Dynamical Mechanism Analysis of Three Neuroregulatory Strategies on the Modulation of Seizures
Abstract
1. Introduction
2. Results
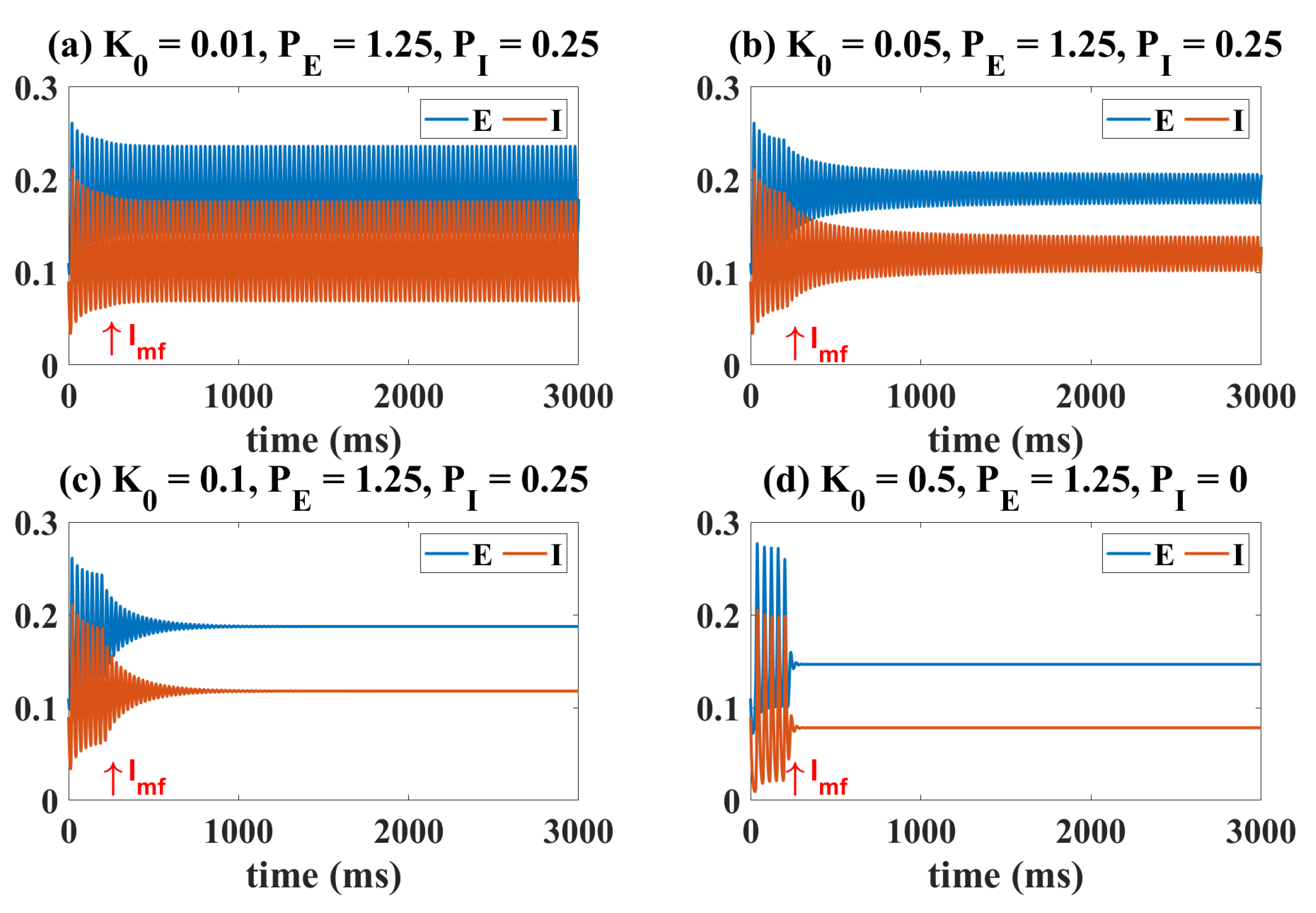
2.1. Epileptiform Activities Regulated by the Model External Inputs
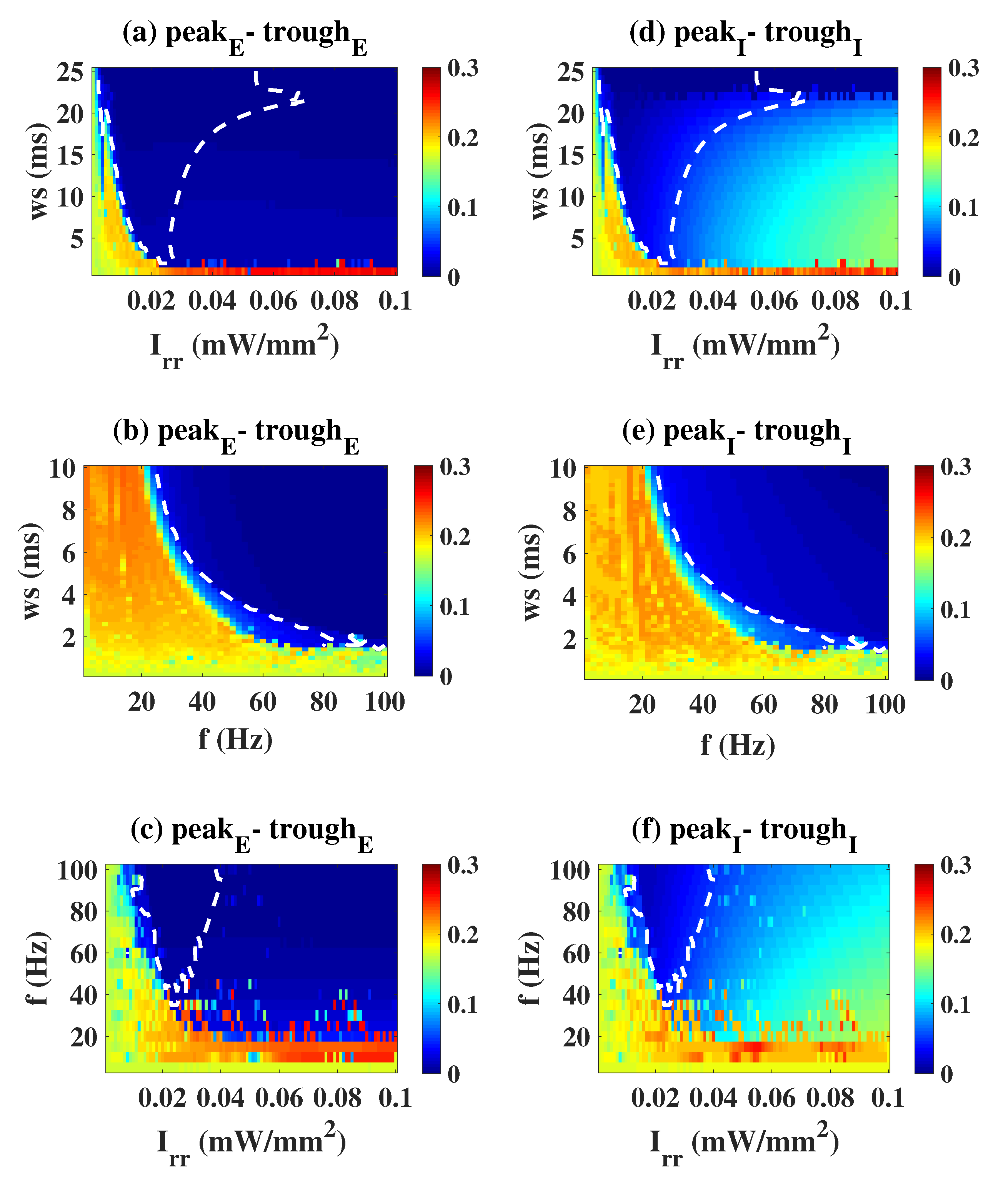
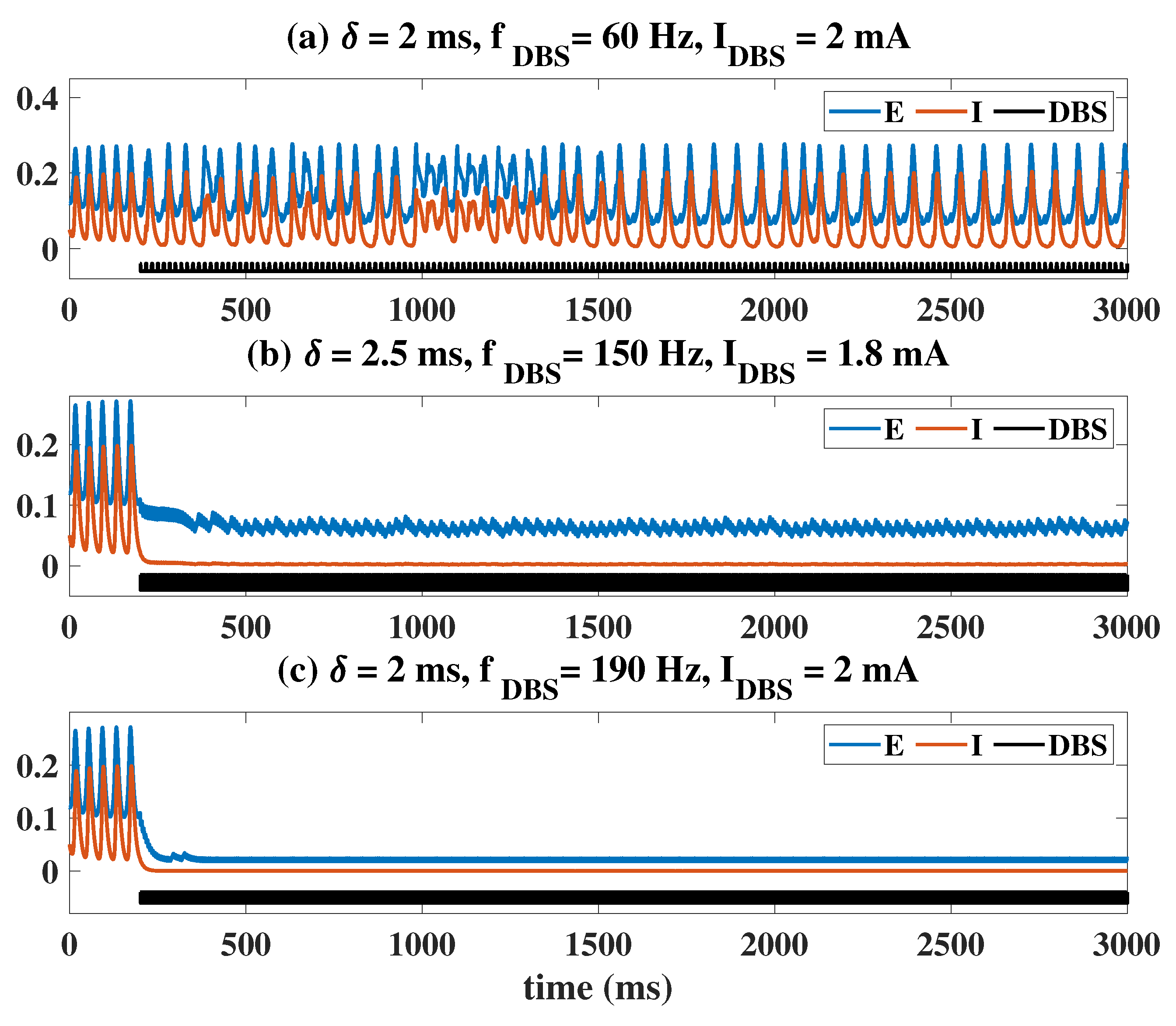
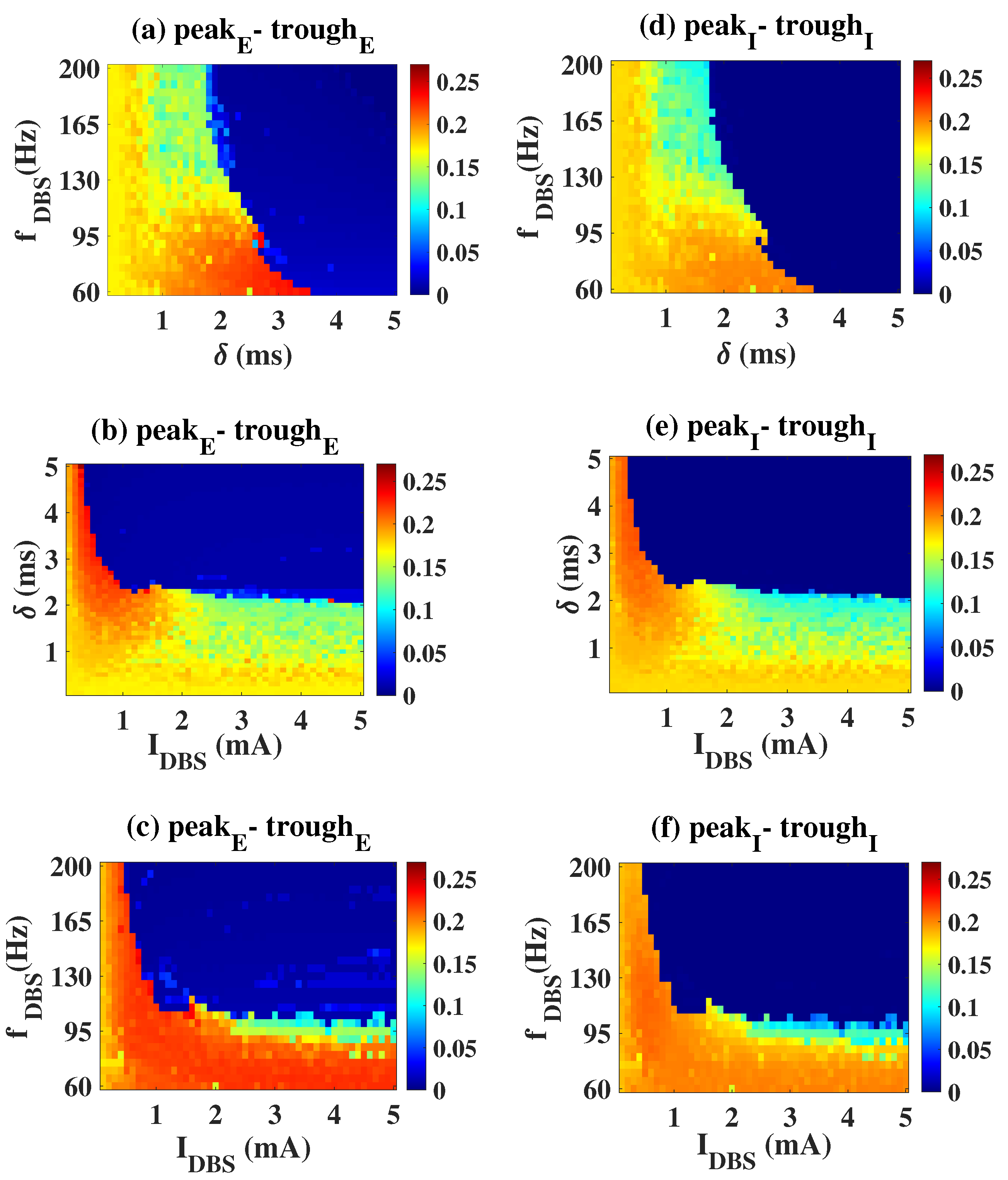
2.2. Epileptiform Activities Regulated by the Three Strategies
2.3. Propagation of Epileptiform Activities in the Coupled Network Structure
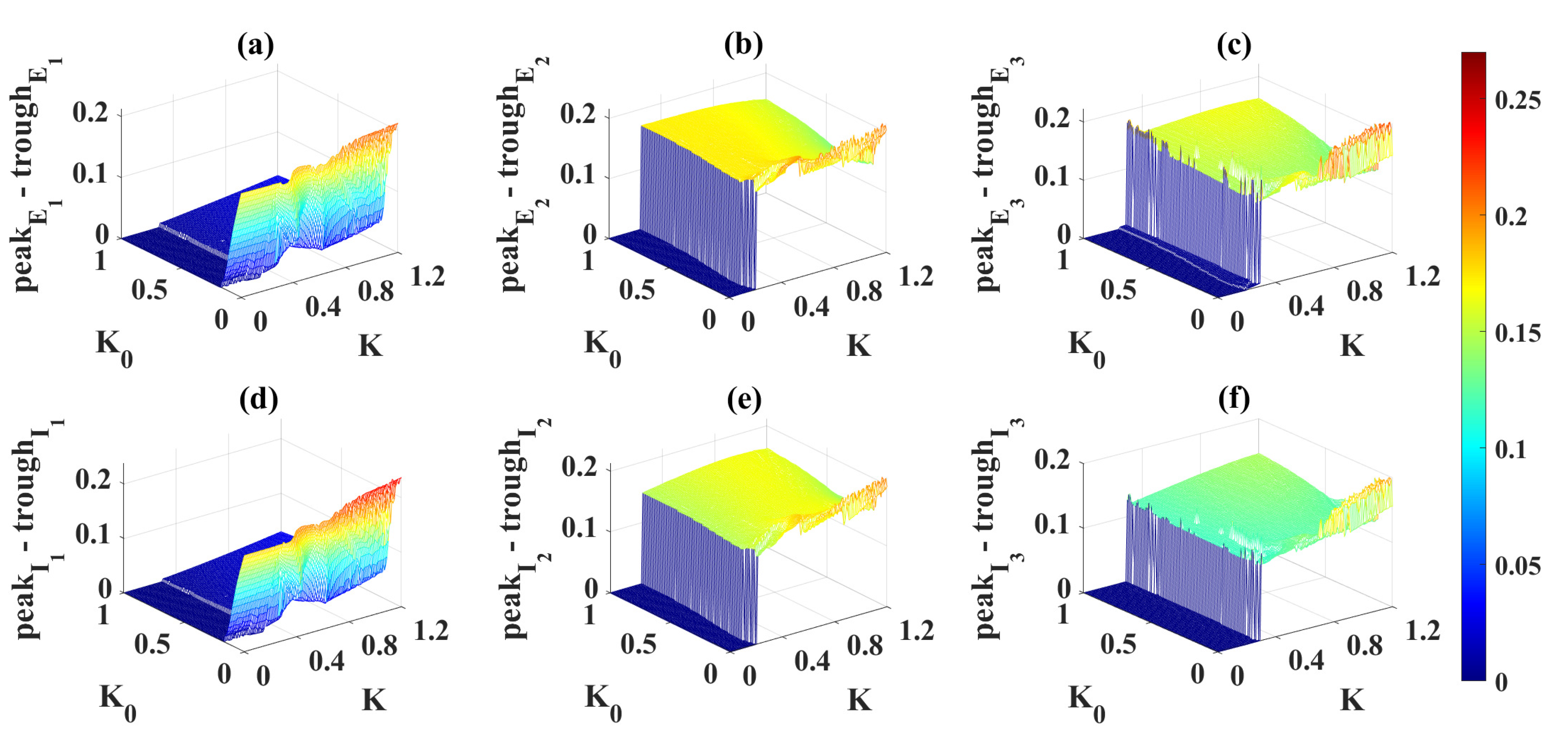
2.4. Modulation Effects in the Coupled Network Structure
3. Discussions
4. Materials and Methods
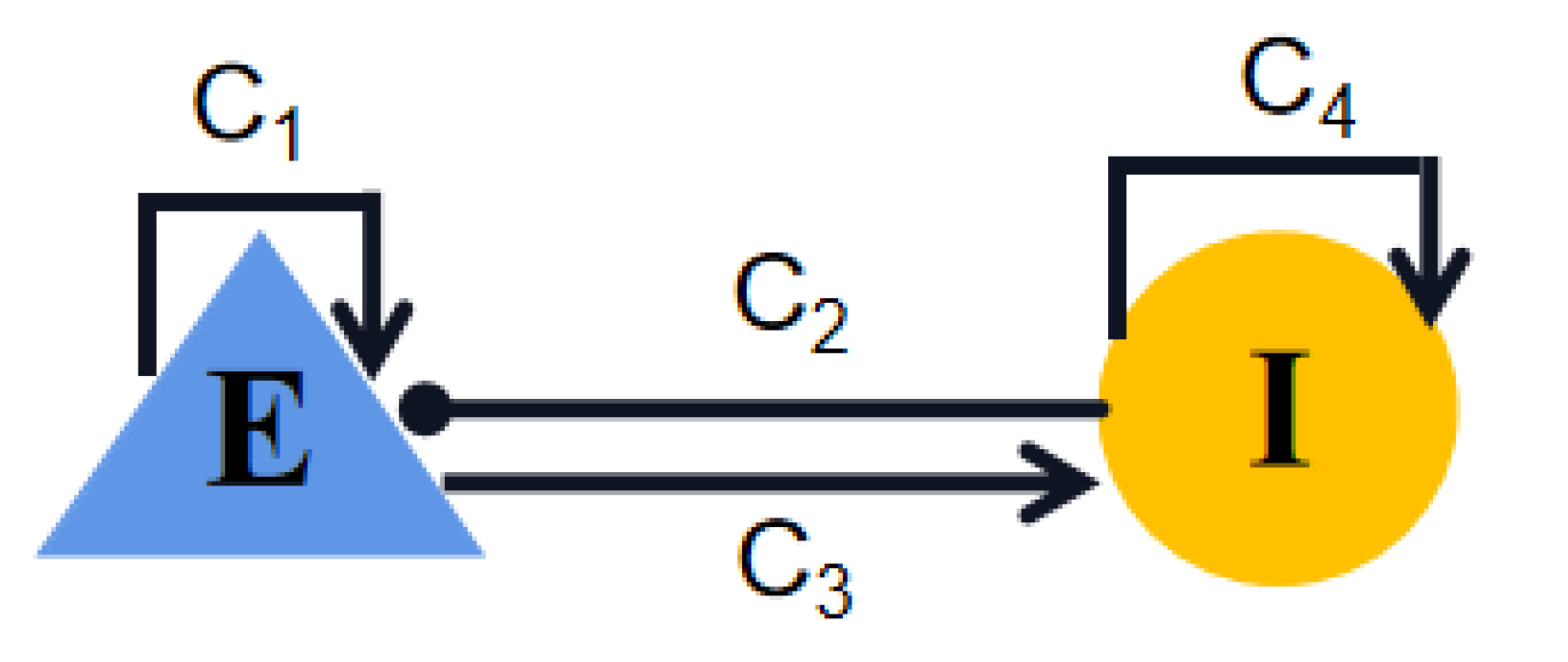
4.1. Wilson–Cowan Model
4.2. Three Neuromodulation Strategies
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wyckhuys, T.; Raedt, R.; Vonck, K.; Wadman, W.; Boon, P. Comparison of hippocampal deep brain stimulation with high (130 Hz) and low frequency (5 Hz) on afterdischarges in kindled rats. Epilepsy Res. 2010, 88, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Zhu, G.Y.; Wang, X.; Shi, L.; Jiang, Y.; Zhang, X.; Zhang, J.G. Deep brain stimulation of the anterior nucleus of the thalamus reverses the gene expression of cytokines and their receptors as well as neuronal degeneration in epileptic rats. Brain Res. 2017, 1657, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Santos-Valencia, F.; Almazán-Alvarado, S.; Rubio-Luviano, A.; Valdés-Cruz, A.; Magdaleno-Madrigal, V.M.; Martínez-Vargas, D. Temporally irregular electrical stimulation to the epileptogenic focus delays epileptogenesis in rats. Brain Stimul. 