Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum
Abstract
1. Introduction
2. Results
2.1. Identification of AP2/ERF Genes in D. catenatum
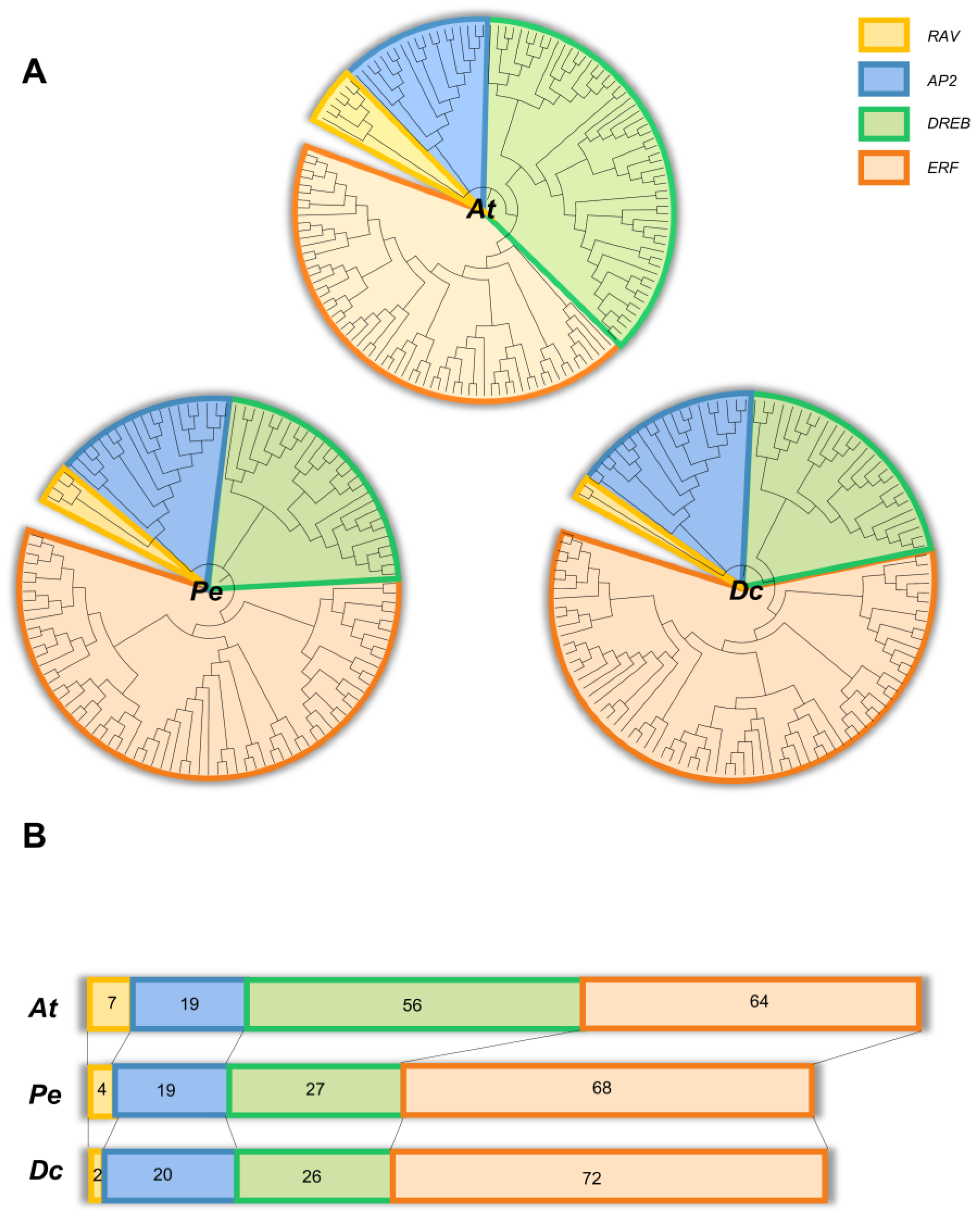
2.2. Phylogenetic Analysis of DcAP2/ERF Families
2.3. Structure Analyses of the AP2/ERF Gene Family in D. catenatum
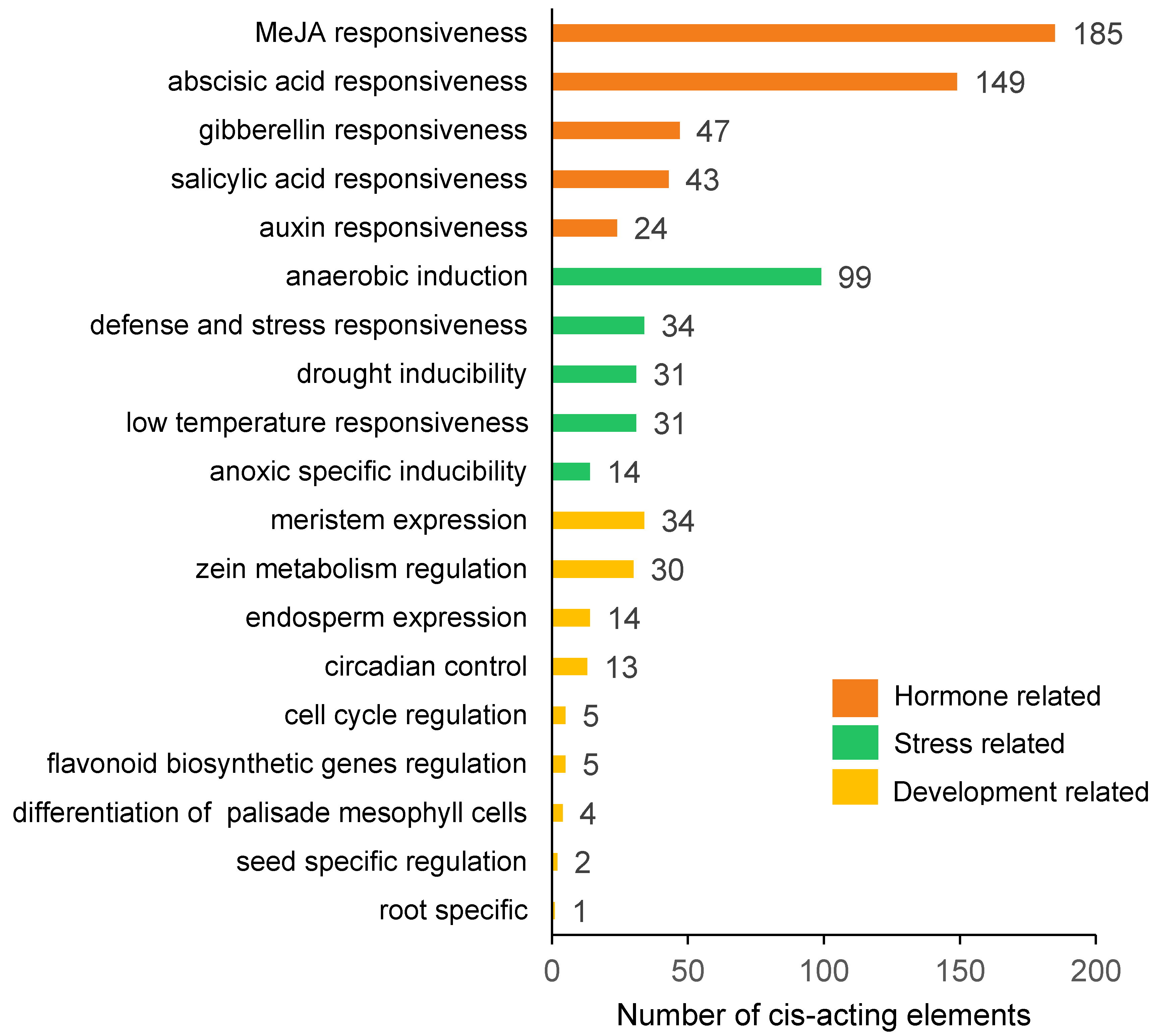
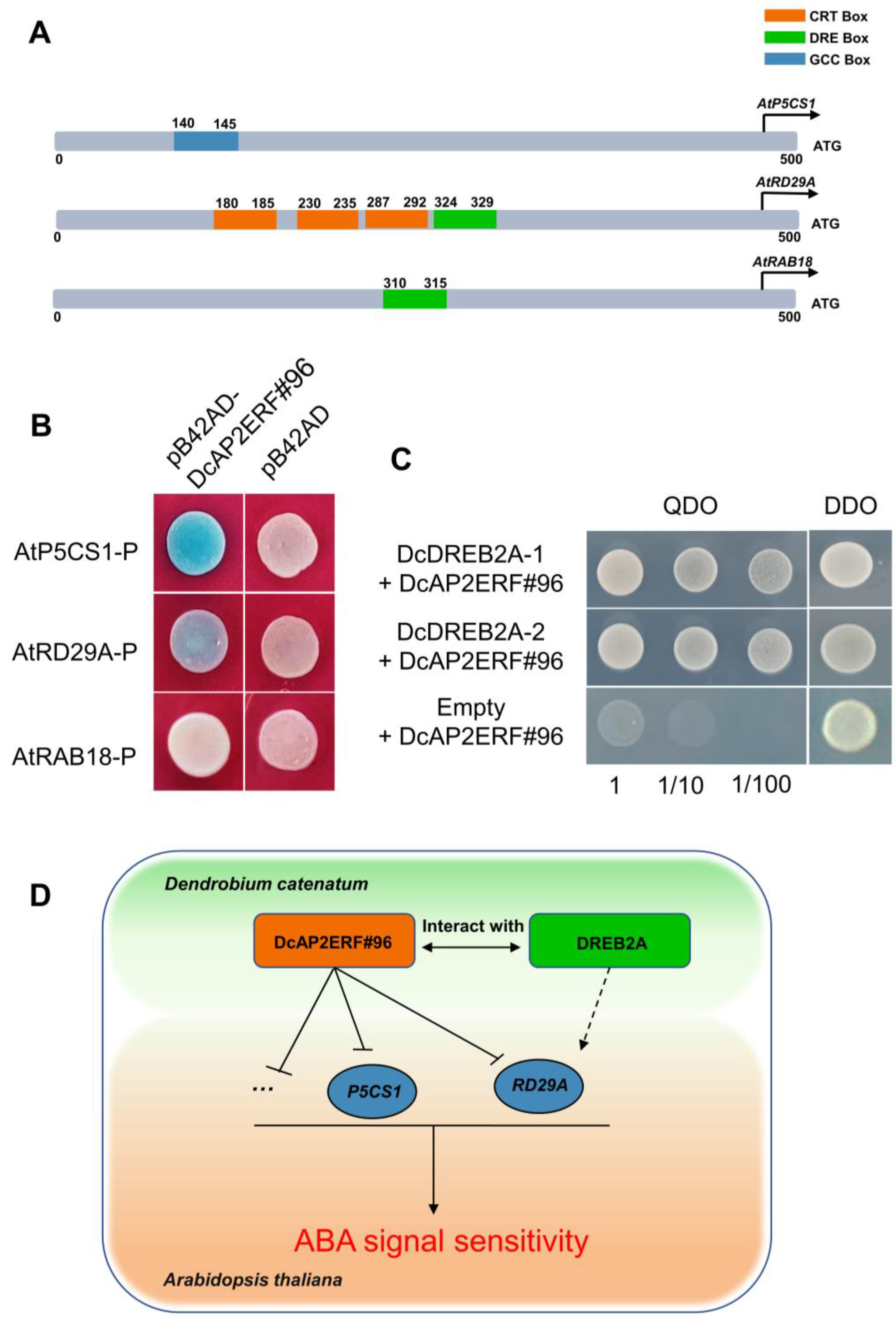
2.4. Cis-Acting Element Analysis of DcAP2/ERF Gene Promoters
2.5. GO Annotation Analysis of DcAP2/ERF Family Proteins
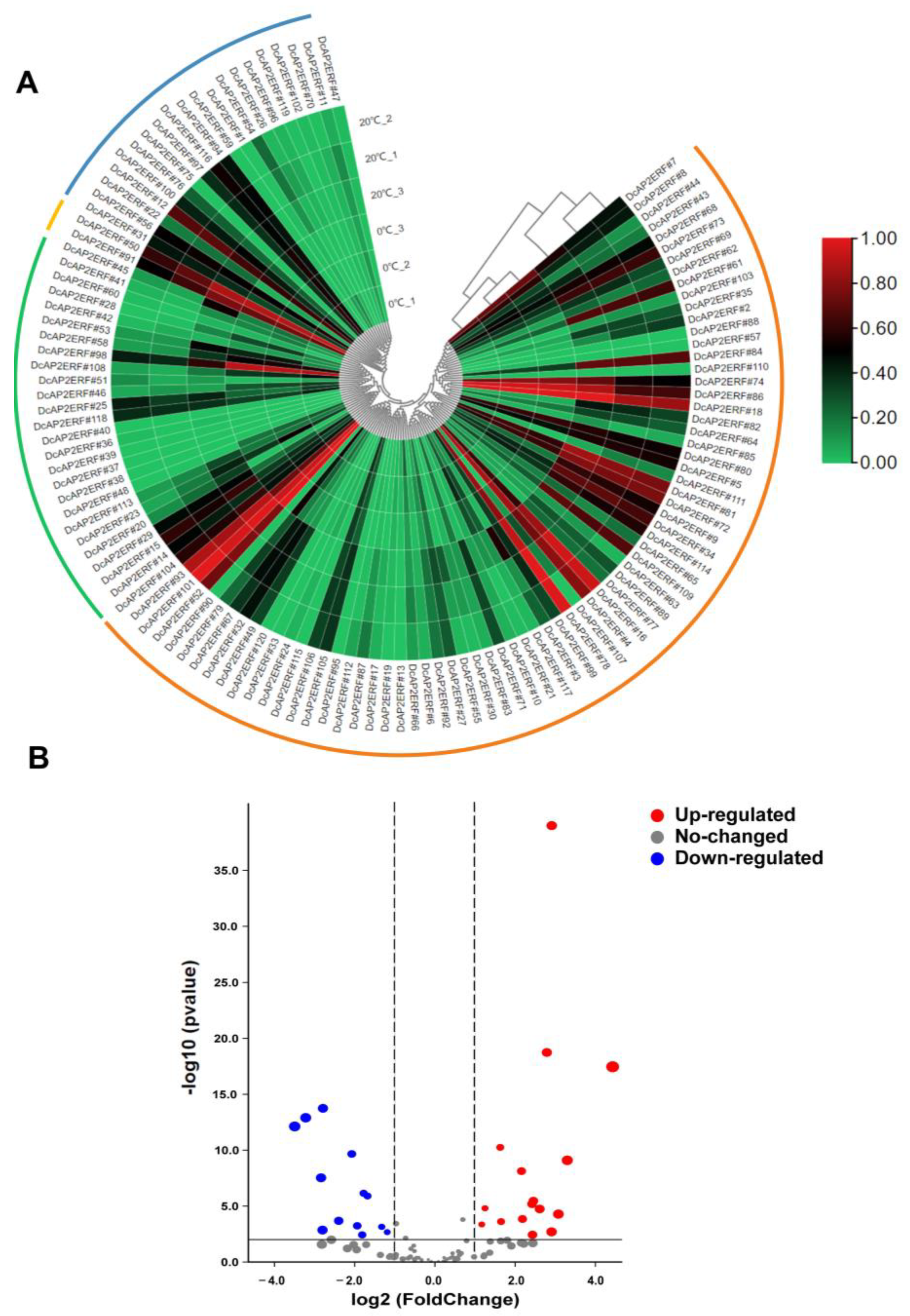
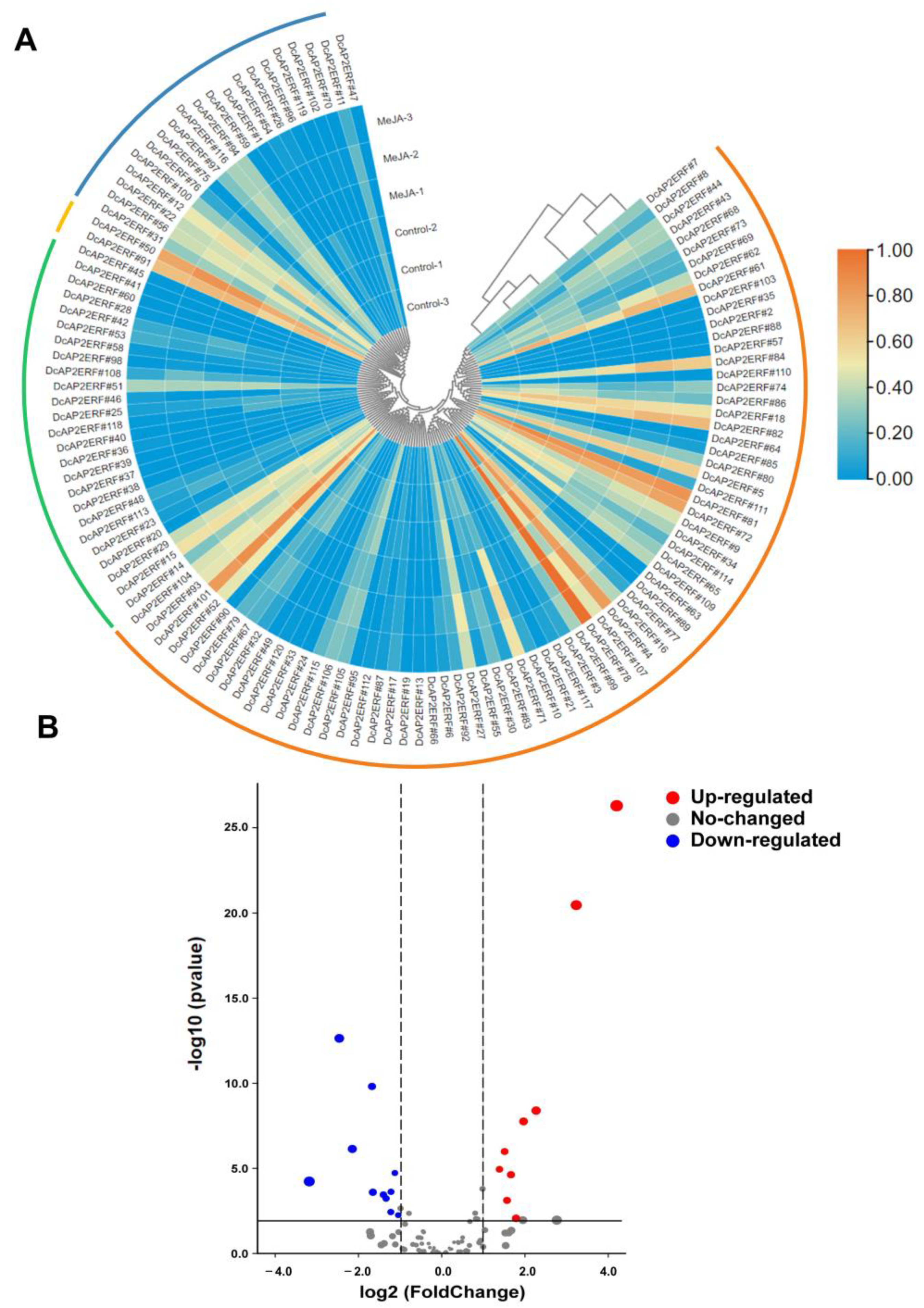
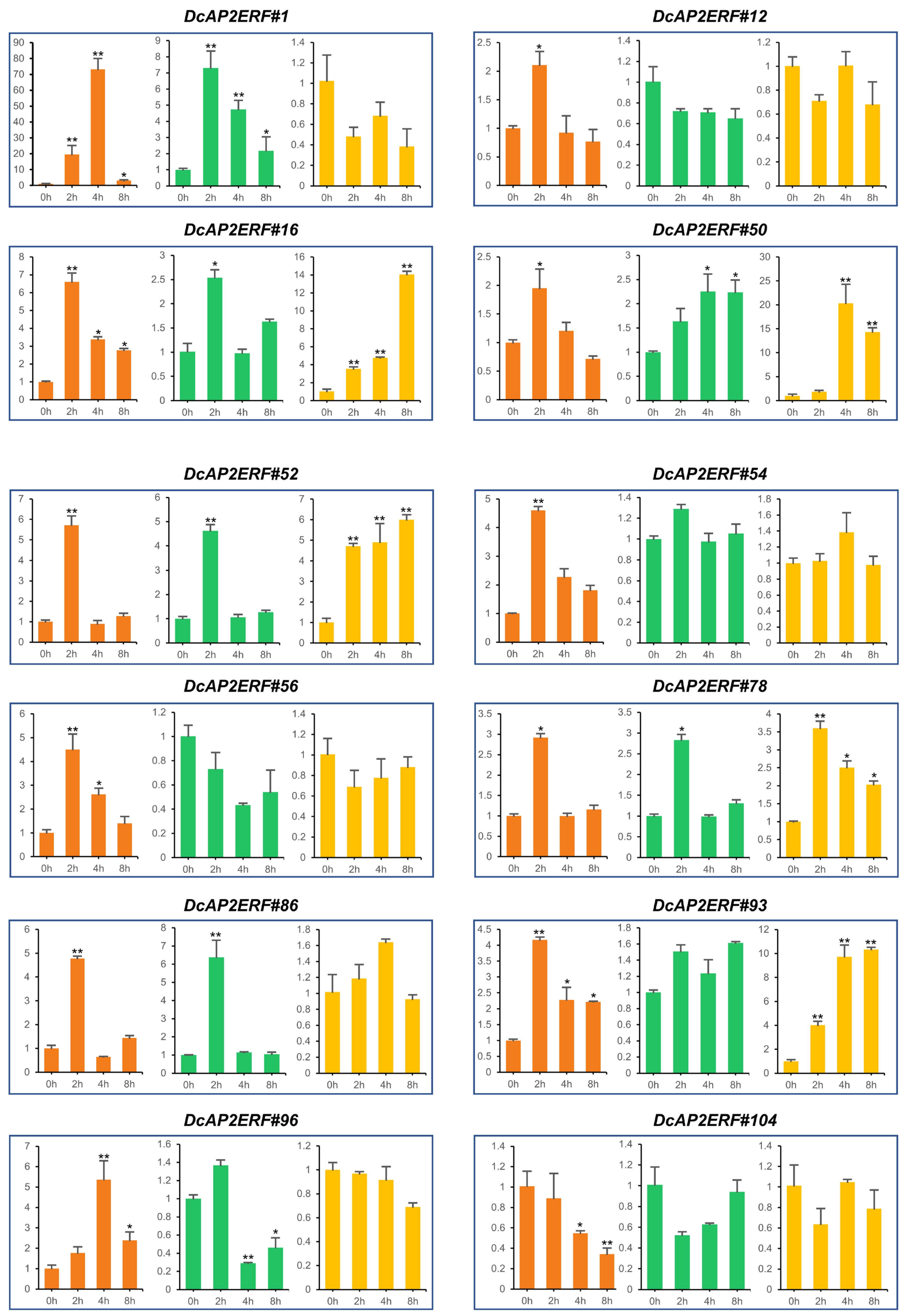
2.6. Expression Patterns of DcAP2/ERF Genes under Different Stresses
