Physiological Impact of a Synthetic Elastic Protein in Arterial Diseases Related to Alterations of Elastic Fibers: Effect on the Aorta of Elastin-Haploinsufficient Male and Female Mice
Abstract
1. Introduction
2. Results
2.1. Body and Heart Weights
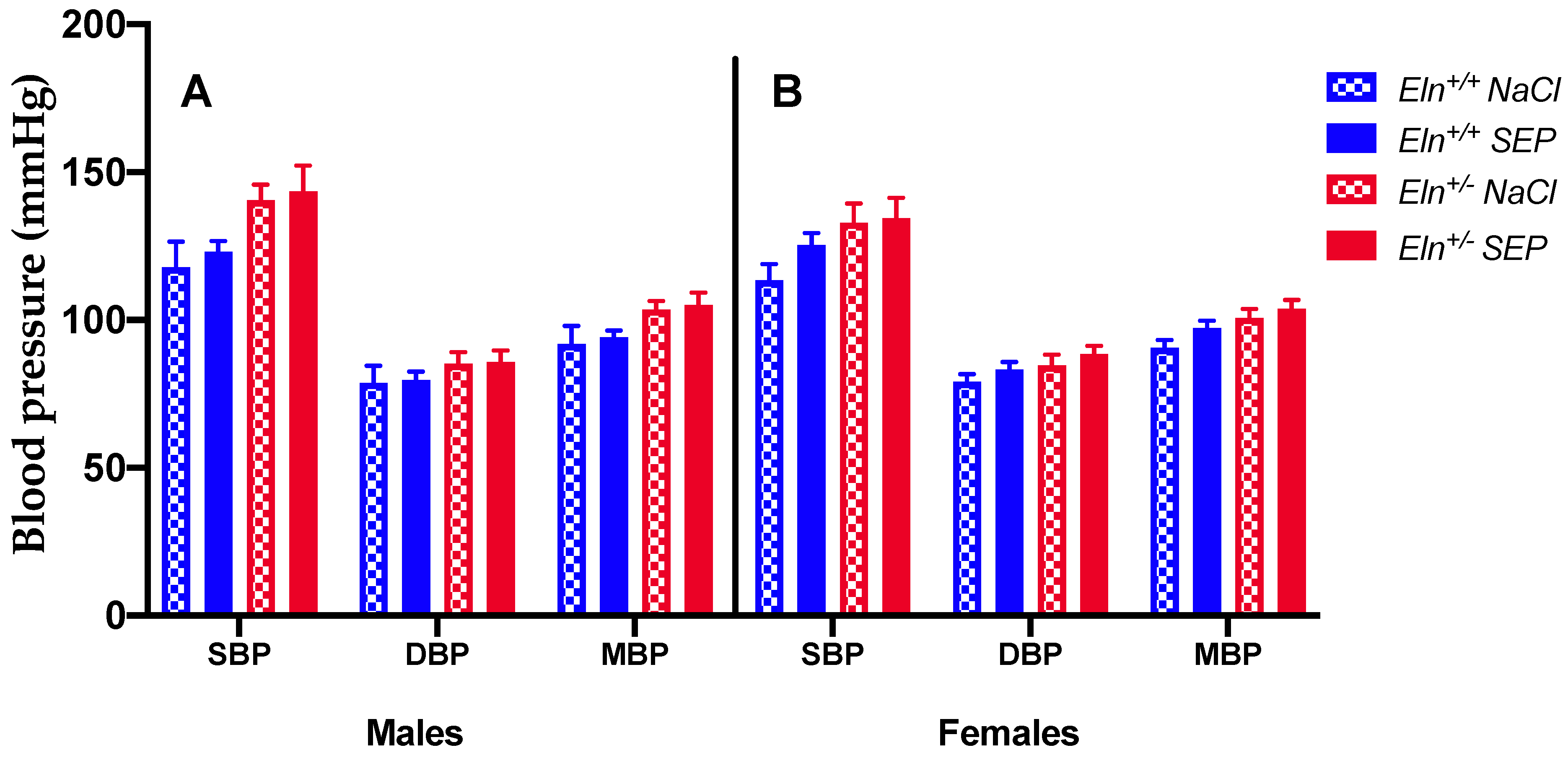
2.2. Blood Pressure
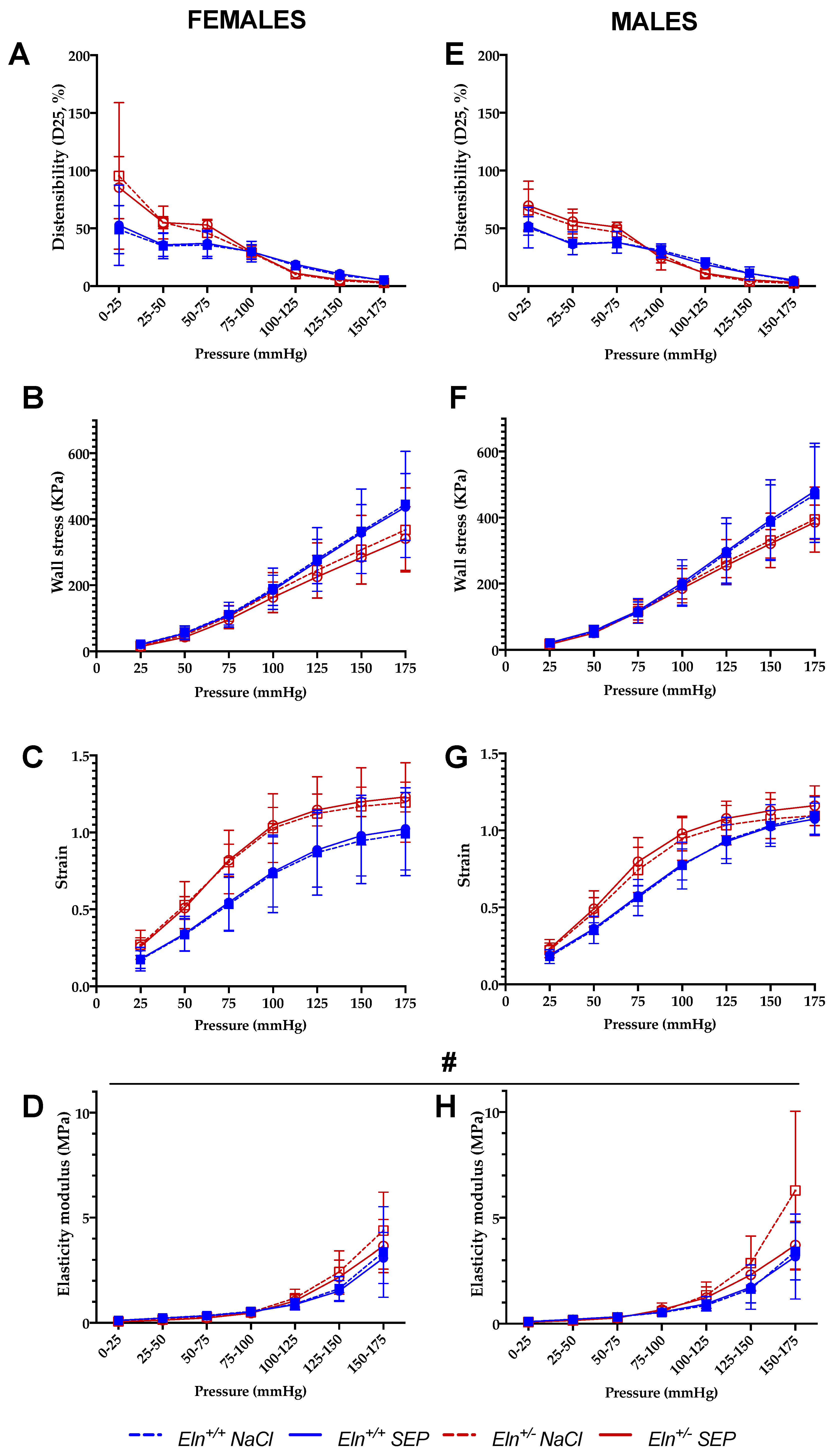
2.3. Biomechanics of the Cannulated Ascending Aorta
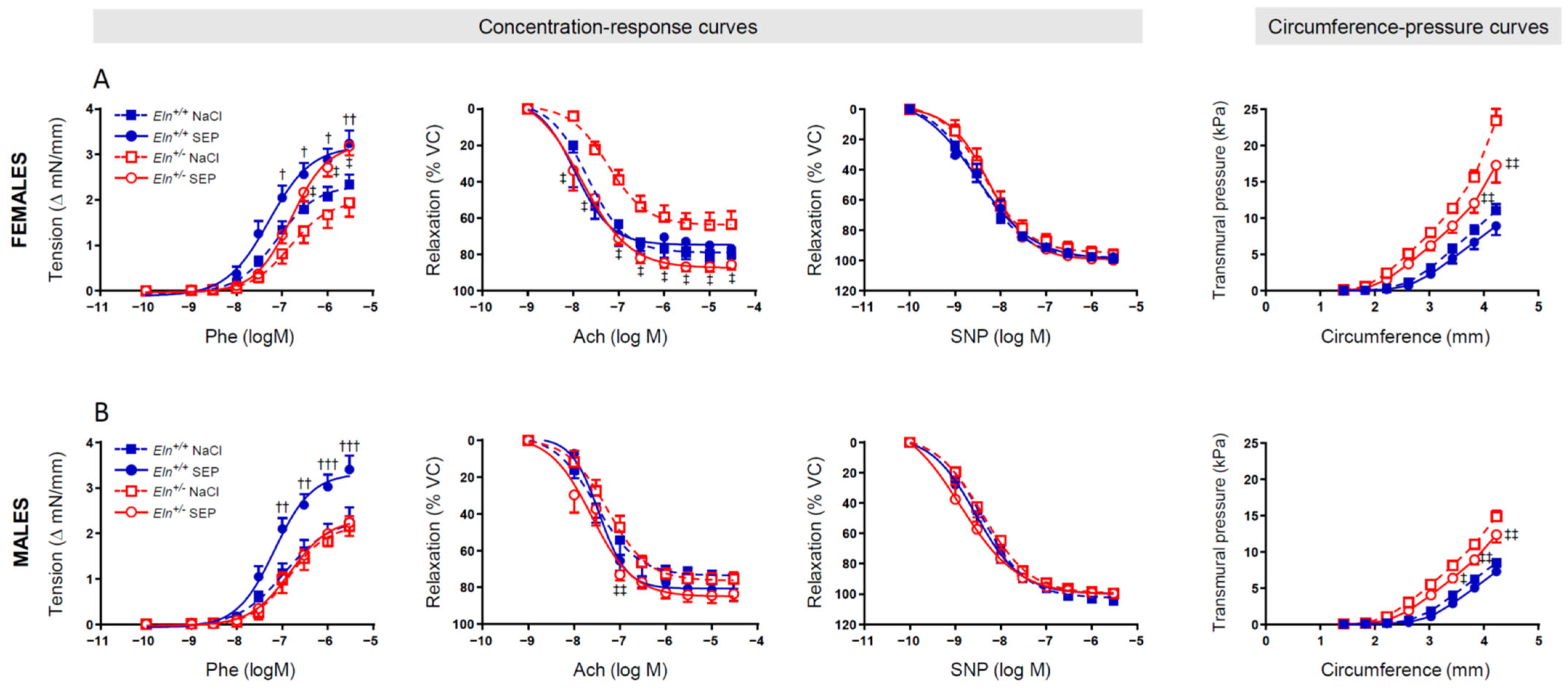
2.4. Ascending Aorta Ring Reactivity and Mechanics
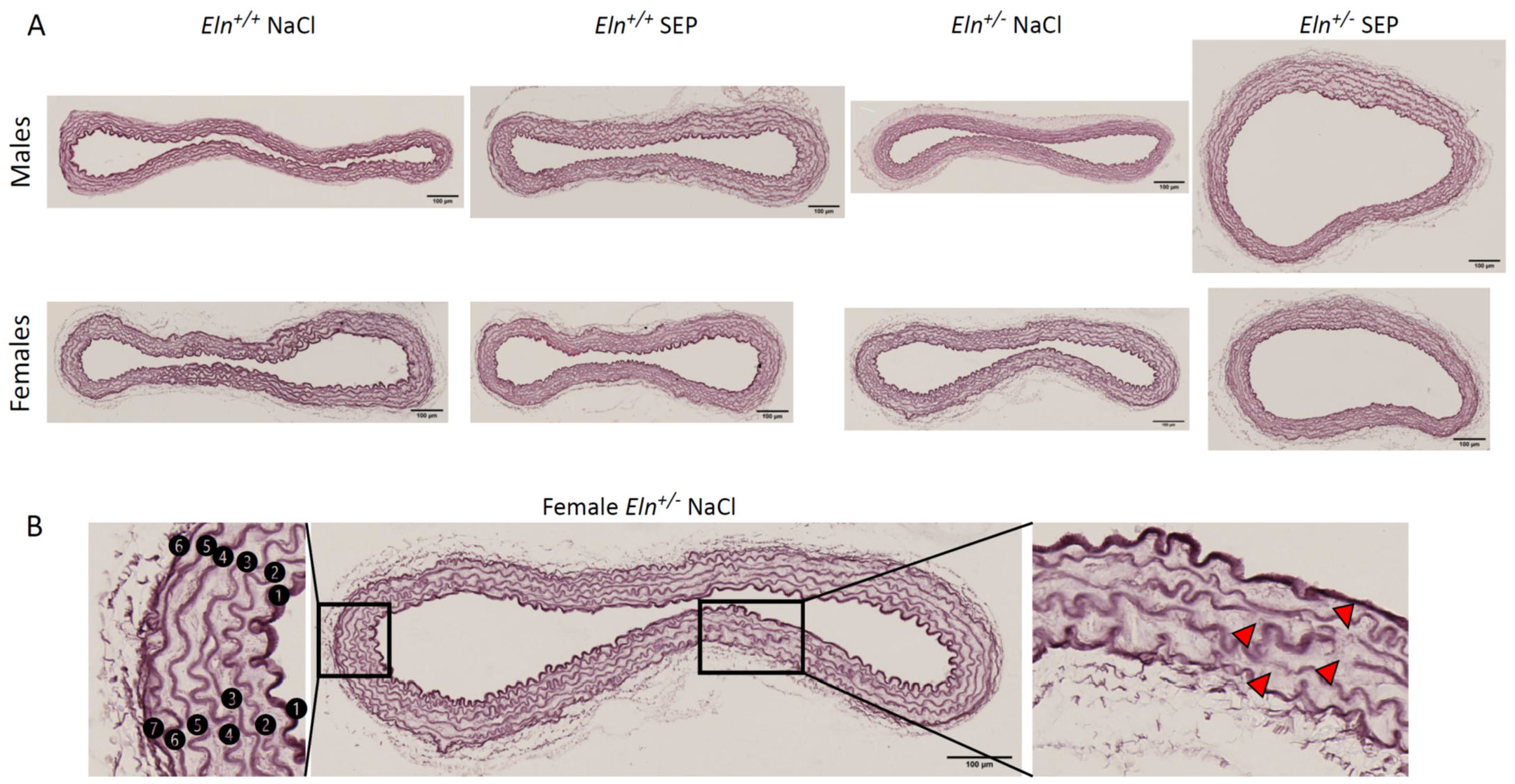
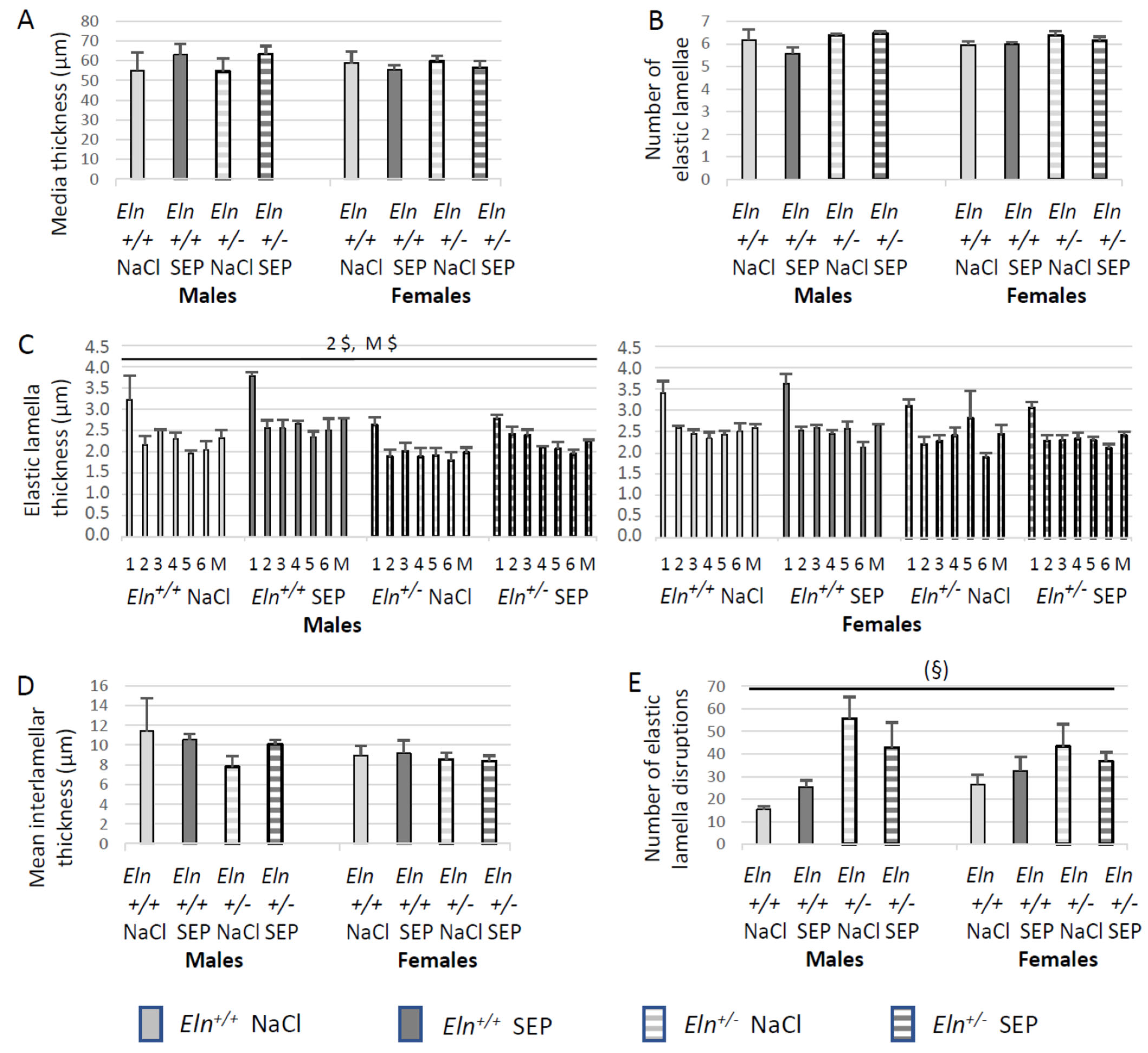
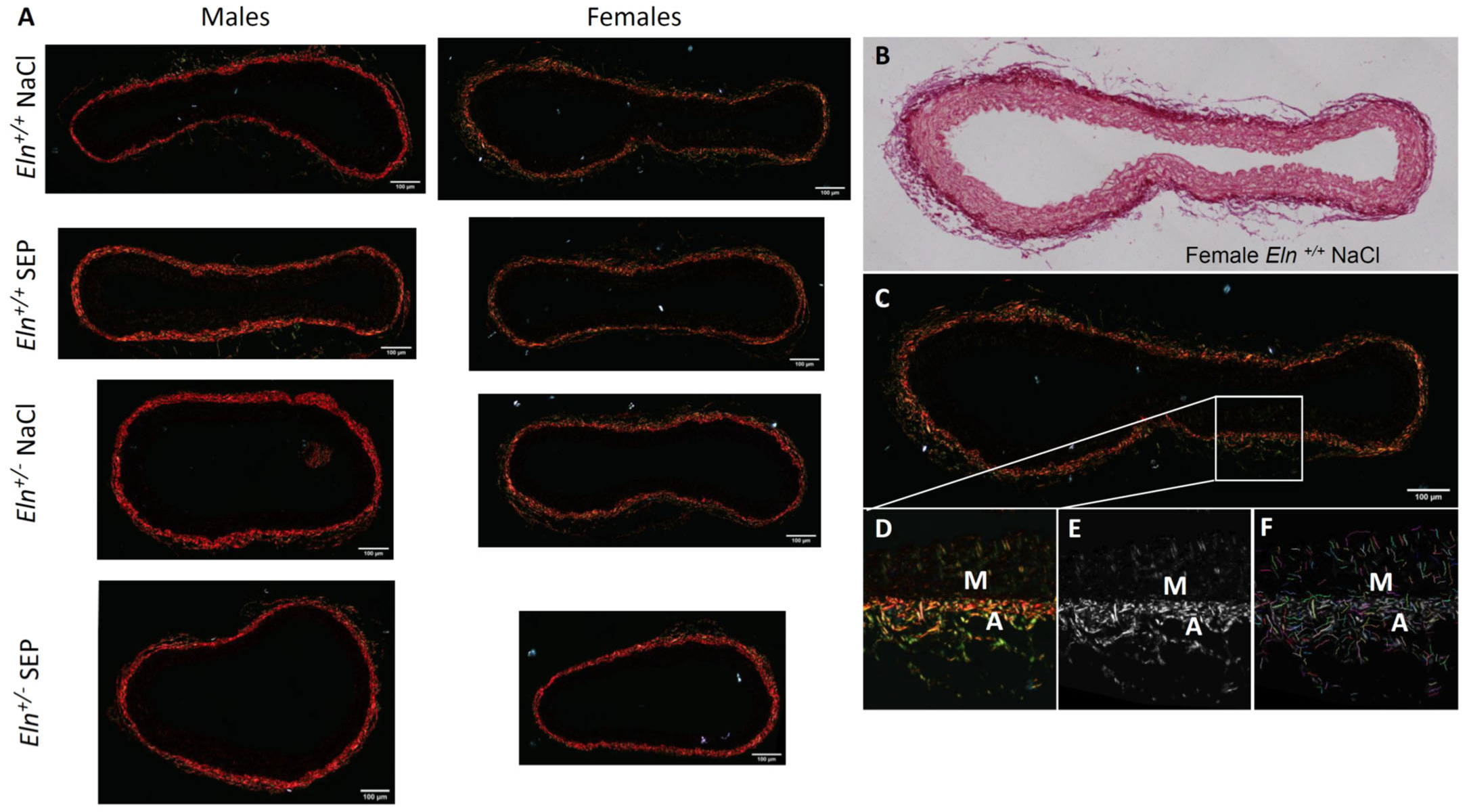
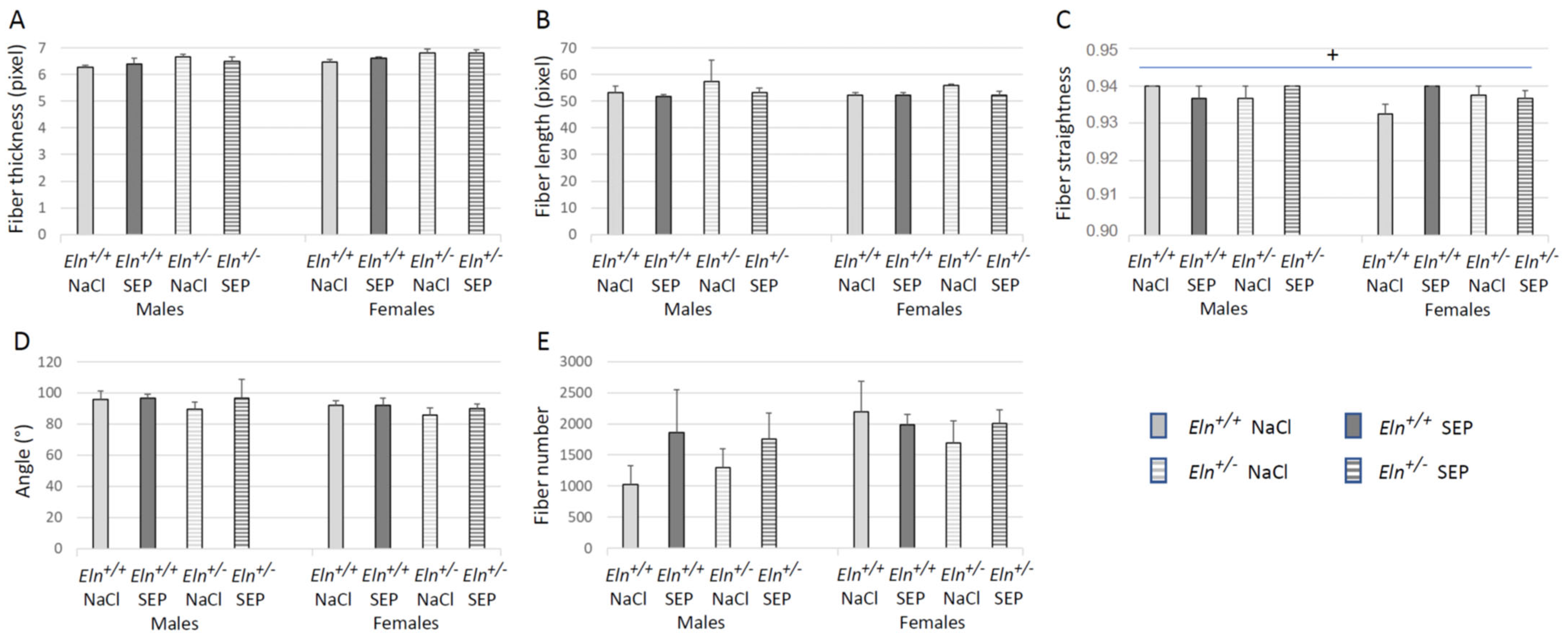
2.5. Aorta Morphology and Structure
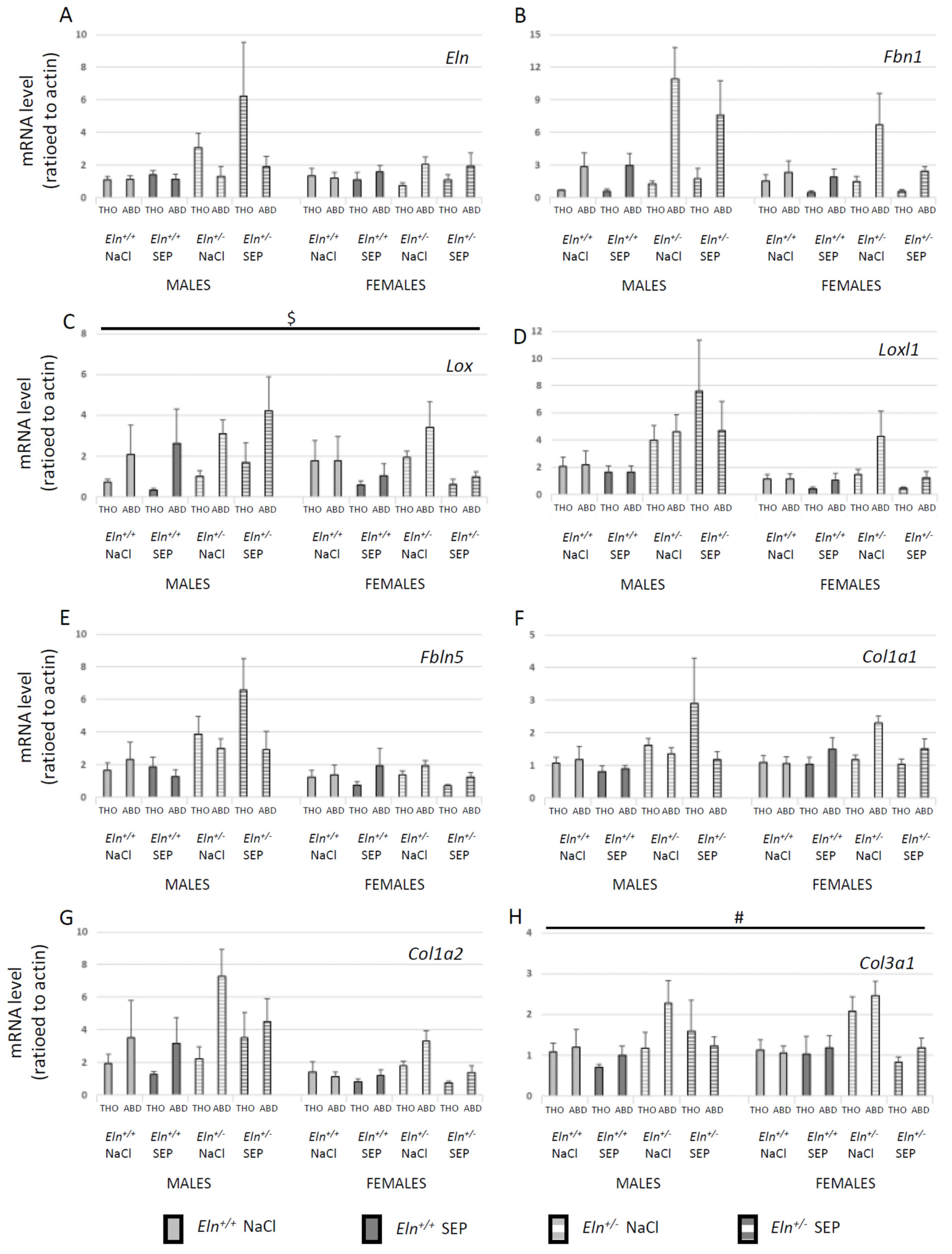
2.6. Aortic Tissue mRNA Levels
2.7. Aortic Tissue Protein Levels
2.8. VSMC Proliferation and Tropoelastin Production
3. Discussion
3.1. Differences between Eln+/+ and Eln+/- Mice
3.2. Impact of SEP Treatment on Blood Pressure and Heart
3.3. Impact of SEP Treatment on Aortic Structure and Mechanics
3.4. Mechanisms of Action of SEP on Aortic Structure and Mechanics: Facts and Hypotheses
3.5. Impact of SEP Treatment on Aortic Reactivity
3.6. Sex-Related Differences in the Response to SEP Treatment
3.7. Questions about the Presence of SEP in the Aorta Wall and Conclusions
4. Materials and Methods
4.1. Animals
4.2. SEP Primary Structure and Production
4.3. SEP Administration
4.4. Body Weight
4.5. Surgical and Post-Surgical Procedures
4.5.1. Blood Pressure
4.5.2. Heart Weight and Hematocrit
4.5.3. Cannulated Ascending Aorta Mechanics—Pressure Arteriography
4.5.4. Ascending Aorta Length, Ring Reactivity and Mechanics—Tension Arteriography
4.5.5. Histological Staining and Image Analysis
Elastic Fiber Staining—Orcein Acid (According to Shikata’s Method)
Collagen Fiber Staining—Picrosirius Red
4.5.6. mRNA Level Analyses—RT-qPCR
DNA and RNA Extraction
Reverse Transcription and Quantitative Real Time PCR Analysis
