Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response
Abstract
1. Introduction
2. Mammalian Circadian System
3. Circadian Rhythms in Innate Immunity
4. Effects of Circadian Disruption on Innate Immunity
4.1. Genetic Circadian Disruption
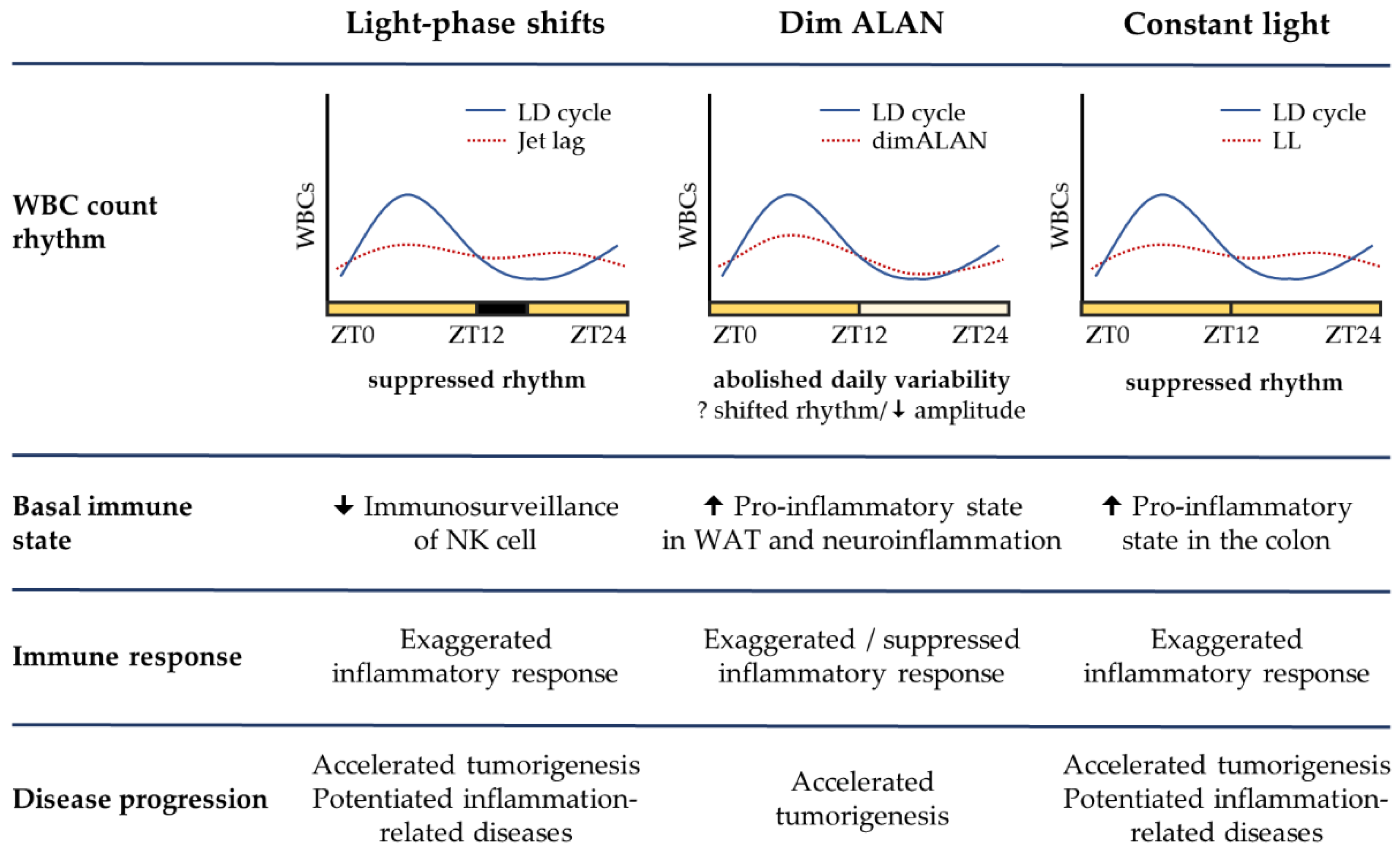
4.2. Light-Phase Shifts
4.3. Dim ALAN
4.4. Constant Light
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Albrecht, U. Timing to perfection: The biology of central and peripheral circadian clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Golombek, D.A.; Rosenstein, R.E. Physiology of circadian entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Astiz, M.; Heinen, K.O.; Rawashdeh, O.; Oster, H. The concept of coupling in the mammalian circadian clock network. J. Mol. Biol. 2020, 432, 3618–3638. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Garbazza, C.; Spitschan, M. Effects of light on human circadian rhythms, sleep and mood. Somnologie 2019, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The ecological impacts of nighttime light pollution: A mechanistic appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef] [PubMed]
- Falchi, F.; Cinzano, P.; Duriscoe, D.; Kyba, C.C.; Elvidge, C.D.; Baugh, K.; Portnov, B.A.; Rybnikova, N.A.; Furgoni, R. The new world atlas of artificial night sky brightness. Sci. Adv. 2016, 2, e1600377. [Google Scholar] [CrossRef]
- Kernbach, M.E.; Miller, C.; Alaasam, V.; Ferguson, S.; Francis, C.D. Introduction to the symposium: Effects of light pollution across diverse natural systems. Integr. Comp. Biol. 2021, 61, 1089–1097. [Google Scholar] [CrossRef]
- Davies, T.W.; Smyth, T. Why artificial light at night should be a focus for global change research in the 21st century. Glob. Chang. Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef]
- Vetter, C. Circadian disruption: What do we actually mean? Eur. J. Neurosci. 2020, 51, 531–550. [Google Scholar] [CrossRef]
- Lunn, R.M.; Blask, D.E.; Coogan, A.N.; Figueiro, M.G.; Gorman, M.R.; Hall, J.E.; Hansen, J.; Nelson, R.J.; Panda, S.; Smolensky, M.H.; et al. Health consequences of electric lighting practices in the modern world: A report on the National Toxicology Program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci. Total Environ. 2017, 607, 1073–1084. [Google Scholar] [CrossRef]
- Stevens, R.G.; Brainard, G.C.; Blask, D.E.; Lockley, S.W.; Motta, M.E. Adverse health effects of nighttime lighting: Comments on American Medical Association policy statement. Am. J. Prev. Med. 2013, 45, 343–346. [Google Scholar] [CrossRef]
- Yazdi, Z.; Sadeghniiat-Haghighi, K.; Loukzadeh, Z.; Elmizadeh, K.; Abbasi, M. Prevalence of sleep disorders and their impacts on occupational performance: A comparison between shift workers and nonshift workers. Sleep Disord. 2014, 2014, 870320. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Milesi, C. Artificial outdoor nighttime lights associate with altered sleep behavior in the American general population. Sleep 2016, 39, 1311–1320. [Google Scholar] [CrossRef]
- Garcia-Saenz, A.; Sánchez de Miguel, A.; Espinosa, A.; Valentin, A.; Aragonés, N.; Llorca, J.; Amiano, P.; Martín Sánchez, V.; Guevara, M.; Capelo, R.; et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study). Environ. Health Perspect. 2018, 126, 047011. [Google Scholar] [CrossRef]
- Wang, X.; Ji, A.; Zhu, Y.; Liang, Z.; Wu, J.; Li, S.Q.; Meng, S.; Zheng, X.Y.; Xie, L.P. A meta-analysis including dose-response relationship between night shift work and the risk of colorectal cancer. Oncotarget 2015, 6, 25046–25060. [Google Scholar] [CrossRef]
- Sun, S.; Cao, W.; Ge, Y.; Ran, J.; Sun, F.; Zeng, Q.; Guo, M.; Huang, J.; Lee, R.S.-Y.; Tian, L.; et al. Outdoor light at night and risk of coronary heart disease among older adults: A prospective cohort study. Eur. Heart J. 2021, 42, 822–830. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association between rotating night shift work and risk of coronary heart disease among women. JAMA-J. Am. Med. Assoc. 2016, 315, 1726–1734. [Google Scholar] [CrossRef]
- Koo, Y.S.; Song, J.-Y.; Joo, E.-Y.; Lee, H.-J.; Lee, E.; Lee, S.-K.; Jung, K.-Y. Outdoor artificial light at night, obesity, and sleep health: Cross-sectional analysis in the KoGES study. Chronobiol. Int. 2016, 33, 301–314. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Honma, S. The mammalian circadian system: A hierarchical multi-oscillator structure for generating circadian rhythm. J. Physiol. Sci. 2018, 68, 207–219. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Buijs, F.N.; León-Mercado, L.; Guzmán-Ruiz, M.; Guerrero-Vargas, N.N.; Romo-Nava, F.; Buijs, R.M. The circadian system: A regulatory feedback network of periphery and brain. Physiology 2016, 31, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Reppert, S.M.; Weaver, D.R. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 2001, 63, 647–676. [Google Scholar] [CrossRef]
- Alessandro, M.S.; Golombek, D.A.; Chiesa, J.J. Protein kinases in the photic signaling of the mammalian circadian clock. Yale J. Biol. Med. 2019, 92, 241–250. [Google Scholar] [PubMed]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef]
- Preitner, N.; Damiola, F.; Luis Lopez, M.; Zakany, J.; Duboule, D.; Albrecht, U.; Schibler, U. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 2002, 110, 251–260. [Google Scholar] [CrossRef]
- Curtis, A.M.; Bellet, M.M.; Sassone-Corsi, P.; O’Neill, L.A. Circadian clock proteins and immunity. Immunity 2014, 40, 178–186. [Google Scholar] [CrossRef]
- Hergenhan, S.; Holtkamp, S.; Scheiermann, C. Molecular interactions between components of the circadian clock and the immune system. J. Mol. Biol. 2020, 432, 3700–3713. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Abele, S.H.; Meadows, K.E.; Medeiros, D.; Silver, A.C. Time is on the immune system’s side, Yes it is. Yale J. Biol. Med. 2019, 92, 225–231. [Google Scholar]
- Waggoner, S.N. Circadian rhythms in immunity. Curr. Allergy Asthma Rep. 2020, 20, 2. [Google Scholar] [CrossRef]
