The Reentry Helix Is Potentially Involved in Cholesterol Sensing of the ABCG1 Transporter Protein
Abstract
1. Introduction
2. Materials and Methods
2.1. Insertion of the ABCG Protein Sequence into the pEGFP-N1 Vector and Expression in Mammalian Cells
- ABCG1S:
- GGCCCTCTGAAGAGGACTCCTCGTCCATGGCCATGGACGAGGAGTCCTCTTCAGAGGGCC
- L430A:
- CCTCATTGGCCTGCTGTACGCGGGGATCGGGAACGAAGCCGGCTTCGTTCCCGATCCCCGCGTACAGCAGGCCAATGAGG
- L626A:
- CCAGAAGTCGGAGGCCATCGCACGGGAGCTGGACGTGGCCACGTCCAGCTCCCGTGCGATGGCCTCCGACTTCTGG
- Y586A:
- CGTACCTACAGTGGATGTCCGCCATCTCCTATGTCAGGTATGGCCATACCTGACATAGGAGATGGCGGACATCCACTGTAGGTACG
- Y655A:
- CCCTCCGCCTCATTGCCGCATTTGTCCTCAGGTACAAAATCCGCGGATTTTGTACCTGAGGACAAATGCGGCAATGAGGCGGAGGG
- Y660A:
- GCCTATTTTGTCCTCAGGGCCAAAATCCGTGCAGAGAGGCCTCTCTGCACGGATTTTGGCCCTGAGGACAAAATAGGC
- F571A:
- GCTCCTGTTCTCGGGGTTCGCCGTCAGCTTCGACACCATCCGGATGGTGTCGAAGCTGACGGCGAACCCCGAGAACAGGAGC
2.2. Expression of ABCG1 and ABCG4 Variants in Insect Cells; Assessment of Their ATPase Activity
2.3. Immunoblot Experiments
2.4. Immunofluorescence Staining
2.5. Detection of Apoptosis
2.6. Structure Visualisation
3. Results
3.1. The Effect of Sterol Compounds on the ATPase Activity of ABCG1 Isoforms
3.2. Effect of Mutations in the Potential Sterol-Binding Sites in ABCG1 on Its ATPase Activity
3.3. Expression and Apoptotic Effect of ABCG1 Mutants in Mammalian Cells
3.4. Positions of Variuos CRAC and SBE Mutation in the ABCG1 Structure
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kerr, I.D.; Hutchison, E.; Gerard, L.; Aleidi, S.M.; Gelissen, I.C. Mammalian ABCG-transporters, sterols and lipids: To bind perchance to transport? Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158860. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kinch, L.N.; Borek, D.M.; Wang, J.; Urbatsch, I.L.; Xie, X.S.; Grishin, N.V.; Cohen, J.C.; Otwinowski, Z.; Hobbs, H.H.; et al. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature 2017, 533, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Manolaridis, I.; Jackson, S.M.; Taylor, N.M.I.; Kowal, J.; Stahlberg, H.; Locher, K.P. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature 2018, 563, 426–430. [Google Scholar] [CrossRef]
