Alkaline Stress Causes Changes in Polyamine Biosynthesis in Thermus thermophilus
Abstract
1. Introduction
2. Results
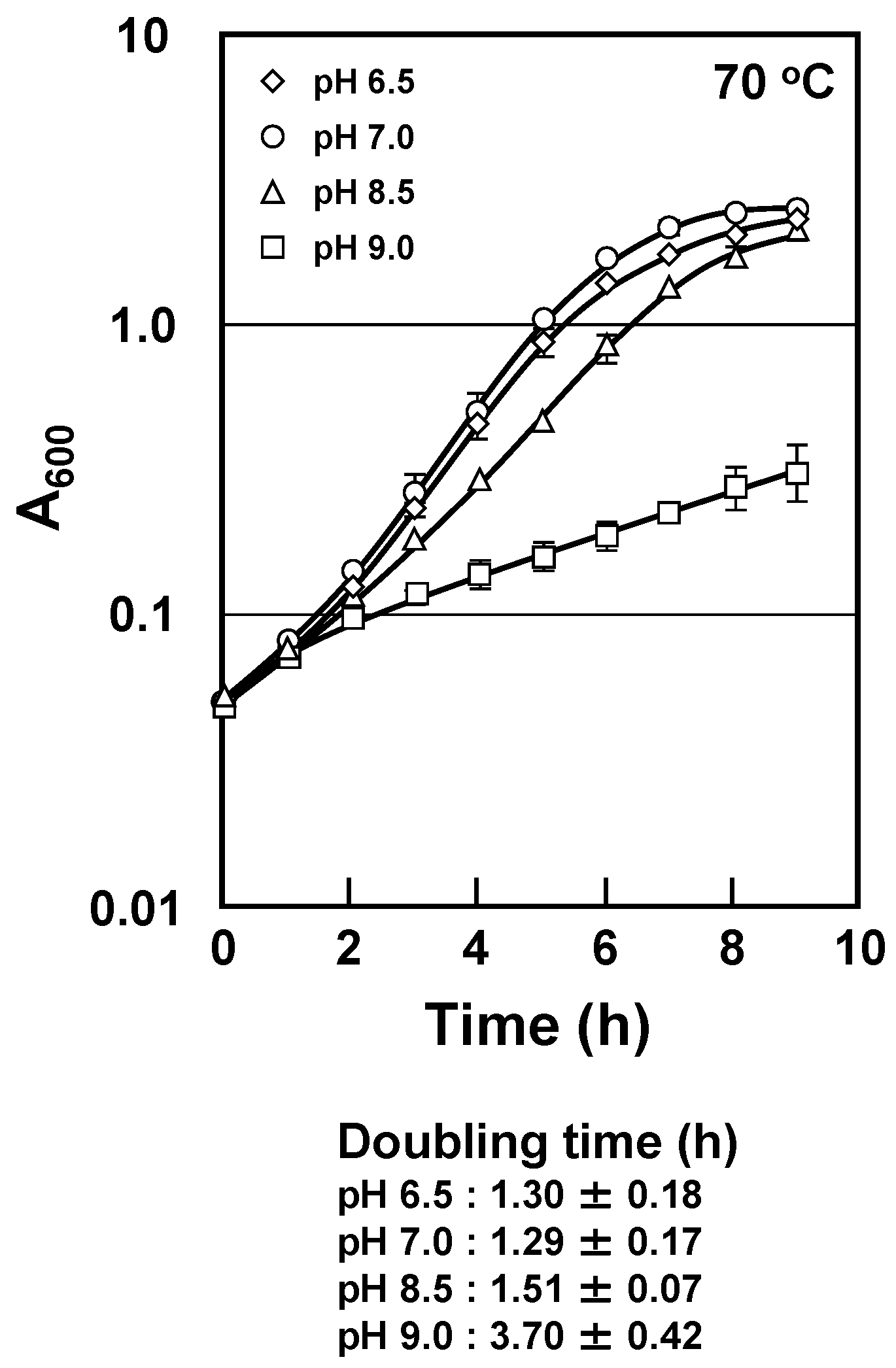
2.1. Changes in Composition of Intracellular Polyamines, Cell Growth and Viability with pH Change
2.2. Changes in Polyamine Biosynthesis in T. thermophilus in Response to Environmental pH
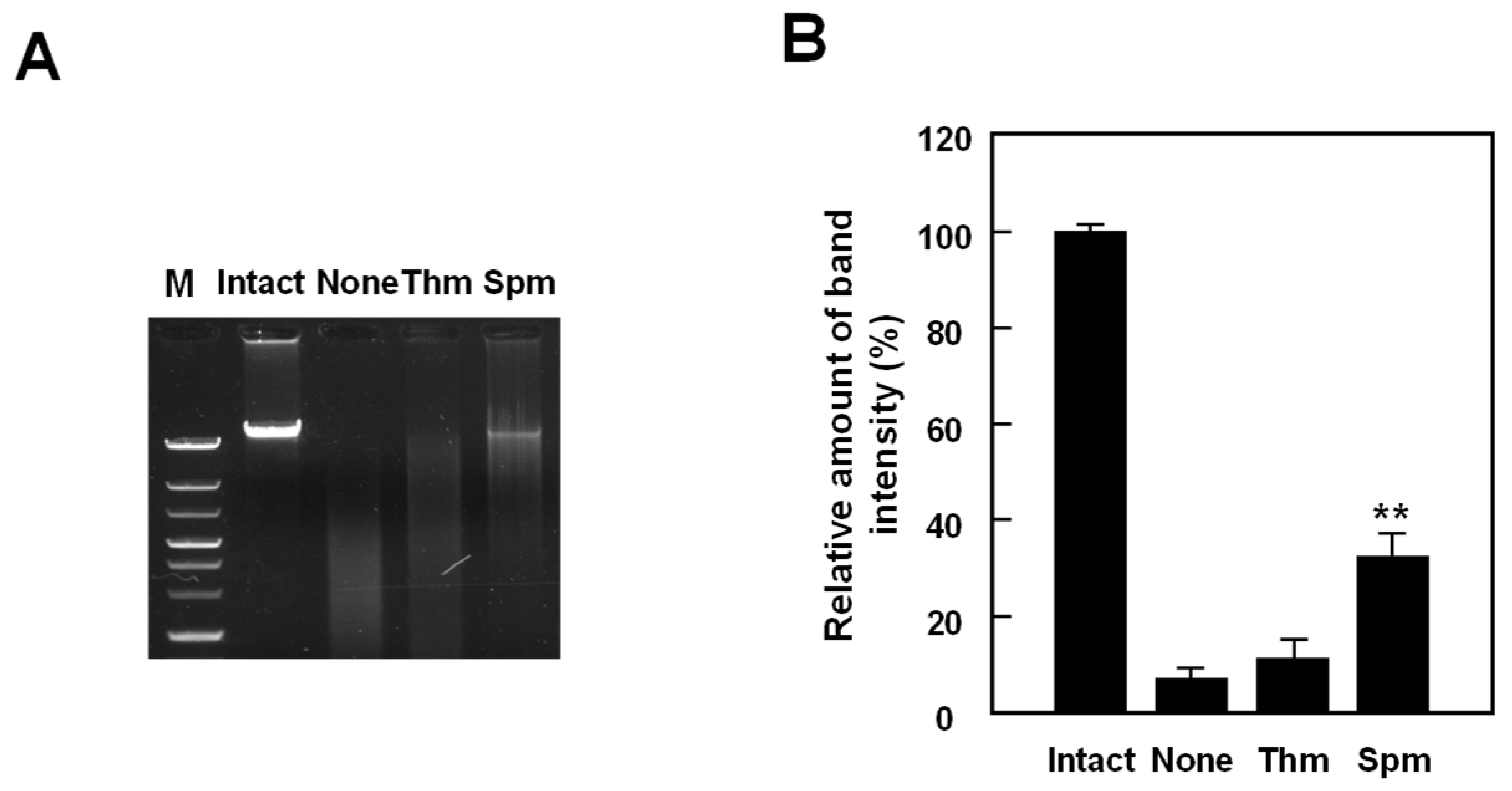
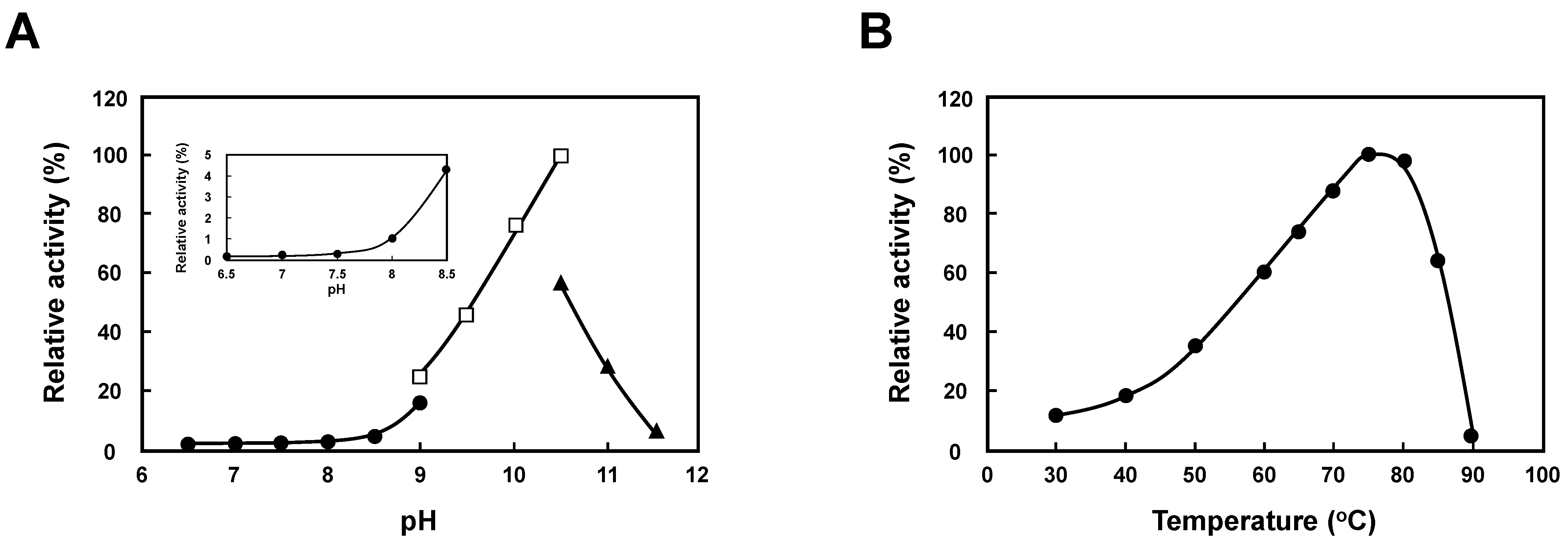
2.3. Substrate-Specificity Change of T. thermophilus SpeB by External pH Change
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Polyamine Analysis
4.3. Disruption of SpeE
4.4. Assay of Double Strand Breaks
4.5. Expression and Purification of SpeB Protein
4.6. Enzymatic Reactions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, H.L.; Hall, J. Endogenous polyamine function the RNA perspective. Nucleic Acids Res. 2014, 42, 11275–11290. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Effects of polyamines on protein synthesis and growth of Escherichia coli. J. Biol. Chem. 2018, 293, 18702–18709. [Google Scholar] [CrossRef]
- Venkiteswaran, S.; Vijayanathan, V.; Shirahata, A.; Thomas, T.; Thomas, T.J. Antisense recognition of the HER-2 mRNA: Effects of phosphorothioate substitution and polyamines on DNA.RNA, RNA.RNA, and DNA.DNA duplex stability. Biochemistry 2005, 44, 303–312. [Google Scholar] [CrossRef]
- Shah, P.; Swaito, E. A multifaceted role for polyamines in bacterial pathogens. Mol. Microbiol. 2008, 68, 4–16. [Google Scholar] [CrossRef]
- Tkachenko, A.G.; Akhova, A.V.; Shumkov, M.S.; Nesterova, L.Y. Polyamines reduce oxidative stress in Escherichia coli cells exposed to bactericidal antibiotics. Res. Microbiol. 2012, 163, 83–91. [Google Scholar] [CrossRef]