4.5.7. Western Blots
4.6. Proliferation and Elastin Production by Cultured VSMCs
4.6.1. Primary Cell Cultures
4.6.2. VSMC Proliferation—MTT Assay
4.6.3. Extracellular Elastin Quantification—ELISA Assay
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
Detailed Surgical Procedure and Methods for Mechanical Study of Cannulated Aortae
Appendix A.2
| mRNA | Forward Primer (5′−3′) | Reverse Primer (5′−3′) |
|---|---|---|
| collagen type I, alpha 1 (Col1a1) | GCGAAGGCAACAGTCGATTC | CCCAAGTTCCGGTGTGACTC |
| collagen type I, alpha 2 (Col1a2) | CAAGCATGTCTGGTTAGGAGAG | AGGACACCCCTTCTACGTTGT |
| collagen type III, alpha 1 (Col3a1) | CAGCTGGCCTTCCTCAGACTT | GCTGTTTTTGCAGTGGTATGTAATG |
| fibrillin1 (Fbn1) | TGCCAGCAGCGAGATGGACGA | TGGCGAGGCTCACGTTGGCTT |
| fibulin 5 (Fbln5) | TTGAGGAAGATGGCATTCACT | GGCTGGTTCACACACTCGT |
| lysyl oxidase (Lox) | AATTCAGCCACTATGACCTGCTTGA | GTAGCGAATGTCACAGCGTACAACA |
| lysyl oxidase-like-1 (Loxl1) | TATGCCTGCACCTCTCACAC | TGTCCGCATTGTATGTGTCAT |
| tropoelastin (Eln) | AAGCTGCTGCTAAGGCTGC | TGCAACTCCTCCACCTGGGAA |
| A: adenine, C: cytosine, G: guanine, T: thymine. | ||
References
- Tucker, W.D.; Mahajan, K. Anatomy, blood vessels. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Greenwald, S.E. Ageing of the Conduit Arteries. J. Pathol. 2007, 211, 157–172. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, M.F.; Hashimoto, J. Mechanical Factors in Arterial Aging: A Clinical Perspective. J. Am. Coll. Cardiol. 2007, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chapter 7: The Cardiovascular System: Blood Vessels and Circulation—Anatomy & Physiology. Available online: https://qut.pressbooks.pub/anatomyandphysiology/chapter/chapter-20-the-cardiovascular-system-blood-vessels-and-circulation/ (accessed on 18 May 2022).
