Vascular Endothelial Growth Factor Receptor 2: Molecular Mechanism and Therapeutic Potential in Preeclampsia Comorbidity with Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2 Infections
Abstract
1. Introduction
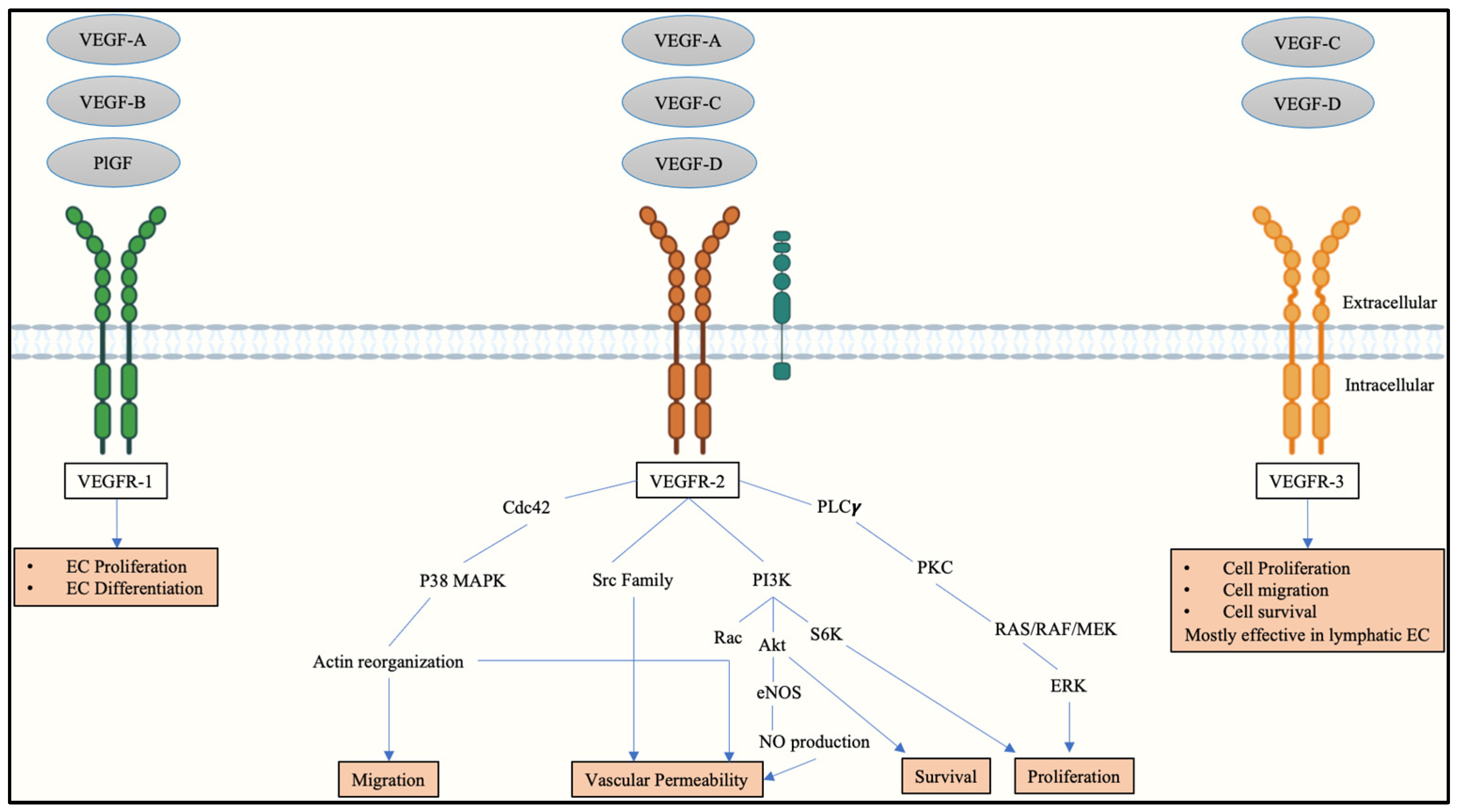
2. Vascular Endothelial Growth Factors and Receptors in Placental Angiogenesis
3. Vascular Endothelial Growth Factor Receptor-2 in a Physiologically Stable Pregnancy
4. Vascular Endothelial Growth Factor Receptor 2 in Preeclampsia
5. Human Immunodeficiency Virus
6. The Role of VEGFR-2 in HIV-Associated Preeclampsia
7. SARS-CoV-2 Infection and the Influence of VEGF and Its Receptors
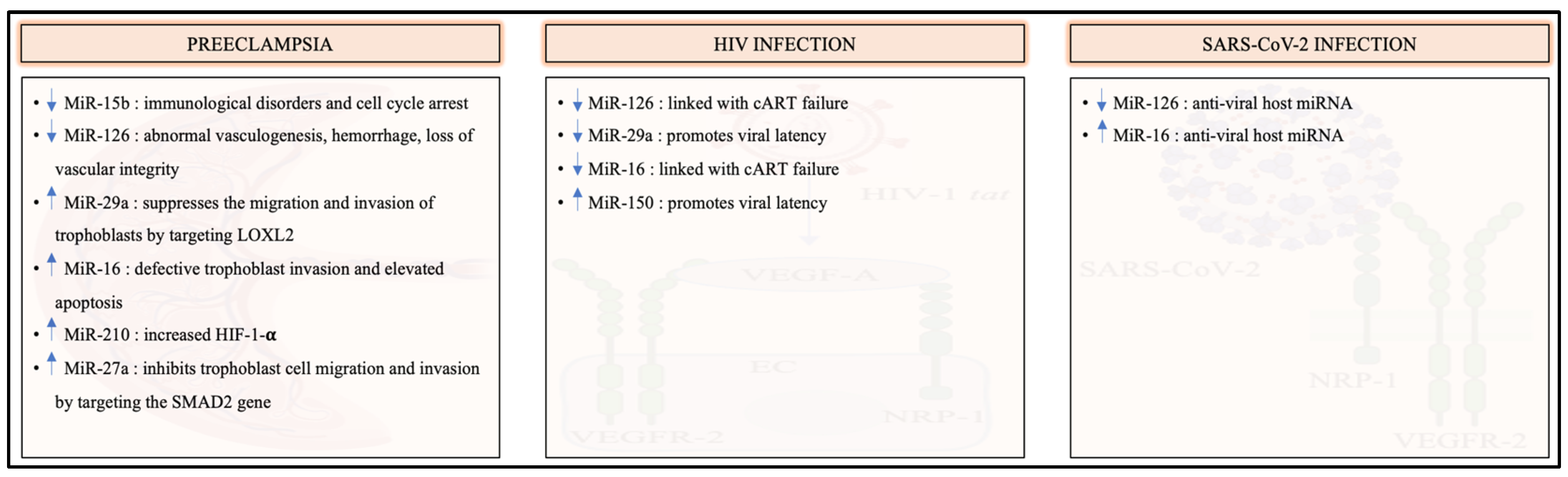
8. The Involvement of VEGFR-2-Specific MicroRNAs in Preeclampsia, HIV and SARS-CoV-2 Infection
9. Conclusions
10. Future Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Trends in Maternal Mortality 2000 to 2017: Estimates by WHO, UNICEF. 2019. Available online: https://documents.worldbank.org/en/publication/documents-reports/documentdetail/793971568908763231/trends-in-maternal-mortality-2000-to-2017-estimates-by-who-unicef-unfpa-world-bank-group-and-the-united-nations-population-division (accessed on 10 September 2022).
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Preeclampsia: Pathophysiology, challeneges, and perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.L.; Seed, P.T.; Hezelgrave, N.L.; De Greeff, A.; Lawley, E.; Conti-Ramsden, F.; Anthony, J.; Steyn, W.; Hall, D.R.; Chappell, L.C. Maternal and perinatal adverse outcomes in women with pre-eclampsia cared for at facility-level in South Africa: A prospective cohort study. J. Glob. Health 2018, 8, 020401. [Google Scholar] [CrossRef] [PubMed]
- Moodley, J.; Onyangunga, O.A.; Maharaj, N.R. Hypertensive disorders in primigravid black South African women: A one-year descriptive analysis. Hypertens. Pregnancy 2016, 35, 529–535. [Google Scholar] [CrossRef] [PubMed]
- UNAIDS. In Danger: UNAIDS Global AIDS Update 2022. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwiHnoqQ-Iz6AhVJEsAKHf61AVgQFnoECAcQAQ&url=https%3A%2F%2Fwww.unaids.org%2Fsites%2Fdefault%2Ffiles%2Fmedia_asset%2F2022-global-aids-update-summary_en.pdf&usg=AOvVaw3TRZwUGcObTLu7IfH3AOWJ (accessed on 10 September 2022).
- Machado, E.S.; Krauss, M.R.; Megazzini, K.; Coutinho, C.M.; Kreitchmann, R.; Melo, V.H.; Pilotto, J.H.; Ceriotto, M.; Hofer, C.B.; Siberry, G.K.; et al. Hypertension, preeclampsia and eclampsia among HIV-infected pregnant women from Latin America and Caribbean countries. J. Infect 2014, 68, 572–580. [Google Scholar] [CrossRef]
- Harris, K.; Yudin, M.H. HIV Infection in Pregnant Women: A 2020 Update. Prenat. Diagn. 2020, 40, 1715–1721. [Google Scholar] [CrossRef]
- Pillay, Y.; Pienaar, S.; Barron, P.; Zondi, T. Impact of COVID-19 on routine primary healthcare services in South Africa. S. Afr. Med. J. 2021, 111, 714–719. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19–11 March 2020; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Naidoo, N.; Moodley, J.; Naicker, T. Maternal endothelial dysfunction in HIV-associated preeclampsia comorbid with COVID-19: A review. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2021, 44, 386–398. [Google Scholar] [CrossRef]
- Abel, T.; Moodley, J.; Naicker, T. The Involvement of MicroRNAs in SARS-CoV-2 Infection Comorbid with HIV-Associated Preeclampsia. Curr. Hypertens. Rep. 2021, 23, 20. [Google Scholar] [CrossRef]
- Clark, D.; Smith, S.; Sharkey, A.; Charnock-Jones, D. Localization of VEGF and expression of its receptors flit and KDR in human placenta throughout pregnancy. Hum. Reprod. 1996, 11, 1090–1098. [Google Scholar] [CrossRef]
- Koch, S.; Claesson-Welsh, L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harb. Perspect. Med. 2012, 2, a006502. [Google Scholar] [CrossRef]
- Tjoa, M.L.; Khankin, E.V.; Rana, S.; Karumanchi, S.A. Angiogenic factors and preeclampsia. Placent. Bed Disord. Basic Sci. Its Transl. Obs. 2010, 31, 229–242. [Google Scholar]