2019, 12, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Valentín, A.; García Navarrete, E.; Chelvarajah, R.; Torres, C.; Navas, M.; Vico, L.; Torres, N.; Pastor, J.; Selway, R.; Sola, R.G.; et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 2013, 54, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Salanova, V.; Witt, T.; Worth, R.; Henry, T.R.; Gross, R.E.; Nazzaro, J.M.; Labar, D.; Sperling, M.R.; Sharan, A.; Sandok, E.; et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 2015, 84, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yu, T.; Wang, D.; Wang, X.; Ni, D.; Zhang, G.; Bartolomei, F.; Wang, Y.; Li, Y. Subthalamic nucleus stimulation modulates motor epileptic activity in humans. Ann. Neurol. 2020, 88, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Q. Stimulation strategies for absence seizures: Targeted therapy of the focus in coupled thalamocortical model. Nonlinear Dyn. 2019, 96, 1649–1663. [Google Scholar] [CrossRef]
- Hu, B.; Chen, S.; Chi, H.; Chen, J.; Yuan, P.; Lai, H.; Dong, W. Controlling absence seizures by tuning activation level of the thalamus and striatum. Chaos Solitons Fractals 2017, 95, 65–76. [Google Scholar] [CrossRef]
- Taylor, P.N.; Baier, G.; Cash, S.S.; Dauwels, J.; Slotine, J.J.; Wang, Y. A model of stimulus induced epileptic spike-wave discharges. In Proceedings of the 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), Singapore, 16–19 April 2013; pp. 53–59. [Google Scholar]
- Sahin, M.; Tie, Y. Non-rectangular waveforms for neural stimulation with practical electrodes. J. Neural Eng. 2007, 4, 227. [Google Scholar] [CrossRef]
- Fan, D.; Wang, Q. Improved control effect of absence seizures by autaptic connections to the subthalamic nucleus. Phys. Rev. E 2018, 98, 052414. [Google Scholar] [CrossRef]