- Behmoaras, J.; Osborne-Pellegrin, M.; Gauguier, D.; Jacob, M.-P. Characteristics of the Aortic Elastic Network and Related Phenotypes in Seven Inbred Rat Strains. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H769–H777. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Glagov, S.; Mathews, M.B. Elastin and Collagen Accumulation in Rabbit Ascending Aorta and Pulmonary Trunk during Postnatal Growth. Correlation of Cellular Synthetic Response with Medial Tension. Circ. Res. 1977, 41, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, H.; Glagov, S. Structural Basis for the Static Mechanical Properties of the Aortic Media. Circ. Res. 1964, 14, 400–413. [Google Scholar] [CrossRef]
- Wolinsky, H.; Glagov, S. A Lamellar Unit of Aortic Medial Structure and Function in Mammals. Circ. Res. 1967, 20, 99–111. [Google Scholar] [CrossRef]
- Fhayli, W.; Boëté, Q.; Harki, O.; Briançon-Marjollet, A.; Jacob, M.-P.; Faury, G. Rise and Fall of Elastic Fibers from Development to Aging. Consequences on Arterial Structure-Function and Therapeutical Perspectives. Matrix Biol. 2019, 84, 41–56. [Google Scholar] [CrossRef]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic Fibres in Health and Disease. Expert Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, C.; Beguin, P.; Lantuejoul, S.; Pepin, J.-L.; Guillermet, C.; Pelli, G.; Burger, F.; Buatois, V.; Ribuot, C.; Baguet, J.-P.; et al. The Inflammatory Preatherosclerotic Remodeling Induced by Intermittent Hypoxia Is Attenuated by RANTES/CCL5 Inhibition. Am. J. Respir. Crit. Care Med. 2011, 184, 724–731. [Google Scholar] [CrossRef]
- Pezet, M.; Jacob, M.-P.; Escoubet, B.; Gheduzzi, D.; Tillet, E.; Perret, P.; Huber, P.; Quaglino, D.; Vranckx, R.; Li, D.Y.; et al. Elastin Haploinsufficiency Induces Alternative Aging Processes in the Aorta. Rejuvenat. Res. 2008, 11, 97–112. [Google Scholar] [CrossRef]