- Pijnenborg, R.; Brosens, I.; Romero, R. Placental Bed Disorders: Basic Science and Its Translation to Obstetrics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Birnhuber, A.; Fließer, E.; Gorkiewicz, G.; Zacharias, M.; Seeliger, B.; David, S.; Welte, T.; Schmidt, J.; Olschewski, H.; Wygrecka, M.; et al. Between inflammation and thrombosis: Endothelial cells in COVID-19. Eur. Respir. J. 2021, 58, 2100377. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Pedroza, J.; Cárdenas-Bedoya, J.; Morán-Moguel, M.C.; Escoto-Delgadillo, M.; Torres-Mendoza, B.M.; Pérez-Ríos, A.M.; González-Enriquez, G.V.; Vázquez-Valls, E. Plasma microRNA expression levels in HIV-1-positive patients receiving antiretroviral therapy. Biosci. Rep. 2020, 40, BSR20194433. [Google Scholar] [CrossRef]
- Garg, A.; Seeliger, B.; Derda, A.A.; Xiao, K.; Gietz, A.; Scherf, K.; Sonnenschein, K.; Pink, I.; Hoeper, M.M.; Welte, T.; et al. Circulating cardiovascular microRNAs in critically ill COVID-19 patients. Eur. J. Heart Fail. 2021, 23, 468–475. [Google Scholar] [CrossRef]
- Rana, S.; Powe, C.E.; Salahuddin, S.; Verlohren, S.; Perschel, F.H.; Levine, R.J.; Lim, K.-H.; Wenger, J.B.; Thadhani, R.; Karumanchi, S.A. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012, 125, 911–919. [Google Scholar] [CrossRef]
- Heinke, J.; Patterson, C.; Moser, M. Life is a pattern: Vascular assembly within the embryo. Front. Biosci. 2012, 4, 2269–2288. [Google Scholar] [CrossRef]
- Karaman, S.; Leppänen, V.-M.; Alitalo, K. Vascular endothelial growth factor signaling in development and disease. Development 2018, 145, dev151019. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Caton, J.S.; Redmer, D.A.; Grazul-Bilska, A.T.; Vonnahme, K.A.; Borowicz, P.P.; Luther, J.S.; Wallace, J.M.; Wu, G.; Spencer, T.E. Evidence for altered placental blood flow and vascularity in compromised pregnancies. J. Physiol. 2006, 572 Pt 1, 51–58. [Google Scholar] [CrossRef]
- Mayhew, T.M.; Charnock-Jones, D.S.; Kaufmann, P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta 2004, 25, 127–139. [Google Scholar] [CrossRef]
- Bakrania, B.A.; Spradley, F.T.; Drummond, H.A.; LaMarca, B.; Ryan, M.J.; Granger, J.P. Preeclampsia: Linking Placental Ischemia with Maternal Endothelial and Vascular Dysfunction. Compr. Physiol. 2020, 11, 1315–1349. [Google Scholar] [PubMed]
- Guo, X.; Yi, H.; Li, T.C.; Wang, Y.; Wang, H.; Chen, X. Role of Vascular Endothelial Growth Factor (VEGF) in Human Embryo Implantation: Clinical Implications. Biomolecules 2021, 11, 253. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Neufeld, G.; Cohen, T.; Gengrinovitch, S.; Poltorak, Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999, 13, 9–22. [Google Scholar] [CrossRef]
- Douglas, N.C.; Tang, H.; Gomez, R.; Pytowski, B.; Hicklin, D.J.; Sauer, C.M.; Kitajewski, J.; Sauer, M.V.; Zimmermann, R.C. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology 2009, 150, 3845–3854. [Google Scholar] [CrossRef]
- Douglas, N.C.; Nakhuda, G.S.; Sauer, M.V.; Zimmermann, R.C. Angiogenesis and ovarian function. J. Fertil. Reprod. 2005, 13, 7–15. [Google Scholar]
- Yeh, J.; Kim, B.S.; Peresie, J. Ovarian vascular endothelial growth factor and vascular endothelial growth factor receptor patterns in reproductive aging. Fertil. Steril. 2008, 89 (Suppl. S5), 1546–1556. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Hartman, T.; Bohlen, P.; Sauer, M.V.; Kitajewski, J. Preovulatory treatment of mice with anti-VEGF receptor 2 antibody inhibits angiogenesis in corpora lutea. Microvasc. Res. 2001, 62, 15–25. [Google Scholar] [CrossRef]
- Pauli, S.A.; Tang, H.; Wang, J.; Bohlen, P.; Posser, R.; Hartman, T.; Sauer, M.V.; Kitajewski, J.; Zimmermann, R.C. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology 2005, 146, 1301–1311. [Google Scholar] [CrossRef][Green Version]