- Park, Y.K.; Bearson, B.; Bang, S.H.; Bang, I.S.; Foster, J.W. Internal pH crisis, lysine decarboxylase and the acid tolerance response of Salmonella typhimurium. Mol. Microbiol. 1996, 20, 605–611. [Google Scholar] [CrossRef]
- Krammer, E.M.; Prévost, M. Function and regulation of acid resistance antiporters. J. Membr. Biol. 2019, 252, 465–481. [Google Scholar] [CrossRef]
- Iwadate, Y.; Ramezanifard, R.; Golubeva, Y.A.; Fenlon, L.A.; Slauch, J.M. PaeA (YtfL) protects from cadaverine and putrescine stress in Salmonella Typhimurium and E. coli. Mol. Microbiol. 2021, 115, 1379–1394. [Google Scholar] [CrossRef]
- Oshima, T.; Imahori, K. Description of Thermus thermophilus (Yoshida and Oshima) comb, nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int. J. Syst. Bacteriol. 1974, 24, 102–112. [Google Scholar] [CrossRef]
- Oshima, T. Unique polyamines produced by an extreme thermophile, Thermus thermophilus. Amino Acids 2007, 33, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Terui, Y.; Ohnuma, M.; Hiraga, K.; Kawashima, E.; Oshima, T. Stabilization of nucleic acids by unusual polyamines produced by an extreme thermophile, Thermus thermophilus. Biochem. J. 2005, 388 Pt 2, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Terui, Y.; Yoshida, T.; Sakamoto, A.; Saito, D.; Oshima, T.; Kawazoe, M.; Yokoyama, S.; Igarashi, K.; Kashiwagi, K. Polyamines protect nucleic acids against depurination. Int. J. Biochem. Cell Biol. 2018, 99, 147–153. [Google Scholar] [CrossRef]