- Wilson, M.T.; Goodwin, D.; Brownjohn, P.W.; Shemmell, J.; Reynolds, J.N. Numerical modelling of plasticity induced by transcranial magnetic stimulation. J. Comput. Neurosci. 2014, 36, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Spencer, D.C.; Weston, J.; Nolan, S.J. Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Tassinari, C.A.; Cincotta, M.; Zaccara, G.; Michelucci, R. Transcranial magnetic stimulation and epilepsy. Clin. Neurophysiol. 2003, 114, 777–798. [Google Scholar] [CrossRef]
- Schwan, H.P. Electrical properties of tissue and cell suspensions. In Advances in Biological and Medical Physics; Elsevier: Amsterdam, The Netherlands, 1957; Volume 5, pp. 147–209. [Google Scholar]
- Kotnik, T.; Bobanović, F.; Miklavcˇicˇ, D. Sensitivity of transmembrane voltage induced by applied electric fields—A theoretical analysis. Bioelectrochemistry Bioenerg. 1997, 43, 285–291. [Google Scholar] [CrossRef]
- Ma, J.; Mi, L.; Zhou, P.; Xu, Y.; Hayat, T. Phase synchronization between two neurons induced by coupling of electromagnetic field. Appl. Math. Comput. 2017, 307, 321–328. [Google Scholar] [CrossRef]
- Du, L.; Cao, Z.; Lei, Y.; Deng, Z. Electrical activities of neural systems exposed to sinusoidal induced electric field with random phase. Sci. China Technol. Sci. 2019, 62, 1141–1150. [Google Scholar] [CrossRef]
- Barry, J.F.; Turner, M.J.; Schloss, J.M.; Glenn, D.R.; Song, Y.; Lukin, M.D.; Park, H.; Walsworth, R.L. Optical magnetic detection of single-neuron action potentials using quantum defects in diamond. Proc. Natl. Acad. Sci. USA 2016, 113, 14133–14138. [Google Scholar] [CrossRef] [PubMed]
