Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment
Abstract
1. Introduction
2. Results
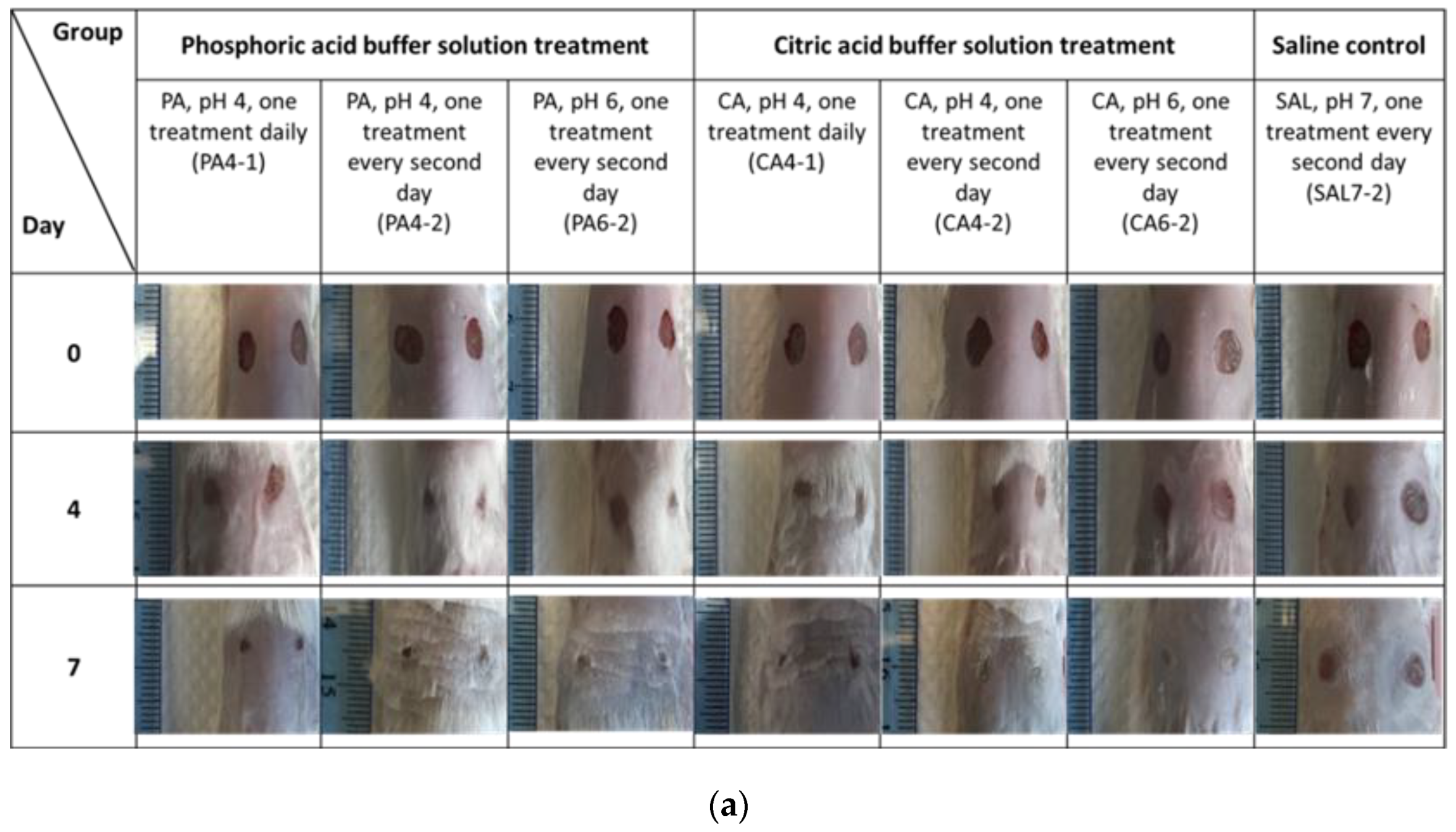
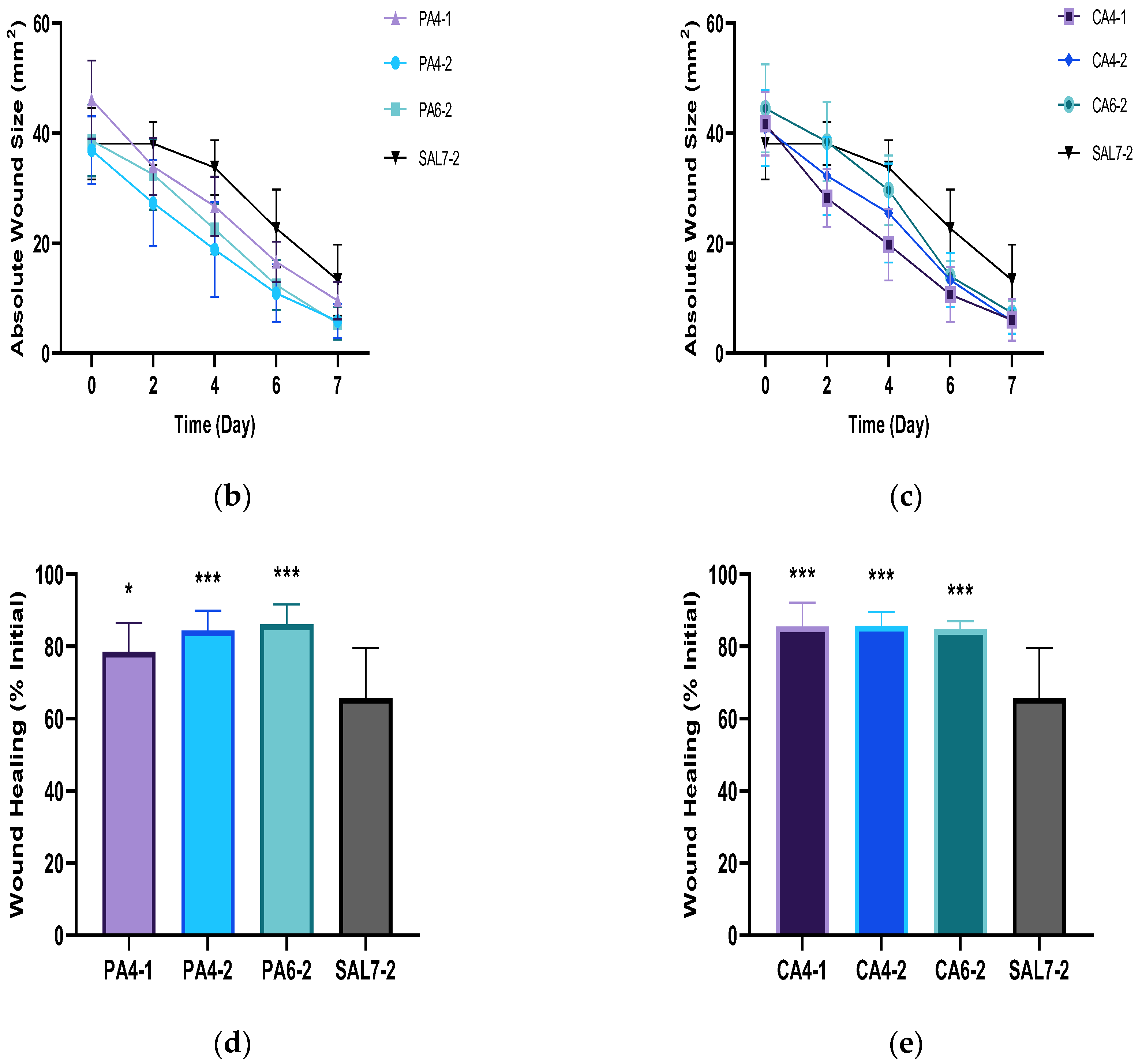
2.1. Acidic Buffers Increased Rate of Wound Closure in Murine Skin
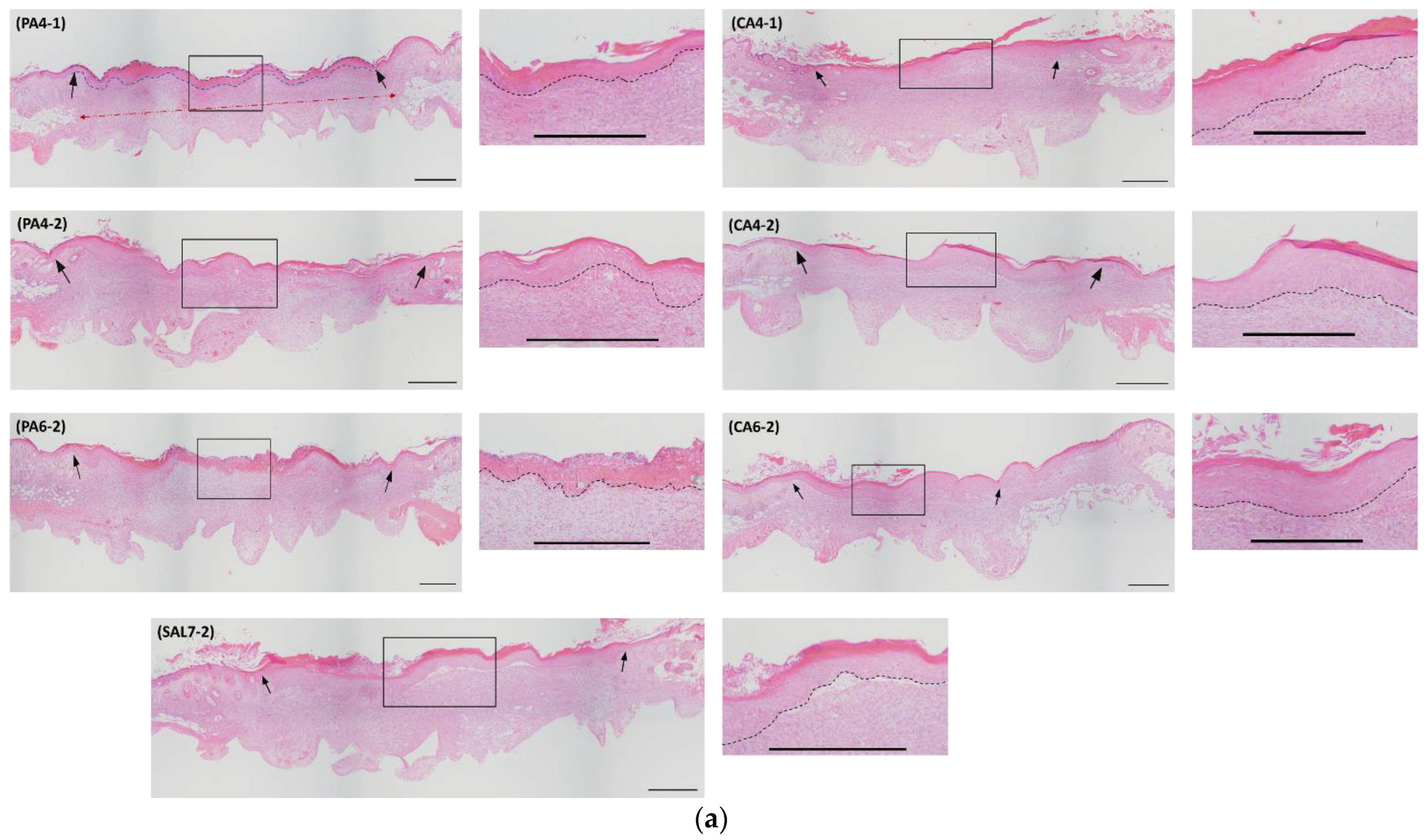
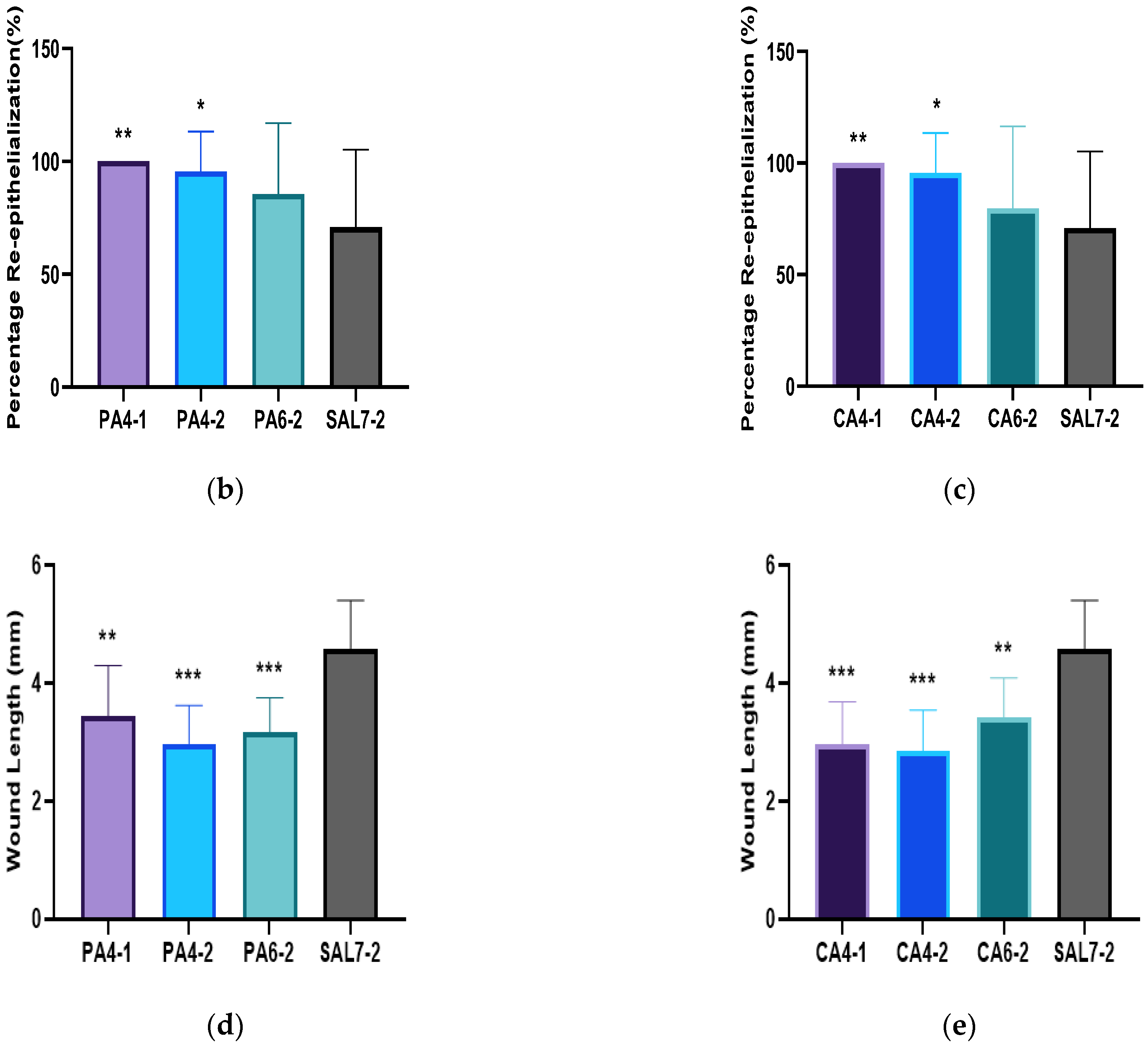
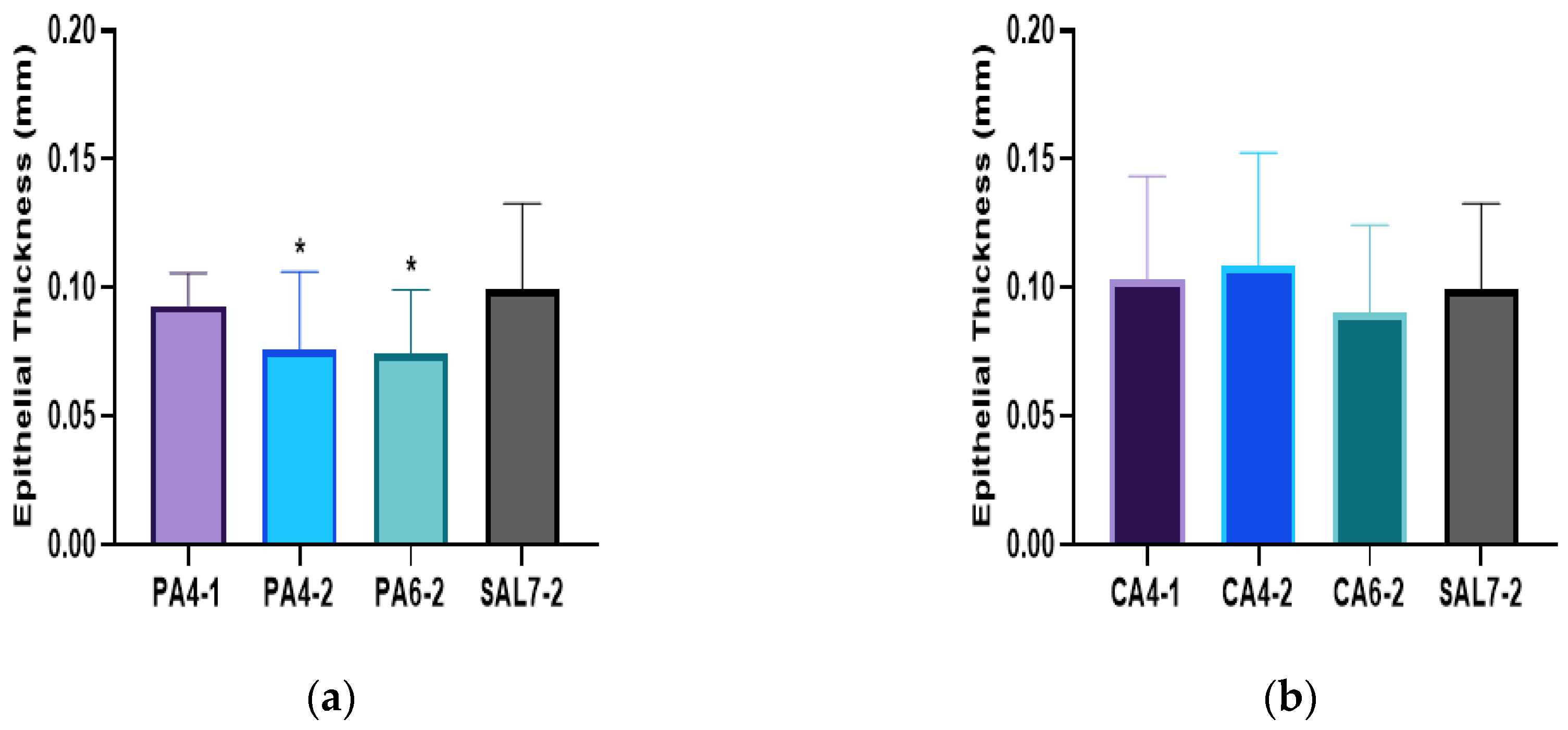
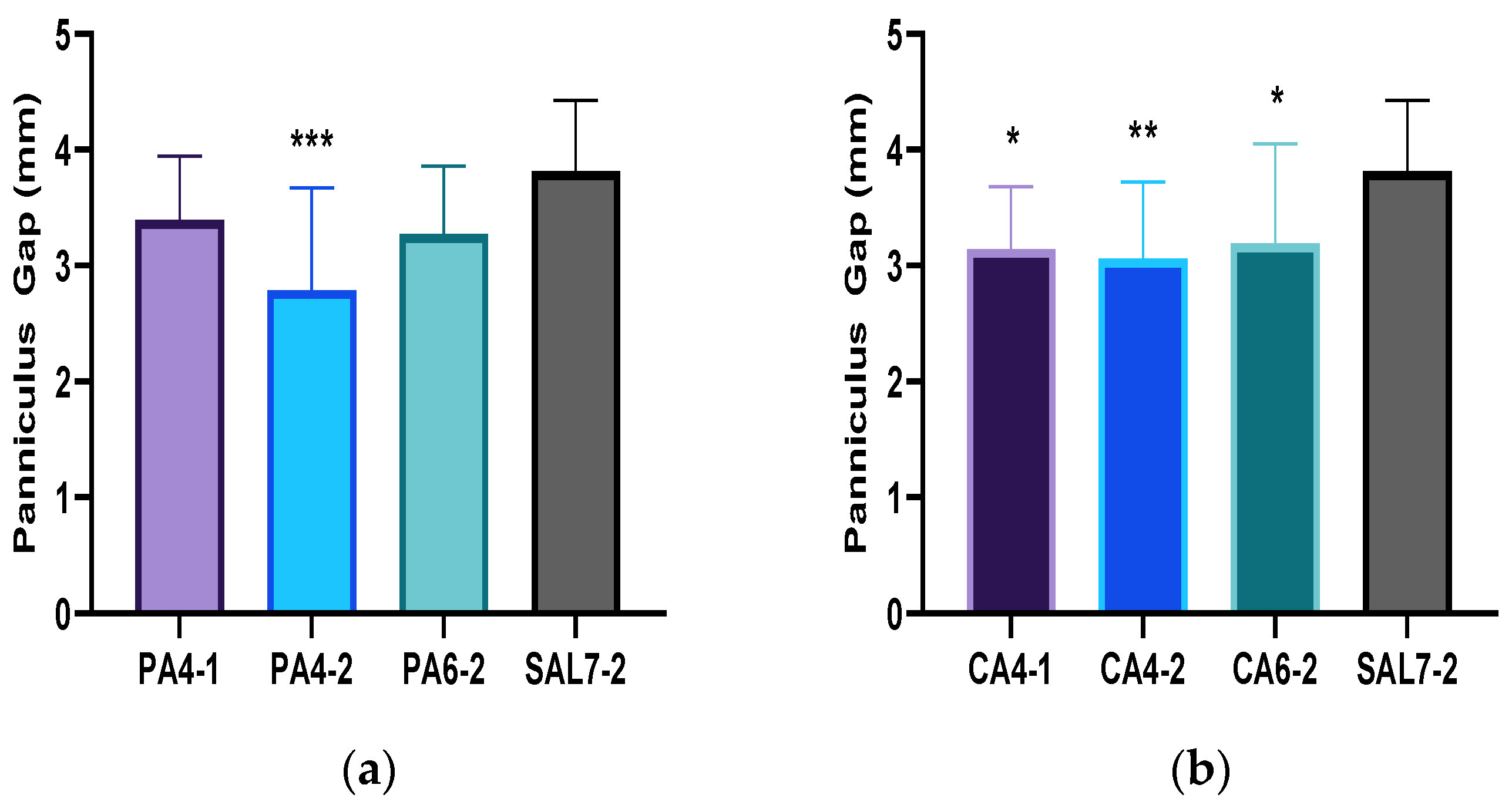
2.2. Acidic Microenvironment Improves Rate of Re-Epithelialization and Wound Contraction
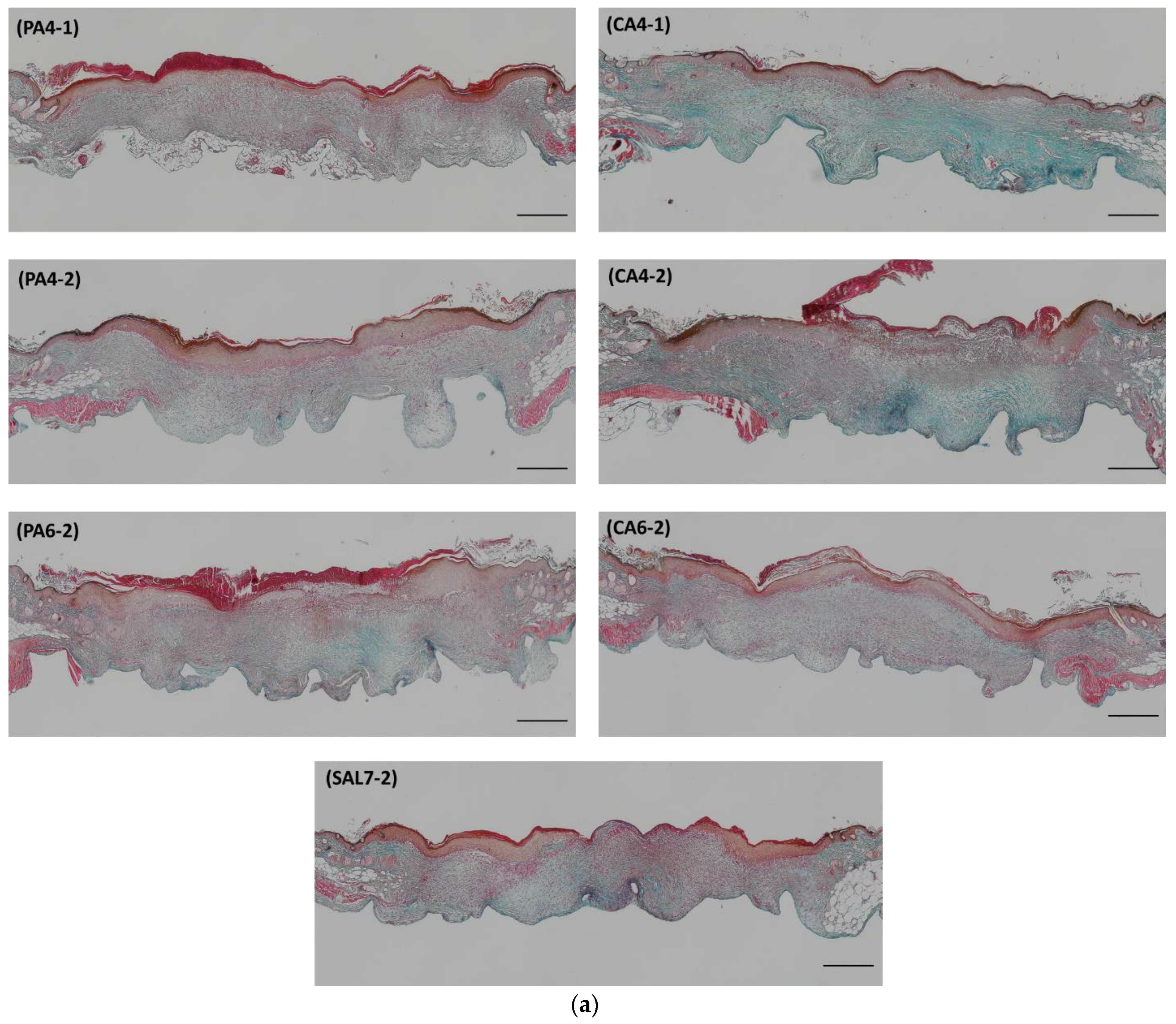
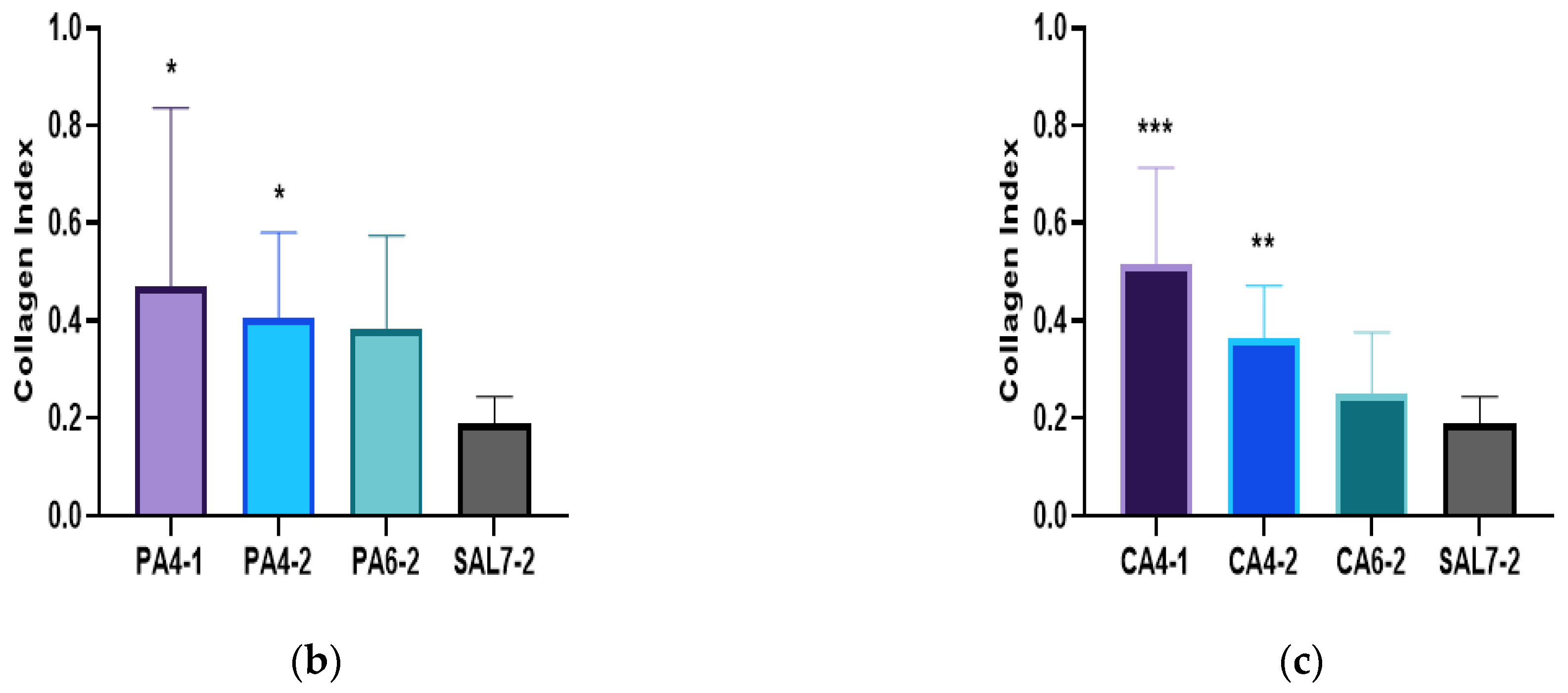
2.3. Acidic Treatments Promote Collagen Deposition in Wounded Tissues
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Topical Buffer Preparation
4.3. Murine Wound Repair Model
4.4. Macroscopic Wound Size Measurement
4.5. Histological Assessment by Hematoxylin & Eosin (H&E)
4.6. Histological Assessment by Masson Trichrome (MT)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2006, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.-M.; Karrer, S.; Heinlin, J.; Landthaler, M.; Babilas, P. The impact of the pH value on skin integrity and cutaneous wound healing. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, J.; Harris, K.; Jubin, K.; Bainbridge, N.; Jordan, N. The effect of pH in modulating skin cell behaviour. Br. J. Dermatol. 2009, 161, 671–673. [Google Scholar] [CrossRef]
- Gethin, G. The significance of surface pH in chronic wounds. Wounds 2007, 3, 52. [Google Scholar]