- Fraser, H.M.; Wulff, C. Angiogenesis in the corpus luteum. Reprod. Biol. Endocrinol. 2003, 1, 88. [Google Scholar] [CrossRef]
- Vuorela, P.; Carpén, O.; Tulppala, M.; Halmesmäki, E. VEGF, its receptors and the tie receptors in recurrent miscarriage. Mol. Hum. Reprod. 2000, 6, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Vailhé, B.; Dietl, J.; Kapp, M.; Toth, B.; Arck, P. Increased blood vessel density in decidua parietalis is associated with spontaneous human first trimester abortion. Hum. Reprod. 1999, 14, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Kashida, S.; Sugino, N.; Takiguchi, S.; Karube, A.; Takayama, H.; Yamagata, Y.; Nakamura, Y.; Kato, H. Regulation and role of vascular endothelial growth factor in the corpus luteum during mid-pregnancy in rats. Biol. Reprod. 2001, 64, 317–323. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Q.; Wang, H.; Lin, H.; Shao, L.; Ni, J.; Duan, E.; Zhu, C. Expression of vascular endothelial growth factor in rat ovary during pregnancy and postpartum. Sci. China C Life Sci. 2002, 45, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Ozaki, H.; Abe, N.; Mori, A.; Sakamoto, K.; Nagamitsu, T.; Nakahara, T.; Ishii, K. Role of vascular endothelial growth factor in maintenance of pregnancy in mice. Endocrinology 2013, 154, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamaguchi, S.; Chida, K.; Shibuya, M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. Embo J. 2001, 20, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004, 25, 581–611. [Google Scholar] [CrossRef]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef]
- Cébe-Suarez, S.; Zehnder-Fjällman, A.; Ballmer-Hofer, K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol. Life Sci. 2006, 63, 601–615. [Google Scholar] [CrossRef]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Magee, L.A.; Singer, J.; Lee, T.; Rey, E.; Asztalos, E.; Hutton, E.; Helewa, M.; Logan, A.G.; Ganzevoort, W.; Welch, R.; et al. The impact of pre-eclampsia definitions on the identification of adverse outcome risk in hypertensive pregnancy—Analyses from the CHIPS trial (Control of Hypertension in Pregnancy Study). BJOG 2021, 128, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Marín, R.; Chiarello, D.I.; Abad, C.; Rojas, D.; Toledo, F.; Sobrevia, L. Oxidative stress and mitochondrial dysfunction in early-onset and late-onset preeclampsia. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165961. [Google Scholar] [CrossRef] [PubMed]
- Shanmugalingam, R.; Hennessy, A.; Makris, A. Aspirin in the prevention of preeclampsia: The conundrum of how, who and when. J. Hum. Hypertens. 2019, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sircar, M.; Thadhani, R.; Karumanchi, S.A. Pathogenesis of preeclampsia. Curr. Opin. Nephrol. Hypertens. 2015, 24, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Brosens, J.J.; Muter, J.; Puttemans, P.; Benagiano, G. Preeclampsia: The role of persistent endothelial cells in uteroplacental arteries. Am. J. Obstet. Gynecol. 2019, 221, 219–226. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Chandel, N.; Jain, V.; Jha, V. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J. Hum. Hypertens 2012, 26, 236–241. [Google Scholar] [CrossRef]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar]
- Flint, E.J.; Cerdeira, A.S.; Redman, C.W.; Vatish, M. The role of angiogenic factors in the management of preeclampsia. Acta Obstet. Gynecol. Scand. 2019, 98, 700–707. [Google Scholar] [CrossRef]
- Knight, M.; Redman, C.W.; Linton, E.A.; Sargent, I.L. Shedding of syncytiotrophoblast microvilli into the maternal circulation in pre-eclamptic pregnancies. Br. J. Obstet. Gynaecol. 1998, 105, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Guney, G.; Taskin, M.I.; Tokmak, A. Increase of circulating inflammatory molecules in preeclampsia, an update. Eur. Cytokine Netw. 2020, 31, 18–31. [Google Scholar] [PubMed]