- Taylor, N.M.I.; Manolaridis, I.; Jackson, S.M.; Kowal, J.; Stahlberg, H.; Locher, K.P. Structure of the human multidrug transporter ABCG2. Nature 2016, 546, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Orlando, B.J.; Liao, M. ABCG2 transports anticancer drugs via a closed-to-open switch. Nat. Commun. 2020, 11, 2264. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.M.; Manolaridis, I.; Kowal, J.; Zechner, M.; Taylor, N.M.I.; Bause, M.; Bauer, S.; Bartholomaeus, R.; Bernhardt, G.; Koenig, B.; et al. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat. Struct. Mol. Biol. 2021, 25, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Skarda, L.; Kowal, J.; Locher, K.P. Structure of the Human Cholesterol Transporter ABCG1. J. Mol. Biol. 2021, 433, 167218. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Long, T.; Qi, X.; Donnelly, L.; Elghobashi-Meinhardt, N.; Esparza, L.; Cohen, J.C.; Xie, X.S.; Hobbs, H.H.; et al. Molecular basis of cholesterol efflux via ABCG subfamily transporters. Proc. Natl. Acad. Sci. USA 2018, 118, e2110483118. [Google Scholar] [CrossRef]
- Tordai, H.; Suhajda, E.; Sillitoe, I.; Nair, S.; Varadi, M.; Hegedus, T. Comprehensive Collection and Prediction of ABC Transmembrane Protein Structures in the AI Era of Structural Biology. Int. J. Mol. Sci. 2022, 23, 8877. [Google Scholar] [CrossRef]
- Engel, T.; Bode, G.; Lueken, A.; Knop, M.; Kannenberg, F.; Nofer, J.R.; Assmann, G.; Seedorf, U. Expression and functional characterization of ABCG1 splice variant ABCG1(666). FEBS Lett. 2006, 580, 4551–4559. [Google Scholar] [CrossRef]
