TFG-β Nuclear Staining as a Potential Relapse Risk Factor in Early-Stage Non-Small-Cell Lung Cancer
Abstract
1. Introduction
2. Results
2.1. Baseline Patient and Tumor Characteristics
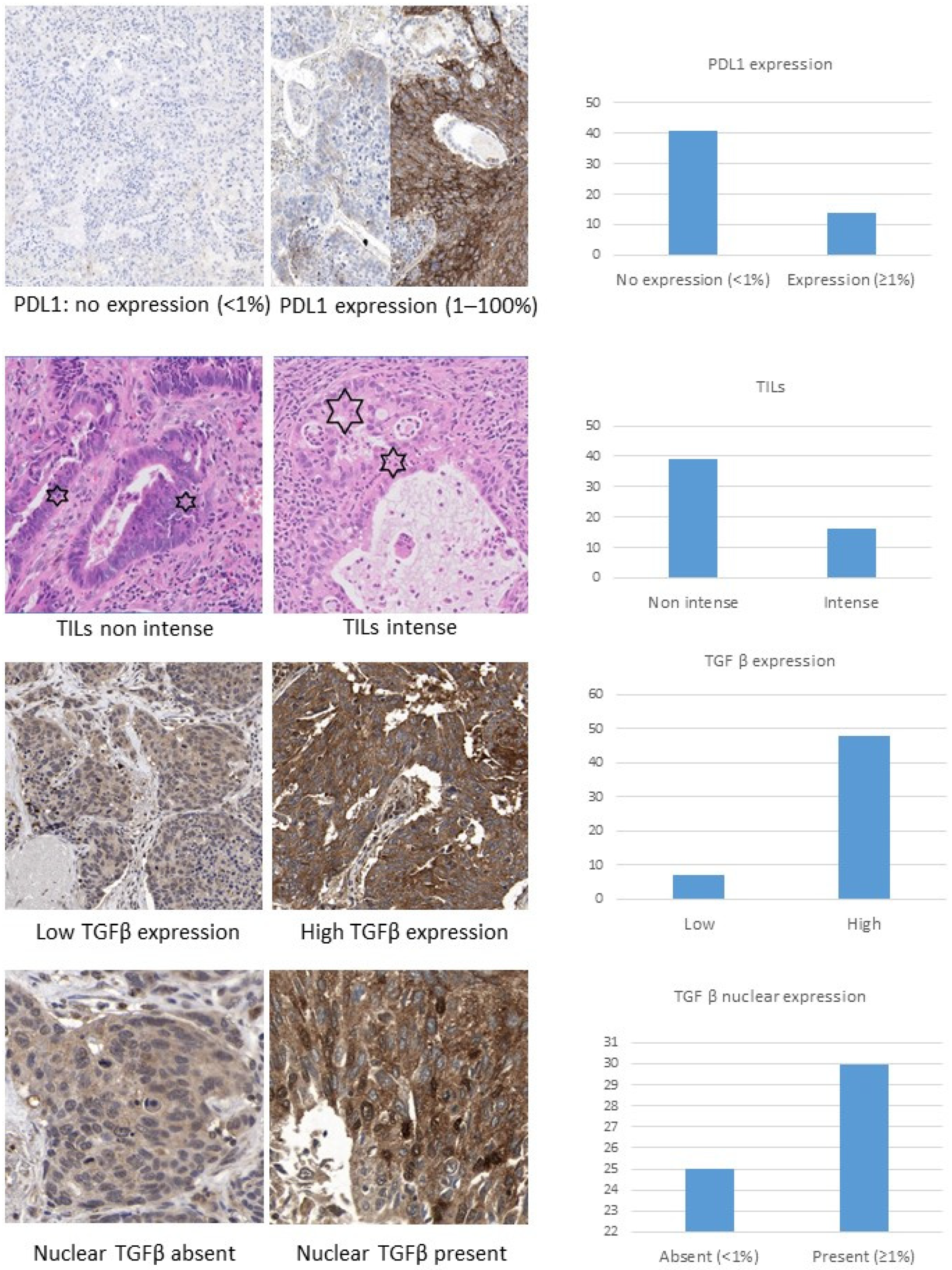
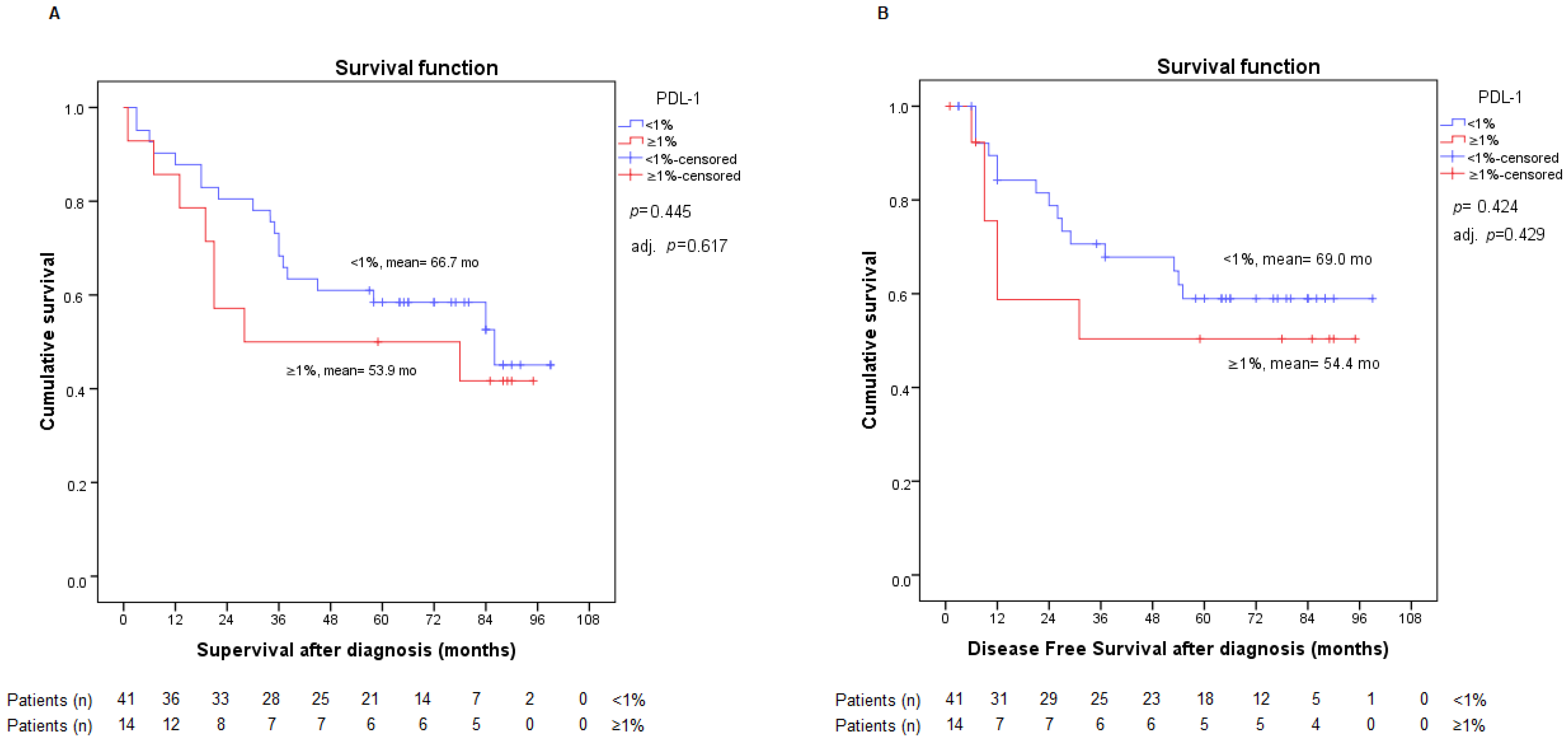
2.2. PD-L1 Expression
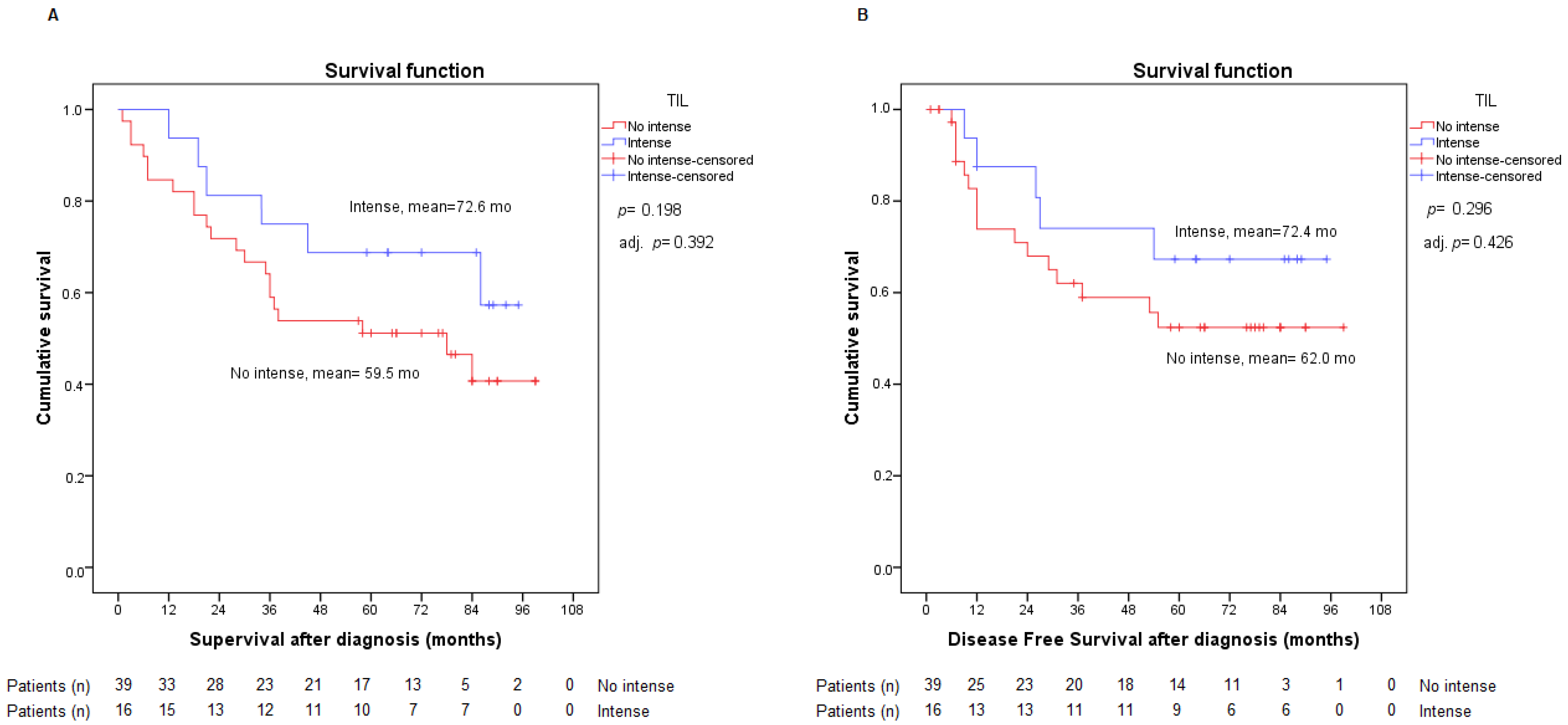
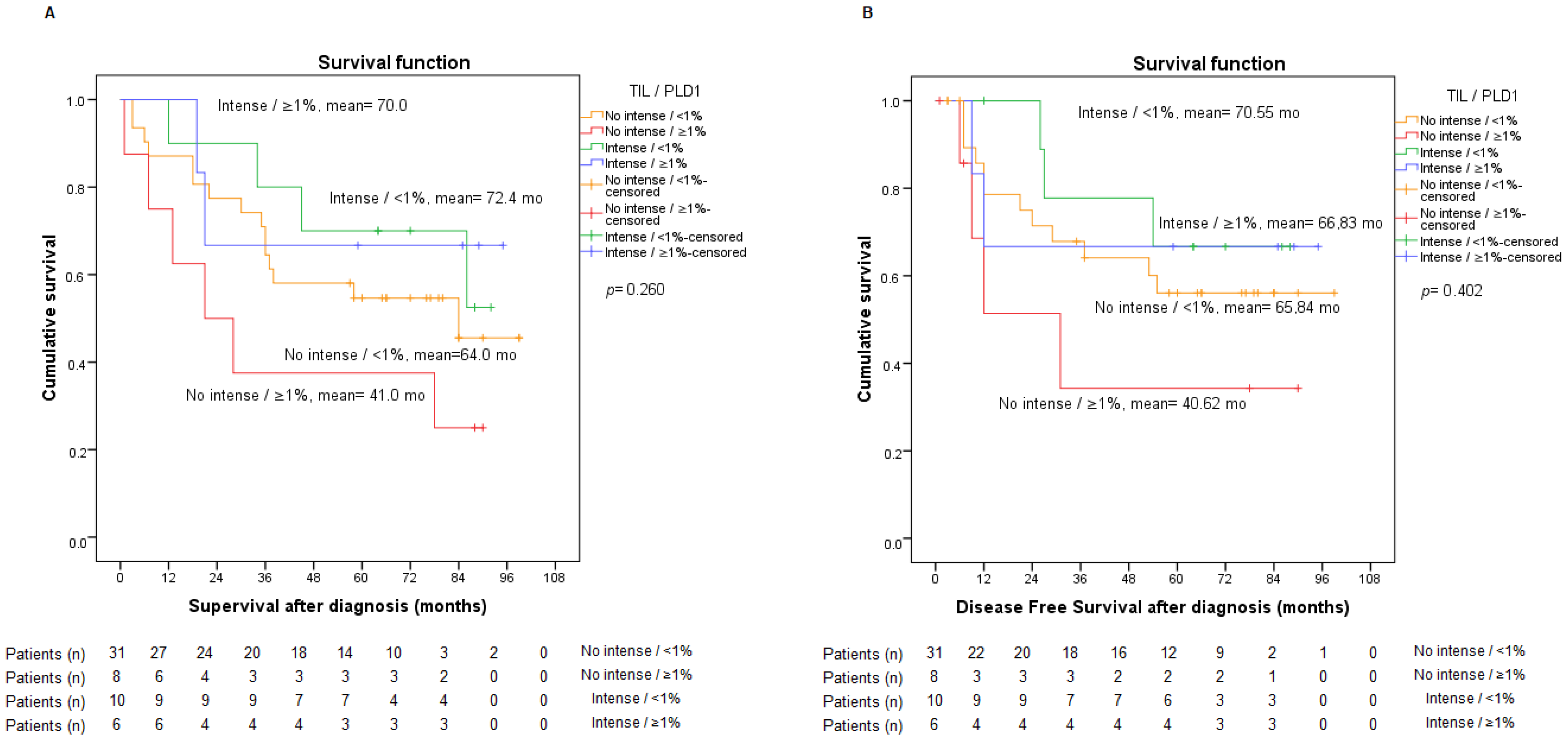
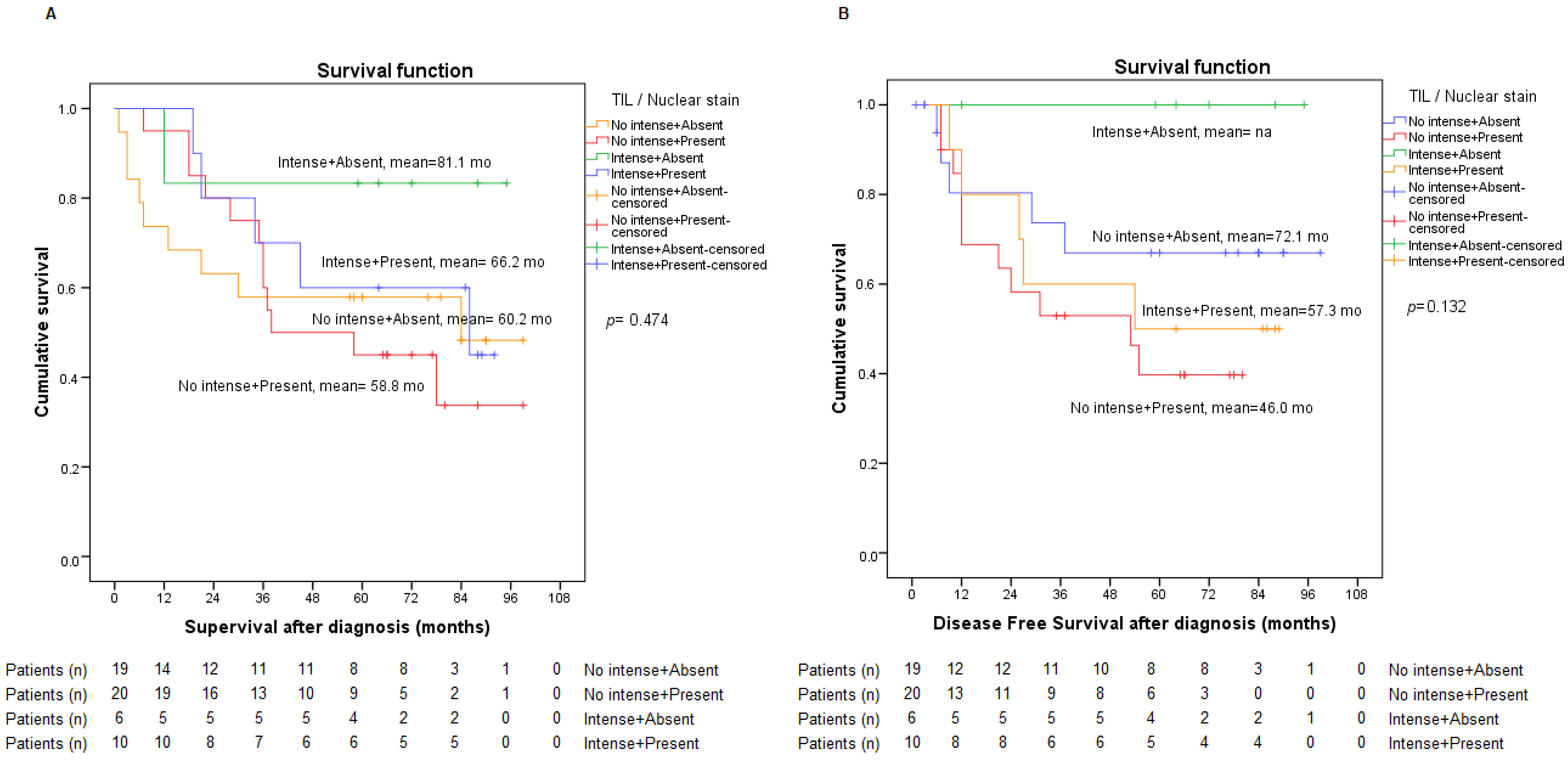
2.3. Presence of TILs
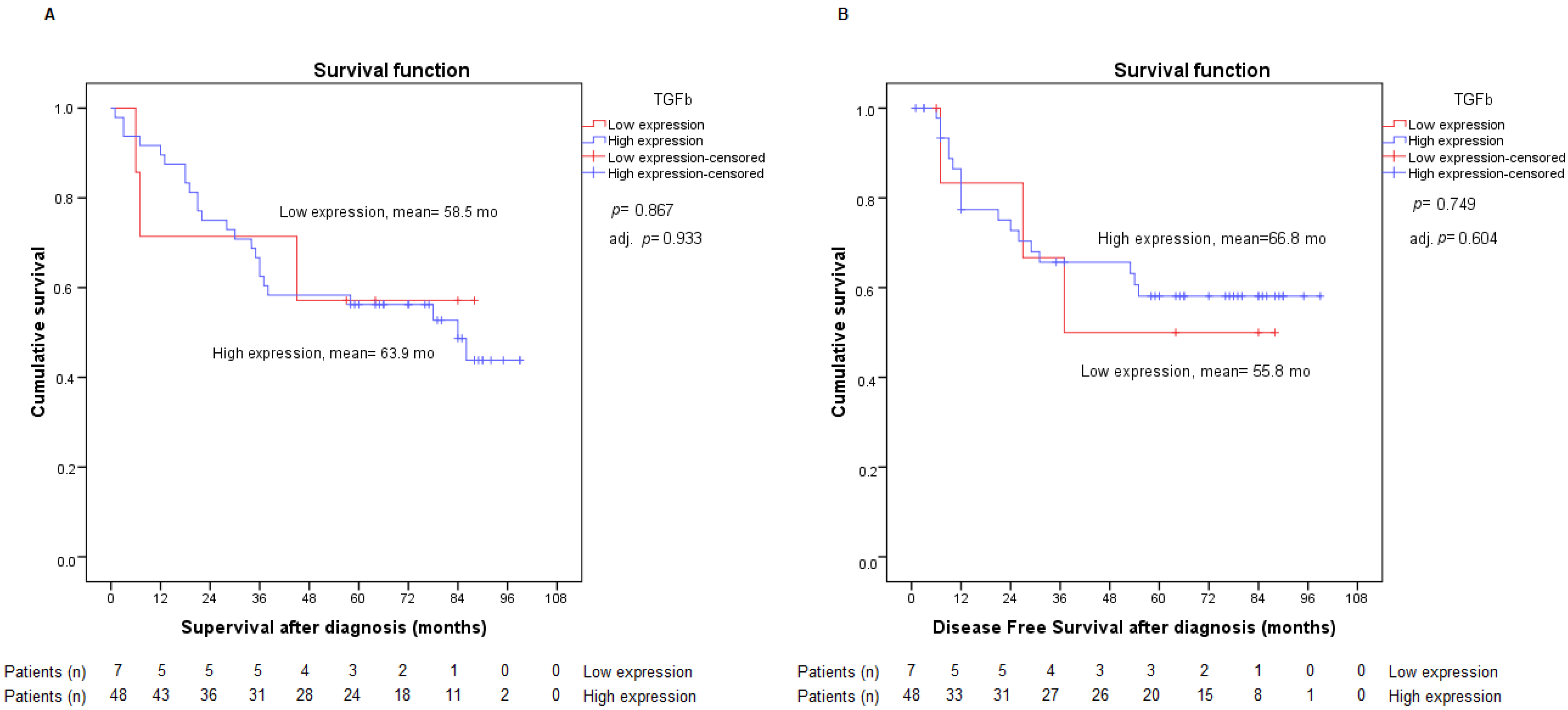
2.4. Assessment of TGF-β Expression
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Assessment of PD-L1 Expression
4.3. Assessment of Tumor-Infiltrating Lymphocytes (TILs)
4.4. Assessment of TGF-β Expression
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- De Koning, H.J.; Van Der Aalst, C.M.; De Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Sadate, A.; Occean, B.V.; Beregi, J.-P.; Hamard, A.; Addala, T.; de Forges, H.; Fabbro-Peray, P.; Frandon, J. Systematic review and meta-analysis on the impact of lung cancer screening by low-dose computed tomography. Eur. J. Cancer 2020, 134, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 8th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Nicholson, A.G.; Yatabe, Y.; Austin, J.H.M.; Beasley, M.B.; Chirieac, L.R.; Dacic, S.; Duhig, E.; Flieder, D.B.; et al. The 2015 World Health Organization Classification of Lung Tumors. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Majem, M.; Juan, O.; Insa, A.; Reguart, N.; Trigo, J.M.; Carcereny, E.; García-Campelo, R.; García, Y.; Guirado, M.; Provencio, M. SEOM clinical guidelines for the treatment of non-small cell lung cancer (2018). Clin. Transl. Oncol. 2019, 21, 3–17. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S.; ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv1–iv21. [Google Scholar] [CrossRef] [PubMed]