- Cobos, I.; Calcagnotto, M.E.; Vilaythong, A.J.; Thwin, M.T.; Noebels, J.L.; Baraban, S.C.; Rubenstein, J.L. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat. Neurosci. 2005, 8, 1059–1068. [Google Scholar] [CrossRef]
- Magloire, V.; Mercier, M.S.; Kullmann, D.M.; Pavlov, I. GABAergic interneurons in seizures: Investigating causality with optogenetics. Neuroscientist 2019, 25, 344–358. [Google Scholar] [CrossRef]
- Selvaraj, P.; Sleigh, J.W.; Freeman, W.J.; Kirsch, H.E.; Szeri, A.J. Open loop optogenetic control of simulated cortical epileptiform activity. J. Comput. Neurosci. 2014, 36, 515–525. [Google Scholar] [CrossRef][Green Version]
- Yizhar, O.; Fenno, L.E.; Davidson, T.J.; Mogri, M.; Deisseroth, K. Optogenetics in neural systems. Neuron 2011, 71, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Krook-Magnuson, E.; Armstrong, C.; Oijala, M.; Soltesz, I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat. Commun. 2013, 4, 1376. [Google Scholar] [CrossRef] [PubMed]
- Ledri, M.; Madsen, M.G.; Nikitidou, L.; Kirik, D.; Kokaia, M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J. Neurosci. 2014, 34, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, P.; Sleigh, J.W.; Kirsch, H.E.; Szeri, A.J. Closed-loop feedback control and bifurcation analysis of epileptiform activity via optogenetic stimulation in a mathematical model of human cortex. Phys. Rev. E 2016, 93, 012416. [Google Scholar] [CrossRef]
- Che, Y. Distributed open-loop optogenetic control of cortical epileptiform activity in a Wilson–Cowan network. In Proceedings of the 2017 8th International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 469–472. [Google Scholar]
