Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio)
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity and Cell Proliferative Activity of PDRN
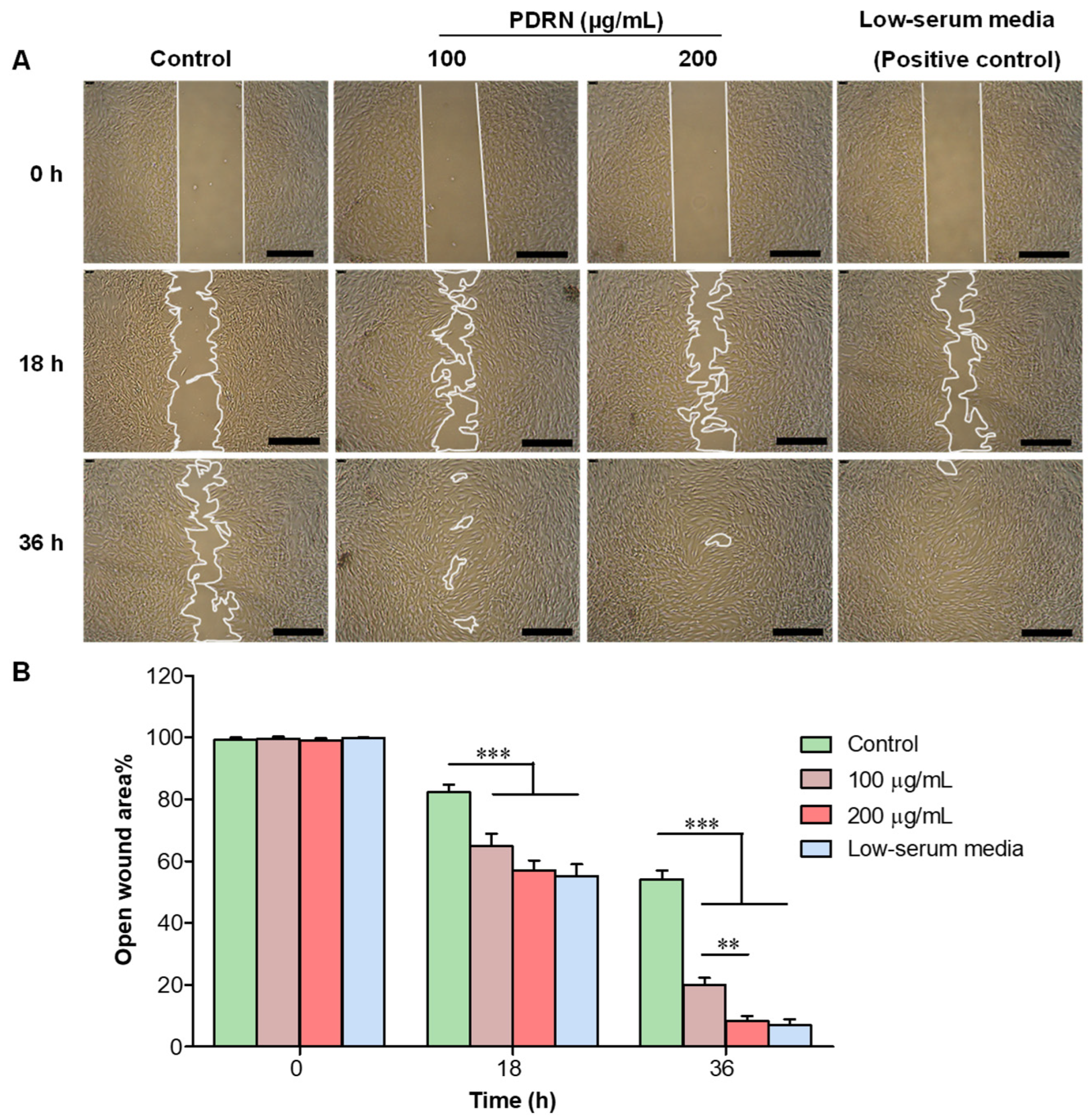
2.2. Effect of PDRN on In Vitro Wound Healing in HDFs
2.3. Effect of PDRN on Corneal Wound Healing upon Acid Injury in Zebrafish
2.4. Histological Analysis of Corneal Re-Epithelialization in Acid-Injured PDRN-Treated Zebrafish
2.5. Effect of PDRN on Goblet Cell Characteristics in Corneal Wound Healing in Zebrafish
2.6. Analysis of mRNA Expression during PDRN Mediated Corneal Wound Healing in Zebrafish
2.6.1. Expression Profiles of Adenosine Receptors (adora2a.1, adora2ab, adora1b, and adora2b)
2.6.2. Expression Profile of tnf-α
2.6.3. Expression Profiles of mmp9 and mmp13
2.6.4. Expression Profile of tgfβ1
2.6.5. Expression Profiles of pax6a and pax6b
2.6.6. Expression Profiles of Heat Shock Proteins (hsp70 and hsp90ab1)
2.6.7. Expression Profiles of klf4
2.6.8. Expression Profiles of Mucins (muc2.1, muc5.1, and muc5.2)
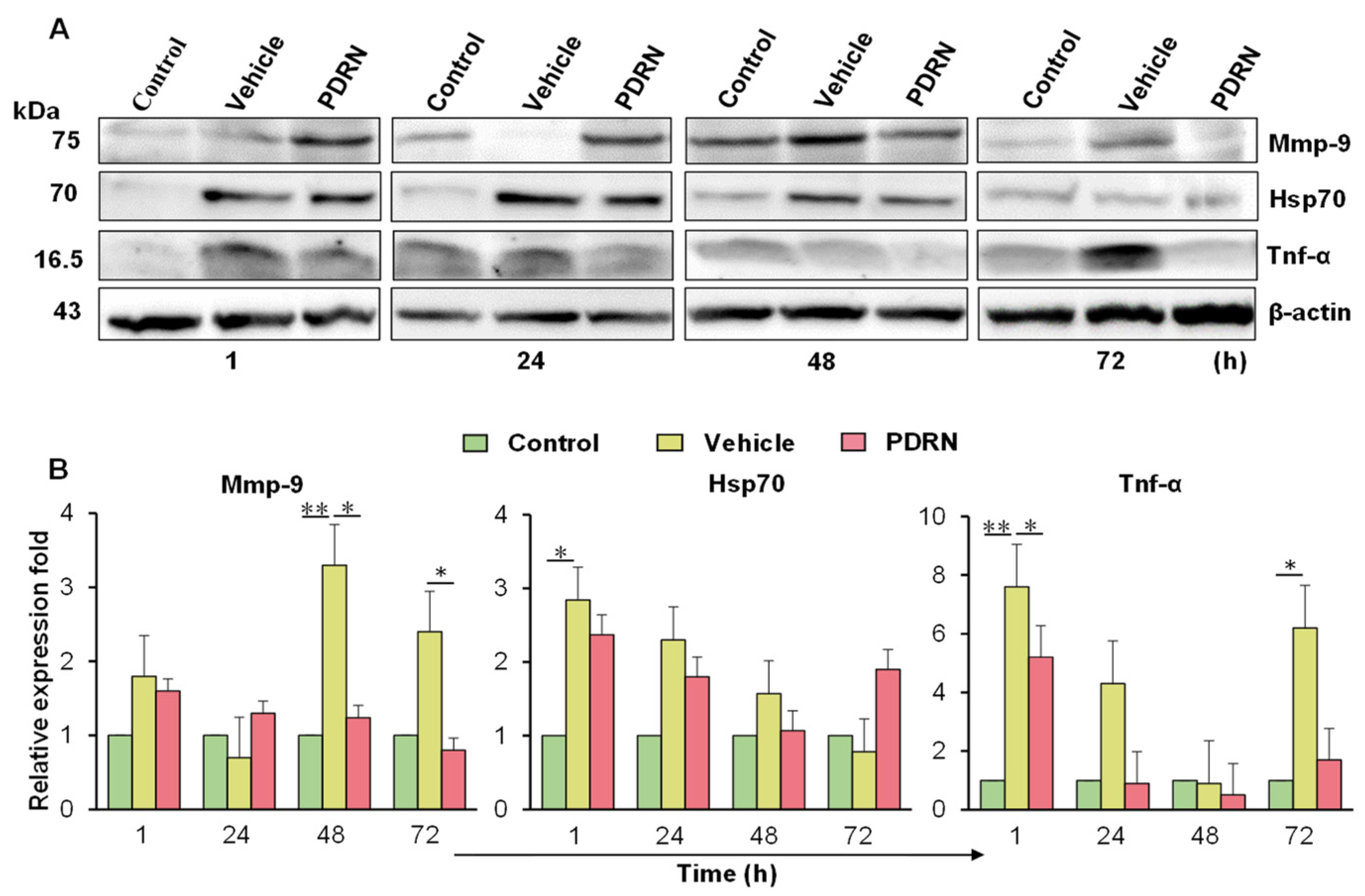
2.7. Immunoblot Analysis of the PDRN-Treated Zebrafish with Corneal Injury
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Cytotoxicity of PDRN on HDFs
4.2. In Vitro Wound Healing Activity of PDRN
4.3. Corneal Injury and Fluorescein Staining of Zebrafish Eye
4.4. Analysis of the Effect of PDRN on Corneal Healing by Histological Analysis
4.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. Analysis of PDRN Effect on Wound Healing Associated Genes
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Böhnke, M.; Masters, B.R. Confocal microscopy of the cornea. Prog. Retin. Eye Res. 1999, 18, 553–628. [Google Scholar] [CrossRef]
- Jester, J.V.; Moller-Pedersen, T.; Huang, J.; Sax, C.M.; Kays, W.T.; Cavangh, H.D.; Petroll, W.M.; Piatigorsky, J. The cellular basis of corneal transparency: Evidence for “corneal crystallins”. J. Cell Sci. 1999, 112, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190–194. [Google Scholar] [CrossRef] [PubMed]
- DelMonte, D.W.; Kim, T. Anatomy and physiology of the cornea. J. Cataract. Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef]
- Pineda, R. World Corneal Blindness. In Foundations of Corneal Disease; Colby, K., Dana, R., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Ljubimov, A.V.; Saghizadeh, M. Progress in corneal wound healing HHS Public Access. Prog. Retin. Eye Res. 2015, 49, 17–45. [Google Scholar] [CrossRef]