2.7. Expression Patterns of DcAP2/ERF Genes in Different Organs
2.8. Expression Pattern of DcAP2/ERFs under PEG6000 Treatment
2.9. DcAP2ERF#96 Encodes a Conserved AP2 Domain Transcription Factor Localized in the Nucleus
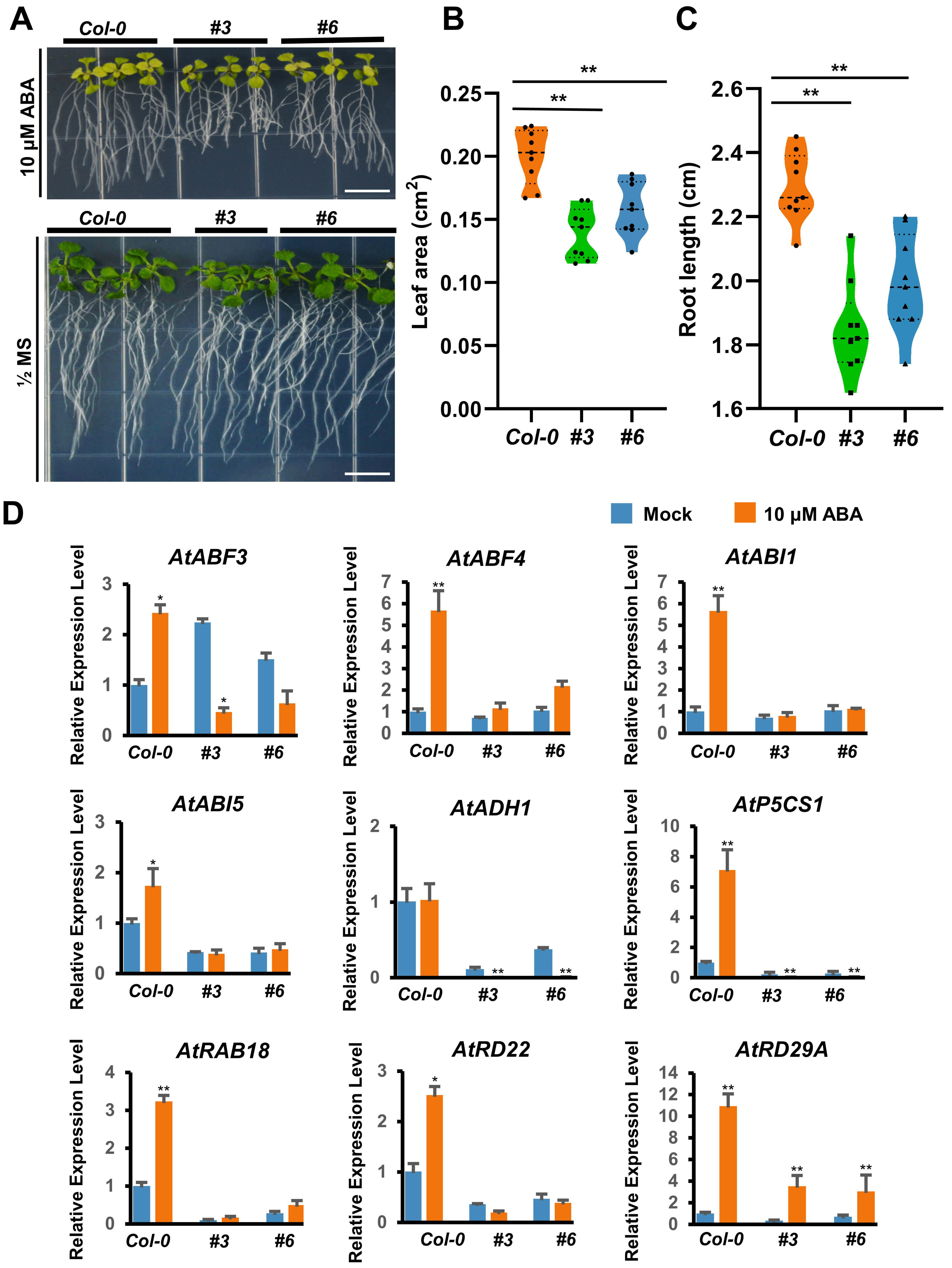
2.10. DcAP2ERF#96 Interacted with DcDREB2A and Negatively Regulate ABA Signaling in Arabidopsis
3. Discussion
4. Materials and Methods
4.1. Identification of AP2/ERF Genes in D. Catenatum
4.2. Phylogenetic Tree Analysis of the DcAP2/ERF Protein
4.3. Gene Structure, Conserved Domain, Motif and Promoter Analyses of AP2/ERF Family Members
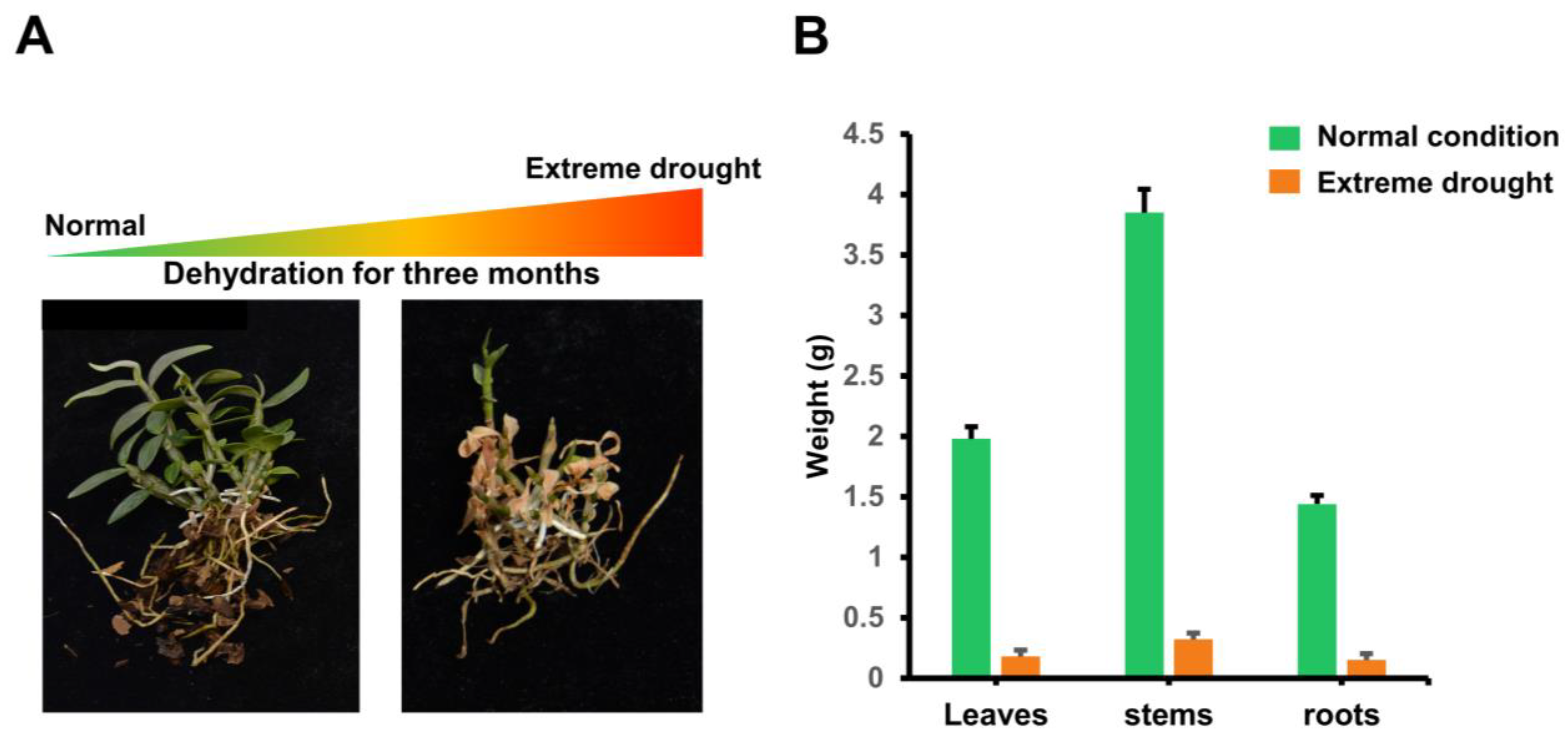
4.4. Plant Materials and Methods of Stress Treatment
