day Fasting Ameliorates Newly Established Sjögren’s Syndrome-like Sialadenitis in Non-Obese Diabetic Mice
Abstract
1. Introduction
2. Results
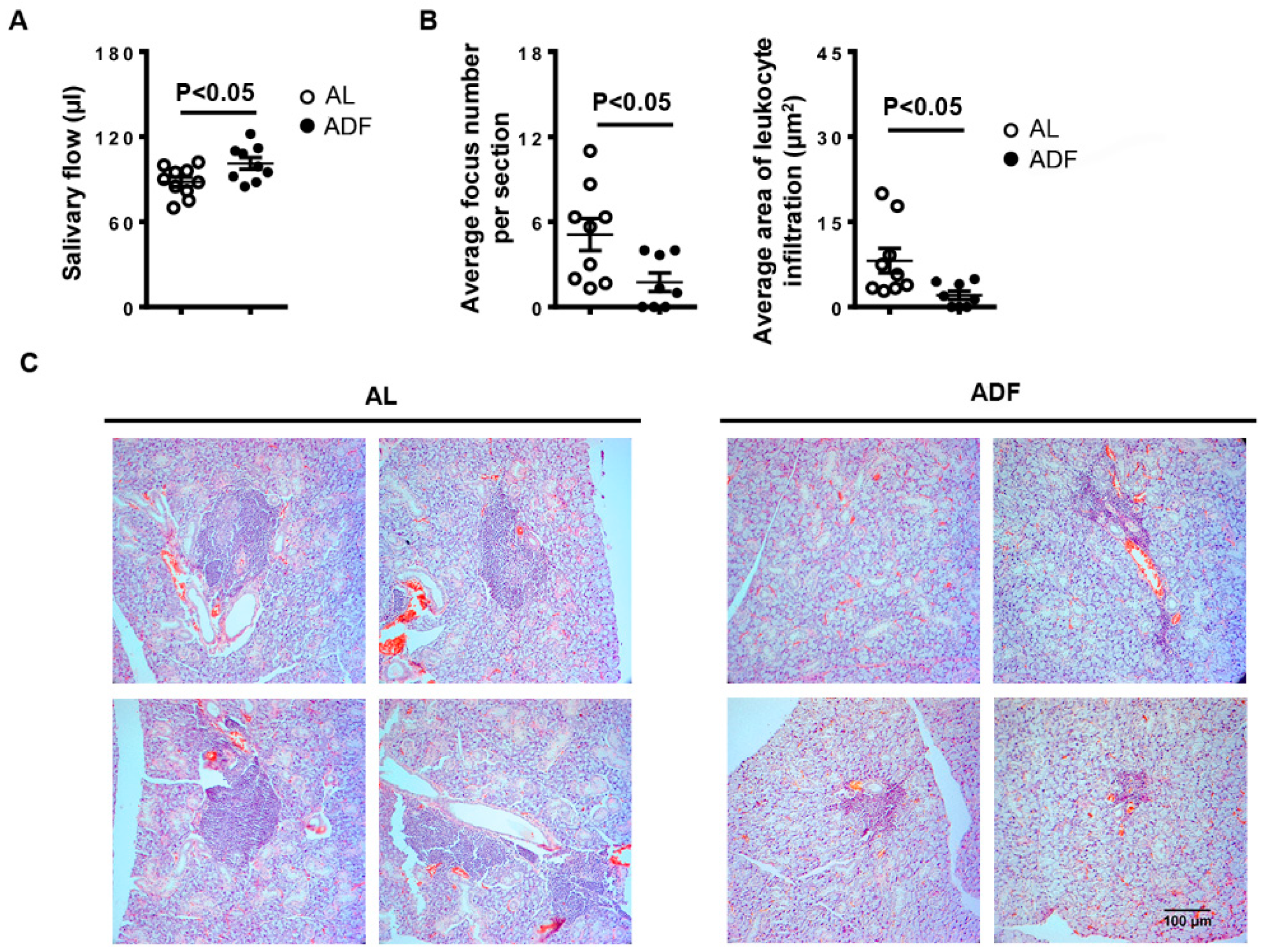
2.1. ADF Improves SS-Associated Hyposalivation and Mitigates Salivary Gland Inflammation in NOD Mice
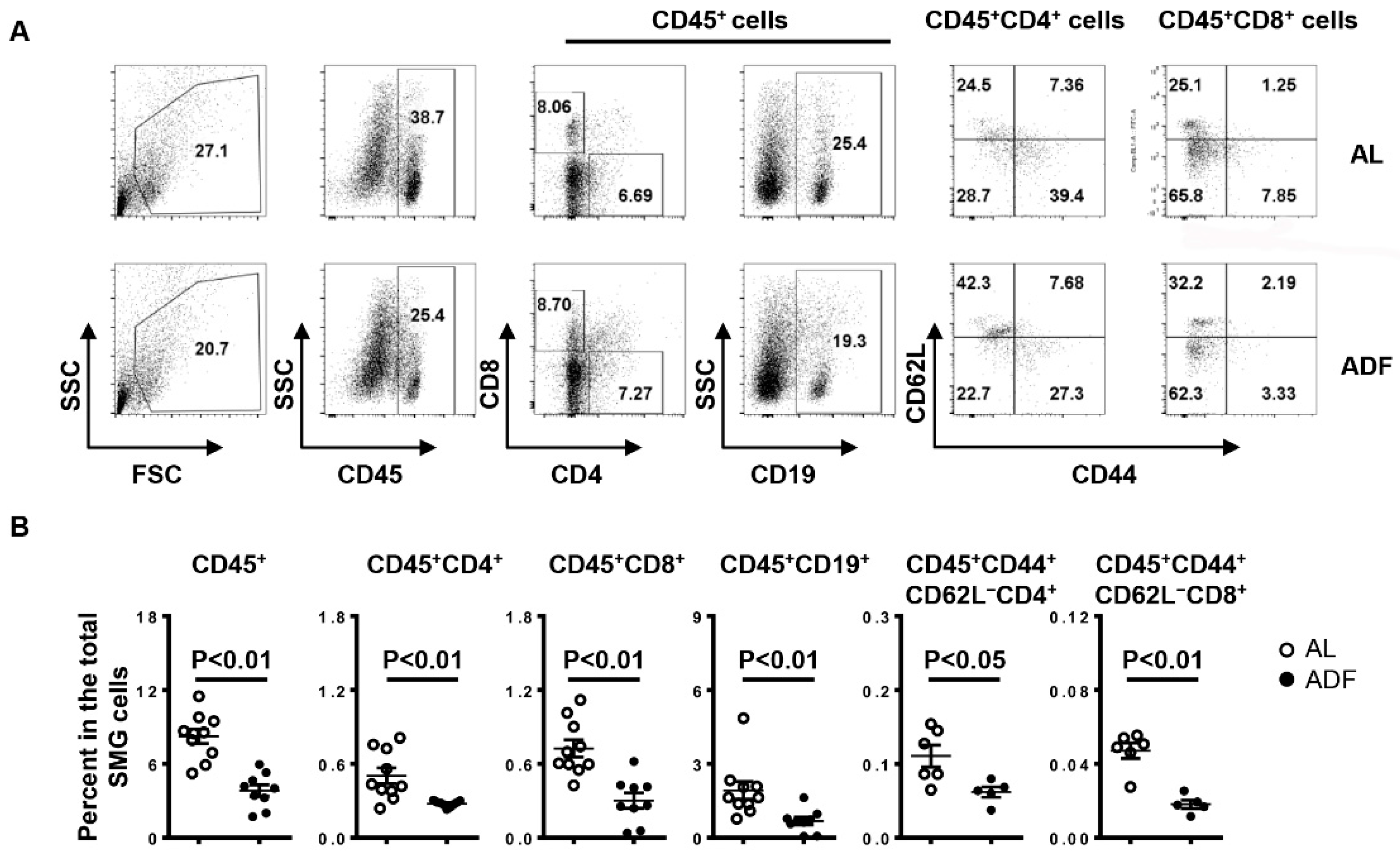
2.2. ADF Decreases T and B Cell Accumulation and Up-Regulates Water Channel Protein Aquaporin 5 (AQP5) and Tight Junction Protein Claudin-1 in the SMGs
2.3. ADF Decreases the Proportion of Th1 and Th17 Cells without Affecting Total CD4 T-, CD8 T Cells and B Cells in the smLNs
2.4. ADF Does Not Significantly Affect Autoantibody Production in the Serum
3. Discussion
4. Materials and Methods
4.1. Mice and Alternate-Day Fasting Intervention
4.2. Measurement of Stimulated Salivary Flow
4.3. Histologic Analysis
4.4. Flow Cytometry
4.5. Real-Time PCR
4.6. Detection of Serum Antinuclear Antibodies (ANA)
4.7. Detection of Anti-M3 Muscarinic Acetylcholine Receptor (M3R)
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, J.O.; Yu, Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. J. Clin. Cell. Immunol. 2013, S1, 11742. [Google Scholar] [CrossRef] [PubMed]
- Fox, P.C. Autoimmune diseases and Sjogren’s syndrome: An autoimmune exocrinopathy. Ann. N. Y. Acad. Sci. 2007, 1098, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Shahane, A. The epidemiology of Sjogren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Rhodus, N.L. Sjogren’s syndrome. Quintessence Int. 1999, 30, 689–699. [Google Scholar]
- Li, H.; Dai, M.; Zhuang, Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity 2004, 21, 551–560. [Google Scholar] [CrossRef]
- Hayakawa, I.; Tedder, T.F.; Zhuang, Y. B-lymphocyte depletion ameliorates Sjogren’s syndrome in Id3 knockout mice. Immunology 2007, 122, 73–79. [Google Scholar] [CrossRef]
- Singh, N.; Cohen, P.L. The T cell in Sjogren’s syndrome: Force majeure, not spectateur. J. Autoimmun. 2012, 39, 229–233. [Google Scholar] [CrossRef]
- Katsifis, G.E.; Moutsopoulos, N.M.; Wahl, S.M. T lymphocytes in Sjogren’s syndrome: Contributors to and regulators of pathophysiology. Clin. Rev. Allergy Immunol. 2007, 32, 252–264. [Google Scholar] [CrossRef]
- Roescher, N.; Tak, P.P.; Illei, G.G. Cytokines in Sjogren’s syndrome: Potential therapeutic targets. Ann. Rheum. Dis. 2010, 69, 945–948. [Google Scholar] [CrossRef]