- Chaurasia, S.S.; Kaur, H.; de Medeiros, F.W.; Smith, S.D.; Wilson, S.E. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res. 2009, 89, 133–139. [Google Scholar] [CrossRef]
- Wilson, S.E.; Mohan, R.R.; Mohan, R.R.; Ambro, R.; Hong, J.; Lee, J. The corneal wound healing response-cytokine mediated interaction of epithelium, stroma and, inflammatory cells. Prog. Retin. Eye Res. 2001, 20, 625–637. [Google Scholar]
- Shoham, A.; Hadziahmetovic, M.; Dunaief, J.L.; Mydlarski, M.B.; Schipper, H.M. Oxidative stress in diseases of the human cornea. Free Radic. Biol. Med. 2008, 45, 1047–1055. [Google Scholar] [CrossRef]
- Bukowiecki, A.; Hos, D.; Cursiefen, C.; Eming, S.A. Wound-healing studies in cornea and skin: Parallels, differences and opportunities. Int. J. Mol. Sci. 2017, 18, 1257. [Google Scholar] [CrossRef]
- Cintron, C.; Covington, H.; Kublin, C.L. Morphogenesis of rabbit corneal stroma. Investig. Ophthalmol. Vis. Sci. 1983, 24, 543–556. [Google Scholar] [CrossRef]
- Mckenna, C.C.; Lwigale, P.Y. Innervation of the mouse cornea during development. Investig. Ophthalmol. Vis. Sci. 2011, 52, 30–35. [Google Scholar] [CrossRef]
- Schumann, S.; Dietrich, E.; Kruse, C.; Grisanti, S.; Ranjbar, M. Establishment of a robust and simple corneal organ culture model to monitor wound healing. J. Clin. Med. 2021, 10, 3486. [Google Scholar] [CrossRef] [PubMed]
- Glenwood, G.; Barbara, W.; MaryJane, R.; Stacy, P.; Damian, G. Corneal Wound Healing Model in New Zealand White Rabbits for Evaluating Persistent Corneal Epithelial Defects. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3903. [Google Scholar]
- Pal-Ghosh, S.; Pajoohesh-Ganji, A.; Brown, M.; Stepp, M.A. A mouse model for the study of recurrent corneal epithelial erosions: α9β1 integrin implicated in progression of the disease. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1775–1788. [Google Scholar] [CrossRef]
- Choi, H.; Phillips, C.; Oh, J.Y.; Stock, E.M.; Kim, D.K.; Won, J.K.; Fulcher, S. Comprehensive Modeling of Corneal Alkali Injury in the Rat Eye. Curr. Eye Res. 2017, 42, 1348–1357. [Google Scholar] [CrossRef]
- Conners, M.S.; Urbano, F.; Vafeas, C.; Stoltz, R.A.; Dunn, M.W.; Schwartzman, M.L. Alkali burn-induced time-dependent synthesis of 12-HETE enantiomers in rabbit corneal epithelium. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2504. [Google Scholar]