- He, W.; Holtkamp, S.; Hergenhan, S.M.; Kraus, K.; de Juan, A.; Weber, J.; Bradfield, P.; Grenier, J.M.P.; Pelletier, J.; Druzd, D.; et al. Circadian expression of migratory factors establishes lineage-specific signatures that guide the homing of leukocyte subsets to tissues. Immunity 2018, 49, 1175–1190.e7. [Google Scholar] [CrossRef]
- Pelegrí, C.; Vilaplana, J.; Castellote, C.; Rabanal, M.; Franch, À.; Castell, M. Circadian rhythms in surface molecules of rat blood lymphocytes. Am. J. Physiol. Cell Physiol. 2003, 284, C67–C76. [Google Scholar] [CrossRef][Green Version]
- Haus, E.; Smolensky, M.H. Biologic rhythms in the immune system. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Pick, R.; He, W.; Chen, C.-S.; Scheiermann, C. Time-of-day-dependent trafficking and function of leukocyte subsets. Trends Immunol. 2019, 40, 524–537. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, L.A.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; van Rooijen, N.; Silberstein, L.E.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Druzd, D.; Matveeva, O.; Ince, L.; Harrison, U.; He, W.; Schmal, C.; Herzel, H.; Tsang, A.H.; Kawakami, N.; Leliavski, A.; et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity 2017, 46, 120–132. [Google Scholar] [CrossRef]
- Gibbs, J.; Ince, L.; Matthews, L.; Mei, J.; Bell, T.; Yang, N.; Saer, B.; Begley, N.; Poolman, T.; Pariollaud, M.; et al. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat. Med. 2014, 20, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Segura, M.; Hidalgo, A. Circadian immune circuits. J. Exp. Med. 2020, 218, e20200798. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Lucas, D.; Battista, M.; Frenette, P.S. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 2008, 452, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Kollet, O.; Vagima, Y.; D’Uva, G.; Golan, K.; Canaani, J.; Itkin, T.; Gur-Cohen, S.; Kalinkovich, A.; Caglio, G.; Medaglia, C.; et al. Physiologic corticosterone oscillations regulate murine hematopoietic stem/progenitor cell proliferation and CXCL12 expression by bone marrow stromal progenitors. Leukemia 2013, 27, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Larbi, K.Y.; Young, R.E.; Nourshargh, S. Migration of leukocytes through the vessel wall and beyond. Thromb. Haemost. 2003, 90, 598–606. [Google Scholar] [CrossRef]
- Nobis, C.C.; Labrecque, N.; Cermakian, N. From immune homeostasis to inflammation, a question of rhythms. Curr. Opin. Physiol. 2018, 5, 90–98. [Google Scholar] [CrossRef]
- Halberg, F.; Johnson, E.A.; Brown, B.W.; Bittner, J.J. Susceptibility rhythm to E. coli endotoxin and bioassay. Proc. Soc. Exp. Biol. Med. 1960, 103, 142–144. [Google Scholar] [CrossRef]
- Marpegan, L.; Leone, M.J.; Katz, M.E.; Sobrero, P.M.; Bekinstein, T.A.; Golombek, D.A. Diurnal variation in endotoxin-induced mortality in mice: Correlation with proinflammatory factors. Chronobiol. Int. 2009, 26, 1430–1442. [Google Scholar] [CrossRef]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, I.I.; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. Core circadian protein CLOCK is a positive regulator of NF-κB–mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E2457–E2465. [Google Scholar] [CrossRef]
- Timmons, G.A.; O’Siorain, J.R.; Kennedy, O.D.; Curtis, A.M.; Early, J.O. Innate rhythms: Clocks at the center of monocyte and macrophage function. Front. Immunol. 2020, 11, 1743. [Google Scholar] [CrossRef]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Fentress, S.J.; Qiu, Y.; Yun, K.; Cox, J.S.; Chawla, A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6Chi inflammatory monocytes. Science 2013, 341, 1483–1488. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, P.S. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef]
- Stenzinger, M.; Karpova, D.; Unterrainer, C.; Harenkamp, S.; Wiercinska, E.; Hoerster, K.; Pfeffer, M.; Maronde, E.; Bonig, H. Hematopoietic-extrinsic cues dictate circadian redistribution of mature and immature hematopoietic cells in blood and spleen. Cells 2019, 8, 1033. [Google Scholar] [CrossRef]
- Oishi, Y.; Hayashi, S.; Isagawa, T.; Oshima, M.; Iwama, A.; Shimba, S.; Okamura, H.; Manabe, I. Bmal1 regulates inflammatory responses in macrophages by modulating enhancer RNA transcription. Sci. Rep. 2017, 7, 7086. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Yuan, X.; Li, F.; Guo, L.; Wu, B. REV-ERBα integrates colon clock with experimental colitis through regulation of NF-κB/NLRP3 axis. Nat. Commun. 2018, 9, 4246. [Google Scholar] [CrossRef]
- Curtis, A.M.; Fagundes, C.T.; Yang, G.; Palsson-McDermott, E.M.; Wochal, P.; McGettrick, A.F.; Foley, N.H.; Early, J.O.; Chen, L.; Zhang, H.; et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proc. Natl. Acad. Sci. USA 2015, 112, 7231–7236. [Google Scholar] [CrossRef]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian clock protein BMAL1 regulates IL-1β in macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef]
- Allen, N.C.; Philip, N.H.; Hui, L.; Zhou, X.; Franklin, R.A.; Kong, Y.; Medzhitov, R. Desynchronization of the molecular clock contributes to the heterogeneity of the inflammatory response. Sci. Signal. 2019, 12, eaau1851. [Google Scholar] [CrossRef]
- Kitchen, G.B.; Cunningham, P.S.; Poolman, T.M.; Iqbal, M.; Maidstone, R.; Baxter, M.; Bagnall, J.; Begley, N.; Saer, B.; Hussell, T.; et al. The clock gene Bmal1 inhibits macrophage motility, phagocytosis, and impairs defense against pneumonia. Proc. Natl. Acad. Sci. USA 2020, 117, 1543–1551. [Google Scholar] [CrossRef]
- Huo, M.; Huang, Y.; Qu, D.; Zhang, H.; Wong, W.T.; Chawla, A.; Huang, Y.; Tian, X.Y. Myeloid Bmal1 deletion increases monocyte recruitment and worsens atherosclerosis. FASEB J. 2017, 31, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Aroca-Crevillén, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzón-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Deriu, E.; Liu, J.Z.; Grimaldi, B.; Blaschitz, C.; Zeller, M.; Edwards, R.A.; Sahar, S.; Dandekar, S.; Baldi, P.; et al. Circadian clock regulates the host response to Salmonella. Proc. Natl. Acad. Sci. USA 2013, 110, 9897–9902. [Google Scholar] [CrossRef] [PubMed]