- Mitsias, D.I.; Tzioufas, A.G.; Veiopoulou, C.; Zintzaras, E.; Tassios, I.K.; Kogopoulou, O.; Moutsopoulos, H.M.; Thyphronitis, G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clin. Exp. Immunol. 2002, 128, 562–568. [Google Scholar] [CrossRef]
- Cha, S.; Brayer, J.; Gao, J.; Brown, V.; Killedar, S.; Yasunari, U.; Peck, A.B. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004, 60, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.Q.; Yin, H.; Lee, B.H.; Carcamo, W.C.; Chiorini, J.A.; Peck, A.B. Pathogenic effect of interleukin-17A in induction of Sjogren’s syndrome-like disease using adenovirus-mediated gene transfer. Arthritis Res. Ther. 2010, 12, R220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, Q. Anti-IL-7 receptor-alpha treatment ameliorates newly established Sjogren’s-like exocrinopathy in non-obese diabetic mice. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2438–2447. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Rui, K.; Deng, J.; Tian, J.; Wang, X.; Wang, S.; Ko, K.H.; Jiao, Z.; Chan, V.S.; Lau, C.S.; et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann. Rheum. Dis. 2015, 74, 1302–1310. [Google Scholar] [CrossRef]
- Amft, N.; Bowman, S.J. Chemokines and cell trafficking in Sjogren’s syndrome. Scand. J. Immunol. 2001, 54, 62–69. [Google Scholar] [CrossRef]
- Ogawa, N.; Ping, L.; Zhenjun, L.; Takada, Y.; Sugai, S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren’s syndrome. Arthritis Rheum. 2002, 46, 2730–2741. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, Q. Disruption of CXCR3 function impedes the development of Sjogren’s syndrome-like xerostomia in non-obese diabetic mice. Lab. Investig. 2018, 98, 620–628. [Google Scholar] [CrossRef]
- Hasegawa, H.; Inoue, A.; Kohno, M.; Muraoka, M.; Miyazaki, T.; Terada, M.; Nakayama, T.; Yoshie, O.; Nose, M.; Yasukawa, M. Antagonist of interferon-inducible protein 10/CXCL10 ameliorates the progression of autoimmune sialadenitis in MRL/lpr mice. Arthritis Rheum. 2006, 54, 1174–1183. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Varady, K.A.; Hellerstein, M.K. Alternate-day fasting and chronic disease prevention: A review of human and animal trials. Am. J. Clin. Nutr. 2007, 86, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kafami, L.; Raza, M.; Razavi, A.; Mirshafiey, A.; Movahedian, M.; Khorramizadeh, M.R. Intermittent feeding attenuates clinical course of experimental autoimmune encephalomyelitis in C57BL/6 mice. Avicenna J. Med. Biotechnol. 2010, 2, 47–52. [Google Scholar] [PubMed]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef]