- Artal Cortés, Á.; Calera Urquizu, L.; Hernando Cubero, J. Adjuvant chemotherapy in non-small cell lung cancer: State-of-the-art. Transl. Lung Cancer Res. 2015, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: A systematic review and meta-analysis of individual participant data. Lancet 2014, 383, 1561–1571. [Google Scholar] [CrossRef]
- Vansteenkiste, J.; Wauters, E.; Reymen, B.; Ackermann, C.; Peters, S.; De Ruysscher, D. Current status of immune checkpoint inhibition in early-stage NSCLC. Ann. Oncol. 2019, 30, 1244–1253. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Tsuboi, M.; He, J.; John, T.; Grohe, C.; Majem, M.; Goldman, J.W.; Laktionov, K.; Kim, S.-W.; Kato, T.; et al. Osimertinib in Resected EGFR -Mutated Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Spicer, J.; Wang, C.; Tanaka, F.; Saylors, G.B.; Chen, K.-N.; Liberman, M.; Vokes, E.E.; Girard, N.; Lu, S.; Provencio, M.; et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39, 8503. [Google Scholar] [CrossRef]
- Wakelee, H.A.; Altorki, N.K.; Zhou, C.; Csőszi, T.; Vynnychenko, I.O.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2021, 39, 8500. [Google Scholar] [CrossRef]
- Provencio, M.; Nadal, E.; Insa, A.; García-Campelo, M.R.; Casal-Rubio, J.; Dómine, M.; Majem, M.; Rodríguez-Abreu, D.; Martínez-Martí, A.; Carpeño, J.D.C. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): An open-label, multicentre, single-arm, phase 2 trial. Lancet 2020, 21, 1413–1422. [Google Scholar] [CrossRef]