- Mariko, B.; Pezet, M.; Escoubet, B.; Bouillot, S.; Andrieu, J.-P.; Starcher, B.; Quaglino, D.; Jacob, M.-P.; Huber, P.; Ramirez, F.; et al. Fibrillin-1 Genetic Deficiency Leads to Pathological Ageing of Arteries in Mice. J. Pathol. 2011, 224, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Duque Lasio, M.L.; Kozel, B.A. Elastin-Driven Genetic Diseases. Matrix Biol. 2018, 71, 144–160. [Google Scholar] [CrossRef] [PubMed]
- Kassai, B.; Bouyé, P.; Gilbert-Dussardier, B.; Godart, F.; Thambo, J.-B.; Rossi, M.; Cochat, P.; Chirossel, P.; Luong, S.; Serusclat, A.; et al. Minoxidil versus Placebo in the Treatment of Arterial Wall Hypertrophy in Children with Williams Beuren Syndrome: A Randomized Controlled Trial. BMC Pediatr. 2019, 19, 170. [Google Scholar] [CrossRef]
- Curran, M.E.; Atkinson, D.L.; Ewart, A.K.; Morris, C.A.; Leppert, M.F.; Keating, M.T. The Elastin Gene Is Disrupted by a Translocation Associated with Supravalvular Aortic Stenosis. Cell 1993, 73, 159–168. [Google Scholar] [CrossRef]
- Ewart, A.K.; Morris, C.A.; Atkinson, D.; Jin, W.; Sternes, K.; Spallone, P.; Stock, A.D.; Leppert, M.; Keating, M.T. Hemizygosity at the Elastin Locus in a Developmental Disorder, Williams Syndrome. Nat. Genet. 1993, 5, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Faury, G.; Taylor, D.G.; Davis, E.C.; Boyle, W.A.; Mecham, R.P.; Stenzel, P.; Boak, B.; Keating, M.T. Novel Arterial Pathology in Mice and Humans Hemizygous for Elastin. J. Clin. Investig. 1998, 102, 1783–1787. [Google Scholar] [CrossRef]
- Faury, G.; Pezet, M.; Knutsen, R.H.; Boyle, W.A.; Heximer, S.P.; McLean, S.E.; Minkes, R.K.; Blumer, K.J.; Kovacs, A.; Kelly, D.P.; et al. Developmental Adaptation of the Mouse Cardiovascular System to Elastin Haploinsufficiency. J. Clin. Investig. 2003, 112, 1419–1428. [Google Scholar] [CrossRef]
- Sproul, E.P.; Argraves, W.S. A Cytokine Axis Regulates Elastin Formation and Degradation. Matrix Biol. 2013, 32, 86–94. [Google Scholar] [CrossRef]
- Hayashi, A.; Suzuki, T.; Wachi, H.; Tajima, S.; Nishikawa, T.; Murad, S.; Pinnell, S.R. Minoxidil Stimulates Elastin Expression in Aortic Smooth Muscle Cells. Arch. Biochem. Biophys. 1994, 315, 137–141. [Google Scholar] [CrossRef]