- Bellet, M.M.; Zocchi, L.; Sassone-Corsi, P. The RelB subunit of NFκB acts as a negative regulator of circadian gene expression. Cell Cycle 2012, 11, 3304–3311. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; Wynne, O.; Levitt, D.; Price, D.; Sarkar, D.K. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1−/− mutant mice. J. Interferon Cytokine Res. 2013, 33, 108–114. [Google Scholar] [CrossRef]
- Arjona, A.; Sarkar, D.K. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J. Interferon Cytokine Res. 2006, 26, 645–649. [Google Scholar] [CrossRef]
- Liu, J.G.; Mankani, G.; Shi, X.Y.; Meyer, M.; Cunningham-Runddles, S.; Ma, X.J.; Sun, Z.S. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infect. Immun. 2006, 74, 4750–4756. [Google Scholar] [CrossRef]
- Silver, A.C.; Arjona, A.; Walker, W.E.; Fikrig, E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 2012, 36, 251–261. [Google Scholar] [CrossRef]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Liu, F.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef]
- Pariollaud, M.; Gibbs, J.E.; Hopwood, T.W.; Brown, S.; Begley, N.; Vonslow, R.; Poolman, T.; Guo, B.; Saer, B.; Jones, D.H.; et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J. Clin. Investig. 2018, 128, 2281–2296. [Google Scholar] [CrossRef]
- Sato, S.; Sakurai, T.; Ogasawara, J.; Takahashi, M.; Izawa, T.; Imaizumi, K.; Taniguchi, N.; Ohno, H.; Kizaki, T. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J. Immunol. 2014, 192, 407–417. [Google Scholar] [CrossRef]
- Griffin, P.; Dimitry, J.M.; Sheehan, P.W.; Lananna, B.V.; Guo, C.; Robinette, M.L.; Hayes, M.E.; Cedeño, M.R.; Nadarajah, C.J.; Ezerskiy, L.A.; et al. Circadian clock protein Rev-erbα regulates neuroinflammation. Proc. Natl. Acad. Sci. USA 2019, 116, 5102–5107. [Google Scholar] [CrossRef]
- Griffin, P.; Sheehan, P.W.; Dimitry, J.M.; Guo, C.; Kanan, M.F.; Lee, J.; Zhang, J.; Musiek, E.S. REV-ERBα mediates complement expression and diurnal regulation of microglial synaptic phagocytosis. eLife 2020, 9, e58765. [Google Scholar] [CrossRef]
- Stapleton, C.M.; Jaradat, M.; Dixon, D.; Kang, H.S.; Kim, S.-C.; Liao, G.; Carey, M.A.; Cristiano, J.; Moorman, M.P.; Jetten, A.M. Enhanced susceptibility of staggerer (RORαsg/sg) mice to lipopolysaccharide-induced lung inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L144–L152. [Google Scholar] [CrossRef]
- Kopmels, B.; Mariani, J.; Delhaye-Bouchaud, N.; Audibert, F.; Fradelizi, D.; Wollman, E.E. Evidence for a hyperexcitability state of staggerer mutant mice macrophages. J. Neurochem. 1992, 58, 192–199. [Google Scholar] [CrossRef]
- Kashiwada, M.; Pham, N.-L.L.; Pewe, L.L.; Harty, J.T.; Rothman, P.B. NFIL3/E4BP4 is a key transcription factor for CD8α+ dendritic cell development. Blood 2011, 117, 6193–6197. [Google Scholar] [CrossRef]
- Gascoyne, D.M.; Long, E.; Veiga-Fernandes, H.; de Boer, J.; Williams, O.; Seddon, B.; Coles, M.; Kioussis, D.; Brady, H.J.M. The basic leucine zipper transcription factor E4BP4 is essential for natural killer cell development. Nat. Immunol. 2009, 10, 1118–1124. [Google Scholar] [CrossRef]
- Geiger, T.L.; Abt, M.C.; Gasteiger, G.; Firth, M.A.; O’Connor, M.H.; Geary, C.D.; O’Sullivan, T.E.; van den Brink, M.R.; Pamer, E.G.; Hanash, A.M.; et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J. Exp. Med. 2014, 211, 1723–1731. [Google Scholar] [CrossRef]
- Kobayashi, T.; Matsuoka, K.; Sheikh, S.Z.; Elloumi, H.Z.; Kamada, N.; Hisamatsu, T.; Hansen, J.J.; Doty, K.R.; Pope, S.D.; Smale, S.T.; et al. NFIL3 is a regulator of IL-12 p40 in macrophages and mucosal immunity. J. Immunol. 2011, 186, 4649. [Google Scholar] [CrossRef]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef]
- Baggs, J.E.; Price, T.S.; DiTacchio, L.; Panda, S.; FitzGerald, G.A.; Hogenesch, J.B. Network features of the mammalian circadian clock. PLoS Biol. 2009, 7, e1000052. [Google Scholar] [CrossRef] [PubMed]
- Delerive, P.; Monté, D.; Dubois, G.; Trottein, F.; Fruchart-Najib, J.; Mariani, J.; Fruchart, J.-C.; Staels, B. The orphan nuclear receptor RORα is a negative regulator of the inflammatory response. EMBO Rep. 2001, 2, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Duez, H.; van der Veen, J.N.; Duhem, C.; Pourcet, B.; Touvier, T.; Fontaine, C.; Derudas, B.; Baugé, E.; Havinga, R.; Bloks, V.W.; et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbα. Gastroenterology 2008, 135, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Vosko, A.M.; Colwell, C.S.; Avidan, A.Y. Jet lag syndrome: Circadian organization, pathophysiology, and management strategies. Nat. Sci. Sleep 2010, 2, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Wickwire, E.M.; Geiger-Brown, J.; Scharf, S.M.; Drake, C.L. Shift work and shift work sleep disorder: Clinical and organizational perspectives. Chest 2017, 151, 1156–1172. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P.; Kosmadopoulos, A. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythm 2021, 37, 3–28. [Google Scholar] [CrossRef]
- Hemmer, A.; Mareschal, J.; Dibner, C.; Pralong, J.A.; Dorribo, V.; Perrig, S.; Genton, L.; Pichard, C.; Collet, T.-H. The effects of shift work on cardio-metabolic diseases and eating patterns. Nutrients 2021, 13, 4178. [Google Scholar] [CrossRef]
- Wang, X.S.; Armstrong, M.E.G.; Cairns, B.J.; Key, T.J.; Travis, R.C. Shift work and chronic disease: The epidemiological evidence. Occup. Med. 2011, 61, 78–89. [Google Scholar] [CrossRef]
- Loef, B.; van Baarle, D.; van der Beek, A.J.; Sanders, E.A.M.; Bruijning-Verhagen, P.; Proper, K.I. Shift work and respiratory infections in health-care workers. Am. J. Epidemiol. 2019, 188, 509–517. [Google Scholar] [CrossRef]