- Tufman, A.; Neumann, J.; Manapov, F.; Sellmer, L.; Jung, A.; Kauffmann-Guerrero, D.; Kahnert, K.; Mertsch, P.; Borgmeier, A.; Semrau, S.; et al. Prognostic and predictive value of PD-L1 expression and tumour infiltrating lymphocytes (TiLs) in locally advanced NSCLC treated with simultaneous radiochemotherapy in the randomized, multicenter, phase III German Intergroup lung Trial (GILT). Lung Cancer 2021, 160, 17–27. [Google Scholar] [CrossRef]
- Lin, G.; Fan, X.; Zhu, W.; Huang, C.; Zhuang, W.; Xu, H.; Lin, X.; Hu, D.; Huang, Y.; Jiang, K.; et al. Prognostic significance of PD-L1 expression and tumor infiltrating lymphocyte in surgically resectable non-small cell lung cancer. Oncotarget 2017, 8, 83986–83994. [Google Scholar] [CrossRef]
- Chen, Z.; Zhao, N.; Wang, Q.; Xi, Y.; Tian, X.; Wu, H.; Xu, Y. PD-L1 Protein Expression and Gene Amplification Correlate with the Clinicopathological Characteristics and Prognosis of Lung Squamous Cell Carcinoma. Cancer Manag. Res. 2021, 13, 6365–6375. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, C.; Wang, X.; Lai, Y.; Zhou, K.; Li, P.; Liu, L.; Che, G. Prognostic value of TGF-β in lung cancer: Systematic review and meta-analysis. BMC Cancer 2019, 19, 691. [Google Scholar] [CrossRef]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccines Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Tuminello, S.; Veluswamy, R.; Lieberman-Cribbin, W.; Gnjatic, S.; Petralia, F.; Wang, P.; Flores, R.; Taioli, E. Prognostic value of immune cells in the tumor microenvironment of early-stage lung cancer: A meta-analysis. Oncotarget 2019, 10, 7142–7155. [Google Scholar] [CrossRef] [PubMed]
- Duchemann, B.; Remon, J.; Naigeon, M.; Cassard, L.; Jouniaux, J.M.; Boselli, L.; Grivel, J.; Auclin, E.; Desnoyer, A.; Besse, B.; et al. Current and future biomarkers for outcomes with immunotherapy in non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 2937–2954. [Google Scholar] [CrossRef]