- Hodgkin, A.L.; Huxley, A.F. The components of membrane conductance in the giant axon of Loligo. J. Physiol. 1952, 116. [Google Scholar] [CrossRef]
- Fitzhugh, R. Mathematical models of threshold phenomena in the nerve membrane. Bull. Math. Biophys. 1955, 17, 257–278. [Google Scholar] [CrossRef]
- Nagumo, J.S.; Arimoto, S.; Yoshizawa, S. An Active Pulse Transmission Line Simulating Nerve Axon. Proc. Ire 1962, 50, 2061–2070. [Google Scholar] [CrossRef]
- Hindmarsh, J.L.; Rose, R.M. A Model of Neuronal Bursting Using Three Coupled First Order Differential Equations. Proc. R. Soc. Lond. B 1984, 221, 87–102. [Google Scholar]
- Wilson, H.R.; Cowan, J.D. Excitatory and Inhibitory Interactions in Localized Populations of Model Neurons. Biophys. J. 1972, 12, 1–24. [Google Scholar] [CrossRef]
- Silva, F.; Hoeks, A.; Smits, H.; Zetterberg, L.H. Model of brain rhythmic activity. Kybernetik 1974, 15, 27–37. [Google Scholar] [CrossRef]
- Liley, D.; Cadusch, P.J.; Dafilis, M.P. A spatially continuous mean field theory of electrocortical activity. Netw. Comput. Neural Syst. 2002, 13, 67–113. [Google Scholar] [CrossRef]
- Suffczynski, P.; Kalitzin, S.; Silva, F. Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Neuroscience 2004, 126, 467–484. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.N.; Baier, G. A spatially extended model for macroscopic spike-wave discharges. J. Comput. Neurosci. 2011, 31, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guo, D.; Wang, T.; Jing, W.; Xia, Y.; Xu, P.; Luo, C.; Valdes-Sosa, P.A.; Yao, D.; Deco, G. Bidirectional Control of Absence Seizures by the Basal Ganglia: A Computational Evidence. PLoS Comput. Biol. 2014, 10, e1003495. [Google Scholar] [CrossRef]