- Tokimitsu, I.; Tajima, S. Inhibition of Elastin Synthesis by High Potassium Salt Is Mediated by Ca2+ Influx in Cultured Smooth Muscle Cells in Vitro: Reciprocal Effects of K+ on Elastin and Collagen Synthesis. J. Biochem. 1994, 115, 536–539. [Google Scholar] [CrossRef]
- Lannoy, M.; Slove, S.; Louedec, L.; Choqueux, C.; Journé, C.; Michel, J.-B.; Jacob, M.-P. Inhibition of ERK1/2 Phosphorylation: A New Strategy to Stimulate Elastogenesis in the Aorta. Hypertension 2014, 64, 423–430. [Google Scholar] [CrossRef]
- Tsoporis, J.; Keeley, F.W.; Lee, R.M.; Leenen, F.H. Arterial Vasodilation and Vascular Connective Tissue Changes in Spontaneously Hypertensive Rats. J. Cardiovasc. Pharmacol. 1998, 31, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, R.H.; Beeman, S.C.; Broekelmann, T.J.; Liu, D.; Tsang, K.M.; Kovacs, A.; Ye, L.; Danback, J.R.; Watson, A.; Wardlaw, A.; et al. Minoxidil Improves Vascular Compliance, Restores Cerebral Blood Flow, and Alters Extracellular Matrix Gene Expression in a Model of Chronic Vascular Stiffness. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H18–H32. [Google Scholar] [CrossRef]
- Slove, S.; Lannoy, M.; Behmoaras, J.; Pezet, M.; Sloboda, N.; Lacolley, P.; Escoubet, B.; Buján, J.; Jacob, M.-P. Potassium Channel Openers Increase Aortic Elastic Fiber Formation and Reverse the Genetically Determined Elastin Deficit in the BN Rat. Hypertension 2013, 62, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Coquand-Gandit, M.; Jacob, M.-P.; Fhayli, W.; Romero, B.; Georgieva, M.; Bouillot, S.; Estève, E.; Andrieu, J.-P.; Brasseur, S.; Bouyon, S.; et al. Chronic Treatment with Minoxidil Induces Elastic Fiber Neosynthesis and Functional Improvement in the Aorta of Aged Mice. Rejuvenat. Res. 2017, 20, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Fhayli, W.; Boyer, M.; Ghandour, Z.; Jacob, M.P.; Andrieu, J.P.; Starcher, B.C.; Estève, E.; Faury, G. Chronic Administration of Minoxidil Protects Elastic Fibers and Stimulates Their Neosynthesis with Improvement of the Aorta Mechanics in Mice. Cell Signal 2019, 62, 109333. [Google Scholar] [CrossRef]