- Naidoo, N.; Moodley, J.; Khaliq, O.P.; Naicker, T. Neuropilin-1 in the pathogenesis of preeclampsia, HIV-1, and SARS-CoV-2 infection: A review. Virus Res. 2022, 319, 198880. [Google Scholar] [CrossRef] [PubMed]
- Lely, A.T.; Salahuddin, S.; Holwerda, K.M.; Karumanchi, S.A.; Rana, S. Circulating lymphangiogenic factors in preeclampsia. Hypertens. Pregnancy 2013, 32, 42–49. [Google Scholar] [CrossRef]
- Dubova, E.A.; Pavlov, K.A.; Borovkova, E.I.; Bayramova, M.A.; Makarov, I.O.; Shchegolev, A.I. Vascular endothelial growth factor and its receptors in the placenta of pregnant women with obesity. Bull. Exp. Biol. Med. 2011, 151, 253–258. [Google Scholar] [CrossRef]
- Escudero, C.; Celis, C.; Saez, T.; San Martin, S.; Valenzuela, F.J.; Aguayo, C.; Bertoglia, P.; Roberts, J.M.; Acurio, J. Increased placental angiogenesis in late and early onset pre-eclampsia is associated with differential activation of vascular endothelial growth factor receptor 2. Placenta 2014, 35, 207–215. [Google Scholar] [CrossRef]
- Atakul, T. Serum Levels of Angiogenic Factors Distinguish Between Women with Preeclampsia and Normotensive Pregnant Women But Not Severity of Preeclampsia in an Obstetric Center in Turkey. Med. Sci. Monit. 2019, 25, 6935–6942. [Google Scholar] [CrossRef]
- Ali, Z.; Khaliq, S.; Zaki, S.; Ahmad, H.U.; Lone, K.P. Altered expression of vascular endothelial growth factor, vascular endothelial growth factor receptor-1, vascular endothelial growth factor receptor-2, and Soluble Fms-like Tyrosine Kinase-1 in peripheral blood mononuclear cells from normal and preeclamptic pregnancies. Chin. J. Physiol. 2019, 62, 117–122. [Google Scholar]
- Cindrova-Davies, T.; Yung, H.W.; Johns, J.; Spasic-Boskovic, O.; Korolchuk, S.; Jauniaux, E.; Burton, G.J.; Charnock-Jones, D.S. Oxidative stress, gene expression, and protein changes induced in the human placenta during labor. Am. J. Pathol. 2007, 171, 1168–1179. [Google Scholar] [CrossRef]
- Nevo, O.; Lee, D.K.; Caniggia, I. Attenuation of VEGFR-2 expression by sFlt-1 and low oxygen in human placenta. PLoS ONE 2013, 8, e81176. [Google Scholar] [CrossRef]
- Munaut, C.; Lorquet, S.; Pequeux, C.; Coulon, C.; Le Goarant, J.; Chantraine, F.; Noël, A.; Goffin, F.; Tsatsaris, V.; Subtil, D. Differential expression of Vegfr-2 and its soluble form in preeclampsia. PLoS ONE 2012, 7, e33475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dellinger, M.T.; Brekken, R.A. Phosphorylation of Akt and ERK1/2 is required for VEGF-A/VEGFR2-induced proliferation and migration of lymphatic endothelium. PLoS ONE 2011, 6, e28947. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Hewett, P.W.; Al-Ani, B.; Sissaoui, S.; Fujisawa, T.; Cudmore, M.J.; Ahmed, A. Autocrine activity of soluble Flt-1 controls endothelial cell function and angiogenesis. Vasc. Cell 2011, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Olszewska-Pazdrak, B.; Hein, T.W.; Olszewska, P.; Carney, D.H. Chronic hypoxia attenuates VEGF signaling and angiogenic responses by downregulation of KDR in human endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C1162-70. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Segal, M.; Croker, B.; Johnson, R.J. A breakthrough in diabetic nephropathy: The role of endothelial dysfunction. Nephrol. Dial. Transpl. 2007, 22, 2775–2777. [Google Scholar] [CrossRef][Green Version]
- Berg, D.; Sonsalla, R.; Kuss, E. Concentrations of 2-methoxyoestrogens in human serum measured by a heterologous immunoassay with an 125I-labelled ligand. Acta Endocrinol. 1983, 103, 282–288. [Google Scholar] [CrossRef]
- Lee, D.K.; Nevo, O. 2-Methoxyestradiol regulates VEGFR-2 and sFlt-1 expression in human placenta. Placenta 2015, 36, 125–130. [Google Scholar] [CrossRef]
- Tooke, L.; Riemer, L.; Matjila, M.; Harrison, M. Antiretrovirals causing severe pre-eclampsia. Pregnancy Hypertens. 2016, 6, 266–268. [Google Scholar] [CrossRef]
- Joint United Nations Programme on HIV/AIDS Global HIV & AIDS Statistics—2022 Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 10 September 2022).