- Chikakane, K.; Takahashi, H. Measurement of skin pH and its significance in cutaneous diseases. Clin. Dermatol. 1995, 13, 299–306. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M. Stratum Corneum pH: Formation and Function of the ‘Acid Mantle’. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Kaufman, T.; Eichenlaub, E.; Angel, M.; Levin, M.; Futrell, J. Topical acidification promotes healing of experimental deep partial thickness skin burns: A randomized double-blind preliminary study. Burns 1985, 12, 84–90. [Google Scholar] [CrossRef]
- Rippke, F.; Schreiner, V.; Schwanitz, H.J. The acidic milieu of the horny layer: New findings on the physiology and pathophysiology of skin pH. Am. J. Clin. Dermatol. 2002, 3, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Power, G.; Moore, Z.; O’Connor, T. Measurement of pH, exudate composition and temperature in wound healing: A systematic review. J. Wound Care 2017, 26, 381–397. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.P. Normal flora. In Medical Microbiology; The University of Texas Medical Branch: Galveston, TX, USA, 1996. [Google Scholar]
- Aly, R.; Shirley, C.; Cunico, B.; Maibach, H.I. Effect of Prolonged Occlusion on the Microbial Flora, pH, Carbon Dioxide and Transepidermal Water Loss on Human Skin. J. Investig. Dermatol. 1978, 71, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, K. The pH changes of pressure ulcers related to the healing process of wounds. Wounds 1992, 4, 16–20. [Google Scholar]