- He, Y.; Yu, H.; Rozeboom, L.; Rivard, C.J.; Ellison, K.; Dziadziuszko, R.; Suda, K.; Ren, S.; Wu, C.; Hou, L.; et al. LAG-3 Protein Expression in Non–Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J. Thorac. Oncol. 2017, 12, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Spira, A.; Raben, D.; Planchard, D.; Cho, B.; Özgüroglu, M.; Daniel, D.; Villegas, A.; Vicente, D.; Hui, R.; et al. Outcomes with durvalumab by tumour PD-L1 expression in unresectable, stage III non-small-cell lung cancer in the PACIFIC trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.R.; Faivre-Finn, C.; Gray, J.E.; Vicente, D.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Garassino, M.C.; Hui, R.; Quantin, X.; et al. Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. J. Clin. Oncol. JCO 2022, 40, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Butter, R.; Hondelink, L.M.; van Elswijk, L.; Blaauwgeers, J.L.; Bloemena, E.; Britstra, R.; Bulkmans, N.; van Gulik, A.L.; Monkhorst, K.; de Rooij, M.J.; et al. The impact of a pathologist’s personality on the interobserver variability and diagnostic accuracy of predictive PD-L1 immunohistochemistry in lung cancer. Lung Cancer 2022, 166, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.-Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Bremnes, R.M.; Busund, L.-T.; Kilvær, T.L.; Andersen, S.; Richardsen, E.; Paulsen, E.E.; Hald, S.; Khanehkenari, M.R.; Cooper, W.A.; Kao, S.C.; et al. The Role of Tumor-Infiltrating Lymphocytes in Development, Progression, and Prognosis of Non–Small Cell Lung Cancer. J. Thorac. Oncol. 