- Stats SA. Mid-Year Population Estimates 2022; Stats SA: Pretoria, South Africa, 2022. [Google Scholar]
- World Health Organization Maternal Health. Available online: https://www.afro.who.int/health-topics/maternal-health (accessed on 30 September 2022).
- Thapa, S.; Shrestha, U. Immune Reconstitution Inflammatory Syndrome. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sebitloane, H.M.; Moodley, J.; Sartorius, B. Associations between HIV, highly active anti-retroviral therapy, and hypertensive disorders of pregnancy among maternal deaths in South Africa 2011–2013. Int. J. Gynecol. Obstet. 2017, 136, 195–199. [Google Scholar] [CrossRef]
- Maartens, G.; Celum, C.; Lewin, S.R. HIV infection: Epidemiology, pathogenesis, treatment, and prevention. Lancet 2014, 384, 258–271. [Google Scholar] [CrossRef]
- Landi, B.; Bezzeccheri, V.; Guerra, B.; Piemontese, M.; Cervi, F.; Cecchi, L.; Margarito, E.; Giannubilo, S.R.; Ciavattini, A.; Tranquilli, A.L. HIV infection in pregnancy and the risk of gestational hypertension and preeclampsia. World J. Cardiovasc. Dis. 2014, 4, 5. [Google Scholar] [CrossRef][Green Version]
- Kalumba, V.M.; Moodley, J.; Naidoo, T.D. Is the prevalence of pre-eclampsia affected by HIV/AIDS? A retrospective case-control study. Cardiovasc. J. Afr. 2013, 24, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Moodley, J. Impact of HIV on the incidence of pre-eclampsia. Cardiovasc. J. Afr. 2013, 24, 5. [Google Scholar] [PubMed]
- Wimalasundera, R.C.; Larbalestier, N.; Smith, J.H.; de Ruiter, A.; Mc, G.T.S.A.; Hughes, A.D.; Poulter, N.; Regan, L.; Taylor, G.P. Pre-eclampsia, antiretroviral therapy, and immune reconstitution. Lancet 2002, 360, 1152–1154. [Google Scholar] [CrossRef]
- Debaisieux, S.; Rayne, F.; Yezid, H.; Beaumelle, B. The ins and outs of HIV-1 Tat. Traffic 2012, 13, 355–363. [Google Scholar] [CrossRef]
- Rusnati, M.; Presta, M. HIV-1 Tat protein and endothelium: From protein/cell interaction to AIDS-associated pathologies. Angiogenesis 2002, 5, 141–151. [Google Scholar] [CrossRef]
- Albini, A.; Soldi, R.; Giunciuclio, D.; Giraudo, E.; Benelli, R.; Primo, L.; Noonan, D.; Salio, M.; Camussi, G.; Rock, W. The angiogenesis induced by HIV–1 Tat protein is mediated by the Flk–1/KDR receptor on vascular endothelial cells. Nat. Med. 1996, 2, 1371–1375. [Google Scholar] [CrossRef]
- Albini, A.; Benelli, R.; Presta, M.; Rusnati, M.; Ziche, M.; Rubartelli, A.; Paglialunga, G.; Bussolino, F.; Noonan, D. HIV-tat protein is a heparin-binding angiogenic growth factor. Oncogene 1996, 12, 289–297. [Google Scholar]
- Albini, A.; Fontanini, G.; Masiello, L.; Tacchetti, C.; Bigini, D.; Luzzi, P.; Noonan, D.M.; Stetler-Stevenson, W.G. Angiogenic potential in vivo by Kaposi’s sarcoma cell-free supernatants and HIV-1 tat product: Inhibition of KS-like lesions by tissue inhibitor of metalloproteinase-2. AIDS 1994, 8, 1237–1244. [Google Scholar] [CrossRef]
- Govender, N.; Naicker, T.; Moodley, J. Maternal imbalance between pro-angiogenic and anti-angiogenic factors in HIV-infected women with pre-eclampsia. Cardiovasc. J. Afr. 2013, 24, 174–179. [Google Scholar] [CrossRef]
- Gavalas, N.G.; Tsiatas, M.; Tsitsilonis, O.; Politi, E.; Ioannou, K.; Ziogas, A.C.; Rodolakis, A.; Vlahos, G.; Thomakos, N.; Haidopoulos, D.; et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 2012, 107, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Browne, J.L.; Schrier, V.J.; Grobbee, D.E.; Peters, S.A.; Klipstein-Grobusch, K. HIV, Antiretroviral Therapy, and Hypertensive Disorders in Pregnancy: A Systematic Review and Meta-analysis. J. Acquir. Immune Defic. Syndr. 2015, 70, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Li, T.; Zhang, S.; Wang, L.; Wu, X.; Liu, J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1629–1635. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 2 September 2022).