- Gelissen, I.C.; Cartland, S.; Brown, A.J.; Sandoval, C.; Kim, M.; Dinnes, D.L.; Lee, Y.; Hsieh, V.; Gaus, K.; Kritharides, L.; et al. Expression and stability of two isoforms of ABCG1 in human vascular cells. Atherosclerosis 2010, 208, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Lan, D.; Chen, W.; Matsuura, F.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc. Natl. Acad. Sci. USA 2004, 101, 9774–9779. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Barrera, G.C.; Nakamura, K.; Baldan, A.; Tarr, P.; Fishbein, M.C.; Frank, J.; Francone, O.L.; Edwards, P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell. Metab. 2005, 1, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Baldan, A.; Tarr, P.; Lee, R.; Edwards, P.A. ATP-binding cassette transporter G1 and lipid homeostasis. Curr. Opin. Lipidol. 2006, 17, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Terasaka, N.; Wang, N.; Yvan-Charvet, L.; Tall, A.R. High-density lipoprotein protects macrophages from oxidized low-density lipoprotein-induced apoptosis by promoting efflux of 7-ketocholesterol via ABCG1. Proc. Natl. Acad. Sci. USA 2007, 104, 15093–15098. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yvan-Charvet, L.; Lutjohann, D.; Mulder, M.; Vanmierlo, T.; Kim, T.W.; Tall, A.R. ATP-binding cassette transporters G1 and G4 mediate cholesterol and desmosterol efflux to HDL and regulate sterol accumulation in the brain. FASEB J. 2008, 22, 1073–1082. [Google Scholar] [CrossRef]
- Bojanic, D.D.; Tarr, P.T.; Gale, G.D.; Smith, D.J.; Bok, D.; Chen, B.; Nusinowitz, S.; Lovgren-Sandblom, A.; Bjorkhem, I.; Edwards, P.A. Differential expression and function of ABCG1 and ABCG4 during development and aging. J. Lipid Res. 2010, 51, 169–181. [Google Scholar] [CrossRef]
- Wang, N.; Ranalletta, M.; Matsuura, F.; Peng, F.; Tall, A.R. LXR-induced redistribution of ABCG1 to plasma membrane in macrophages enhances cholesterol mass efflux to HDL. Arter. Thromb. Vasc. Biol. 2006, 26, 1310–1316. [Google Scholar] [CrossRef]