4.5. RNA Extraction and Quantitative/Real-Time-PCR (qRT-PCR) Analysis
4.6. Expression Pattern Analysis of DcAP2/ERF Genes
4.7. Gene Ontology Annotation Analysis
4.8. Subcellular Localization of DcAP2ERF#96
4.9. Yeast One-Hybrid (Y1H) and Yeast Two-Hybrid (Y2H) Assays
4.10. Acquisition and Handling of Arabidopsis Transgenic Lines
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutterson, N.; Reuber, T.L. Regulation of disease resistance pathways by AP2/ERF transcription factors. Curr. Opin. Plant Biol. 2004, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Ortiz, G.; Huq, E.; Quail, P.H. The Arabidopsis Basic/Helix-Loop-Helix Transcription Factor Family. Plant Cell 2003, 15, 1749–1770. [Google Scholar] [CrossRef] [PubMed]
- Dubos, C.; Stracke, R.; Grotewold, E.; Weisshaar, B.; Martin, C.; Lepiniec, L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010, 15, 573–581. [Google Scholar] [CrossRef]
- Yang, X.; Tuskan, G.A.; Cheng, Z.-M. Divergence of the Dof Gene Families in Poplar, Arabidopsis, and Rice Suggests Multiple Modes of Gene Evolution after Duplication. Plant Physiol. 2006, 142, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Networks of WRKY Transcription Factors in Defense Signaling. Available online: networks-of-wrky-transcription-factors-in-defense-signaling-5d5wsqb4ip.pdf (accessed on 1 November 2022).