- Raveaud, S.; Mezin, P.; Lavanchy, N.; Starcher, B.; Mecham, R.P.; Verdetti, J.; Faury, G. Effects of Chronic Treatment with a Low Dose of Nicorandil on the Function of the Rat Aorta during Ageing. Clin. Exp. Pharmacol. Physiol. 2009, 36, 988–994. [Google Scholar] [CrossRef]
- Bouhedja, M.; Peres, B.; Fhayli, W.; Ghandour, Z.; Boumendjel, A.; Faury, G.; Khelili, S. Design, Synthesis and Biological Evaluation of Novel Ring-Opened Cromakalim Analogues with Relaxant Effects on Vascular and Respiratory Smooth Muscles and as Stimulators of Elastin Synthesis. Eur. J. Med. Chem. 2018, 144, 774–796. [Google Scholar] [CrossRef]
- Bouider, N.; Fhayli, W.; Ghandour, Z.; Boyer, M.; Harrouche, K.; Florence, X.; Pirotte, B.; Lebrun, P.; Faury, G.; Khelili, S. Design and Synthesis of New Potassium Channel Activators Derived from the Ring Opening of Diazoxide: Study of Their Vasodilatory Effect, Stimulation of Elastin Synthesis and Inhibitory Effect on Insulin Release. Bioorg. Med. Chem. 2015, 23, 1735–1746. [Google Scholar] [CrossRef]
- Fhayli, W.; Boëté, Q.; Kihal, N.; Cenizo, V.; Sommer, P.; Boyle, W.A.; Jacob, M.-P.; Faury, G. Dill Extract Induces Elastic Fiber Neosynthesis and Functional Improvement in the Ascending Aorta of Aged Mice with Reversal of Age-Dependent Cardiac Hypertrophy and Involvement of Lysyl Oxidase-Like-1. Biomolecules 2020, 10, 173. [Google Scholar] [CrossRef]
- Nonaka, R.; Sato, F.; Wachi, H. Domain 36 of Tropoelastin in Elastic Fiber Formation. Biol. Pharm. Bull. 2014, 37, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Bax, D.V.; Rodgers, U.R.; Bilek, M.M.M.; Weiss, A.S. Cell Adhesion to Tropoelastin Is Mediated via the C-Terminal GRKRK Motif and Integrin AlphaVbeta3. J. Biol. Chem. 2009, 284, 28616–28623. [Google Scholar] [CrossRef] [PubMed]