- Vaughan, A.M.; Oram, J.F. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J. Lipid Res. 2006, 47, 2433–2443. [Google Scholar] [CrossRef]
- Gelissen, I.C.; Harris, M.; Rye, K.A.; Quinn, C.; Brown, A.J.; Kockx, M.; Cartland, S.; Packianathan, M.; Kritharides, L.; Jessup, W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arter. Thromb. Vasc. Biol. 2006, 26, 534–540. [Google Scholar] [CrossRef]
- Kobayashi, A.; Takanezawa, Y.; Hirata, T.; Shimizu, Y.; Misasa, K.; Kioka, N.; Arai, H.; Ueda, K.; Matsuo, M. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J. Lipid Res. 2006, 47, 1791–1802. [Google Scholar] [CrossRef] [PubMed]
- Sano, O.; Kobayashi, A.; Nagao, K.; Kumagai, K.; Kioka, N.; Hanada, K.; Ueda, K.; Matsuo, M. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J. Lipid Res. 2007, 48, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Kimura, Y.; Kioka, N.; Matsuo, M.; Ueda, K. ATPase activity of human ABCG1 is stimulated by cholesterol and sphingomyelin. J. Lipid Res. 2013, 54, 496–502. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Kerksiek, A.; Gebert, K.; Annema, W.; Sibler, R.; Radosavljevic, S.; Lutjohann, D.; Rohrer, L.; von Eckardstein, A. HDL inhibits endoplasmic reticulum stress-induced apoptosis of pancreatic beta-cells in vitro by activation of Smoothened. J. Lipid Res. 2020, 61, 492–504. [Google Scholar] [CrossRef] [PubMed]
- Tarling, E.J.; Bojanic, D.D.; Tangirala, R.K.; Wang, X.; Lovgren-Sandblom, A.; Lusis, A.J.; Bjorkhem, I.; Edwards, P.A. Impaired development of atherosclerosis in Abcg1-/- Apoe-/- mice: Identification of specific oxysterols that both accumulate in Abcg1-/- Apoe-/- tissues and induce apoptosis. Arter. Thromb. Vasc. Biol. 2010, 30, 1174–1180. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Kannenberg, F.; Fobker, M.; Nofer, J.R.; Bode, G.; Lueken, A.; Assmann, G.; Seedorf, U. Expression of ATP binding cassette-transporter ABCG1 prevents cell death by transporting cytotoxic 7beta-hydroxycholesterol. FEBS Lett. 2007, 581, 1673–1680. [Google Scholar] [CrossRef]