- Mohren, D.C.L.; Jansen, N.W.H.; Kant, I.; Galama, J.M.D.; van den Brandt, P.A.; Swaen, G.M.H. Prevalence of common infections among employees in different work schedules. J. Occup. Environ. Med. 2002, 44, 1003–1011. [Google Scholar] [CrossRef]
- Fatima, Y.; Bucks, R.S.; Mamun, A.A.; Skinner, I.; Rosenzweig, I.; Leschziner, G.; Skinner, T.C. Shift work is associated with increased risk of COVID-19: Findings from the UK Biobank cohort. J. Sleep Res. 2021, 30, e13326. [Google Scholar] [CrossRef]
- Lu, L.-F.; Wang, C.-P.; Tsai, I.T.; Hung, W.-C.; Yu, T.-H.; Wu, C.-C.; Hsu, C.-C.; Lu, Y.-C.; Chung, F.-M.; Jean, M.-C.Y. Relationship between shift work and peripheral total and differential leukocyte counts in Chinese steel workers. J. Occup. Health 2016, 58, 81–88. [Google Scholar] [CrossRef]
- Wirth, M.D.; Andrew, M.E.; Burchfiel, C.M.; Burch, J.B.; Fekedulegn, D.; Hartley, T.A.; Charles, L.E.; Violanti, J.M. Association of shiftwork and immune cells among police officers from the Buffalo Cardio-Metabolic Occupational Police Stress study. Chronobiol. Int. 2017, 34, 721–731. [Google Scholar] [CrossRef]
- Atwater, A.Q.; Immergluck, L.C.; Davidson, A.J.; Castanon-Cervantes, O. Shift work predicts increases in lipopolysaccharide-binding protein, interleukin-10, and leukocyte counts in a cross-sectional study of healthy volunteers carrying low-grade systemic inflammation. Int. J. Environ. Res. Public Health 2021, 18, 13158. [Google Scholar] [CrossRef]
- Okamoto, H.; Tsunoda, T.; Teruya, K.; Takeda, N.; Uemura, T.; Matsui, T.; Fukazawa, S.; Ichikawa, K.; Takemae, R.; Tsuchida, K.; et al. An occupational health study of emergency physicians in Japan: Health assessment by immune variables (CD4, CD8, CD56, and NK cell activity) at the beginning of work. J. Occup. Health 2008, 50, 136–146. [Google Scholar] [CrossRef]
- Kervezee, L.; Cuesta, M.; Cermakian, N.; Boivin, D.B. Simulated night shift work induces circadian misalignment of the human peripheral blood mononuclear cell transcriptome. Proc. Natl. Acad. Sci. USA 2018, 115, 5540–5545. [Google Scholar] [CrossRef]
- Cuesta, M.; Boudreau, P.; Dubeau-Laramée, G.; Cermakian, N.; Boivin, D.B. Simulated night shift disrupts circadian rhythms of immune functions in humans. J. Immunol. 2016, 196, 2466. [Google Scholar] [CrossRef]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian disruption and metabolic disease: Findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef]
- Opperhuizen, A.L.; van Kerkhof, L.W.M.; Proper, K.I.; Rodenburg, W.; Kalsbeek, A. Rodent models to study the metabolic effects of shiftwork in humans. Front. Pharmacol. 2015, 6, 50. [Google Scholar] [CrossRef]
- Casiraghi, L.P.; Oda, G.A.; Chiesa, J.J.; Friesen, W.O.; Golombek, D.A. Forced desynchronization of activity rhythms in a model of chronic jet lag in mice. J. Biol. Rhythm 2012, 27, 59–69. [Google Scholar] [CrossRef]
- Iwamoto, A.; Kawai, M.; Furuse, M.; Yasuo, S. Effects of chronic jet lag on the central and peripheral circadian clocks in CBA/N mice. Chronobiol. Int. 2014, 31, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, M.; Chan, X.Y.; Tan, S.Y.; Subramaniam, S.; Fan, Y.; Loh, E.; Chang, K.T.E.; Tan, T.C.; Chen, Q. Uncovering the mystery of opposite circadian rhythms between mouse and human leukocytes in humanized mice. Blood 2017, 130, 1995–2005. [Google Scholar] [CrossRef]
- Crespo, M.; Gonzalez-Teran, B.; Nikolic, I.; Mora, A.; Folgueira, C.; Rodríguez, E.; Leiva-Vega, L.; Pintor-Chocano, A.; Fernández-Chacón, M.; Ruiz-Garrido, I.; et al. Neutrophil infiltration regulates clock-gene expression to organize daily hepatic metabolism. eLife 2020, 9, e59258. [Google Scholar] [CrossRef] [PubMed]
- Adams, K.L.; Castanon-Cervantes, O.; Evans, J.A.; Davidson, A.J. Environmental circadian disruption elevates the IL-6 response to lipopolysaccharide in blood. J. Biol. Rhythm 2013, 28, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Brager, A.J.; Ehlen, J.C.; Castanon-Cervantes, O.; Natarajan, D.; Delisser, P.; Davidson, A.J.; Paul, K.N. Sleep loss and the inflammatory response in mice under chronic environmental circadian disruption. PLoS ONE 2013, 8, e63752. [Google Scholar] [CrossRef]
- Castanon-Cervantes, O.; Wu, M.W.; Ehlen, J.C.; Paul, K.; Gamble, K.L.; Johnson, R.L.; Besing, R.C.; Menaker, M.; Gewirtz, A.T.; Davidson, A.J. Dysregulation of inflammatory responses by chronic circadian disruption. J. Immunol. 2010, 185, 5796–5805. [Google Scholar] [CrossRef]
- Mul Fedele, M.L.; Aiello, I.; Caldart, C.S.; Golombek, D.A.; Marpegan, L.; Paladino, N. Differential thermoregulatory and inflammatory patterns in the circadian response to LPS-induced septic shock. Front. Cell. Infect. Microbiol. 2020, 10, 100. [Google Scholar] [CrossRef]
- Kim, S.-M.; Neuendorff, N.; Alaniz, R.C.; Sun, Y.; Chapkin, R.S.; Earnest, D.J. Shift work cycle-induced alterations of circadian rhythms potentiate the effects of high-fat diet on inflammation and metabolism. FASEB J. 2018, 32, 3085–3095. [Google Scholar] [CrossRef]
- Schilperoort, M.; van den Berg, R.; Bosmans, L.A.; van Os, B.W.; Dollé, M.E.T.; Smits, N.A.M.; Guichelaar, T.; van Baarle, D.; Koemans, L.; Berbée, J.F.P.; et al. Disruption of circadian rhythm by alternating light-dark cycles aggravates atherosclerosis development in APOE*3-Leiden.CETP mice. J. Pineal Res. 2020, 68, e12614. [Google Scholar] [CrossRef]
- Aiello, I.; Fedele, M.L.M.; Román, F.; Marpegan, L.; Caldart, C.; Chiesa, J.J.; Golombek, D.A.; Finkielstein, C.V.; Paladino, N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020, 6, eaaz4530. [Google Scholar] [CrossRef]
- Zeng, X.; Liang, C.; Yao, J. Chronic shift-lag promotes NK cell ageing and impairs immunosurveillance in mice by decreasing the expression of CD122. J. Cell. Mol. Med. 2020, 24, 14583–14595. [Google Scholar] [CrossRef]
- Logan, R.W.; Zhang, C.; Murugan, S.; O’Connell, S.; Levitt, D.; Rosenwasser, A.M.; Sarkar, D.K. Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J. Immunol. 2012, 188, 2583–2591. [Google Scholar] [CrossRef]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, P.; Gao, W.; Zhou, Q.; Feng, T.; Tian, X. Circadian clock: A regulator of the immunity in cancer. Cell Commun. Signal. 2021, 19, 37. [Google Scholar] [CrossRef]