- Sheffield, J. Coronavirus and Pregnancy: What You Should Know. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/coronavirus-and-covid-19-what-pregnant-women-need-to-know (accessed on 1 September 2022).
- Fell, D.B.; Dhinsa, T.; Alton, G.D.; Török, E.; Dimanlig-Cruz, S.; Regan, A.K.; Sprague, A.E.; Buchan, S.A.; Kwong, J.C.; Wilson, S.E.; et al. Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. JAMA 2022, 327, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Balfe, P.; Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Warren, F.; Crook, D.W.; Jeffery, K.; Matthews, P.C.; Klerman, E.B.; et al. Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers. J. Biol. Rhythm. 2022, 37, 124–129. [Google Scholar] [CrossRef]
- Budhram, S.; Vannevel, V.; Botha, T.; Chauke, L.; Bhoora, S.; Balie, G.M.; Odell, N.; Lombaard, H.; Wise, A.; Georgiou, C.; et al. Maternal characteristics and pregnancy outcomes of hospitalized pregnant women with SARS-CoV-2 infection in South Africa: An International Network of Obstetric Survey Systems-based cohort study. Int. J. Gynaecol. Obstet. 2021, 155, 455–465. [Google Scholar] [CrossRef]
- Villar, J.; Ariff, S.; Gunier, R.B.; Thiruvengadam, R.; Rauch, S.; Kholin, A.; Roggero, P.; Prefumo, F.; do Vale, M.S.; Cardona-Perez, J.A.; et al. Maternal and Neonatal Morbidity and Mortality Among Pregnant Women With and Without COVID-19 Infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021, 175, 817–826. [Google Scholar] [CrossRef]
- Basu, J.K.; Chauke, L.; Magoro, T. Maternal mortality from COVID 19 among South African pregnant women. J. Matern. Fetal. Neonatal. Med. 2021, 1–3. [Google Scholar] [CrossRef]
- Goldshtein, I.; Steinberg, D.M.; Kuint, J.; Chodick, G.; Segal, Y.; Shapiro Ben David, S.; Ben-Tov, A. Association of BNT162b2 COVID-19 Vaccination During Pregnancy With Neonatal and Early Infant Outcomes. JAMA Pediatr. 2022, 176, 470–477. [Google Scholar] [CrossRef]
- Bookstein Peretz, S.; Regev, N.; Novick, L.; Nachshol, M.; Goffer, E.; Ben-David, A.; Asraf, K.; Doolman, R.; Levin, E.G.; Regev Yochay, G.; et al. Short-term outcome of pregnant women vaccinated with BNT162b2 mRNA COVID-19 vaccine. Ultrasound. Obs. Gynecol. 2021, 58, 450–456. [Google Scholar] [CrossRef]
- Valcarce, V.; Stafford, L.S.; Neu, J.; Cacho, N.; Parker, L.; Mueller, M.; Burchfield, D.J.; Li, N.; Larkin, J., III. Detection of SARS-CoV-2-Specific IgA in the Human Milk of COVID-19 Vaccinated Lactating Health Care Workers. Breastfeed. Med. 2021, 16, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Tripathi, D.M.; Yadav, A. The Enigma of Endothelium in COVID-19. Front. Physiol. 2020, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; van der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial activation and dysfunction in COVID-19: From basic mechanisms to potential therapeutic approaches. Signal Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Shanes, E.D.; Mithal, L.B.; Otero, S.; Azad, H.A.; Miller, E.S.; Goldstein, J.A. Placental Pathology in COVID-19. Am. J. Clin. Pathol. 2020, 154, 23–32. [Google Scholar] [CrossRef]
- Giardini, V.; Carrer, A.; Casati, M.; Contro, E.; Vergani, P.; Gambacorti-Passerini, C. Increased sFLT-1/PlGF ratio in COVID-19: A novel link to angiotensin II-mediated endothelial dysfunction. Am. J. Hematol. 2020, 95, e188–e191. [Google Scholar] [CrossRef]
- Hernández-Pacheco, J.A.; Rosales-Zamudio, C.I.; Borboa-Olivares, H.; Espejel-Núñez, A.; Parra-Hernández, S.; Estrada-Gutiérrez, G.; Camargo-Marín, L.; Medina-Bastidas, D.; Guzmán-Huerta, M. The sFlt-1/PlGF ratio as a triage tool to identify superimposed preeclampsia in women with chronic hypertension in emergency rooms. Pregnancy Hypertens. 2020, 21, 38–42. [Google Scholar] [CrossRef]
- Phoswa, W.N.; Khaliq, O.P. Is pregnancy a risk factor of COVID-19? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 605–609. [Google Scholar] [CrossRef]
- Xu, X.H.; Tang, L.C.; Hao, F.; Jin, L.P. Upregulation of miR-29a suppressed the migration and invasion of trophoblasts by directly targeting LOXL2 in preeclampsia. J. Hypertens. 2021, 39, 1642–1651. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gao, Y.; Li, Z.; Miao, Y.; Huang, Z.; Liu, X.; Xie, L.; Li, H.; Wen, W.; Zheng, Y.; et al. The noncoding and coding transcriptional landscape of the peripheral immune response in patients with COVID-19. Clin. Transl. Med. 2020, 10, e200. [Google Scholar] [CrossRef] [PubMed]