- Seres, L.; Cserepes, J.; Elkind, N.B.; Torocsik, D.; Nagy, L.; Sarkadi, B.; Homolya, L. Functional ABCG1 expression induces apoptosis in macrophages and other cell types. Biochim. Biophys. Acta 2008, 1778, 2378–2387. [Google Scholar] [CrossRef]
- Hegyi, Z.; Homolya, L. Functional Cooperativity between ABCG4 and ABCG1 Isoforms. PLoS ONE 2016, 11, e0156516. [Google Scholar] [CrossRef]
- Epand, R.F.; Thomas, A.; Brasseur, R.; Vishwanathan, S.A.; Hunter, E.; Epand, R.M. Juxtamembrane protein segments that contribute to recruitment of cholesterol into domains. Biochemistry 2006, 45, 6105–6114. [Google Scholar] [CrossRef]
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef]
- Gal, Z.; Hegedus, C.; Szakacs, G.; Varadi, A.; Sarkadi, B.; Ozvegy-Laczka, C. Mutations of the central tyrosines of putative cholesterol recognition amino acid consensus (CRAC) sequences modify folding, activity, and sterol-sensing of the human ABCG2 multidrug transporter. Biochim. Biophys. Acta 2015, 1848, 477–487. [Google Scholar] [CrossRef]
- Velamakanni, S.; Janvilisri, T.; Shahi, S.; van Veen, H.W. A functional steroid-binding element in an ATP-binding cassette multidrug transporter. Mol. Pharm. 2008, 73, 12–17. [Google Scholar] [CrossRef]
- Cserepes, J.; Szentpetery, Z.; Seres, L.; Ozvegy-Laczka, C.; Langmann, T.; Schmitz, G.; Glavinas, H.; Klein, I.; Homolya, L.; Varadi, A.; et al. Functional expression and characterization of the human ABCG1 and ABCG4 proteins: Indications for heterodimerization. Biochem. Biophys. Res. Commun. 2004, 320, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sarkadi, B.; Price, E.M.; Boucher, R.C.; Germann, U.A.; Scarborough, G.A. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 1992, 267, 4854–4858. [Google Scholar] [CrossRef]