- Nagoba, B.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic Environment and Wound Healing: A Review. Wounds Compend. Clin. Res. Pract. 2015, 27, 5–11. [Google Scholar]
- Percival, S.L.; McCarty, S.; Hunt, J.A.; Woods, E.J. The effects of pH on wound healing, biofilms, and antimicrobial efficacy. Wound Repair Regen. 2014, 22, 174–186. [Google Scholar] [CrossRef]
- Constant, E. Chemical acidification of wounds: An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Plast. Reconstr. Surg. 1974, 54, 114. [Google Scholar] [CrossRef]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef]
- Hopf, H.W.; Rollins, M.D. Wounds: An Overview of the Role of Oxygen. Antioxid. Redox Signal. 2007, 9, 1183–1192. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.; Prantl, L.; Karrer, S.; Landthaler, M.; Babilas, P. Oxygen in acute and chronic wound healing. Br. J. Dermatol. 2010, 163, 257–268. [Google Scholar] [CrossRef]
- Leveen, H.H.; Falk, G.; Borek, B.; Diaz, C.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann. Surg. 1973, 178, 745. [Google Scholar] [CrossRef] [PubMed]
- Lengheden, A.; Jansson, L. PH effects on experimental wound healing of human fibroblasts in vitro. Eur. J. Oral Sci. 1995, 103, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Sim, P.; Song, Y.; Yang, G.N.; Cowin, A.J.; Garg, S. In Vitro Wound Healing Properties of Novel Acidic Treatment Regimen in Enhancing Metabolic Activity and Migration of Skin Cells. Int. J. Mol. Sci. 2022, 23, 7188. [Google Scholar] [CrossRef] [PubMed]