- Milo, R.; Shen-Orr, S.; Itzkovitz, S.; Kashtan, N.; Chklovskii, D.; Alon, U. Network motifs: Simple building blocks of complex networks. Science 2002, 298, 824–827. [Google Scholar] [CrossRef]
- Gollo, L.L.; Breakspear, M. The frustrated brain: From dynamics on motifs to communities and networks. Philos. Trans. R. Soc. Biol. Sci. 2014, 369, 20130532. [Google Scholar] [CrossRef]
- Cao, Y.; Ren, K.; Su, F.; Deng, B.; Wei, X.; Wang, J. Suppression of seizures based on the multi-coupled neural mass model. Chaos Interdiscip. J. Nonlinear Sci. 2015, 25, 103120. [Google Scholar] [CrossRef]
- Jirsa, V.K.; Stacey, W.C.; Quilichini, P.P.; Ivanov, A.I.; Bernard, C. On the nature of seizure dynamics. Brain 2014, 137, 2210–2230. [Google Scholar] [CrossRef]
- Heers, M.; Helias, M.; Hedrich, T.; Dümpelmann, M.; Schulze-Bonhage, A.; Ball, T. Spectral bandwidth of interictal fast epileptic activity characterizes the seizure onset zone. Neuroimage Clin. 2018, 17, 865–872. [Google Scholar] [CrossRef]
- Tsuboyama, M.; Kaye, H.L.; Rotenberg, A. Review of transcranial magnetic stimulation in epilepsy. Clin. Ther. 2020, 42, 1155–1168. [Google Scholar] [CrossRef]
- Yousif, N.; Bain, P.G.; Nandi, D.; Borisyuk, R. A population model of deep brain stimulation in movement disorders from circuits to cells. Front. Hum. Neurosci. 2020, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Duchet, B.; Weerasinghe, G.; Bick, C.; Bogacz, R. Optimizing deep brain stimulation based on isostable amplitude in essential tremor patient models. J. Neural Eng. 2021, 18, 046023. [Google Scholar] [CrossRef] [PubMed]