- Keikha, R.; Hashemi-Shahri, S.M.; Jebali, A. The relative expression of miR-31, miR-29, miR-126, and miR-17 and their mRNA targets in the serum of COVID-19 patients with different grades during hospitalization. Eur. J. Med. Res. 2021, 26, 75. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, X.; Sun, Q.; Dai, X.; Cai, Y. MicroRNA-16 is involved in the pathogenesis of pre-eclampsia via regulation of Notch2. J. Cell Physiol. 2020, 235, 4530–4544. [Google Scholar] [CrossRef] [PubMed]
- Hazem, R.M.; Mohamed, A.A.; Ghareb, N.; Mehanna, E.T.; Mesbah, N.M.; Abo-Elmatty, D.M.; Elgawish, M.S. Anti-cancer activity of two novel heterocyclic compounds through modulation of VEGFR and miR-122 in mice bearing Ehrlich ascites carcinoma. Eur. J. Pharmacol. 2021, 892, 173747. [Google Scholar] [CrossRef]
- Fish, J.E.; Santoro, M.M.; Morton, S.U.; Yu, S.; Yeh, R.F.; Wythe, J.D.; Ivey, K.N.; Bruneau, B.G.; Stainier, D.Y.; Srivastava, D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell 2008, 15, 272–284. [Google Scholar] [CrossRef]
- Sardar, R.; Satish, D.; Birla, S.; Gupta, D. Comparative analyses of SAR-CoV2 genomes from different geographical locations and other coronavirus family genomes reveals unique features potentially consequential to host-virus interaction and pathogenesis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Houzet, L.; Yeung, M.L.; de Lame, V.; Desai, D.; Smith, S.M.; Jeang, K.T. MicroRNA profile changes in human immunodeficiency virus type 1 (HIV-1) seropositive individuals. Retrovirology 2008, 5, 118. [Google Scholar] [CrossRef]
- Ahluwalia, J.K.; Khan, S.Z.; Soni, K.; Rawat, P.; Gupta, A.; Hariharan, M.; Scaria, V.; Lalwani, M.; Pillai, B.; Mitra, D.; et al. Human cellular microRNA hsa-miR-29a interferes with viral nef protein expression and HIV-1 replication. Retrovirology 2008, 5, 117. [Google Scholar] [CrossRef]
- Nersisyan, S.; Engibaryan, N.; Gorbonos, A.; Kirdey, K.; Makhonin, A.; Tonevitsky, A. Potential role of cellular miRNAs in coronavirus-host interplay. PeerJ 2020, 8, e9994. [Google Scholar] [CrossRef]
- Bounds, K.R.; Chiasson, V.L.; Pan, L.J.; Gupta, S.; Chatterjee, P. MicroRNAs: New Players in the Pathobiology of Preeclampsia. Front. Cardiovasc. Med. 2017, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Romero, R.; Kim, J.S.; Tarca, A.L.; Montenegro, D.; Pineles, B.L.; Kim, E.; Lee, J.; Kim, S.Y.; Draghici, S.; et al. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: Siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am. J. Pathol. 2011, 179, 590–602. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, V.; Ralfkiaer, U.; Pedersen, K.K.; Hove, M.; Koplev, S.; Brændstrup, P.; Ryder, L.P.; Madsen, H.O.; Gerstoft, J.; Grønbæk, K.; et al. MicroRNA-210, MicroRNA-331, and MicroRNA-7 Are Differentially Regulated in Treated HIV-1-Infected Individuals and Are Associated With Markers of Systemic Inflammation. J. Acquir. Immune Defic. Syndr. 2017, 74, e104–e113. [Google Scholar] [CrossRef]
- Zeng, Y.; Wei, L.; Lali, M.S.; Chen, Y.; Yu, J.; Feng, L. miR-150-5p mediates extravillous trophoblast cell migration and angiogenesis functions by regulating VEGF and MMP9. Placenta 2020, 93, 94–100. [Google Scholar] [CrossRef]
- Dubey, R.C.; Alam, N.B.; Gaur, R. miR-150-mediated increase in glucose uptake in HIV-infected cells. J. Med. Virol. 2021, 93, 6377–6382. [Google Scholar] [CrossRef]
- Lasabová, Z.; Vazan, M.; Zibolenova, J.; Svecova, I. Overexpression of miR-21 and miR-122 in preeclamptic placentas. Neuro Endocrinol. Lett. 2015, 36, 695–699. [Google Scholar] [PubMed]
- Moghoofei, M.; Najafipour, S.; Mostafaei, S.; Tavakoli, A.; Bokharaei-Salim, F.; Ghorbani, S.; Javanmard, D.; Ghaffari, H.; Monavari, S.H. MicroRNAs Profiling in HIV, HCV, and HIV/HCV Co-Infected Patients. Curr. HIV Res. 2021, 19, 27–34. [Google Scholar] [PubMed]
- Zheng, W.; Chen, A.; Yang, H.; Hong, L. MicroRNA-27a inhibits trophoblast cell migration and invasion by targeting SMAD2: Potential role in preeclampsia. Exp. Ther. Med. 2020, 20, 2262–2269. [Google Scholar] [CrossRef]
- Yuan, Z.; Petree, J.R.; Lee, F.E.; Fan, X.; Salaita, K.; Guidot, D.M.; Sadikot, R.T. Macrophages exposed to HIV viral protein disrupt lung epithelial cell integrity and mitochondrial bioenergetics via exosomal microRNA shuttling. Cell Death Dis. 2019, 10, 580. [Google Scholar] [CrossRef]
- Zhang, Y.; Fei, M.; Xue, G.; Zhou, Q.; Jia, Y.; Li, L.; Xin, H.; Sun, S. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: New insights into molecular mechanisms for the disease. J. Cell Mol. Med. 2012, 16, 249–259. [Google Scholar] [CrossRef]