- Bian, F.; Xiao, Y.; Zaheer, M.; Volpe, E.A.; Pflugfelder, S.C.; Li, D.Q.; De Paiva, C.S. Inhibition of NLRP3 inflammasome pathway by butyrate improves corneal wound healing in corneal alkali burn. Int. J. Mol. Sci. 2017, 18, 562. [Google Scholar] [CrossRef]
- Anderson, C.; Zhou, Q.; Wang, S. An Alkali-burn injury model of corneal neovascularization in the mouse. J. Vis. Exp. 2014, 86, e51159. [Google Scholar] [CrossRef]
- Barabino, S.; Dana, M.R. Animal models of dry eye: A critical assessment of opportunities and limitations. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1641–1646. [Google Scholar] [CrossRef]
- Evangelho, K.; Mastronardi, C.A.; De-La-Torre, A. Experimental models of glaucoma: A powerful translational tool for the future development of new therapies for glaucoma in humans—A review of the literature. Medicina 2019, 55, 280. [Google Scholar] [CrossRef]
- Sun, Z.; Amsterdam, A.; Pazour, G.J.; Cole, D.G.; Miller, M.S.; Hopkins, N. A genetic screen in zebrafish indentifies cilia genes as a principal cause of cystic kidney. Development 2004, 131, 4085–4093. [Google Scholar] [CrossRef] [PubMed]
- Kleinjan, D.A.; Bancewicz, R.M.; Gautier, P.; Dahm, R.; Schonthaler, H.B.; Damante, G.; Seawright, A.; Hever, A.M.; Yeyati, P.L.; Van Heyningen, V.; et al. Subfunctionalization of duplicated zebrafish pax6 genes by cis-regulatory divergence. PLoS Genet. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed]
- Bibliowicz, J.; Tittle, R.K.; Gross, J.M. Toward a better understanding of human eye disease: Insights from the zebrafish, Danio rerio. Prog. Mol. Biol. Transl. Sci. 2011, 100, 287–330. [Google Scholar] [CrossRef]
- Bohnsack, B.L.; Kasprick, D.S.; Kish, P.E.; Goldman, D.; Kahana, A. A zebrafish model of Axenfeld-Rieger syndrome reveals that pitx2 regulation by Retinoic Acid is essential for ocular and craniofacial development. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kawahara, G.; Gundry, S.R.; Chen, A.T.; Lencer, W.I.; Zhou, Y.; Zon, L.I.; Kunkel, L.M.; Beggs, A.H. The zebrafish dag1 mutant: A novel genetic model for dystroglycanopathies. Hum. Mol. Genet. 2011, 20, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.C.; Yee, R.W.; Norcom, E.; Burgess, H.; Avanesov, A.S.; Barrish, J.P.; Malicki, J. The zebrafish cornea: Structure and development. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4341–4348. [Google Scholar] [CrossRef][Green Version]
- Ikkala, K.; Stratoulias, V.; Michon, F. Unilateral Zebrafish Corneal Injury Induces Bilateral Cell Plasticity Supporting Wound Closure. Sci. Rep. 2022, 12, 1–20. [Google Scholar] [CrossRef]