- Sakamoto, A.; Tamakoshi, M.; Moriya, T.; Oshima, T.; Takao, K.; Sugita, Y.; Fruchi, T.; Niitsu, M.; Uemura, T.; Igarashi, K.; et al. Polyamines produced by an extreme thermophile are essential for cell growth at high temperature. J. Biochem. 2022, 172, 109–115. [Google Scholar] [CrossRef]
- Ranawat, P.; Rawat, S. Stress response physiology of thermophiles. Arch. Microbiol. 2017, 199, 391–414. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. With pH range tolerance in extremophiles: Towards understanding an important phenomenon for future biotechnology. Appl. Microbiol. Biotechnol. 2016, 100, 2499–2510. [Google Scholar] [CrossRef]
- Kondo, N.; Nishikubo, T.; Wakamatsu, T.; Ishikawa, H.; Nakagawa, N.; Kuramitsu, S.; Masui, R. Insights into different dependence of dNTP triphosphohydrolase on metal ion species from intracellular ion concentrations in Thermus thermophilus. Extremophiles 2008, 12, 217–223. [Google Scholar] [CrossRef]
- Ohnuma, M.; Terui, Y.; Tamakoshi, M.; Mitome, H.; Niitsu, M.; Samejima, K.; Kawashima, E.; Oshima, T. N1-Aminopropylagmatine, a new polyamine produced as a key intermediate in polyamine biosynthesis of an extreme thermophile, Thermus thermophilus. J. Biol. Chem. 2005, 280, 30073–30082. [Google Scholar] [CrossRef]
- Oshima, T. Enigmas of biosyntheses of unusual polyamines in an extreme thermophile, Thermus thermophilus. Plant Physiol. Biochem. 2010, 48, 521–526. [Google Scholar] [CrossRef]
- Ohnuma, M.; Ganbe, T.; Terui, Y.; Niitsu, M.; Sato, T.; Tanaka, N.; Tamakoshi, M.; Samejima, K.; Kumasaka, T.; Oshima, T. Crystal structures and enzymatic properties of a triamine/agmatine aminopropyltransferase from Thermus thermophilus. J. Mol. Biol. 2011, 408, 971–986. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, K.; Kashiwagi, K. Functional roles of polyamines and their metabolite acrolein in eukaryote cells. Amino Acids 2021, 53, 1473–1492. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.D. The Genus Thermus in Thermophilic Microorganisum and Life at High Temperatures; Springer Series in Microbiology; Springer Nature: Berlin/Heidelberg, Germany, 1978; pp. 72–91. [Google Scholar]
- Lutnaes, B.F.; Strand, A.; Petursdottir, S.K.; Liaaen-Jensen, S. Carotenoids of thermophilic bacteria Rhodothermus marinus from submarine Icelandic hot springs. Biochem. Syst. Ecol. 2004, 32, 455–468. [Google Scholar] [CrossRef]
- Cui, J.; Pottosin, I.; Lamade, E.; Tcherkez, G. What is the role of putrescine accumulated under potassium deficiency? Plant Cell Environ. 2020, 43, 1331–1347. [Google Scholar] [CrossRef]
- Michael, A.J. Polyamine function in archaea and bacteria. J. Biol. Chem. 2018, 293, 18693–18701. [Google Scholar] [CrossRef]
- Hitomi, J.; Park, J.S.; Nishiyama, M.; Horinouchi, S.; Beppu, T. Substrate-dependent change in the pH-activity profile of alkaline endo-1,4-beta-glucanase from an alkaline Bacillus sp. J. Biochem. 1994, 116, 554–559. [Google Scholar] [CrossRef]
- Denu, J.M.; Fitzpatrick, P.F. pH and kinetic isotope effects on the oxidative half-reaction of D-amino-acid oxidase. J. Biol. Chem. 1994, 269, 15054–15059. [Google Scholar] [CrossRef]
- Foster, J.W. Escherichia coli acid resistance: Tales of an amateur acidophile. Nat. Rev. Microbiol. 2004, 2, 898–907. [Google Scholar] [CrossRef]
- Tanaka, T.; Kawano, N.; Oshima, T. Cloning of 3-isopropylmalate dehydrogenase gene of an extreme thermophile and partial purification of the gene product. J. Biochem. 1981, 89, 677–682. [Google Scholar] [CrossRef]