- Duchet, B.; Weerasinghe, G.; Cagnan, H.; Brown, P.; Bick, C.; Bogacz, R. Phase-dependence of response curves to deep brain stimulation and their relationship: From essential tremor patient data to a Wilson–Cowan model. J. Math. Neurosci. 2020, 10, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.R.; Cowan, J.D. A mathematical theory of the functional dynamics of cortical and thalamic nervous tissue. Kybernetik 1973, 13, 55–80. [Google Scholar] [CrossRef]
- Nikolic, K.; Grossman, N.; Grubb, M.S.; Burrone, J.; Toumazou, C.; Degenaar, P. Photocycles of channelrhodopsin-2. Photochem. Photobiol. 2009, 85, 400–411. [Google Scholar] [CrossRef]
- Stefanescu, R.A.; Shivakeshavan, R.; Khargonekar, P.P.; Talathi, S.S. Computational modeling of channelrhodopsin-2 photocurrent characteristics in relation to neural signaling. Bull. Math. Biol. 2013, 75, 2208–2240. [Google Scholar] [CrossRef]
- Lv, M.; Ma, J. Multiple modes of electrical activities in a new neuron model under electromagnetic radiation. Neurocomputing 2016, 205, 375–381. [Google Scholar] [CrossRef]
- Ermentrout, B.; Mahajan, A. Simulating, analyzing, and animating dynamical systems: A guide to XPPAUT for researchers and students. Appl. Mech. Rev. 2020, 56, B53. [Google Scholar] [CrossRef]



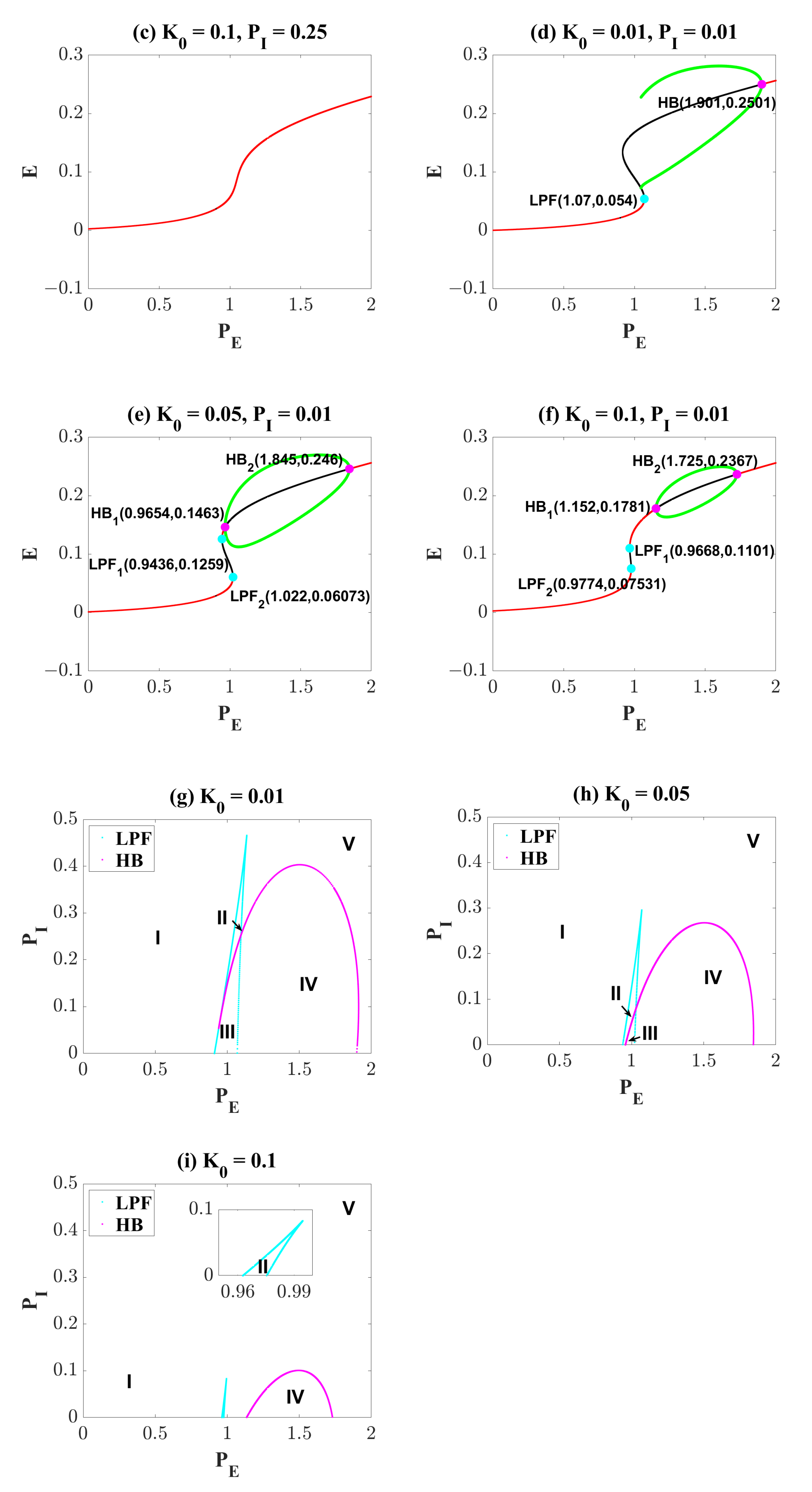
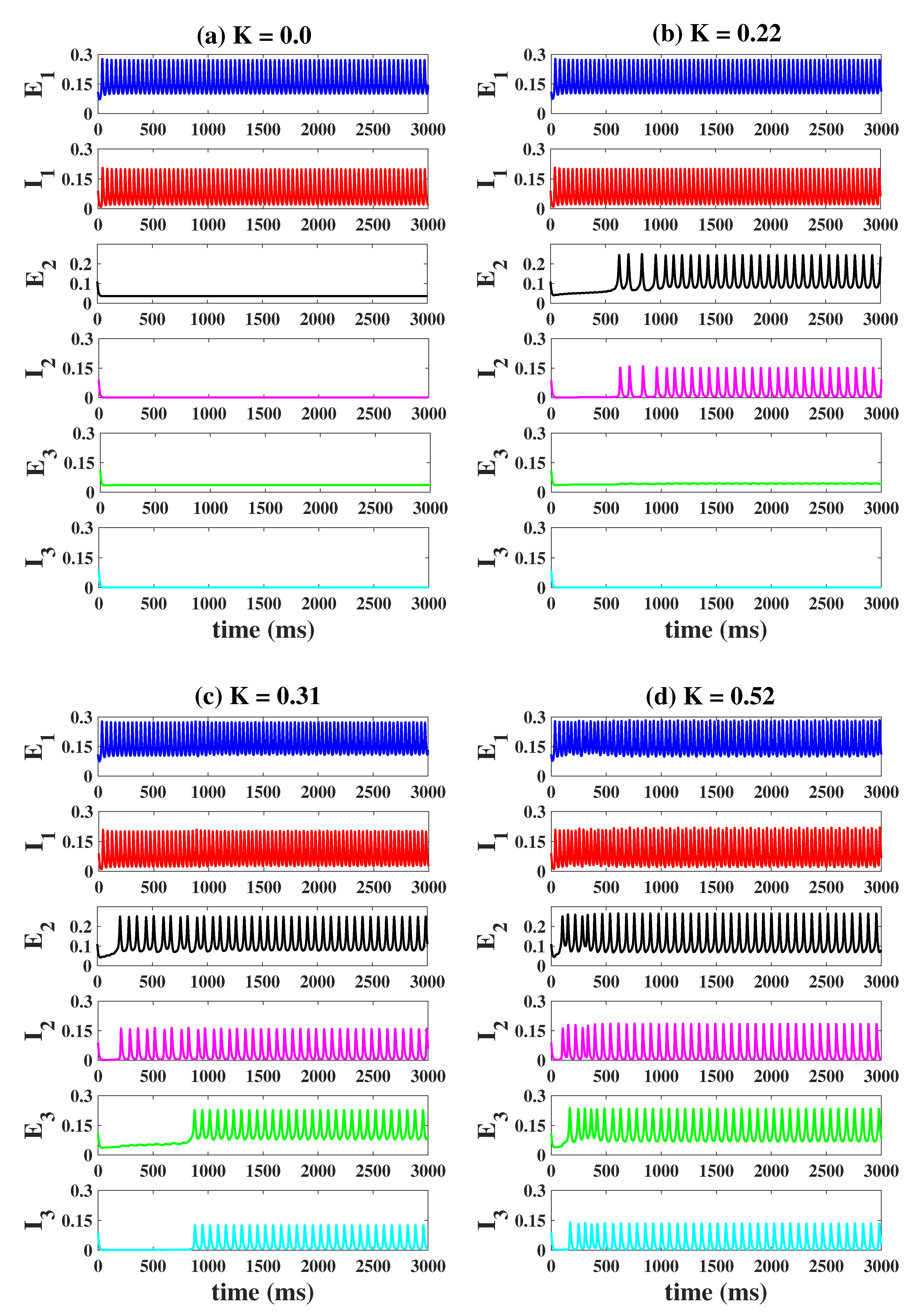
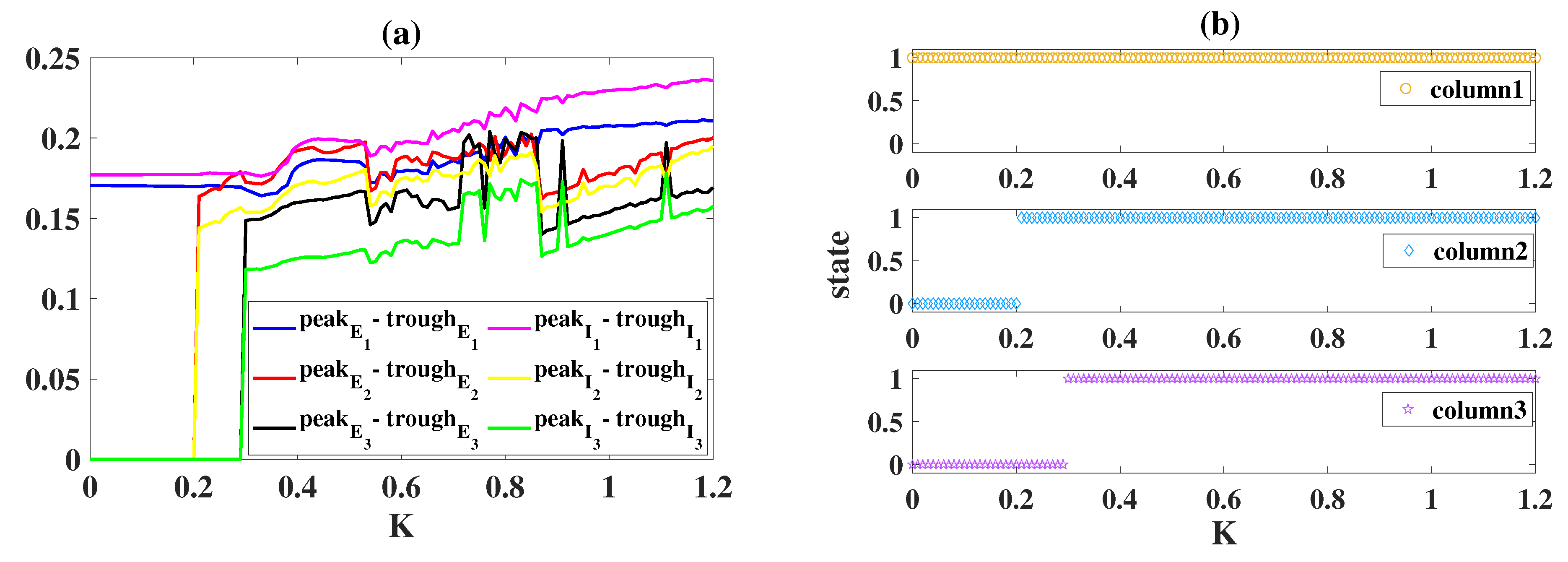

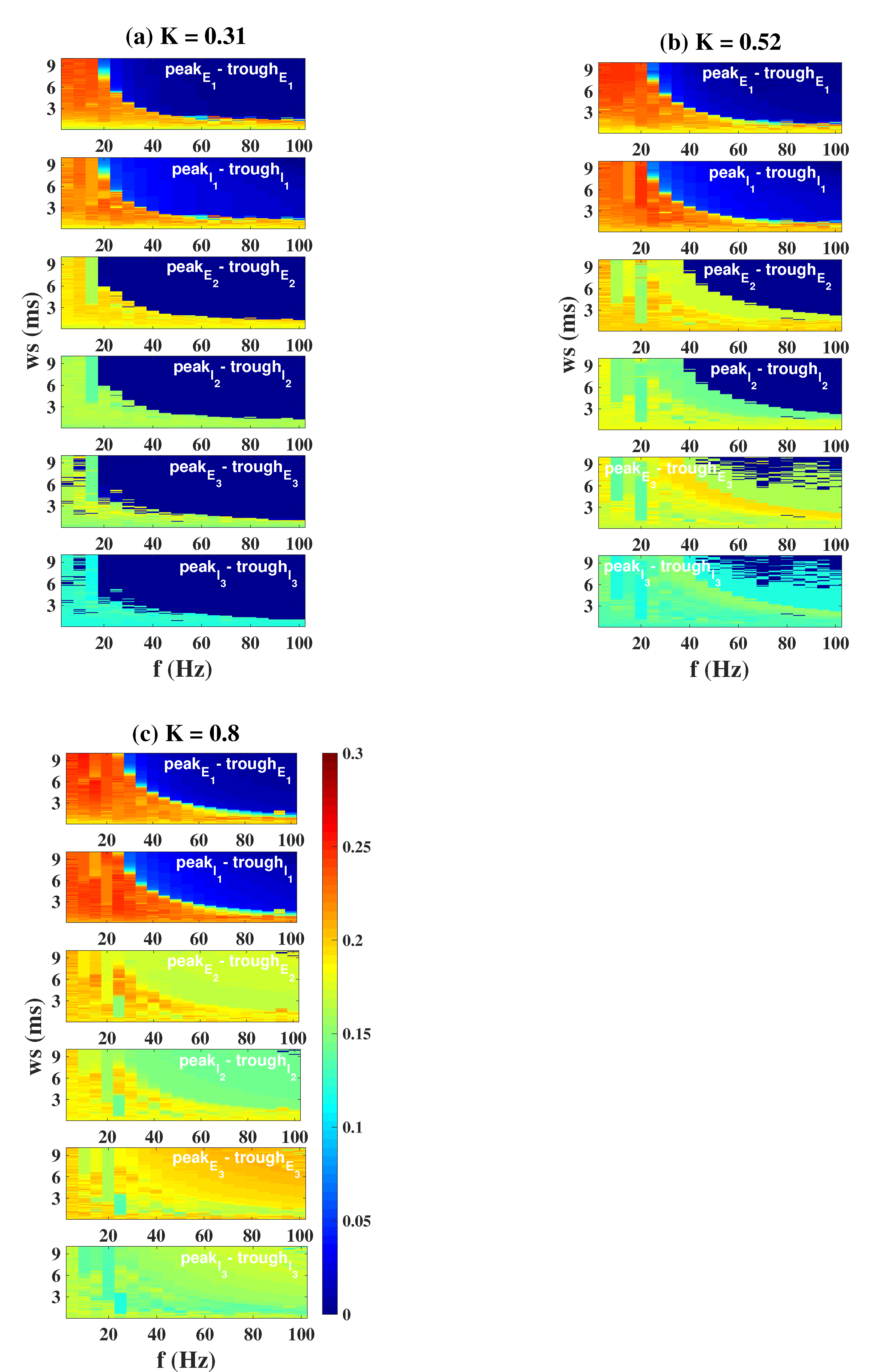

| Symbol | Interpretation | Value |
|---|---|---|
| activation rates of process | ||
| activation rates of process | ||
| transition rate of | ||
| transition rate of | ||
| transition rate of | ||
| transition rate of | ||