- Orban, T.I.; Seres, L.; Ozvegy-Laczka, C.; Elkind, N.B.; Sarkadi, B.; Homolya, L. Combined localization and real-time functional studies using a GFP-tagged ABCG2 multidrug transporter. Biochem. Biophys. Res. Commun. 2008, 367, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Telbisz, A.; Muller, M.; Ozvegy-Laczka, C.; Homolya, L.; Szente, L.; Varadi, A.; Sarkadi, B. Membrane cholesterol selectively modulates the activity of the human ABCG2 multidrug transporter. Biochim. Biophys. Acta 2007, 1768, 2698–2713. [Google Scholar] [CrossRef]
- Homolya, L. Medically Important Alterations in Transport Function and Trafficking of ABCG2. Int. J. Mol. Sci. 2021, 22, 2786. [Google Scholar] [CrossRef]
- Khunweeraphong, N.; Szollosi, D.; Stockner, T.; Kuchler, K. The ABCG2 multidrug transporter is a pump gated by a valve and an extracellular lid. Nat. Commun. 2019, 10, 5433. [Google Scholar] [CrossRef]
- Ilias, A.; Urban, Z.; Seidl, T.L.; Le Saux, O.; Sinko, E.; Boyd, C.D.; Sarkadi, B.; Varadi, A. Loss of ATP-dependent transport activity in pseudoxanthoma elasticum-associated mutants of human ABCC6 (MRP6). J. Biol. Chem. 2002, 277, 16860–16867. [Google Scholar] [CrossRef]
- Ishikawa, T.; Kasamatsu, S.; Hagiwara, Y.; Mitomo, H.; Kato, R.; Sumino, Y. Expression and functional characterization of human ABC transporter ABCG2 variants in insect cells. Drug Metab. Pharmacokinet. 2003, 18, 194–202. [Google Scholar] [CrossRef]
- Pal, A.; Mehn, D.; Molnar, E.; Gedey, S.; Meszaros, P.; Nagy, T.; Glavinas, H.; Janaky, T.; von Richter, O.; Bathori, G.; et al. Cholesterol potentiates ABCG2 activity in a heterologous expression system: Improved in vitro model to study function of human ABCG2. J. Pharmacol. Exp. Ther. 2007, 321, 1085–1094. [Google Scholar] [CrossRef]
- Bakos, E.; Homolya, L. Portrait of multifaceted transporter, the multidrug resistance-associated protein 1 (MRP1/ABCC1). Pflug. Arch. Eur. J. Physiol. 2007, 453, 621–641. [Google Scholar] [CrossRef] [PubMed]