- Faury, G. Role of the Elastin-Laminin Receptor in the Cardiovascular System. Pathol. Biol. 1998, 46, 517–526. [Google Scholar] [PubMed]
- Rodgers, U.R.; Weiss, A.S. Integrin Alpha v Beta 3 Binds a Unique Non-RGD Site near the C-Terminus of Human Tropoelastin. Biochimie 2004, 86, 173–178. [Google Scholar] [CrossRef]
- Wahart, A.; Hocine, T.; Albrecht, C.; Henry, A.; Sarazin, T.; Martiny, L.; El Btaouri, H.; Maurice, P.; Bennasroune, A.; Romier-Crouzet, B.; et al. Role of Elastin Peptides and Elastin Receptor Complex in Metabolic and Cardiovascular Diseases. FEBS J. 2019, 286, 2980–2993. [Google Scholar] [CrossRef]
- Yamashiro, Y.; Yanagisawa, H. The Molecular Mechanism of Mechanotransduction in Vascular Homeostasis and Disease. Clin. Sci. 2020, 134, 2399–2418. [Google Scholar] [CrossRef]
- Chen, J.; Yu, J.; Yuan, R.; Li, N.; Li, C.; Zhang, X. MTOR Inhibitor Improves Testosterone-Induced Myocardial Hypertrophy in Hypertensive Rats. J. Endocrinol. 2021, 252, 179–193. [Google Scholar] [CrossRef]
- Robinet, A.; Millart, H.; Oszust, F.; Hornebeck, W.; Bellon, G. Binding of Elastin Peptides to S-Gal Protects the Heart against Ischemia/Reperfusion Injury by Triggering the RISK Pathway. FASEB J. 2007, 21, 1968–1978. [Google Scholar] [CrossRef]
- Azevedo, J.; Arroja, I.; Jacques, A.; Santos, I.; Amado, P.; Marques, J.C.; Araújo, V. A double ambulatory product (blood pressure and heart rate), mild arterial hypertension and left ventricular hypertrophy. Rev. Port. Cardiol. 1993, 12, 663–673, 602. [Google Scholar]
- Sahu, S.P.; Liu, Q.; Prasad, A.; Hasan, S.M.A.; Liu, Q.; Rodriguez, M.X.B.; Mukhopadhyay, O.; Burk, D.; Francis, J.; Mukhopadhyay, S.; et al. Characterization of Fibrillar Collagen Isoforms in Infarcted Mouse Hearts Using Second Harmonic Generation Imaging. Biomed. Opt. Express 2020, 12, 604–618. [Google Scholar] [CrossRef]