2016, 11, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Horne, Z.D.; Jack, R.; Gray, Z.T.; Siegfried, J.M.; Wilson, D.O.; Yousem, S.A.; Nason, K.S.; Landreneau, R.J.; Luketich, J.D.; Schuchert, M.J. Increased levels of tumor-infiltrating lymphocytes are associated with improved recurrence-free survival in stage 1A non-small-cell lung cancer. J. Surg. Res. 2011, 171, 1–5. [Google Scholar] [CrossRef]
- Barlesi, F.; Greillier, L.; Monville, F.; Foa, C.; le Treut, J.; Audigier-Valette, C.; Vély, F.; Garcia, S.; Sabatier, F.; Ciccolini, J.; et al. LBA53 Precision immuno-oncology for advanced non-small cell lung cancer (NSCLC) patients (pts) treated with PD1/L1 immune checkpoint inhibitors (ICIs): A first analysis of the PIONeeR study. Ann. Oncol. 2020, 31, S1183. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Ock, C.-Y.; Keam, B.; Kim, S.; Lee, J.-S.; Kim, M.; Kim, T.M.; Jeon, Y.K.; Kim, D.-W.; Chung, D.H.; Heo, D.S. Pan-Cancer Immunogenomic Perspective on the Tumor Microenvironment Based on PD-L1 and CD8 T-Cell Infiltration. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2261–2270. [Google Scholar] [CrossRef] [PubMed]
- Tschernia, N.P.; Gulley, J.L. Tumor in the Crossfire: Inhibiting TGF-β to Enhance Cancer Immunotherapy. BioDrugs 2022, 36, 153–180. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Kim, T.M.; Vicente, D.; Felip, E.; Lee, D.H.; Lee, K.H.; Lin, C.-C.; Flor, M.J.; Di Nicola, M.; Alvarez, R.M.; et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2020, 15, 1210–1222. [Google Scholar] [CrossRef]
- Xue, Y.-J.; Lu, Q.; Sun, Z.-X. CD147 overexpression is a prognostic factor and a potential therapeutic target in bladder cancer. Med. Oncol. 2011, 28, 1363–1372. [Google Scholar] [CrossRef]
- Huang, A.-L.; Liu, S.-G.; Qi, W.-J.; Zhao, Y.-F.; Li, Y.-M.; Lei, B.; Sheng, W.-J.; Shen, H. TGF-β1 Protein Expression in Non-Small Cell Lung Cancers is Correlated with Prognosis. Asian Pac. J. Cancer Prev. 2014, 15, 8143–8147. [Google Scholar] [CrossRef]