- Telbisz, A.; Hegedus, C.; Varadi, A.; Sarkadi, B.; Ozvegy-Laczka, C. Regulation of the function of the human ABCG2 multidrug transporter by cholesterol and bile acids: Effects of mutations in potential substrate and steroid binding sites. Drug Metab. Dispos. Biol. Fate Chem. 2014, 42, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, C.; Elliott, J.I.; Sardini, A.; Litman, T.; Stieger, B.; Meier, P.J.; Higgins, C.F. Functional analysis of candidate ABC transporter proteins for sitosterol transport. Biochim. Biophys. Acta 2002, 1567, 133–142. [Google Scholar] [CrossRef][Green Version]
- Graf, G.A.; Yu, L.; Li, W.P.; Gerard, R.; Tuma, P.L.; Cohen, J.C.; Hobbs, H.H. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J. Biol. Chem. 2003, 278, 48275–48282. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, A.; Repa, J.J.; Lobaccaro, J.M.; Bronson, A.; Mangelsdorf, D.J.; Edwards, P.A. Human white/murine ABC8 mRNA levels are highly induced in lipid-loaded macrophages. A transcriptional role for specific oxysterols. J. Biol. Chem. 2000, 275, 14700–14707. [Google Scholar] [CrossRef]
- Kim, W.S.; Chan, S.L.; Hill, A.F.; Guillemin, G.J.; Garner, B. Impact of 27-hydroxycholesterol on amyloid-beta peptide production and ATP-binding cassette transporter expression in primary human neurons. J. Alzheimer’s Dis. JAD 2009, 16, 121–131. [Google Scholar] [CrossRef]
- Sharpe, L.J.; Rao, G.; Jones, P.M.; Glancey, E.; Aleidi, S.M.; George, A.M.; Brown, A.J.; Gelissen, I.C. Cholesterol sensing by the ABCG1 lipid transporter: Requirement of a CRAC motif in the final transmembrane domain. Biochim. Biophys. Acta 2015, 1851, 956–964. [Google Scholar] [CrossRef]
- Dergunov, A.D.; Savushkin, E.V.; Dergunova, L.V.; Litvinov, D.Y. Significance of Cholesterol-Binding Motifs in ABCA1, ABCG1, and SR-B1 Structure. J. Membr. Biol. 2019, 252, 41–60. [Google Scholar] [CrossRef]
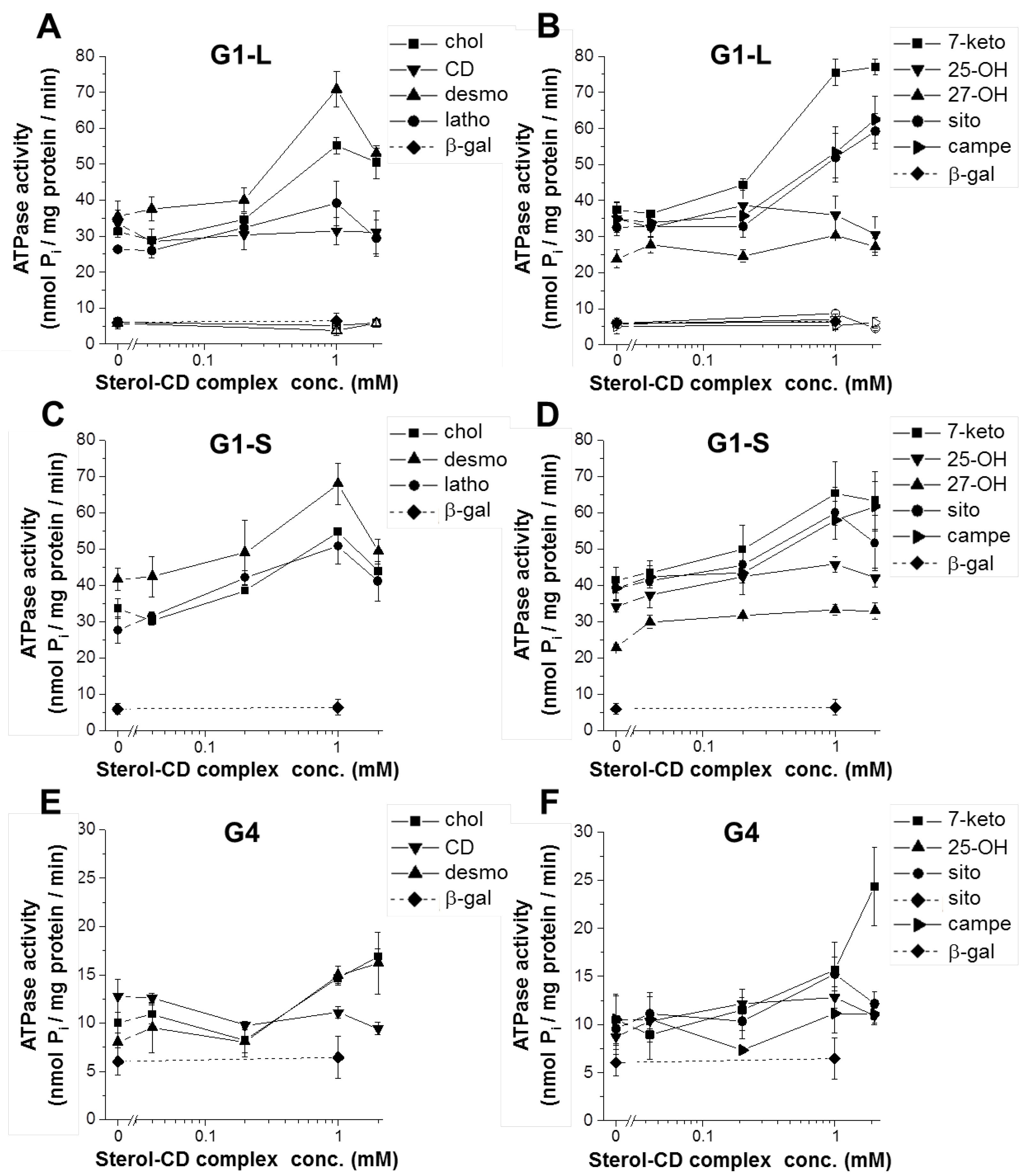
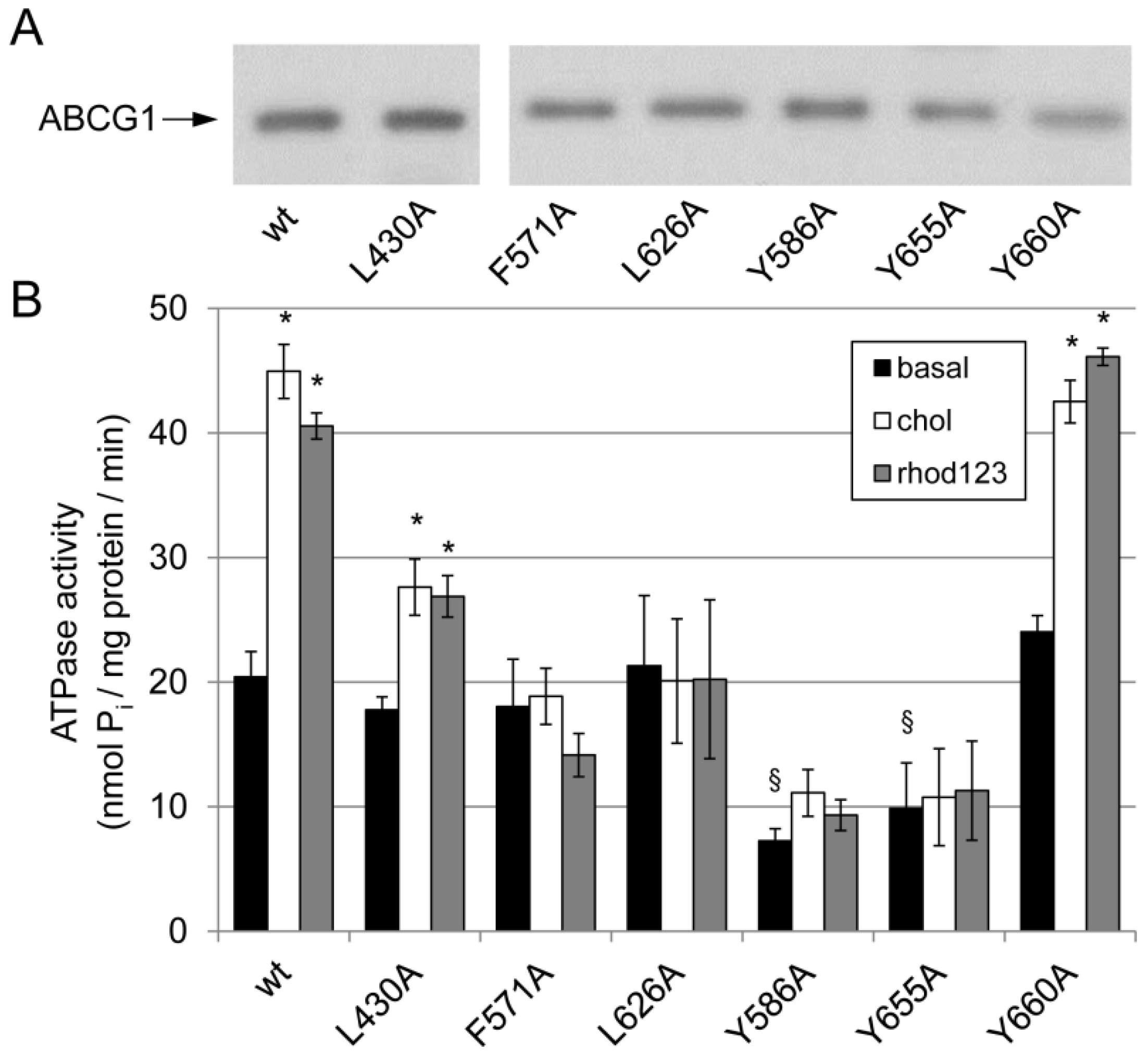