- Huang, J.; Wang, F.; Argyris, E.; Chen, K.; Liang, Z.; Tian, H.; Huang, W.; Squires, K.; Verlinghieri, G.; Zhang, H. Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 2007, 13, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Agudo, J.; Ruzo, A.; Tung, N.; Salmon, H.; Leboeuf, M.; Hashimoto, D.; Becker, C.; Garrett-Sinha, L.A.; Baccarini, A.; Merad, M.; et al. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014, 15, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Wan, L.; Jie, Z.; Zhu, X.; Yin, J.; Cao, H. Upregulated miR-27a-3p Indicates a Poor Prognosis in Pancreatic Carcinoma Patients and Promotes the Angiogenesis and Migration by Epigenetic Silencing of GATA6 and Activating VEGFA/VEGFR2 Signaling Pathway. Onco Targets Ther. 2019, 12, 11241–11254. [Google Scholar] [CrossRef]
- Chan, L.S.; Yue, P.Y.; Wong, Y.Y.; Wong, R.N. MicroRNA-15b contributes to ginsenoside-Rg1-induced angiogenesis through increased expression of VEGFR-2. Biochem. Pharmacol. 2013, 86, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Huang, M.; Lv, M.; He, Y.; Duan, C.; Zhang, L.; Chen, J. Circular RNA MYLK as a competing endogenous RNA promotes bladder cancer progression through modulating VEGFA/VEGFR2 signaling pathway. Cancer Lett. 2017, 403, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Chamorro-Jorganes, A.; Araldi, E.; Penalva, L.O.; Sandhu, D.; Fernández-Hernando, C.; Suárez, Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells via targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, H.; Gokmen, E.; Bozkurt, G.; Kocaturk, T.; Ergin, K. Effects of sunitinib and bevacizumab on VEGF and miRNA levels on corneal neovascularization. Cutan. Ocul. Toxicol. 2018, 37, 191–195. [Google Scholar] [CrossRef]
- Shi, L.; Kim, A.J.; Chang, R.C.; Chang, J.Y.; Ying, W.; Ko, M.L.; Zhou, B.; Ko, G.Y. Deletion of miR-150 Exacerbates Retinal Vascular Overgrowth in High-Fat-Diet Induced Diabetic Mice. PLoS ONE 2016, 11, e0157543. [Google Scholar] [CrossRef]

| MicroRNA | PE | HIV | COVID-19 | References |
|---|---|---|---|---|
| miR-15b | Downregulated | Downregulated | Upregulated | [18,112] |
| miR-126 | Downregulated | Downregulated | Downregulated | [18,19] |
| miR-29a | Upregulated | Downregulated | Downregulated | [111,113] |
| miR-16 | Upregulated | Downregulated | Upregulated | [18,114] |
| MicroRNA | PE | HIV | References |
|---|---|---|---|
| miR-210 | Upregulated | Upregulated | [121,122,123] |
| miR-150 | Upregulated | Upregulation | [124,125] |
| miR-122 | Upregulated | Upregulated | [126,127] |
| miR-27a | Upregulated | Upregulated | [128,129] |
| MicroRNA | VEGFR-2 Expression | References |
|---|---|---|
| miR-15b | Downregulation | [134] |
| miR-126 | Upregulation | [132] |
| miR-29a | Downregulation | [135] |
| miR-122 | Upregulation | [115] |
| miR-27a | Upregulation | [133] |
| miR-16 | Downregulation | [136] |
| miR-210 | Downregulation | [137] |
| miR-150 | Downregulation | [138] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abel, T.; Moodley, J.; Khaliq, O.P.; Naicker, T. Vascular Endothelial Growth Factor Receptor 2: Molecular Mechanism and Therapeutic Potential in Preeclampsia Comorbidity with Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2 Infections. Int. J. Mol. Sci. 2022, 23, 13752. https://doi.org/10.3390/ijms232213752
Abel T, Moodley J, Khaliq OP, Naicker T. Vascular Endothelial Growth Factor Receptor 2: Molecular Mechanism and Therapeutic Potential in Preeclampsia Comorbidity with Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2 Infections. International Journal of Molecular Sciences. 2022; 23(22):13752. https://doi.org/10.3390/ijms232213752
Chicago/Turabian StyleAbel, Tashlen, Jagidesa Moodley, Olive P. Khaliq, and Thajasvarie Naicker. 2022. "Vascular Endothelial Growth Factor Receptor 2: Molecular Mechanism and Therapeutic Potential in Preeclampsia Comorbidity with Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2 Infections" International Journal of Molecular Sciences 23, no. 22: 13752. https://doi.org/10.3390/ijms232213752
APA StyleAbel, T., Moodley, J., Khaliq, O. P., & Naicker, T. (2022). Vascular Endothelial Growth Factor Receptor 2: Molecular Mechanism and Therapeutic Potential in Preeclampsia Comorbidity with Human Immunodeficiency Virus and Severe Acute Respiratory Syndrome Coronavirus 2 Infections. International Journal of Molecular Sciences, 23(22), 13752. https://doi.org/10.3390/ijms232213752