- Javle, M.; Li, Y.; Tan, D.; Dong, X.; Chang, P.; Kar, S.; Li, D. Biomarkers of TGF-β signaling pathway and prognosis of pancreatic cancer. PLoS ONE 2014, 9, e85942. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, M.C.; Jha, P.; Pathak, P.; Suri, V.; Sarkar, C.; Chosdol, K.; Suri, A.; Kale, S.S.; Mahapatra, A.K. Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro-Oncology 2013, 15, 718–726. [Google Scholar] [CrossRef]
- Huynh, L.; Hipolito, C.; Dijke, P.T. A Perspective on the Development of TGF-β Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef]
- Krohn, D. PD-L1 IHC 22C3 pharmDx, Interpretation Manual, NSCLC 1% 50%. 2021. Available online: https://www.agilent.com/cs/library/usermanuals/public/29158_pd-l1-ihc-22C3-pharmdx-nsclc-interpretation-manual.pdf (accessed on 26 June 2022).

| Total (n = 55) | PD-L1 | TIL | TGF-β | TGF-Β Nuclear Staining | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1% (n = 41) | ≥1% (n = 14) | p-Value | Non-Intense (n = 39) | Intense (n = 16) | p-Value | Weak Expression (n = 7) | High Expression (n = 48) | p-Value | Absent (n = 25) | Present (n = 30) | p-Value | ||
| Sex, n (%) | 1.000 | 0.165 | 0.577 | 1.000 | |||||||||
| Male | 49 (89.1) | 36 (87.8) | 13 (92.9) | 33 (84.6) | 16 (100) | 6 (85.7) | 43 (89.6) | 22 (88.0) | 27 (90.0) | ||||
| Female | 6 (10.9) | 5 (12.2) | 1 (7.1) | 6 (15.4) | 0 (0) | 1 (14.3) | 5 (10.4) | 3 (12.0) | 3 (10.0) | ||||
| Age, mean (SD) | 63.93 (9.74) | 64.76 (10.17) | 61.50 (8.19) | 0.284 | 64.08 (9.73) | 63.56 (10.07) | 0.861 | 67.57 (11.23) | 63.40 (9.52) | 0.294 | 65.48 (10.19) | 62.63 (9.32) | 0.285 |