- Feng, K.; Hou, X.-L.; Xing, G.-M.; Liu, J.-X.; Duan, A.-Q.; Xu, Z.-S.; Li, M.-Y.; Zhuang, J.; Xiong, A.-S. Advances in AP2/ERF super-family transcription factors in plant. Crit. Rev. Biotechnol. 2020, 40, 750–776. [Google Scholar] [CrossRef]
- Okamuro, J.K.; Caster, B.; Villarroel, R.; Van Montagu, M.; Jofuku, K.D. The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis. Proc. Natl. Acad. Sci. USA 1997, 94, 7076–7081. [Google Scholar] [CrossRef]
- Ohme-Takagi, M.; Shinshi, H. Ethylene-lnducible DNA Binding Proteins That lnteract with an Ethylene-Responsive Element. Plant Cell 1995, 7, 173–182. [Google Scholar]
- Allen, M.D.; Yamasaki, K.; Ohme-Takagi, M.; Tateno, M.; Suzuki, M. A novel mode of DNA recognition by a β-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. EMBO J. 1998, 17, 5484–5496. [Google Scholar] [CrossRef]
- Riechmann, J.L.; Meyerowitz, E. The AP2/EREBP Family of Plant Transcription Factors. Biol Chem. 1998, 379, 633–646. [Google Scholar]
- Nakano, T.; Suzuki, K.; Fujimura, T.; Shinshi, H. Genome-Wide Analysis of the ERF Gene Family in Arabidopsis and Rice. Plant Physiol. 2006, 140, 411–432. [Google Scholar] [CrossRef]
- François, L.; Verdenaud, M.; Fu, X.; Ruleman, D.; Dubois, A.; Vandenbussche, M.; Bendahmane, A.; Raymond, O.; Just, J.; Bendahmane, M. A miR172 target-deficient AP2-like gene correlates with the double flower phenotype in roses. Sci. Rep. 2018, 8, 12912. [Google Scholar] [CrossRef]
- Matías-Hernández, L.; Aguilar-Jaramillo, A.E.; Marín-González, E.; Suárez-López, P.; Pelaz, S. RAV genes: Regulation of floral induction and beyond. Ann. Bot. 2014, 114, 1459–1470. [Google Scholar] [CrossRef]
- Liu, Q.; Kasuga, M.; Sakuma, Y.; Abe, H.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Two Transcription Factors, DREB1 and DREB2, with an EREBP/AP2 DNA Binding Domain Separate Two Cellular Signal Transduction Pathways in Drought- and Low- Temperature-Responsive Gene Expression, Respectively, in Arabidopsis. Plant Cell 1998, 10, 1391–1406. [Google Scholar] [CrossRef]
- Zhang, T.; Cui, Z.; Li, Y.; Kang, Y.; Song, X.; Wang, J.; Zhou, Y. Genome-Wide Identification and Expression Analysis of MYB Transcription Factor Superfamily in Dendrobium catenatum. Front. Genet. 2021, 12, 714696. [Google Scholar] [CrossRef]
- Xing, H.; Jiang, Y.; Zou, Y.; Long, X.; Wu, X.; Ren, Y.; Li, Y.; Li, H.-L. Genome-wide investigation of the AP2/ERF gene family in ginger: Evolution and expression profiling during development and abiotic stresses. BMC Plant Biol. 2021, 21, 561. [Google Scholar] [CrossRef]
- Riaz, M.W.; Lu, J.; Shah, L.; Yang, L.; Chen, C.; Mei, X.D.; Xue, L.; Manzoor, M.A.; Abdullah, M.; Rehman, S.; et al. Expansion and Molecular Characterization of AP2/ERF Gene Family in Wheat (Triticum aestivum L.). Front. Genet. 2021, 12, 632155. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Zhang, Q.; Liu, Q.; Li, L.; Sun, C.; Wang, K.; Wang, Y.; Zhao, M.; Li, H.; et al. Structural variation, functional differentiation and expression characteristics of the AP2/ERF gene family and its response to cold stress and methyl jasmonate in Panax ginseng C.A. Meyer. PLoS ONE 2020, 15, e0226055. [Google Scholar] [CrossRef]
- Ghorbani, R.; Zakipour, Z.; Alemzadeh, A.; Razi, H. Genome-wide analysis of AP2/ERF transcription factors family in Brassica napus. Physiol. Mol. Biol. Plants 2020, 26, 1463–1476. [Google Scholar] [CrossRef]
- Stockinger, E.J.; Gilmour, S.J.; Thomashow, M.F. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc. Natl. Acad. Sci. USA 1997, 94, 1035–1040. [Google Scholar] [CrossRef]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J.; Salazar, M.P.; Houghton, J.M.; Thomashow, M.F. Low temperature regulation of theArabidopsisCBF family of AP2 transcriptional activators as an early step in cold-inducedCORgene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef]
- Qin, F.; Kakimoto, M.; Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Tran, L.-S.P.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Regulation and functional analysis of ZmDREB2A in response to drought and heat stresses in Zea mays L: ZmDREB2A in drought and heat stress response. Plant J. 2007, 50, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, M.; Chen, X.; Xu, Z.; Li, L.; Guo, J.; Ma, Y. Isolation and characterization of a novel EAR-motif-containing gene GmERF4 from soybean (Glycine max L.). Mol. Biol. Rep. 2010, 37, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-K.; Jung, H.; Jang, G.; Jeong, J.S.; Kim, Y.S.; Ha, S.-H.; Do Choi, Y.; Kim, J.-K. Overexpression of the OsERF71 Transcription Factor Alters Rice Root Structure and Drought Resistance. Plant Physiol. 2016, 172, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Cai, Y.; Chen, W.; Gao, Y.; Jin, J.; Wang, H.; Feng, S.; Lu, J. Development, Identification, and Application of a Germplasm Specific SCAR Marker for Dendrobium officinale Kimura et Migo. Front. Plant Sci. 2021, 12, 669458. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zou, L.-H.; Zheng, B.-Q.; Tian, Y.-Q.; Wang, Y. Transcriptomic profiling for prolonged drought in Dendrobium catenatum. Sci. Data 2018, 5, 180233. [Google Scholar] [CrossRef]
- Zeng, X.; Ling, H.; Chen, X.; Guo, S. Genome-wide identification, phylogeny and function analysis of GRAS gene family in Dendrobium catenatum (Orchidaceae). Gene 2019, 705, 5–15. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Xu, Q.; Bian, C.; Tsai, W.-C.; Yeh, C.-M.; Liu, K.-W.; Yoshida, K.; Zhang, L.-S.; Chang, S.-B.; Chen, F.; et al. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Zhang, G.-Q.; Liu, K.-W.; Li, Z.; Lohaus, R.; Hsiao, Y.-Y.; Niu, S.-C.; Wang, J.-Y.; Lin, Y.-C.; Xu, Q.; Chen, L.-J.; et al. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Niu, Z.; Zhu, F.; Fan, Y.; Li, C.; Zhang, B.; Zhu, S.; Hou, Z.; Wang, M.; Yang, J.; Xue, Q.; et al. The chromosome-level reference genome assembly for Dendrobium officinale and its utility of functional genomics research and molecular breeding study. Acta Pharm. Sin. B 2021, 11, 2080–2092. [Google Scholar] [CrossRef]