- Aly, S.M. Role of intermittent fasting on improving health and reducing diseases. Int. J. Health Sci. (Qassim) 2014, 8, 1–2. [Google Scholar] [CrossRef]
- Razeghi Jahromi, S.; Ghaemi, A.; Alizadeh, A.; Sabetghadam, F.; Moradi Tabriz, H.; Togha, M. Effects of Intermittent Fasting on Experimental Autoimune Encephalomyelitis in C57BL/6 Mice. Iran. J. Allergy Asthma Immunol. 2016, 15, 212–219. [Google Scholar] [PubMed]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-Mimicking Diet Modulates Microbiota and Promotes Intestinal Regeneration to Reduce Inflammatory Bowel Disease Pathology. Cell Rep. 2019, 26, 2704–2719.e2706. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, A.R.; Jolly, C.A.; Zaman, K.; Fernandes, G. Calorie restriction decreases proinflammatory cytokines and polymeric Ig receptor expression in the submandibular glands of autoimmune prone (NZB x NZW)F1 mice. J. Clin. Immunol. 2000, 20, 354–361. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Yu, Q. Plasmacytoid dendritic cells promote the pathogenesis of Sjogren’s syndrome. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166302. [Google Scholar] [CrossRef] [PubMed]
- Boumba, D.; Skopouli, F.N.; Moutsopoulos, H.M. Cytokine mRNA expression in the labial salivary gland tissues from patients with primary Sjogren’s syndrome. Br. J. Rheumatol. 1995, 34, 326–333. [Google Scholar] [CrossRef]
- Katsifis, G.E.; Rekka, S.; Moutsopoulos, N.M.; Pillemer, S.; Wahl, S.M. Systemic and local interleukin-17 and linked cytokines associated with Sjogren’s syndrome immunopathogenesis. Am. J. Pathol. 2009, 175, 1167–1177. [Google Scholar] [CrossRef]
- Hong, S.M.; Lee, J.; Jang, S.G.; Song, Y.; Kim, M.; Lee, J.; Cho, M.L.; Kwok, S.K.; Park, S.H. Intermittent Fasting Aggravates Lupus Nephritis through Increasing Survival and Autophagy of Antibody Secreting Cells in MRL/lpr Mice. Int. J. Mol. Sci. 2020, 21, 8477. [Google Scholar] [CrossRef] [PubMed]
- Vakrakou, A.G.; Polyzos, A.; Kapsogeorgou, E.K.; Thanos, D.; Manoussakis, M.N. Impaired anti-inflammatory activity of PPARgamma in the salivary epithelia of Sjogren’s syndrome patients imposed by intrinsic NF-kappaB activation. J. Autoimmun. 2018, 86, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, B.; Wang, Y.; Wei, L. Anti-inflammatory effect of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) on non-obese diabetic mice with Sjogren’s syndrome. Int. J. Clin. Exp. Pathol. 2014, 7, 4886–4894. [Google Scholar] [PubMed]