- Oliver, V.F.; Van Bysterveldt, K.A.; Cadzow, M.; Steger, B.; Romano, V.; Markie, D.; Hewitt, A.W.; MacKey, D.A.; Willoughby, C.E.; Sherwin, T.; et al. A COL17A1 Splice-Altering Mutation Is Prevalent in Inherited Recurrent Corneal Erosions. Ophthalmology 2016, 123, 709–722. [Google Scholar] [CrossRef]
- Colin, S.P.; Colin, H.B. The fish cornea: Adaptation for different aquatic environment. In Sensory Biology of Jawed Fishes-New Insights; Kapoor, B.G., Hara, T.J., Eds.; Science Publishers Inc.: New York, NY, USA, 2001; p. 57. [Google Scholar]
- Heur, M.; Jiao, S.; Schindler, S.; Crump, J.G. Regenerative potential of the zebrafish corneal endothelium. Exp. Eye Res. 2013, 106, 1–4. [Google Scholar] [CrossRef]
- Julia, R.; Anderson, A.; Pascal, L. Corneal injury and repair in the zebrafish. Exp. Biol. 2015, 29, 1–10. [Google Scholar] [CrossRef]
- Choi, J.-S.; Joo, C.-K. Polydeoxyribonucleotide (PDRN) inhibits corneal inflammation in experimental rat keratoconjunctivitis sicca model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5730. [Google Scholar]
- Lazzarotto, M.; Tomasello, E.M.; Caporossi, A. Clinical Evaluation of Corneal Epithelialization after Photorefractive Keratectomy in Patients Treated with Polydeoxyribonucleotide (PDRN) Eye Drops: A Randomized, Double-blind, Placebo-controlled Trial. Eur. J. Ophthalmol. 2004, 14, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Tracey-White, D.; Webster, A.; Moosajee, M. The zebrafish eye-a paradigm for investigating human ocular genetics. Eye 2017, 31, 68–86. [Google Scholar] [CrossRef] [PubMed]
- Fadool, J.M.; Dowling, J.E. Zebrafish: A model system for the study of eye genetics. Prog. Retin. Eye Res. 2008, 27, 89–110. [Google Scholar] [CrossRef]
- Morris, A.C. The genetics of ocular disorders: Insights from the zebrafish. Birth Defects Res. Part C-Embryo Today Rev. 2011, 93, 215–228. [Google Scholar] [CrossRef]
- Link, B.A.; Collery, R.F. Zebrafish Models of Retinal Disease. Annu. Rev. Vis. Sci. 2015, 1, 125–153. [Google Scholar] [CrossRef]
- Kujawski, S.; Crespo, C.; Luz, M.; Yuan, M.; Winkler, S.; Knust, E. Loss of Crb2b-lf leads to anterior segment defects in old zebrafish. Biol. Open 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Kwon, T.R.; Han, S.W.; Kim, J.H.; Lee, B.C.; Kim, J.M.; Hong, J.Y.; Kim, B.J. Polydeoxyribonucleotides Improve Diabetic Wound Healing in Mouse Animal Model for Experimental Validation. Ann. Dermatol. 2019, 31, 403–413. [Google Scholar] [CrossRef]