- Filipski, E.; Delaunay, F.; King, V.M.; Wu, M.-W.; Claustrat, B.; Gréchez-Cassiau, A.; Guettier, C.; Hastings, M.H.; Francis, L.V. Effects of chronic jet lag on tumor progression in mice. Cancer Res. 2004, 64, 7879–7885. [Google Scholar] [CrossRef]
- Pierce, S.; Geanes, E.S.; Bradley, T. Targeting natural killer cells for improved immunity and control of the adaptive immune response. Front. Cell. Infect. Microbiol. 2020, 10, 231. [Google Scholar] [CrossRef]
- Kim, S.; Iizuka, K.; Aguila, H.L.; Weissman, I.L.; Yokoyama, W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 2000, 97, 2731–2736. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Aktas, E.; Kucuksezer, U.C.; Bilgic, S.; Erten, G.; Deniz, G. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009, 254, 149–154. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.J.; Sellix, M.T.; Daniel, J.; Yamazaki, S.; Menaker, M.; Block, G.D. Chronic jet-lag increases mortality in aged mice. Curr. Biol. 2006, 16, R914–R916. [Google Scholar] [CrossRef] [PubMed]
- Inokawa, H.; Umemura, Y.; Shimba, A.; Kawakami, E.; Koike, N.; Tsuchiya, Y.; Ohashi, M.; Minami, Y.; Cui, G.; Asahi, T.; et al. Chronic circadian misalignment accelerates immune senescence and abbreviates lifespan in mice. Sci. Rep. 2020, 10, 2569. [Google Scholar] [CrossRef] [PubMed]
- Kochan, D.Z.; Ilnytskyy, Y.; Golubov, A.; Deibel, S.H.; McDonald, R.J.; Kovalchuk, O. Circadian disruption-induced microRNAome deregulation in rat mammary gland tissues. Oncoscience 2015, 2, 428–442. [Google Scholar] [CrossRef] [PubMed]
- Sánchez de Miguel, A.; Bennie, J.; Rosenfeld, E.; Dzurjak, S.; Gaston, K.J. First estimation of global trends in nocturnal power emissions reveals acceleration of light pollution. Remote Sens. 2021, 13, 3311. [Google Scholar] [CrossRef]
- Cajochen, C.; Frey, S.; Anders, D.; Späti, J.; Bues, M.; Pross, A.; Mager, R.; Wirz-Justice, A.; Stefani, O. Evening exposure to a light-emitting diodes (LED)-backlit computer screen affects circadian physiology and cognitive performance. J. Appl. Physiol. 2011, 110, 1432–1438. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Foppen, E.; Kalsbeek, A.; Zeman, M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 2022, 13, 973461. [Google Scholar] [CrossRef]
- Stenvers, D.J.; van Dorp, R.; Foppen, E.; Mendoza, J.; Opperhuizen, A.L.; Fliers, E.; Bisschop, P.H.; Meijer, J.H.; Kalsbeek, A.; Deboer, T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016, 6, 35662. [Google Scholar] [CrossRef]
- Okuliarova, M.; Dzirbikova, Z.; Rumanova, V.S.; Foppen, E.; Kalsbeek, A.; Zeman, M. Disrupted circadian control of hormonal rhythms and anticipatory thirst by dim light at night. Neuroendocrinology 2022, 112, 1116–1128. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Fonken, L.K.; Aubrecht, T.G.; Meléndez-Fernández, O.H.; Weil, Z.M.; Nelson, R.J. Dim light at night disrupts molecular circadian rhythms and increases body weight. J. Biol. Rhythm 2013, 28, 262–271. [Google Scholar] [CrossRef]
- Shuboni, D.; Yan, L. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience 2010, 170, 1172–1178. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Dauchy, E.M.; Tirrell, R.P.; Hill, C.R.; Davidson, L.K.; Greene, M.W.; Tirrell, P.C.; Wu, J.; Sauer, L.A.; Blask, D.E. Dark-phase light contamination disrupts circadian rhythms in plasma measures of endocrine physiology and metabolism in rats. Comp. Med. 2010, 60, 348–356. [Google Scholar]
- Molcan, L.; Sutovska, H.; Okuliarova, M.; Senko, T.; Krskova, L.; Zeman, M. Dim light at night attenuates circadian rhythms in the cardiovascular system and suppresses melatonin in rats. Life Sci. 2019, 231, 116568. [Google Scholar] [CrossRef]
- Moaraf, S.; Heiblum, R.; Okuliarová, M.; Hefetz, A.; Scharf, I.; Zeman, M.; Barnea, A. Evidence that artificial light at night induces structure-specific changes in brain plasticity in a diurnal bird. Biomolecules 2021, 11, 1069. [Google Scholar] [CrossRef]
- Moaraf, S.; Vistoropsky, Y.; Pozner, T.; Heiblum, R.; Okuliarová, M.; Zeman, M.; Barnea, A. Artificial light at night affects brain plasticity and melatonin in birds. Neurosci. Lett. 2020, 716, 134639. [Google Scholar] [CrossRef]
- Grubisic, M.; Haim, A.; Bhusal, P.; Dominoni, D.M.; Gabriel, K.M.A.; Jechow, A.; Kupprat, F.; Lerner, A.; Marchant, P.; Riley, W.; et al. Light pollution, circadian photoreception, and melatonin in vertebrates. Sustainability 2019, 11, 6400. [Google Scholar] [CrossRef]
- Okuliarova, M.; Mazgutova, N.; Majzunova, M.; Rumanova, V.S.; Zeman, M. Dim light at night impairs daily variation of circulating immune cells and renal immune homeostasis. Front. Immunol. 2021, 11, 614960. [Google Scholar] [CrossRef]
- Fonken, L.K.; Lieberman, R.A.; Weil, Z.M.; Nelson, R.J. Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 2013, 154, 3817–3825. [Google Scholar] [CrossRef]
- Bumgarner, J.R.; Walker, W.H.; Liu, J.A.; Walton, J.C.; Nelson, R.J. Dim light at night exposure induces cold hyperalgesia and mechanical allodynia in male mice. Neuroscience 2020, 434, 111–119. [Google Scholar] [CrossRef]
- Fonken, L.K.; Weil, Z.M.; Nelson, R.J. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav. Immun. 2013, 34, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Fonken, L.K.; Bedrosian, T.A.; Zhang, N.; Weil, Z.M.; DeVries, A.C.; Nelson, R.J. Dim light at night impairs recovery from global cerebral ischemia. Exp. Neurol. 2019, 317, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H.; Kvadas, R.M.; May, L.E.; Liu, J.A.; Bumgarner, J.R.; Walton, J.C.; DeVries, A.C.; Dauchy, R.T.; Blask, D.E.; Nelson, R.J. Artificial light at night reduces anxiety-like behavior in female mice with exacerbated mammary tumor growth. Cancers 2021, 13, 4860. [Google Scholar] [CrossRef] [PubMed]