- Nagoba, B.S.; Gandhi, R.C.; Wadher, B.J.; Potekar, R.; Kolhe, S.M. Microbiological, histopathological and clinical changes in chronic infected wounds after citric acid treatment. J. Med. Microbiol. 2008, 57, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F. Fibroblasts, myofibroblasts, and wound contraction. J. Cell Biol. 1994, 124, 401–404. [Google Scholar] [CrossRef]
- Chang, P.C.; Sulik, G.I.; Soong, H.K.; Parkinson, W.C. Galvanotropic and galvanotaxic responses of corneal endothelial cells. J. Formos. Med. Assoc. 1996, 95. [Google Scholar]
- Soong, H.K.; Parkinson, W.C.; Bafna, S.; Sulik, G.L.; Huang, S.C. Movements of cultured corneal epithelial cells and stromal fibroblasts in electric fields. Investig. Ophthalmol. Vis. Sci. 1990, 31. [Google Scholar]
- Nagoba, B.; Davane, M.; Gandhi, R.; Wadher, B.; Suryawanshi, N.; Selkar, S. Treatment of skin and soft tissue infections caused by Pseudomonas aeruginosa —A review of our experiences with citric acid over the past 20 years. Wound Med. 2017, 19, 5–9. [Google Scholar] [CrossRef]
- Malu, R.G.; Nagoba, B.S.; Jaju, C.R.; Suryawanshi, N.M.; Mali, S.A.; Goyal, V.S.; Misal, N.S. Topical use of citric acid for wound bed preparation. Int. Wound J. 2014, 13, 709–712. [Google Scholar] [CrossRef]
- Tandon, S.; Singh, B.; Kapoor, S.; Mangal, S. Comparison of Effect of pH Modulation on Wound Healing with Topical Application of Citric Acid Versus Superoxide Ions. Niger. J. Surg. 2020, 26, 122–126. [Google Scholar]