- Joshua, E.J.; Barbara, E.C. Corned Staining After Instillation of Topical Anesthetic (SSII). Investig. Ophthalmol. Vis. Sci. 1988, 29, 1096–1099. [Google Scholar]
- Ellina, A.M.; Peter, W.J.M.; Adrian, C.W.; Vitaliy, V.K. On the Barrier Properties of the Cornea: A Microscopy Study of the Penetration of Fluorescently Labeled Nanoparticles, Polymers, and Sodium Fluorescein. Mol. Pharm. 2014, 11, 3556–3564. [Google Scholar] [CrossRef]
- Caffery, B.E.; Josephson, J.E. Corneal staining after sequential instillations of fluorescein over 30 days. Optom. Vis. Sci. 1991, 68, 467–469. [Google Scholar] [CrossRef] [PubMed]
- Kasus, J.A.; Noor, M.S.; Griffith, G.L.; Hinsley, H.; Mathias, L.; Pereira, H.A. A multifunctional peptide based on the neutrophil immune defense molecule, CAP37, has antibacterial and wound-healing properties. J. Leukoc. Biol. 2015, 97, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Sumioka, T.; Ichikawa, K.; Sano, H.; Nambu, A.; Kobayashi, K.; Uchida, K.; Suzuki, Y.; Tominaga, M.; Reinach, P.S.; et al. Sensory nerve supports epithelial stem cell function in healing of corneal epithelium in mice: The role of trigeminal nerve transient receptor potential vanilloid 4. Lab. Investig. 2019, 99, 210–230. [Google Scholar] [CrossRef]
- Hertsenberg, A.J.; Funderburgh, J.L. Stem Cells in the Cornea. Prog. Mol. Biol. Transl. Sci. 2015, 134, 25–41. [Google Scholar] [CrossRef]
- Puri, S.; Sun, M.; Mutoji, K.N.; Gesteira, T.F.; Coulson-Thomas, V.J. Epithelial Cell Migration and Proliferation Patterns During Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency. Investig. Ophthalmol. Vis. Sci. 2020, 61, 1–15. [Google Scholar] [CrossRef]
- Wijnholds, J. “Basal Cell Migration” in Regeneration of the Corneal Wound-Bed. Stem Cell Rep. 2019, 12, 3–5. [Google Scholar] [CrossRef]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef]
- Thellung, S.; Florio, T.; Maragliano, A.; Cattarini, G.; Schettini, G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: Involvement of A2 purinergic receptor subtypes. Life Sci. 1999, 64, 1661–1674. [Google Scholar] [CrossRef]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef]
- Yochai, S.; Jacob, P.; Victoria, D.; Joseph, F.P.; Abraham, S. Increased Expression of Inflammatory Cytokines and Matrix Metalloproteinases in Pseudophakic Corneal Edema. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1940–1947. [Google Scholar] [CrossRef][Green Version]