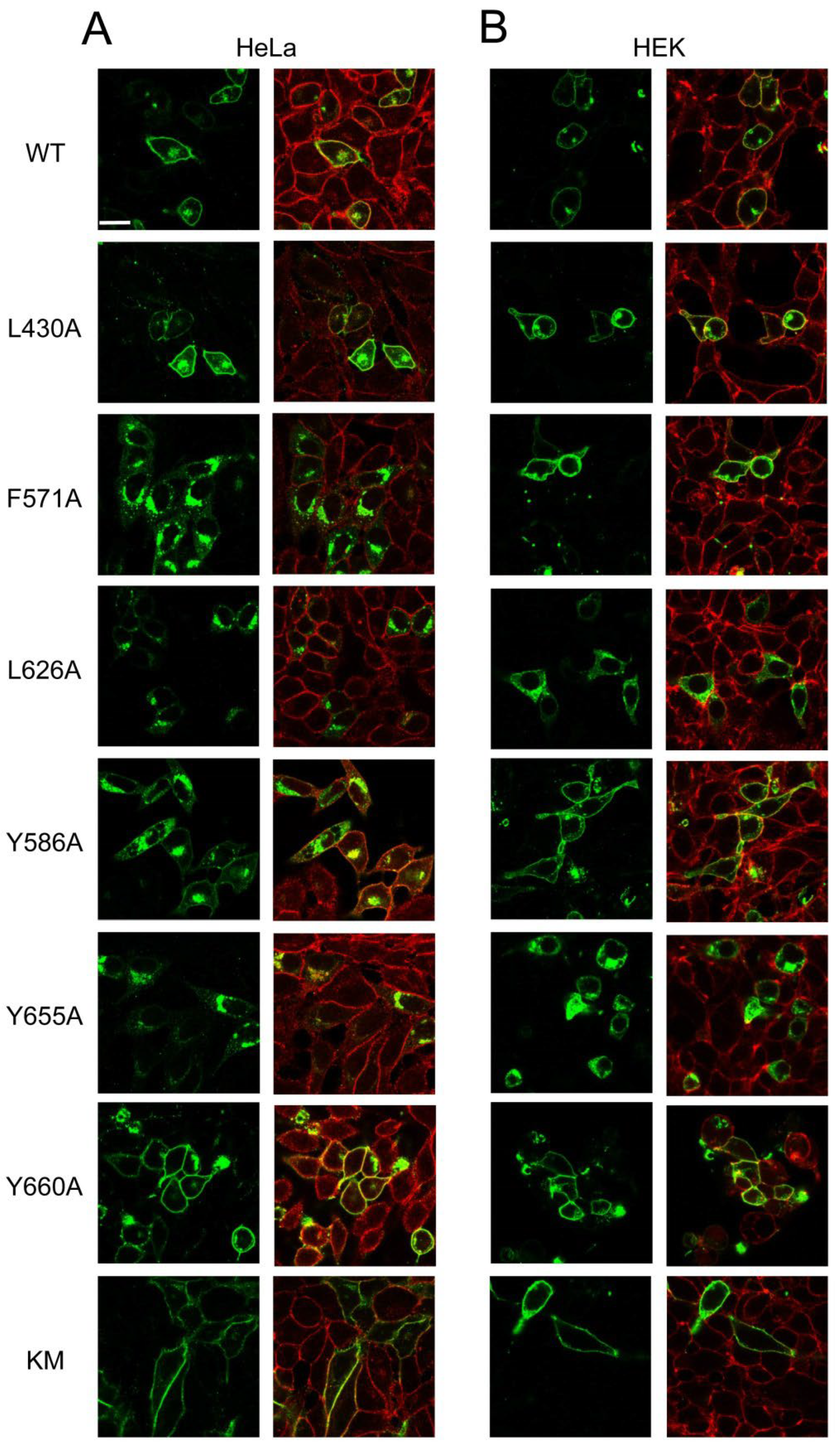

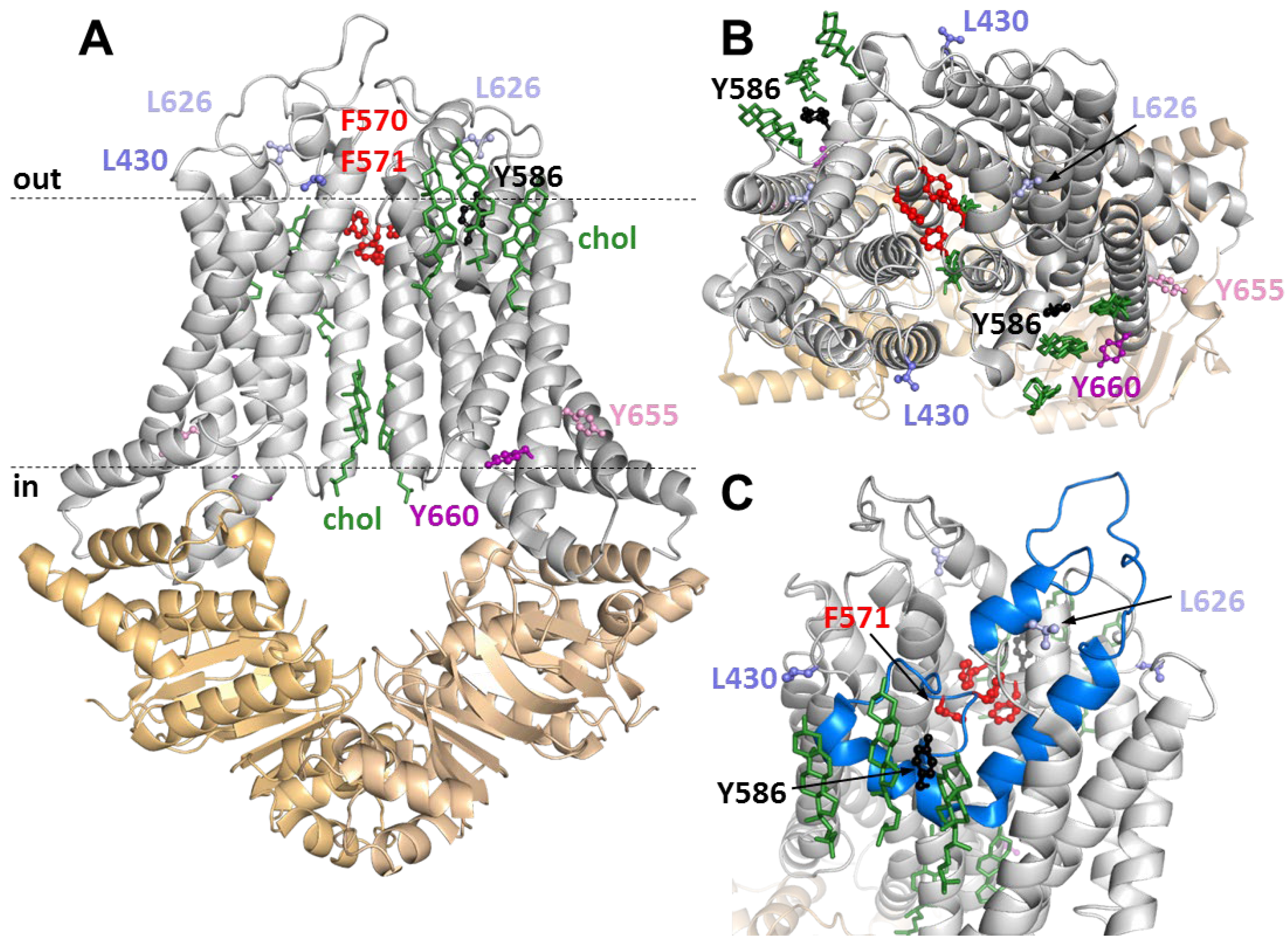
| G1-L | G1-S | G4 | |
|---|---|---|---|
| basal activity (nmol Pi/mg protein/min) | 32.07 ± 0.94 § | 33.15 ± 1.51 § | 10.78 ± 0.62 § |
| peak stimulation (fold change) | |||
| cyclodextrin | 0.94 ± 0.06 | 1.02 ± 0.00 | 0.89 ± 0.09 |
| cholesterol | 1.76 ± 0.06 * | 1.64 ± 0.11 * | 1.72 ± 0.20 * |
| desmosterol | 2.02 ± 0.12 * | 1.63 ± 0.10 *† | 1.91 ± 0.25 * |
| lathosterol | 1.51 ± 0.31 | 1.92 ± 0.22 * | n.d. |
| 7-ketocholesterol | 2.02 ± 0.06 * | 1.56 ± 0.12 *† | 2.18 ± 0.25 * |
| 25-OH-cholesterol | 1.02 ± 0.06 | 1.34 ± 0.07 *† | 1.55 ± 0.21 *† |
| 27-OH-cholesterol | 1.31 ± 0.17 | 1.50 ± 0.12 * | n.d. |
| β-sitosterol | 1.81 ± 0.09 * | 1.52 ± 0.09 *† | 1.63 ± 0.25 * |
| campesterol | 1.84 ± 0.26 * | 1.57 ± 0.07 * | 1.24 ± 0.42 |
| rhodamine 123 | 1.91 ± 0.06 * | 1.71 ± 0.09 * | 1.08 ± 0.00 † |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegyi, Z.; Hegedűs, T.; Homolya, L. The Reentry Helix Is Potentially Involved in Cholesterol Sensing of the ABCG1 Transporter Protein. Int. J. Mol. Sci. 2022, 23, 13744. https://doi.org/10.3390/ijms232213744
Hegyi Z, Hegedűs T, Homolya L. The Reentry Helix Is Potentially Involved in Cholesterol Sensing of the ABCG1 Transporter Protein. International Journal of Molecular Sciences. 2022; 23(22):13744. https://doi.org/10.3390/ijms232213744
Chicago/Turabian StyleHegyi, Zoltán, Tamás Hegedűs, and László Homolya. 2022. "The Reentry Helix Is Potentially Involved in Cholesterol Sensing of the ABCG1 Transporter Protein" International Journal of Molecular Sciences 23, no. 22: 13744. https://doi.org/10.3390/ijms232213744
APA StyleHegyi, Z., Hegedűs, T., & Homolya, L. (2022). The Reentry Helix Is Potentially Involved in Cholesterol Sensing of the ABCG1 Transporter Protein. International Journal of Molecular Sciences, 23(22), 13744. https://doi.org/10.3390/ijms232213744