- Mizushima, S.; Machida, Y.; Kitahara, K. Quantitative studied on glycolytic enzymes in Lactobacillus plantarum. J. Bacteriol. 1963, 86, 1295–1300. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Yano, T.; Kuramitsu, S.; Kagamiyama, H. Disruption of Thermus thermophilus genes by homologous recombination using a thermostable kanamycin-resistant marker. FEBS Lett. 2001, 506, 231–234. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, T.; Sakamoto, A.; Kashiwagi, K.; Igarashi, K.; Moriya, T.; Oshima, T.; Terui, Y. Alkaline Stress Causes Changes in Polyamine Biosynthesis in Thermus thermophilus. Int. J. Mol. Sci. 2022, 23, 13523. https://doi.org/10.3390/ijms232113523
Kobayashi T, Sakamoto A, Kashiwagi K, Igarashi K, Moriya T, Oshima T, Terui Y. Alkaline Stress Causes Changes in Polyamine Biosynthesis in Thermus thermophilus. International Journal of Molecular Sciences. 2022; 23(21):13523. https://doi.org/10.3390/ijms232113523
Chicago/Turabian StyleKobayashi, Teruyuki, Akihiko Sakamoto, Keiko Kashiwagi, Kazuei Igarashi, Toshiyuki Moriya, Tairo Oshima, and Yusuke Terui. 2022. "Alkaline Stress Causes Changes in Polyamine Biosynthesis in Thermus thermophilus" International Journal of Molecular Sciences 23, no. 21: 13523. https://doi.org/10.3390/ijms232113523
APA StyleKobayashi, T., Sakamoto, A., Kashiwagi, K., Igarashi, K., Moriya, T., Oshima, T., & Terui, Y. (2022). Alkaline Stress Causes Changes in Polyamine Biosynthesis in Thermus thermophilus. International Journal of Molecular Sciences, 23(21), 13523. https://doi.org/10.3390/ijms232113523