- Mihaylova, M.M.; Cheng, C.W.; Cao, A.Q.; Tripathi, S.; Mana, M.D.; Bauer-Rowe, K.E.; Abu-Remaileh, M.; Clavain, L.; Erdemir, A.; Lewis, C.A.; et al. Fasting Activates Fatty Acid Oxidation to Enhance Intestinal Stem Cell Function during Homeostasis and Aging. Cell Stem Cell 2018, 22, 769–778.e764. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S.; Seydoux, J.; Peters, J.M.; Gonzalez, F.J.; Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Investig. 1999, 103, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Konig, B.; Rauer, C.; Rosenbaum, S.; Brandsch, C.; Eder, K.; Stangl, G.I. Fasting Upregulates PPARalpha Target Genes in Brain and Influences Pituitary Hormone Expression in a PPARalpha Dependent Manner. PPAR Res. 2009, 2009, 801609. [Google Scholar] [CrossRef] [PubMed]
- Klotz, L.; Burgdorf, S.; Dani, I.; Saijo, K.; Flossdorf, J.; Hucke, S.; Alferink, J.; Nowak, N.; Beyer, M.; Mayer, G.; et al. The nuclear receptor PPAR gamma selectively inhibits Th17 differentiation in a T cell-intrinsic fashion and suppresses CNS autoimmunity. J. Exp. Med. 2009, 206, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Ewert, P.; Aguilera, S.; Alliende, C.; Kwon, Y.J.; Albornoz, A.; Molina, C.; Urzua, U.; Quest, A.F.; Olea, N.; Perez, P.; et al. Disruption of tight junction structure in salivary glands from Sjogren’s syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010, 62, 1280–1289. [Google Scholar] [CrossRef]
- Baker, O.J.; Camden, J.M.; Redman, R.S.; Jones, J.E.; Seye, C.I.; Erb, L.; Weisman, G.A. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. Am. J. Physiol. Cell Physiol. 2008, 295, C1191–C1201. [Google Scholar] [CrossRef]
- Lai, Z.; Yin, H.; Cabrera-Perez, J.; Guimaro, M.C.; Afione, S.; Michael, D.G.; Glenton, P.; Patel, A.; Swaim, W.D.; Zheng, C.; et al. Aquaporin gene therapy corrects Sjogren’s syndrome phenotype in mice. Proc. Natl. Acad. Sci. USA 2016, 113, 5694–5699. [Google Scholar] [CrossRef]
- Culp, D.J.; Quivey, R.Q.; Bowen, W.H.; Fallon, M.A.; Pearson, S.K.; Faustoferri, R. A mouse caries model and evaluation of aqp5-/-knockout mice. Caries Res. 2005, 39, 448–454. [Google Scholar] [CrossRef]