| thermal recovery rate of | ||
| quantum efficiency of light absorption in c1 state | 0.8535 | |
| quantum efficiency of light absorption in c2 state | 0.14 | |
| F | photon flux at a given time | |
| effective cross section of a ChR2 channel | m | |
| light irradiance | 0–10 mW/mm | |
| light wavelength | 470 nm | |
| scaling factor of photon loss | 1.3 | |
| h | Planck’s constant | Js |
| c | light speed | m/s |
| activation time constant of ChR2 | 1.3 ms | |
| activation function of ChR2 | ||
| max conductance of ChR2 | mS/cm | |
| ratio of conductances of | 0.1 | |
| reversal potential of ChR2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Shen, Z.; Zhao, Y.; Du, L.; Deng, Z. Dynamical Mechanism Analysis of Three Neuroregulatory Strategies on the Modulation of Seizures. Int. J. Mol. Sci. 2022, 23, 13652. https://doi.org/10.3390/ijms232113652
Zhang H, Shen Z, Zhao Y, Du L, Deng Z. Dynamical Mechanism Analysis of Three Neuroregulatory Strategies on the Modulation of Seizures. International Journal of Molecular Sciences. 2022; 23(21):13652. https://doi.org/10.3390/ijms232113652
Chicago/Turabian StyleZhang, Honghui, Zhuan Shen, Yuzhi Zhao, Lin Du, and Zichen Deng. 2022. "Dynamical Mechanism Analysis of Three Neuroregulatory Strategies on the Modulation of Seizures" International Journal of Molecular Sciences 23, no. 21: 13652. https://doi.org/10.3390/ijms232113652
APA StyleZhang, H., Shen, Z., Zhao, Y., Du, L., & Deng, Z. (2022). Dynamical Mechanism Analysis of Three Neuroregulatory Strategies on the Modulation of Seizures. International Journal of Molecular Sciences, 23(21), 13652. https://doi.org/10.3390/ijms232113652