- Prabhu, V.; Prasadi, S.; Shivani, A.; Gore, A.; Pawar, V. Does wound pH modulation with 3% citric acid solution dressing help in wound healing: A pilot study. Saudi Surg. J. 2014, 2, 38. [Google Scholar] [CrossRef]
- Yang, Q.; Liang, H.; Maulu, S.; Ge, X.; Ren, M.; Xie, J.; Xi, B. Dietary phosphorus affects growth, glucolipid metabolism, antioxidant activity and immune status of juvenile blunt snout bream (Megalobrama amblycephala). Anim. Feed Sci. Technol. 2021, 274, 114896. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Liu, Y.J.; Mai, K.S.; Tian, L.X. Effect of dietary phosphorus levels on growth, body composition, muscle and bone mineral concentrations for orange-spotted grouper Epinephelus coioides reared in floating cages. J. World Aquac. Soc. 2004, 35, 427–435. [Google Scholar] [CrossRef]
- Chen, X.; Lv, Q.; Liu, Y.; Deng, W. Effect of Food Additive Citric Acid on The Growth of Human Esophageal Carcinoma Cell Line EC109. Cell J. 2016, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Bodner, G.M. Metabolism Part II: The tricarboxylic acid (TCA), citric acid, or Krebs cycle. J. Chem. Educ. 1986, 63. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-Canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef]
- Chen, J.S.; Longaker, M.T.; Gurtner, G.C. Murine models of human wound healing. Methods Mol. Biol. 2013, 1037, 265–274. [Google Scholar]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Hachem, J.-P.; Behne, M.; Aronchik, I.; Demerjian, M.; Feingold, K.; Elias, P.; Mauro, T. Extracellular pH Controls NHE1 Expression in Epidermis and Keratinocytes: Implications for Barrier Repair. J. Investig. Dermatol. 2005, 125, 790–797. [Google Scholar] [CrossRef]