- Blask, D.E.; Dauchy, R.T.; Dauchy, E.M.; Mao, L.; Hill, S.M.; Greene, M.W.; Belancio, V.P.; Sauer, L.A.; Davidson, L. Light exposure at night disrupts host/cancer circadian regulatory dynamics: Impact on the Warburg effect, lipid signaling and tumor growth prevention. PLoS ONE 2014, 9, e102776. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Weil, Z.M.; Nelson, R.J. Chronic dim light at night provokes reversible depression-like phenotype: Possible role for TNF. Mol. Psychiatry 2013, 18, 930–936. [Google Scholar] [CrossRef]
- Bedrosian, T.A.; Fonken, L.K.; Walton, J.C.; Nelson, R.J. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 2011, 7, 468–471. [Google Scholar] [CrossRef]
- Fonken, L.K.; Haim, A.; Nelson, R.J. Dim light at night increases immune function in nile grass rats, a diurnal rodent. Chronobiol. Int. 2012, 29, 26–34. [Google Scholar] [CrossRef]
- Fonken, L.K.; Kitsmiller, E.; Smale, L.; Nelson, R.J. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythm 2012, 27, 319–327. [Google Scholar] [CrossRef]
- Rumanova, V.S.; Okuliarova, M.; Zeman, M. Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int. J. Mol. Sci. 2020, 21, 5478. [Google Scholar] [CrossRef]
- Ohta, H.; Yamazaki, S.; McMahon, D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005, 8, 267–269. [Google Scholar] [CrossRef]
- Polidarová, L.; Houdek, P.; Sumová, A. Chronic disruptions of circadian sleep regulation induce specific proinflammatory responses in the rat colon. Chronobiol. Int. 2017, 34, 1273–1287. [Google Scholar] [CrossRef]
- Guerrero-Vargas, N.N.; Navarro-Espíndola, R.; Guzmán-Ruíz, M.A.; Basualdo, M.D.C.; Espitia-Bautista, E.; López-Bago, A.; Lascurain, R.; Córdoba-Manilla, C.; Buijs, R.M.; Escobar, C. Circadian disruption promotes tumor growth by anabolic host metabolism; experimental evidence in a rat model. BMC Cancer 2017, 17, 625. [Google Scholar] [CrossRef]
- Coomans, C.P.; van den Berg, S.A.A.; Houben, T.; van Klinken, J.-B.; van den Berg, R.; Pronk, A.C.M.; Havekes, L.M.; Romijn, J.A.; van Dijk, K.W.; Biermasz, N.R.; et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013, 27, 1721–1732. [Google Scholar] [CrossRef]
- Lucassen, E.A.; Coomans, C.P.; van Putten, M.; de Kreij, S.R.; van Genugten, J.H.L.T.; Sutorius, R.P.M.; de Rooij, K.E.; van der Velde, M.; Verhoeve, S.L.; Smit, J.W.A.; et al. Environmental 24-hr cycles are essential for health. Curr. Biol. 2016, 26, 1843–1853. [Google Scholar] [CrossRef]
- Báez-Ruiz, A.; Guerrero-Vargas, N.N.; Cázarez-Márquez, F.; Sabath, E.; Basualdo, M.d.C.; Salgado-Delgado, R.; Escobar, C.; Buijs, R.M. Food in synchrony with melatonin and corticosterone relieves constant light disturbed metabolism. J. Endocrinol. 2017, 235, 167–178. [Google Scholar] [CrossRef]
- Gale, J.E.; Cox, H.I.; Qian, J.Y.; Block, G.D.; Colwell, C.S.; Matveyenko, A.V. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythm 2011, 26, 423–433. [Google Scholar] [CrossRef]
- Claustrat, B.; Valatx, J.L.; Harthé, C.; Brun, J. Effect of constant light on prolactin and corticosterone rhythms evaluated using a noninvasive urine sampling protocol in the rat. Horm. Metab. Res. 2008, 40, 398–403. [Google Scholar] [CrossRef]
- Deprés-Brummer, P.; Bourin, P.; Pages, N.; Metzger, G.; Lévi, F. Persistent T lymphocyte rhythms despite suppressed circadian clock outputs in rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R1891–R1899. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, M.; Su, T.; Gao, T.; Yu, W. Constant light exposure aggravates POMC-mediated muscle wasting associated with hypothalamic alteration of circadian clock and SIRT1 in endotoxemia rats. Biochem. Biophys. Res. Commun. 2019, 508, 811–817. [Google Scholar] [CrossRef]
- Perfilyeva, Y.V.; Abdolla, N.; Ostapchuk, Y.O.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Expansion of CD11b+Ly6Ghigh and CD11b+CD49d+ myeloid cells with suppressive potential in mice with chronic inflammation and light-at-night-induced circadian disruption. Inflamm. Res. 2017, 66, 711–724. [Google Scholar] [CrossRef]
- Katoh, H.; Watanabe, M. Myeloid-derived suppressor cells and therapeutic strategies in cancer. Mediat. Inflamm. 2015, 2015, 159269. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Immune Challenge | Effects | Refs. |
|---|---|---|---|
| Bmal1−/− mice (global KO) | Lost daily rhythms in the circulating numbers of white blood cells and their progenitors | [43,54] | |
| Bmal1−/− mice (global KO) | KLA (in vitro) | Disturbed transcriptome response to TLR4 activation in BMDMs (enhanced and prolonged response of Il-1β, iNos and Hif1α) | [55] |
| Bmal1−/− mice (global KO) | ↑ severity of DSS-induced colitis | [56] | |
| ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) | Lost daily variability in Ly6Chigh monocyte counts in the blood, spleen, and bone marrow | [52] | |
| ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) | TG-induced peritoneal inflammation | ↑ peritoneal recruitment of Ly6Chigh monocytes and amplified CCL2, CCL8, IL-1β, and IL-6 response | [52] |
| ArntlLoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) | Listeria monocytogenes infection | ↓ survival and ↑ serum levels of IL-1β, IL-6, IFNγ, and CCL2 | [52] |
| Bmal1−/−Lys-MCre mice (myeloid-specific Bmal1 KO) | LPS 25 mg/kg (i.p.) | Lost protection to LPS-induced lethality at ZT0 compared to ZT12 | [57] |
| Bmal1−/−Lys-MCre mice (myeloid-specific Bmal1 KO) | LPS 100 ng/mL (in vitro) | ↑ LPS-induced production of IL-6, TNFα, CXCL1 and CCL2 and ↓ levels of IL-10 in BMDMs ↑ pro-inflammatory microRNA miR-155 induction upon LPS in BMDMs | [57] |
| Bmal1LoxP/LoxPLyz2Cre mice (myeloid-specific Bmal1 KO) | LPS 100 ng/mL (in vitro) or LPS 5 mg/kg (i.p.) | ↓ NRF2 response in LPS stimulated BMDMs ↑ basal and LPS stimulated ROS levels, ↑ LPS stimulated IL-1β and HIF1α levels in BMDMs ↑ serum IL-1β response to in vivo LPS stimulation | [58] |
| Bmal1FloxP/FloxP;LysMCre mice (myeloid-specific Bmal1 KO) | LPS 10 or 100 ng/mL (in vitro) | Lost daily variability in IL-12p40-producing cells in LPS-stimulated peritoneal macrophages | [59] |