| Histology, n (%) | 0.097 | 0.626 | 0.686 | 0.095 | |||||||||
| squamous cell carcinoma | 23 (41.8) | 14 (34.1) | 9 (64.3) | 15 (38.5) | 8 (50.0) | 2 (28.6) | 21 (43.8) | 14 (56.0) | 9 (30.0) | ||||
| non-squamous | 32 (58.2) | 27 (65.9) | 5 (35.7) | 24 (61.5) | 8 (50.0) | 5 (71.4) | 27 (56.3) | 11 (44.0) | 21 (70.0) | ||||
| Staging, n (%) | 0.189 | 0.585 | 1.000 | 0.864 | |||||||||
| I | 33 (60.0) | 27 (65.9) | 6 (42.9) | 22 (56.4) | 11 (68.8) | 4 (57.1) | 29 (60.4) | 14 (56.0) | 19 (63.3) | ||||
| II | 14 (25.5) | 10 (24.4) | 4 (28.6) | 10 (25.6) | 4 (25.0) | 2 (28.6) | 12 (25.0) | 7 (28.0) | 7 (23.3) | ||||
| III | 8 (14.5) | 4 (9.8) | 4 (28.6) | 7 (17.9) | 1 (6.3) | 1 (14.3) | 7 (14.6) | 4 (16.0) | 4 (13.3) | ||||
| Type of surgery, n (%) | 0.099 | 0.698 | 0.211 | 0.542 | |||||||||
| Limited resection (segmentectomy or wedge resection) | 4 (7.3) | 4 (9.8) | 0 (0) | 3 (7.7) | 1 (6.3) | 1 (14.3) | 3 (6.3) | 3 (12.0) | 1 (3.3) | ||||
| Lobectomy | 39 (70.9) | 31 (75.6) | 8 (57.1) | 26 (66.7) | 13 (81.3) | 6 (85.7) | 33 (68.8) | 17 (68.0) | 22 (73.3) | ||||
| Pneumonectomy | 12 (21.8) | 6 (14.6) | 6 (42.9) | 10 (25.6) | 2 (12.5) | 0 (0) | 12 (25.0) | 5 (20.0) | 7 (23.3) | ||||
| EGFR mutation, n (%) | 0.742 | 0.343 | 0.761 | 0.039 | |||||||||
| Positive | 2 (3.6) | 2 (4.9) | 0 (0) | 1 (2.6) | 1 (6.3) | 0 (0) | 2 (4.2) | 0 (0) | 2 (6.7) | ||||
| wt | 31 (56.4) | 24 (58.5) | 7 (50.0) | 24 (61.5) | 7 (43.8) | 5 (71.4) | 26 (54.2) | 11 (44.0) | 20 (66.7) | ||||
| Unknown | 22 (40.0) | 15 (36.6) | 7 (50.0) | 14 (35.9) | 8 (50.0) | 2 (28.6) | 20 (41.7) | 14 (56.0) | 8 (26.7) | ||||
| Chemotherapy, n (%) | 0.099 | 0.527 | 0.346 | 0.541 | |||||||||
| No | 33 (60.0) | 27 (65.9) | 6 (42.9) | 25 (64.1) | 8 (50.0) | 4 (57.1) | 29 (60.4) | 17 (68.0) | 16 (53.3) | ||||