- Julie, T.D.; Astrid, L.G.; Ulpu, S.K.; Gillian, M.; Peng, T.K. Human Corneal Epithelial Cells Require MMP-1 for HGF-Mediated Migration on Collagen I. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1048–1055. [Google Scholar] [CrossRef]
- Blanco-Mezquita, J.T.; Hutcheon, A.E.; Zieske, J.D. Role of Thrombospondin-1 in Repair of Penetrating Corneal Wounds. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6262–6268. [Google Scholar] [CrossRef] [PubMed]
- Mauris, J.; Woodward, A.M.; Cao, Z.; Panjwani, N.; Argüeso, P. Molecular basis for MMP9 induction and disruption of epithelial cell-cell contacts by galectin-3. J. Cell Sci. 2014, 127 Pt 14, 3141–3148. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.E.; Cook, J.R.; Mohan, R. Proteolytic mechanisms in corneal ulceration and repair. Arch. Dermatol. Res. 1998, 290, S12–S23. [Google Scholar] [CrossRef]
- Ottino, P.; Taheri, F.; Bazan, H.E.P. Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: Imbalance between the gene expression of MMP-9 and TIMP-1 and -2. Exp. Eye Res. 2002, 74, 393–402. [Google Scholar] [CrossRef]
- Gordon, G.M.; Austin, J.S.; Sklar, A.L.; Feuer, W.J.; Lagier, A.J.; Fini, M.E. Comprehensive gene expression profiling and functional analysis of matrix metalloproteinases and TIMPs, and identification of ADAM-10 gene expression, in a corneal model of epithelial resurfacing. J. Cell. Physiol. 2011, 226, 1461–1470. [Google Scholar] [CrossRef]
- Ye, H.Q.; Maeda, M.; Yu, F.S.X.; Azar, D.T. Differential expression of MT1-MMP (MMP-14) and collagenase III (MMP-13) genes in normal and wounded rat corneas. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2894–2899. [Google Scholar]
- Haber, M.; Cao, Z.; Panjwani, N.; Bedenice, D.; Li, W.W.; Provost, P.J. Effects of growth factors (EGF, PDGF-BB and ββ1) on cultured equine epithelial cells and keratocytes: Implications for wound healing. Vet. Ophthalmol. 2003, 6, 211–217. [Google Scholar] [CrossRef]
- Jens, L.A.; Thomas, L.; Niels, E. Keratocyte migration and peptide growth factors: The effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-ß on human keratocyte migration in a collagen gel. Curr. Eye Res. 1997, 16, 605–613. [Google Scholar] [CrossRef]
- Koroma, B.M.; Yang, J.M.; Sundin, O.H. The Pax-6 homeobox gene is expressed throughout the corneal and conjunctival epithelia. Investig. Ophthalmol. Vis. Sci. 1997, 38, 108–120. [Google Scholar]