- Shrestha, A.; Fendel, A.; Nguyen, T.H.; Adebabay, A.; Kullik, A.S.; Benndorf, J.; Leon, J.; Naz, A.A. Natural diversity uncovers P5CS1 regulation and its role in drought stress tolerance and yield sustainability in barley. Plant Cell Environ. 2022, pce.14445. [Google Scholar] [CrossRef]
- Kasuga, M.; Liu, Q.; Miura, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999, 17, 287–291. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 synergistically regulate TaP5CS1/TaP5CR1—mediated proline biosynthesis to enhance drought tolerance in wheat. New Phytol. 2022, nph.18549. [Google Scholar] [CrossRef]
- Sheng, L.; Ma, C.; Chen, Y.; Gao, H.; Wang, J. Genome-Wide Screening of AP2 Transcription Factors Involving in Fruit Color and Aroma Regulation of Cultivated Strawberry. Genes 2021, 12, 530. [Google Scholar] [CrossRef]
- Wessler, S.R. Homing into the origin of the AP2 DNA binding domain. Trends Plant Sci. 2005, 10, 54–56. [Google Scholar] [CrossRef]
- Goyal, N.; Bhatia, G.; Sharma, S.; Garewal, N.; Upadhyay, A.; Upadhyay, S.K.; Singh, K. Genome-wide characterization revealed role of NBS-LRR genes during powdery mildew infection in Vitis vinifera. Genomics 2020, 112, 312–322. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of an Arabidopsis Transcription Factor, DREB2A, Involved in Drought-Responsive Gene Expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Roy, S.W.; Penny, D. A Very High Fraction of Unique Intron Positions in the Intron-Rich Diatom Thalassiosira pseudonana Indicates Widespread Intron Gain. Mol. Biol. Evol. 2007, 24, 1447–1457. [Google Scholar] [CrossRef]
- Magnani, E.; Sjölander, K.; Hake, S. From Endonucleases to Transcription Factors: Evolution of the AP2 DNA Binding Domain in Plants. Plant Cell 2004, 16, 2265–2277. [Google Scholar] [CrossRef]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef]
- Zou, C.; Sun, K.; Mackaluso, J.D.; Seddon, A.E.; Jin, R.; Thomashow, M.F.; Shiu, S.-H. Cis-regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2011, 108, 14992–14997. [Google Scholar] [CrossRef]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of Stomatal Closure in Plants Exposed to Drought and Cold Stress. In Survival Strategies in Extreme Cold and Desiccation; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Advances in Experimental Medicine and Biology; Springer Singapore: Singapore, 2018; Volume 1081, pp. 215–232. ISBN 9789811312434. [Google Scholar]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Jung, I.; Shin, S.-J.; Park, J.; Rhee, S.; Kim, J.-K.; Jung, W.; Kwon, H.-B.; Kim, S. Transcriptional Network Analysis Reveals Drought Resistance Mechanisms of AP2/ERF Transgenic Rice. Front. Plant Sci. 2017, 8, 1044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Hu, R.; Liu, D.; Liu, X.; Wang, J.; Xiang, X.; Li, Y. The AP2 transcription factor NtERF172 confers drought resistance by modifying NtCAT. Plant Biotechnol. J. 2020, 18, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Go, Y.S.; Suh, M.C. Cuticular wax biosynthesis is positively regulated by WRINKLED4, an AP2/ERF-type transcription factor, in Arabidopsis stems. Plant J. 2016, 88, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. 20 years of the SMART protein domain annotation resource. Nucleic Acids Res. 2018, 46, D493–D496. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.-C.; Meng, Y.-Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Wu, Z.-G.; Jiang, W.; Chen, S.-L.; Mantri, N.; Tao, Z.-M.; Jiang, C.-X. Insights from the Cold Transcriptome and Metabolome of Dendrobium officinale: Global Reprogramming of Metabolic and Gene Regulation Networks during Cold Acclimation. Front. Plant Sci. 2016, 7, 1653. [Google Scholar] [CrossRef]
- Li, C.; Shen, Q.; Cai, X.; Lai, D.; Wu, L.; Han, Z.; Zhao, T.; Chen, D.; Si, J. JA signal-mediated immunity of Dendrobium catenatum to necrotrophic Southern Blight pathogen. BMC Plant Biol. 2021, 21, 360. [Google Scholar] [CrossRef]
- Chai, D.; Lee, S.M.; Ng, J.H.; Yu, H. l-Methionine sulfoximine as a novel selection agent for genetic transformation of orchids. J. Biotechnol. 2007, 131, 466–472. [Google Scholar] [CrossRef]


| ID | LOC | Rename | Length (aa) | Mw (Da) | PI | Aliphatic Index | GRAVY | Location |
|---|---|---|---|---|---|---|---|---|
| XM_020816501.1 | LOC110092113 | DcAP2ERF#1 | 393 | 43,675.56 | 9.27 | 59.49 | −0.678 | mitochondria |
| XM_020816597.2 | LOC110092178 | DcAP2ERF#2 | 117 | 13,244.55 | 5.3 | 61.88 | −0.913 | nucleus |
| XM_020817122.2 | LOC110092532 | DcAP2ERF#3 | 205 | 22,653.27 | 7.92 | 67.61 | −0.666 | nucleus |
| XM_020817685.2 | LOC110092963 | DcAP2ERF#4 | 344 | 38,910.42 | 4.89 | 62.21 | −0.691 | nucleus |
| XM_020817704.2 | LOC110092982 | DcAP2ERF#5 | 199 | 21,704.64 | 9.36 | 71.16 | −0.545 | nucleus |
| XM_020817821.2 | LOC110093058 | DcAP2ERF#6 | 142 | 15,996.03 | 5.31 | 67.39 | −0.497 | mitochondria |
| XM_020818820.2 | LOC110093822 | DcAP2ERF#7 | 273 | 30,323.27 | 5.12 | 77.95 | −0.467 | cytoplasm |
| XM_020818851.2 | LOC110093837 | DcAP2ERF#8 | 273 | 30,499.49 | 5.28 | 71.5 | −0.49 | cytoplasm |
| XM_020818859.2 | LOC110093843 | DcAP2ERF#9 | 240 | 26,225.9 | 9.69 | 75.71 | −0.383 | nucleus |
| XM_020818985.2 | LOC110093926 | DcAP2ERF#10 | 378 | 40,998.34 | 6.34 | 56.9 | −0.635 | nucleus |
| XM_020819386.2 | LOC110094210 | DcAP2ERF#11 | 431 | 47,967.91 | 6.35 | 62.48 | −0.686 | nucleus |
| XM_020820818.2 | LOC110095317 | DcAP2ERF#12 | 487 | 52,737.33 | 6.81 | 59.16 | −0.591 | nucleus |
| XM_020820987.1 | LOC110095445 | DcAP2ERF#13 | 258 | 28,269.68 | 7.78 | 66.67 | −0.537 | nucleus |
| XM_020821323.2 | LOC110095684 | DcAP2ERF#14 | 155 | 17,183.33 | 9.98 | 65.03 | −0.815 | nucleus |