- Ma, T.; Song, Y.; Gillespie, A.; Carlson, E.J.; Epstein, C.J.; Verkman, A.S. Defective secretion of saliva in transgenic mice lacking aquaporin-5 water channels. J. Biol. Chem. 1999, 274, 20071–20074. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Xing, L.; Cerise, J.; Wang, E.H.; Jabbari, A.; de Jong, A.; Petukhova, L.; Christiano, A.M.; Clynes, R. CXCR3 Blockade Inhibits T Cell Migration into the Skin and Prevents Development of Alopecia Areata. J. Immunol. 2016, 197, 1089–1099. [Google Scholar] [CrossRef]
- Chaturvedi, V.; Ertelt, J.M.; Jiang, T.T.; Kinder, J.M.; Xin, L.; Owens, K.J.; Jones, H.N.; Way, S.S. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J. Clin. Investig. 2015, 125, 1713–1725. [Google Scholar] [CrossRef]
- Uppaluri, R.; Sheehan, K.C.; Wang, L.; Bui, J.D.; Brotman, J.J.; Lu, B.; Gerard, C.; Hancock, W.W.; Schreiber, R.D. Prolongation of cardiac and islet allograft survival by a blocking hamster anti-mouse CXCR3 monoclonal antibody. Transplantation 2008, 86, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Giarratana, N.; Penna, G.; Adorini, L. Animal models of spontaneous autoimmune disease: Type 1 diabetes in the nonobese diabetic mouse. Methods Mol. Biol. 2007, 380, 285–311. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Onodera, S.; Deng, S.; Alnujaydi, B.; Yu, Q.; Zhou, J. Alternate-Day Fasting Ameliorates Newly Established Sjögren’s Syndrome-like Sialadenitis in Non-Obese Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 13791. https://doi.org/10.3390/ijms232213791
Li D, Onodera S, Deng S, Alnujaydi B, Yu Q, Zhou J. Alternate-Day Fasting Ameliorates Newly Established Sjögren’s Syndrome-like Sialadenitis in Non-Obese Diabetic Mice. International Journal of Molecular Sciences. 2022; 23(22):13791. https://doi.org/10.3390/ijms232213791
Chicago/Turabian StyleLi, Dongfang, Shoko Onodera, Shu Deng, Bashaer Alnujaydi, Qing Yu, and Jing Zhou. 2022. "Alternate-Day Fasting Ameliorates Newly Established Sjögren’s Syndrome-like Sialadenitis in Non-Obese Diabetic Mice" International Journal of Molecular Sciences 23, no. 22: 13791. https://doi.org/10.3390/ijms232213791
APA StyleLi, D., Onodera, S., Deng, S., Alnujaydi, B., Yu, Q., & Zhou, J. (2022). Alternate-Day Fasting Ameliorates Newly Established Sjögren’s Syndrome-like Sialadenitis in Non-Obese Diabetic Mice. International Journal of Molecular Sciences, 23(22), 13791. https://doi.org/10.3390/ijms232213791