| LysM-Bmal1−/− mice (myeloid-specific Bmal1 KO) | Streptococcus pneumoniae or Staphylococcus aureus infection | Protection against pneumococcal infection ↑ phagocytic activity in peritoneal and alveolar macrophages | [60] |
| LysM-Bmal1−/− mice (myeloid-specific Bmal1 KO) | LPS 1 mg/kg (i.p.) | Lost daily variability in IL-6 response to LPS in peritoneal macrophages | [51] |
| BmallLoxP/LoxPLyz2Cre mice (ApoE−/− background) (myeloid-specific Bmal1 KO) | ↑ size of atherosclerotic lesions ↑ recruitment of Ly6Chigh monocytes and accumulation of pro-inflammatory M1 macrophages in atherosclerotic lesions | [61] | |
| Bmal1ΔN mice (neutrophil-specific deletion of Bmal1) | Lost daily variability in neutrophil proteome, granule content and NET formation | [62] | |
| Clock−/− mice (global KO) | TNFα 2 ng/mL or CBLB502 100 ng/mL (in vitro) | ↓ NF-κB activation upon TNFα treatment in MEFs and upon bacterial flagellin (CBLB502) treatment in hepatocytes | [49] |
| Clock mutant mice | LPS 1 µg/mL or S. Typhimurium (in vitro) | ↓ expression of pro-inflammatory genes Il-6, Il-1β, Tnfα, Cxcl1, Ifnβ, and Ccl2 and ↓ TNFα and IL-6 response in BMDMs | [63] |
| Clock mutant mice | Salmonella infection (in vivo) | Impaired rhythmicity in bacterial colonization in the gut and reduced pro-inflammatory gene expression | [63] |
| Clock mutant mice | LPS 1 µg/mL (in vitro) | ↓ LPS-induced expression of Il-6, Il-1β and Cxcl1 in MEFs ↑ RELB and p100/52 protein levels in MEFs independent of LPS | [64] |
| Per1tm1Drw mutant mice | Modified circadian rhythms of perforin, granzyme B and IFNγ in the splenic NK cells | [65] | |
| mPer2Brdml mutant mice | Lost daily IFNγ rhythms (splenic mRNA and protein expression, and serum levels) in the spleen | [66] | |
| mPer2Brdml mutant mice | LPS 25 mg/kg (i.p.) | ↑ survival upon lethal dose of LPS and suppressed daily rhythm in susceptibility to endotoxic shock ↓ serum IFNγ and IL-1β levels and ↓ IFNγ production by splenic NK cells in response to LPS | [67] |
| mPer2Brdml mutant mice | TLR9 ligand (in vitro) | ↓ TNFα and IL-12 production in challenged peritoneal macrophages and ↓ Tlr9 expression | [68] |
| Cry1−/−Cry2−/− mice and fibroblasts (double KO) | Constitutive activation NF-κB via PKA signaling in fibroblasts ↑ constitutive expression of pro-inflammatory molecules in the hypothalamus and fibroblasts (Il-6, Tnfα and iNos), and in the BMDMs (Il-6, Cxcl1 and iNos) ↑ inflammatory response of BMDMs to LPS (TNFα and IL-6) | [69] | |
| Rev-erbα−/− mice (global KO) | LPS 1 mg/kg (i.p.) LPS 1 µg/mL (in vitro) | Lost circadian response of IL-6 to LPS challenge in vivo and in vitro using isolated PECs | [51] |
| Rev-erbα−/− mice (global KO) | aerosolized LPS 2 mg/mL | ↑ neutrophil numbers and CXCL1, CXCL2 and CXCL5 levels in BAL fluid | [70] |
| Rev-erbα−/− mice (global KO) | LPS 100 ng/mL (ex vivo) | ↑ cytokine and chemokine response to LPS (Il-6, Ccl2 and Ccl5 expression) in alveolar macrophages | [70] |
| Rev-erbα−/− mice (global KO) | LPS 1 µg/mL (in vitro) | ↑ basal and LPS-stimulated Ccl2 gene expression in peritoneal macrophages | [71] |
| Rev-erbα−/− mice (global KO) | ↑ basal NF-κB signaling and pro-inflammatory microglial activation in the hippocampus ↑ LPS-induced neuroinflammation | [72] | |
| Rev-erbα−/− mice (global KO) | ↑ complement transcripts (C4b and C3) in the hippocampus | [73] | |
| Rev-erbα−/− mice (global KO) | DSS-induced colitis | ↑ severity of DSS-induced colitis ↑ colonic levels of NLRP3, IL-1β, and IL-18 in DSS-induced colitis suppressed daily rhythm of Nlrp3 in the colon | [56] |
| Rev-erbα−/− mice (global KO) | LPS 100 ng/mL (in vitro) | ↑ LPS-induced protein levels of NLRP3 and IL-1β in peritoneal macrophages | [56] |
| staggerer (RORαsg/sg) mice | intra-tracheal LPS 2 µg/50 µL | ↑ susceptibility to LPS-induced airway inflammation ↑ neutrophil counts and cytokine levels IL-1β, IL-6, and MIP-2 in BAL fluid | [74] |
| staggerer (RORαsg/sg) mice | LPS 5 µg/mL (in vitro) | ↑ Il-1β, Il-1α, and Tnfα expression in LPS-stimulated splenocytes | [75] |
| Nfil3−/− mice (global KO) | Lack of CD8α+ cDC population in the lymphoid organs | [76] | |
| Nfil3−/− mice (global KO) | Lack of NK cells and impaired NK-cell mediated cytotoxicity | [77] | |
| Nfil3−/− mice (global KO) | Clostridium difficile infection | ↓ numbers of innate lymphoid cells in the intestinal mucosa ↓ immune defence against acute intestinal bacterial infection with Clostridium difficile | [78] |
| Nfil3−/− mice (global KO) | LPS 10 ng/mL (in vitro) | ↑ LPS-induced Il-12b expression and IL-12p40 release from BMDMs Spontaneous expression of Il-12b in colonic CD11b+ LPMCs | [79] |
| Nfil3−/− mice (global KO) | LPS 10 ng/mL (in vitro) | ↑ proportion of IL-12p40 producing macrophages in response to LPS and ↑ expression of Ccr2 in unstimulated BMDMs | [59] |
| Species | Shift Paradigm | Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Humanized NSG mice | 8 h PA/2 days for 10 days | Eliminated circadian rhythm of mouse and human blood leukocytes | [102] | |
| Lyzs-Cre mice | LD reverse/5 days for 3 weeks | Eliminated circadian rhythm of neutrophil hepatic infiltration and ↑ triacylglycerol levels in the liver | [103] | |
| C57BL/6J mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization | LPS 50 μg/mL (ex vivo) or 1 µg/mL (in vitro) | ↑ LPS-induced IL-6 response of the whole blood and incubated PECs, preserving a daily rhythm in this immune response | [104] [105] |
| C57BL/6J Per2Luc mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization | LPS 10 µg/mL (in vitro) | ↑ LPS-induced IL-6 response of incubated PECs | [106] |
| C57BL/6J Per2Luc mice | 6 h PA/7 days for 4 weeks and 1 week of re-synchronization | LPS 12.5 mg/kg (i.p.) | Persistent hypothermia, ↑ mortality rate, ↑ response of pro-inflammatory cytokines (IL-1β, GM-CSF, IL-12, IL-13) to LPS | [106] |