| XM_020821563.2 | LOC110095855 | DcAP2ERF#15 | 134 | 14,806.86 | 9.71 | 64.18 | −0.525 | cytoplasm |
| XM_020821729.2 | LOC110095987 | DcAP2ERF#16 | 285 | 31,434.6 | 5.44 | 81.47 | −0.432 | nucleus |
| XM_020822089.2 | LOC110096240 | DcAP2ERF#17 | 219 | 24,140.1 | 9.14 | 59.77 | −0.591 | cytoplasm |
| XM_020822222.2 | LOC110096335 | DcAP2ERF#18 | 231 | 24,602.71 | 9.2 | 68.18 | −0.349 | nucleus |
| XM_020822428.2 | LOC110096456 | DcAP2ERF#19 | 275 | 29,678.79 | 6.46 | 60.51 | −0.476 | nucleus |
| XM_020823171.2 | LOC110096991 | DcAP2ERF#20 | 147 | 16,174.86 | 5 | 64.35 | −0.612 | nucleus |
| XM_020823245.2 | LOC110097036 | DcAP2ERF#21 | 210 | 22,952.7 | 8.41 | 67.57 | −0.503 | nucleus |
| XM_020823401.2 | LOC110097153 | DcAP2ERF#22 | 478 | 53,136.39 | 6.52 | 66.95 | −0.597 | nucleus |
| XM_020823475.2 | LOC110097214 | DcAP2ERF#23 | 181 | 20,350.63 | 5.36 | 61.16 | −0.646 | nucleus |
| XM_020823706.2 | LOC110097366 | DcAP2ERF#24 | 166 | 18,041.33 | 6.43 | 60.6 | −0.581 | cytoplasm |
| XM_020824867.2 | LOC110098141 | DcAP2ERF#25 | 201 | 21,854.61 | 5.83 | 69.1 | −0.4 | nucleus |
| XM_020825001.2 | LOC110098242 | DcAP2ERF#26 | 347 | 39,167.06 | 5.64 | 56.57 | −0.847 | nucleus |
| XM_020825318.2 | LOC110098477 | DcAP2ERF#27 | 323 | 35,258.22 | 8.32 | 58.08 | −0.645 | nucleus |
| XM_020825410.2 | LOC110098547 | DcAP2ERF#28 | 193 | 20,380.73 | 4.49 | 70 | −0.454 | nucleus |
| XM_020825656.2 | LOC110098714 | DcAP2ERF#29 | 174 | 19,198.48 | 9.82 | 58.51 | −0.936 | nucleus |
| XM_020825700.2 | LOC110098779 | DcAP2ERF#30 | 215 | 23,420.82 | 6.44 | 56.88 | −0.686 | nucleus |
| XM_020826383.2 | LOC110099287 | DcAP2ERF#31 | 233 | 26,407.91 | 9.26 | 66.95 | −0.771 | nucleus |
| XM_020826509.2 | LOC110099386 | DcAP2ERF#32 | 239 | 26,222.62 | 9.21 | 74.44 | −0.535 | nucleus |
| XM_020826512.1 | LOC110099389 | DcAP2ERF#33 | 253 | 28,355.92 | 6.35 | 75.89 | −0.687 | nucleus |
| XM_020826786.2 | LOC110099586 | DcAP2ERF#34 | 255 | 28,810.6 | 6.22 | 65.73 | −0.436 | cytoplasm |
| XM_020827298.2 | LOC110099956 | DcAP2ERF#35 | 170 | 19,093.62 | 5.69 | 86.06 | −0.573 | cytoplasm |
| XM_020827868.2 | LOC110100389 | DcAP2ERF#36 | 229 | 24,589.95 | 8.91 | 76.77 | −0.172 | nucleus |
| XM_020827870.1 | LOC110100391 | DcAP2ERF#37 | 164 | 18,123.61 | 4.86 | 86.34 | −0.033 | cytoplasm |
| XM_020827871.2 | LOC110100392 | DcAP2ERF#38 | 164 | 18,035.27 | 4.59 | 85.67 | −0.157 | cytoplasm |
| XM_020827872.1 | LOC110100393 | DcAP2ERF#39 | 178 | 19,751.31 | 4.35 | 83.99 | −0.19 | cytoplasm |
| XM_020827873.1 | LOC110100394 | DcAP2ERF#40 | 122 | 13,445.6 | 9.27 | 96.89 | 0.025 | cytoplasm |
| XM_020827909.2 | LOC110100414 | DcAP2ERF#41 | 179 | 19,288.48 | 4.51 | 69.44 | −0.491 | nucleus |
| XM_020828052.2 | LOC110100522 | DcAP2ERF#42 | 165 | 18,544.94 | 8.11 | 65.88 | −0.66 | cytoplasm |
| XM_020828120.2 | LOC110100557 | DcAP2ERF#43 | 268 | 30,011.64 | 5.2 | 67.8 | −0.532 | nucleus |
| XM_020828122.2 | LOC110100558 | DcAP2ERF#44 | 289 | 31,823.9 | 5.4 | 74.05 | −0.407 | cytoplasm |
| XM_020828709.2 | LOC110100983 | DcAP2ERF#45 | 155 | 16,800.78 | 4.83 | 69.48 | −0.427 | nucleus |
| XM_020828734.2 | LOC110100999 | DcAP2ERF#46 | 210 | 23,031.59 | 6.44 | 63.76 | −0.604 | nucleus |
| XM_020829170.2 | LOC110101314 | DcAP2ERF#47 | 733 | 81,463.46 | 6.82 | 64.6 | −0.577 | nucleus |
| XM_020829570.2 | LOC110101604 | DcAP2ERF#48 | 174 | 18,520.72 | 6.82 | 66.32 | −0.37 | nucleus |
| XM_020829885.2 | LOC110101821 | DcAP2ERF#49 | 244 | 27,139.94 | 9.23 | 79.22 | −0.437 | nucleus |
| XM_020830439.2 | LOC110102213 | DcAP2ERF#50 | 338 | 36,717.66 | 9.39 | 69.85 | −0.426 | nucleus |
| XM_020831356.2 | LOC110102864 | DcAP2ERF#51 | 191 | 21,091.72 | 9.12 | 60.94 | −0.654 | nucleus |
| XM_020831553.2 | LOC110103007 | DcAP2ERF#52 | 333 | 36,879.23 | 5.83 | 71.02 | −0.514 | nucleus |
| XM_020831585.2 | LOC110103039 | DcAP2ERF#53 | 205 | 22,548.56 | 6.92 | 63.85 | −0.415 | nucleus |
| XM_020831635.2 | LOC110103078 | DcAP2ERF#54 | 350 | 39,719.18 | 8.63 | 62.74 | −0.763 | nucleus |
| XM_020831820.2 | LOC110103210 | DcAP2ERF#55 | 319 | 34,146.41 | 5.23 | 55.49 | −0.573 | nucleus |
| XM_020832156.2 | LOC110103436 | DcAP2ERF#56 | 233 | 26,398.05 | 9.59 | 61.55 | −0.839 | nucleus |
| XM_020832246.1 | LOC110103500 | DcAP2ERF#57 | 216 | 23,871.26 | 4.65 | 63.7 | −0.469 | mitochondria |
| XM_020832253.2 | LOC110103508 | DcAP2ERF#58 | 188 | 20,706.09 | 5.44 | 55.74 | −0.648 | nucleus |
| XM_020832331.2 | LOC110103559 | DcAP2ERF#59 | 333 | 37,914.71 | 6.23 | 52.82 | −0.984 | nucleus |
| XM_020832378.2 | LOC110103593 | DcAP2ERF#60 | 182 | 19,679.18 | 5.35 | 84.95 | −0.175 | nucleus |
| XM_020834156.2 | LOC110104876 | DcAP2ERF#61 | 226 | 24,995.95 | 5.17 | 76.42 | −0.376 | nucleus |
| XM_020834164.2 | LOC110104880 | DcAP2ERF#62 | 138 | 15,094.93 | 5.44 | 76.45 | −0.46 | cytoplasm |
| XM_020834254.2 | LOC110104943 | DcAP2ERF#63 | 317 | 35,188.21 | 5.56 | 66.81 | −0.568 | nucleus |
| XM_020834863.1 | LOC110105385 | DcAP2ERF#64 | 146 | 16,196.67 | 10.1 | 66.99 | −0.646 | nucleus |
| XM_020835088.2 | LOC110105539 | DcAP2ERF#65 | 351 | 39,673.69 | 9.75 | 58.38 | −0.915 | nucleus |
| XM_020835287.2 | LOC110105686 | DcAP2ERF#66 | 237 | 26,183.23 | 7.06 | 69.58 | −0.675 | nucleus |
| XM_020835818.2 | LOC110106077 | DcAP2ERF#67 | 388 | 42,370.13 | 9.25 | 57.24 | −0.649 | nucleus |
| XM_020836334.2 | LOC110106428 | DcAP2ERF#68 | 193 | 21,736.5 | 6.19 | 66.27 | −0.59 | cytoplasm |
| XM_020836335.2 | LOC110106429 | DcAP2ERF#69 | 259 | 28,586.49 | 8.96 | 81.85 | −0.388 | nucleus |
| XM_020837398.2 | LOC110107204 | DcAP2ERF#70 | 628 | 69,816.29 | 6.17 | 59.54 | −0.671 | nucleus |
| XM_020837735.2 | LOC110107464 | DcAP2ERF#71 | 388 | 41,489.16 | 7.81 | 55.67 | −0.557 | nucleus |
| XM_020838142.2 | LOC110107770 | DcAP2ERF#72 | 266 | 28,625.14 | 8.89 | 73.46 | −0.421 | nucleus |
| XM_020838192.2 | LOC110107803 | DcAP2ERF#73 | 251 | 27,642.36 | 5.67 | 71.95 | −0.498 | nucleus |