| Adjuvant | 15 (27.3) | 8 (19.5) | 7 (50.0) | 10 (25.6) | 5 (31.3) | 1 (14.3) | 14 (29.2) | (24.0) | 9 (30.0) | ||||
| Neo Adjuvant | 7 (12.7) | 6 (14.6) | 1 (7.1) | 4 (10.3) | 3 (18.8) | 2 (28.6) | 5 (10.4) | 2 (8.0) | 5 (16.7) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cárdenas-Quesada, N.; Díaz-Beltrán, L.; Rosa-Garrido, C.; Márquez-Lobo, B.; Sabio-González, A.; Luque-Barona, R.J.; Núñez, M.I.; Sánchez-Rovira, P. TFG-β Nuclear Staining as a Potential Relapse Risk Factor in Early-Stage Non-Small-Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 13780. https://doi.org/10.3390/ijms232213780
Cárdenas-Quesada N, Díaz-Beltrán L, Rosa-Garrido C, Márquez-Lobo B, Sabio-González A, Luque-Barona RJ, Núñez MI, Sánchez-Rovira P. TFG-β Nuclear Staining as a Potential Relapse Risk Factor in Early-Stage Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences. 2022; 23(22):13780. https://doi.org/10.3390/ijms232213780
Chicago/Turabian StyleCárdenas-Quesada, Nuria, Leticia Díaz-Beltrán, Carmen Rosa-Garrido, Bélgica Márquez-Lobo, Adela Sabio-González, Rafael J. Luque-Barona, María Isabel Núñez, and Pedro Sánchez-Rovira. 2022. "TFG-β Nuclear Staining as a Potential Relapse Risk Factor in Early-Stage Non-Small-Cell Lung Cancer" International Journal of Molecular Sciences 23, no. 22: 13780. https://doi.org/10.3390/ijms232213780
APA StyleCárdenas-Quesada, N., Díaz-Beltrán, L., Rosa-Garrido, C., Márquez-Lobo, B., Sabio-González, A., Luque-Barona, R. J., Núñez, M. I., & Sánchez-Rovira, P. (2022). TFG-β Nuclear Staining as a Potential Relapse Risk Factor in Early-Stage Non-Small-Cell Lung Cancer. International Journal of Molecular Sciences, 23(22), 13780. https://doi.org/10.3390/ijms232213780