| C57BL/6J mice | 6 h PA/2 days for 3 weeks | LPS 20 mg/kg (i.p.) | 80% mortality rate independent of time of LPS administration, ↑ hypothermic and serum TNFα response | [107] |
| C57BL/6J Per2Luc mice on HFD | LD reverse/5 days for 10 weeks | ↑ adipose tissue macrophage infiltration and pro-inflammatory M1 polarization associated with amplified expression of Il-1β, Il-6 and Tnfα ↑ pro-inflammatory activation of BMDMs with ↑ LPS-induced expression of Il-1β, Il-6, and Tnfα | [108] | |
| APOE*3-Leiden.CETP mice on HFD | LD reverse/7 days for 10 and 15 weeks | ↑ atherosclerosis development in the aortic root ↑ lesion macrophage content and ↑ vascular expression of markers for inflammation (Nfκb1, Tnfα, iNos), oxidative stress (Sod1, Gpx1, Hif1α, Nox2), and leukocyte recruitment (Icam1, Ccr2, CCL2) at ZT0 Phase-shifted rhythms of circulating leukocytes | [109] | |
| C57BL/6J mice | 8 h PA/2–3 days for 8 weeks | ↑ severity of DSS-induced colitis | [56] | |
| C57BL/6J mice | 6 h PA/2 days for 3 weeks | B16F0 nonmetastatic melanoma cells (s.c.) | ↑ mortality rate, ↑ tumor growth rate, lost daily variability and M1/M2 macrophage ratio in melanoma tumors | [110] |
| C57BL/6J mice | LD reverse/4 days for 12 weeks | ↓ NK cell numbers in the spleen and lungs ↓ expression of CD107a and IFNγ in non-stimulated and activated splenic NK cells ↑ lung metastasis of B16 melanoma | [111] | |
| Fischer rats | 6 h PA/2 days for 3 weeks and 1 week in DD | ↓ rhythm and cytotoxicity of splenic NK cells ↓ or shifted circadian rhythms of perforin, granzyme B and IFNγ in NK cells | [112] | |
| Fischer rats | 6 h PA/2 days for 3 weeks and 1 week in DD | MADB106 tumor cells (i.v.) | ↑ lung tumor frequency (after 6–8 weeks in LD) and ↑ cytolytic activity of NK cells (24 h post stimulation) | [112] |
| Species | ALAN Intensity and Duration | Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Wistar rats | L: 150 lx; dimL: 2 lx for 2 and 5 weeks | Impaired daily variation in the numbers of circulating monocytes and T cells ↓ numbers of blood monocytes ↑ expression of macrophage marker Cd68 and ↓ Ccl2 expression in the kidney | [138] | |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 4 weeks | ↑ expression of Mac-1 and Tnfα in WAT Exacerbated peripheral inflammation associated with HFD | [139] | |
| CFW mice | L: 125 lx; dimL: 5 lx for 4 weeks | ↑ Il-6 expression in the medulla associated with cold hyperalgesia and mechanical allodynia | [140] | |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 4 weeks | LPS 0.5 mg/kg (i.p.) | Exaggerated changes in body temperature and prolonged sickness responses to LPS ↑ LPS-induced expression of Tnfα and Il-6 in microglia | [141] |
| Swiss Webster mice | L: 150 lx; dimL: 5 lx for 1 week | Model of global cerebral ischemia | ↑ mortality rate 7 days following injury ↑ neuroinflammation 24 h following injury (amplified Tnfα mRNA levels in the hippocampus) | [142] |
| C3H mice | L: 150 lx; dimL: 5 lx for 3 weeks | FM3A mammary carcinoma cells | ↓ latency to tumor onset and ↑ tumor volume | [143] |
| Nude rats | L: 345 lx; dimL: 0.2 lx for 6 weeks | MCF-7 human breast cancer xenografts | ↑ tumor growth rate | [144] |
| Siberian hamsters | L: 150 lx; dimL: 5 lx for 4 weeks | ↑ Tnfα and ↓ Bdnf expression in the hippocampus ↓ hippocampal dendritic spine density | [145] | |
| Siberian hamsters | L: 150 lx; dimL: 5 lx for 4 weeks | LPS 0.4 mg/kg (i.p.) or DNFB treatment | ↓ plasma bactericidal capacity following LPS ↓ delayed-type hypersensitivity response to DNFB | [146] |
| Nile grass rats | L: 150 lx; dimL: 5 lx for 3 weeks | ↑ basal plasma bactericidal capacity ↑ delayed-type hypersensitivity response to DNFB | [147] |
| Species | LL Intensity and Duration | Immune Challenge | Effects | Ref. |
|---|---|---|---|---|
| Sprague-Dawley rats | 300 lx for 17 weeks and 16 weeks of re-synchronization | Lost 24 h rhythm in blood leukocytes after 11/16 weeks in LL, (the rhythm was not restored after 16 weeks of re-synchronization in LD regime) ↓ NK cell counts in the blood | [158] | |
| Wistar rats | 150 lx for 4 weeks | Activated pro-inflammatory state (↑ expression of Stat3, Il-1α and Il-17ra) in the colonic mucosa | [151] | |
| Wistar rats | 200–250 lx for 5 weeks | LPS 2 µg/kg (i.v.) | ↑ plasma TNFα response and sickness symptoms upon LPS | [152] |
| Wistar rats | 200–250 lx for 5 weeks | C6 tumor cells (s.c.) | ↑ tumor growth ↑ tumor infiltration of monocytes/macrophages | [152] |
| Sprague-Dawley rats | 200 lx for 7 days | Endotoxemia model (daily i.p. LPS injection for 7 days) | ↑ hypothalamic expression of Il-1β and Tnfα | [159] |
| C56BL/6J mice | 105 lx for 24 weeks | LPS 50 µg/kg (i.v.) | Transient ↑ of neutrophil and ↓ of lymphocyte numbers in the blood was associated with enhanced inflammatory response to LPS (↑ IL-1β, TNFα, IL-6, and ↓ IL-10 plasma levels) after 8 weeks of LL No immune changes after 24 weeks of LL | [154] |
| CD-1 mice | 750 lx for 4 weeks | Complete Freund’s adjuvant (100 µL) | ↑ proportion of myeloid-derived suppressor cells in the spleen under LL was potentiated by chronic inflammation ↑ plasma TGF-β1 levels and ↑ chronic inflammation induced elevation of IL-6 levels | [160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerigova, V.; Zeman, M.; Okuliarova, M. Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 13722. https://doi.org/10.3390/ijms232213722
Jerigova V, Zeman M, Okuliarova M. Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. International Journal of Molecular Sciences. 2022; 23(22):13722. https://doi.org/10.3390/ijms232213722
Chicago/Turabian StyleJerigova, Viera, Michal Zeman, and Monika Okuliarova. 2022. "Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response" International Journal of Molecular Sciences 23, no. 22: 13722. https://doi.org/10.3390/ijms232213722
APA StyleJerigova, V., Zeman, M., & Okuliarova, M. (2022). Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. International Journal of Molecular Sciences, 23(22), 13722. https://doi.org/10.3390/ijms232213722