| XM_020838224.2 | LOC110107825 | DcAP2ERF#74 | 188 | 20,344.91 | 9.3 | 54.68 | −0.677 | cytoplasm |
| XM_020838231.2 | LOC110107829 | DcAP2ERF#75 | 481 | 53,471.97 | 7.64 | 71.81 | −0.516 | nucleus |
| XM_020838234.2 | LOC110107830 | DcAP2ERF#76 | 476 | 52,887.25 | 7.64 | 71.13 | −0.535 | nucleus |
| XM_020838388.2 | LOC110107938 | DcAP2ERF#77 | 295 | 32,730.65 | 5.57 | 67.83 | −0.523 | nucleus |
| XM_020838721.2 | LOC110108187 | DcAP2ERF#78 | 377 | 42,334.33 | 5 | 60.34 | −0.746 | nucleus |
| XM_020838765.2 | LOC110108219 | DcAP2ERF#79 | 376 | 41,253.26 | 6.28 | 59.26 | −0.623 | nucleus |
| XM_020838815.2 | LOC110108252 | DcAP2ERF#80 | 205 | 21,740.44 | 9.34 | 67.27 | −0.423 | nucleus |
| XM_020838948.2 | LOC110108340 | DcAP2ERF#81 | 250 | 26,683.77 | 8.56 | 64.16 | −0.449 | nucleus |
| XM_020839309.2 | LOC110108607 | DcAP2ERF#82 | 177 | 19,603.15 | 7.81 | 59.77 | −0.459 | cytoplasm |
| XM_020839427.2 | LOC110108677 | DcAP2ERF#83 | 381 | 41,657.98 | 5.13 | 58.71 | −0.519 | nucleus |
| XM_020840002.2 | LOC110109092 | DcAP2ERF#84 | 143 | 15,701.74 | 9.51 | 62.94 | −0.524 | nucleus |
| XM_020840101.2 | LOC110109163 | DcAP2ERF#85 | 146 | 16,192.68 | 10.1 | 66.99 | −0.652 | nucleus |
| XM_020840120.2 | LOC110109181 | DcAP2ERF#86 | 256 | 26,949.36 | 9.2 | 69.1 | −0.32 | nucleus |
| XM_020840182.2 | LOC110109222 | DcAP2ERF#87 | 372 | 40,701.75 | 4.68 | 59.87 | −0.761 | nucleus |
| XM_020840524.2 | LOC110109455 | DcAP2ERF#88 | 263 | 28,948.72 | 5.12 | 48.37 | −0.681 | nucleus |
| XM_020840628.2 | LOC110109529 | DcAP2ERF#89 | 323 | 35,656.47 | 4.85 | 62.29 | −0.542 | nucleus |
| XM_020840999.2 | LOC110109796 | DcAP2ERF#90 | 194 | 21,956.89 | 6.31 | 72.99 | −0.591 | nucleus |
| XM_020841375.2 | LOC110110072 | DcAP2ERF#91 | 326 | 35,893.82 | 9.64 | 78.34 | −0.444 | nucleus |
| XM_020841449.2 | LOC110110123 | DcAP2ERF#92 | 190 | 21,631.67 | 8.98 | 65.32 | −0.522 | nucleus |
| XM_020841821.2 | LOC110110379 | DcAP2ERF#93 | 307 | 34,245.39 | 5.85 | 70.26 | −0.536 | nucleus |
| XM_020842046.2 | LOC110110526 | DcAP2ERF#94 | 377 | 41,376.99 | 5.87 | 62.65 | −0.665 | nucleus |
| XM_020842532.1 | LOC110110883 | DcAP2ERF#95 | 249 | 27,664.27 | 9.46 | 70.92 | −0.62 | nucleus |
| XM_020843531.2 | LOC110111594 | DcAP2ERF#96 | 428 | 47,796.64 | 5.64 | 59.53 | −0.581 | cytoplasm |
| XM_020844032.2 | LOC110111961 | DcAP2ERF#97 | 393 | 43,606.04 | 9.3 | 62.39 | −0.647 | nucleus |
| XM_020844150.2 | LOC110112064 | DcAP2ERF#98 | 213 | 23,769.37 | 5 | 54.23 | −0.665 | cytoplasm |
| XM_020844655.2 | LOC110112434 | DcAP2ERF#99 | 180 | 19,766.05 | 6.63 | 61.28 | −0.763 | nucleus |
| XM_020844798.2 | LOC110112545 | DcAP2ERF#100 | 482 | 53,271.16 | 6.47 | 68.05 | −0.595 | nucleus |
| XM_020844875.2 | LOC110112594 | DcAP2ERF#101 | 311 | 34,278.27 | 5.29 | 70.96 | −0.433 | nucleus |
| XM_020844987.2 | LOC110112684 | DcAP2ERF#102 | 640 | 69,342.86 | 6.03 | 61.78 | −0.474 | nucleus |
| XM_020845292.2 | LOC110112910 | DcAP2ERF#103 | 198 | 21,803.57 | 5.71 | 69.49 | −0.397 | nucleus |
| XM_020845879.2 | LOC110113332 | DcAP2ERF#104 | 160 | 17,799.22 | 9.78 | 62.19 | −0.819 | nucleus |
| XM_020846166.2 | LOC110113533 | DcAP2ERF#105 | 271 | 30,721.33 | 5.26 | 56.24 | −0.851 | nucleus |
| XM_020846170.2 | LOC110113537 | DcAP2ERF#106 | 384 | 42,198.39 | 4.73 | 54.24 | −0.736 | nucleus |
| XM_020846319.2 | LOC110113656 | DcAP2ERF#107 | 350 | 39,815.18 | 4.89 | 55.54 | −0.903 | nucleus |
| XM_020847119.2 | LOC110114282 | DcAP2ERF#108 | 198 | 21,677.03 | 6.84 | 59.29 | −0.67 | nucleus |
| XM_020847550.2 | LOC110114618 | DcAP2ERF#109 | 262 | 28,562.38 | 6.78 | 76.45 | −0.426 | cytoplasm |
| XM_020847791.2 | LOC110114791 | DcAP2ERF#110 | 268 | 29,590.17 | 5.9 | 56.16 | −0.538 | nucleus |
| XM_020848468.2 | LOC110115279 | DcAP2ERF#111 | 138 | 14,972.24 | 7.86 | 84.93 | −0.132 | nucleus |
| XM_020848863.2 | LOC110115597 | DcAP2ERF#112 | 271 | 30,293.53 | 5.19 | 57.68 | −0.744 | nucleus |
| XM_020849558.2 | LOC110116094 | DcAP2ERF#113 | 205 | 21,591.06 | 7.7 | 57.85 | −0.299 | mitochondria |
| XM_020850163.2 | LOC110116551 | DcAP2ERF#114 | 251 | 28,723.56 | 9.24 | 67.21 | −0.648 | cytoplasm |
| XM_028691742.1 | LOC110112950 | DcAP2ERF#115 | 279 | 31,067.05 | 6.16 | 69.21 | −0.6 | nucleus |
| XM_028692461.1 | LOC110096264 | DcAP2ERF#116 | 329 | 36,673.85 | 5.29 | 64.62 | −0.631 | cytoplasm |
| XM_028694856.1 | LOC110099516 | DcAP2ERF#117 | 182 | 20,153.72 | 9.35 | 64.45 | −0.713 | nucleus |
| XM_028696782.1 | LOC110098115 | DcAP2ERF#118 | 201 | 21,811.55 | 5.83 | 68.11 | −0.396 | nucleus |
| XM_028698356.1 | LOC110103792 | DcAP2ERF#119 | 628 | 67,877 | 5.8 | 60.25 | −0.61 | nucleus |
| XM_028699460.1 | LOC110115745 | DcAP2ERF#120 | 248 | 26,592.45 | 9.01 | 51.57 | −0.605 | nucleus |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Cai, M.; Zhang, S.; Chai, J.; Sun, M.; Wang, Y.; Xie, Q.; Chen, Y.; Wang, H.; Chen, T. Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum. Int. J. Mol. Sci. 2022, 23, 13603. https://doi.org/10.3390/ijms232113603
Han Y, Cai M, Zhang S, Chai J, Sun M, Wang Y, Xie Q, Chen Y, Wang H, Chen T. Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum. International Journal of Molecular Sciences. 2022; 23(21):13603. https://doi.org/10.3390/ijms232113603
Chicago/Turabian StyleHan, Yuliang, Maohong Cai, Siqi Zhang, Jiawen Chai, Mingzhe Sun, Yingwei Wang, Qinyu Xie, Youheng Chen, Huizhong Wang, and Tao Chen. 2022. "Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum" International Journal of Molecular Sciences 23, no. 21: 13603. https://doi.org/10.3390/ijms232113603
APA StyleHan, Y., Cai, M., Zhang, S., Chai, J., Sun, M., Wang, Y., Xie, Q., Chen, Y., Wang, H., & Chen, T. (2022). Genome-Wide Identification of AP2/ERF Transcription Factor Family and Functional Analysis of DcAP2/ERF#96 Associated with Abiotic Stress in Dendrobium catenatum. International Journal of Molecular Sciences, 23(21), 13603. https://doi.org/10.3390/ijms232113603