- Chan, A.; Mauro, T. Acidification in the epidermis and the role of secretory phospholipases. Derm.-Endocrinol. 2011, 3, 84–90. [Google Scholar] [CrossRef]
- Abels, C.; Masur, C.; Daehnhardt-Pfeiffer, S.; Daehnhardt, D.; Knie, U. 413 Formulation with low pH decreases skin pH, restores disrupted epidermal barrier and improves lipid lamellae structure. J. Investig. Dermatol. 2017, 137, S71. [Google Scholar] [CrossRef][Green Version]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, R.; Urata, M.M.; Ito, Y.; Bringas, P.; Chai, Y. Functional Significance of Smad2 in Regulating Basal Keratinocyte Migration During Wound Healing. J. Investig. Dermatol. 2005, 125, 1302–1309. [Google Scholar] [CrossRef]
- Miscianinov, V.; Martello, A.; Rose, L.; Parish, E.; Cathcart, B.; Mitić, T.; Gray, G.A.; Meloni, M.; Zen, A.A.H.; Caporali, A. MicroRNA-148b Targets the TGF-β Pathway to Regulate Angiogenesis and Endothelial-to-Mesenchymal Transition during Skin Wound Healing. Mol. Ther. 2018, 26, 1996–2007. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, V.; Etich, J.; Pitzler, L.; Frie, C.; Koch, M.; Fischer, M.; Rappl, G.; Abken, H.; Tomasek, J.J.; Brachvogel, B. Identification of a myofibroblast-specific expression signature in skin wounds. Matrix Biol. 2018, 65, 59–74. [Google Scholar] [CrossRef]