- Dorà, N.; Ou, J.; Kucerova, R.; Parisi, I.; West, J.D.; Collinson, J.M. PAX6 dosage effects on corneal development, growth, and wound healing. Dev. Dyn. 2008, 237, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Piatigorsky, J. Overexpression of Pax6 in mouse cornea directly alters corneal epithelial cells: Changes in immune function, vascularization, and differentiation. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4158–4168. [Google Scholar] [CrossRef] [PubMed]
- Swamynathan, S.K.; Katz, J.P.; Kaestner, K.H.; Ashery-Padan, R.; Crawford, M.A.; Piatigorsky, J. Conditional Deletion of the Mouse Klf4 Gene Results in Corneal Epithelial Fragility, Stromal Edema, and Loss of Conjunctival Goblet Cells. Mol. Cell. Biol. 2007, 27, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Swamynathan, S.K. Ocular surface development and gene expression. J. Ophthalmol. 2013, 2013, 103947. [Google Scholar] [CrossRef]
- Peterson, C.W.M.; Carter, R.T.; Bentley, E.; Murphy, C.J.; Chandler, H.L. Heat-shock protein expression in canine corneal wound healing. Vet. Ophthalmol. 2016, 19, 262–266. [Google Scholar] [CrossRef]
- Edirisinghe, S.L.; Rajapaksha, D.C.; Nikapitiya, C.; Oh, C.; Lee, K.A.; Kang, D.H.; De Zoysa, M. Spirulina maxima derived marine pectin promotes the in vitro and in vivo regeneration and wound healing in zebrafish. Fish Shellfish. Immunol. 2020, 107, 414–425. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edirisinghe, S.L.; Nikapitiya, C.; Dananjaya, S.H.S.; Park, J.; Kim, D.; Choi, D.; De Zoysa, M. Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). Int. J. Mol. Sci. 2022, 23, 13525. https://doi.org/10.3390/ijms232113525
Edirisinghe SL, Nikapitiya C, Dananjaya SHS, Park J, Kim D, Choi D, De Zoysa M. Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). International Journal of Molecular Sciences. 2022; 23(21):13525. https://doi.org/10.3390/ijms232113525
Chicago/Turabian StyleEdirisinghe, Shan Lakmal, Chamilani Nikapitiya, S. H. S. Dananjaya, Jungho Park, Dukgyu Kim, Dongrack Choi, and Mahanama De Zoysa. 2022. "Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio)" International Journal of Molecular Sciences 23, no. 21: 13525. https://doi.org/10.3390/ijms232113525
APA StyleEdirisinghe, S. L., Nikapitiya, C., Dananjaya, S. H. S., Park, J., Kim, D., Choi, D., & De Zoysa, M. (2022). Effect of Polydeoxyribonucleotide (PDRN) Treatment on Corneal Wound Healing in Zebrafish (Danio rerio). International Journal of Molecular Sciences, 23(21), 13525. https://doi.org/10.3390/ijms232113525