- Zhang, Y.E. Mechanistic insight into contextual TGF-β signaling. Curr. Opin. Cell Biol. 2018, 51, 1–7. [Google Scholar] [CrossRef]
- Helary, C.; Ovtracht, L.; Coulomb, B.; Godeau, G.; Giraudguille, M. Dense fibrillar collagen matrices: A model to study myofibroblast behaviour during wound healing. Biomaterials 2006, 27, 4443–4452. [Google Scholar] [CrossRef]
- Hinz, B.; Gabbiani, G. Mechanisms of force generation and transmission by myofibroblasts. Curr. Opin. Biotechnol. 2003, 14, 538–546. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Sinha, M.; Sen, C.K.; Singh, K.; DAS, A.; Ghatak, S.; Rhea, B.; Blackstone, B.; Powell, H.M.; Khanna, S.; Roy, S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat. Commun. 2018, 9, 1–19. [Google Scholar] [CrossRef]
- Thangavel, P.; Vilvanathan, S.P.; Kuttalam, I.; Lonchin, S. Topical administration of pullulan gel accelerates skin tissue regeneration by enhancing collagen synthesis and wound contraction in rats. Int. J. Biol. Macromol. 2020, 149, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Avizheh, L.; Peirouvi, T.; Diba, K.; Fathi-Azarbayjani, A. Electrospun wound dressing as a promising tool for the therapeutic delivery of ascorbic acid and caffeine. Ther. Deliv. 2019, 10, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Rajanikant, G.; Rao, K.M. Ascorbic acid increases healing of excision wounds of mice whole body exposed to different doses of γ-radiation. Burns 2007, 33, 484–494. [Google Scholar] [CrossRef]
- Shu, D.Y.; Lovicu, F.J. Myofibroblast transdifferentiation: The dark force in ocular wound healing and fibrosis. Prog. Retin. Eye Res. 2017, 60, 44–65. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Vetteth, S.; Periyasamy, S.M.; Kanj, M.; Fedorova, L.; Khouri, S.; Kahaleh, M.B.; Xie, Z.; Malhotra, D.; Kolodkin, N.I.; et al. Central Role for the Cardiotonic Steroid Marinobufagenin in the Pathogenesis of Experimental Uremic Cardiomyopathy. Hypertension 2006, 47, 488–495. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sim, P.; Strudwick, X.L.; Song, Y.; Cowin, A.J.; Garg, S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. Int. J. Mol. Sci. 2022, 23, 13655. https://doi.org/10.3390/ijms232113655
Sim P, Strudwick XL, Song Y, Cowin AJ, Garg S. Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. International Journal of Molecular Sciences. 2022; 23(21):13655. https://doi.org/10.3390/ijms232113655
Chicago/Turabian StyleSim, Pivian, Xanthe L. Strudwick, YunMei Song, Allison J. Cowin, and Sanjay Garg. 2022. "Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment" International Journal of Molecular Sciences 23, no. 21: 13655. https://doi.org/10.3390/ijms232113655
APA StyleSim, P., Strudwick, X. L., Song, Y., Cowin, A. J., & Garg, S. (2022). Influence of Acidic pH on Wound Healing In Vivo: A Novel Perspective for Wound Treatment. International Journal of Molecular Sciences, 23(21), 13655. https://doi.org/10.3390/ijms232113655