- McNulty, M.; Mahmud, A.; Spiers, P.; Feely, J. Collagen Type-I Degradation Is Related to Arterial Stiffness in Hypertensive and Normotensive Subjects. J. Hum. Hypertens. 2006, 20, 867–873. [Google Scholar] [CrossRef]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin Is an Essential Determinant of Arterial Morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Urbán, Z.; Riazi, S.; Seidl, T.L.; Katahira, J.; Smoot, L.B.; Chitayat, D.; Boyd, C.D.; Hinek, A. Connection between Elastin Haploinsufficiency and Increased Cell Proliferation in Patients with Supravalvular Aortic Stenosis and Williams-Beuren Syndrome. Am. J. Hum. Genet. 2002, 71, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Karnik, S.K.; Brooke, B.S.; Bayes-Genis, A.; Sorensen, L.; Wythe, J.D.; Schwartz, R.S.; Keating, M.T.; Li, D.Y. A Critical Role for Elastin Signaling in Vascular Morphogenesis and Disease. Development 2003, 130, 411–423. [Google Scholar] [CrossRef]
- Li, W.; Li, Q.; Qin, L.; Ali, R.; Qyang, Y.; Tassabehji, M.; Pober, B.R.; Sessa, W.C.; Giordano, F.J.; Tellides, G. Rapamycin Inhibits Smooth Muscle Cell Proliferation and Obstructive Arteriopathy Attributable to Elastin Deficiency. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1028–1035. [Google Scholar] [CrossRef]
- Wachi, H.; Seyama, Y.; Yamashita, S.; Suganami, H.; Uemura, Y.; Okamoto, K.; Yamada, H.; Tajima, S. Stimulation of Cell Proliferation and Autoregulation of Elastin Expression by Elastin Peptide VPGVG in Cultured Chick Vascular Smooth Muscle Cells. FEBS Lett. 1995, 368, 215–219. [Google Scholar] [CrossRef]
- Debret, R.; Faye, C.; Sohier, J.; Sommer, P. Polypeptide Derive de la Tropoelastine et Materiau Biocompatible le Comprenant. WIPO Patent WO2017/194761 A, 16 November 2017. [Google Scholar]
- Le Page, A.; Khalil, A.; Vermette, P.; Frost, E.H.; Larbi, A.; Witkowski, J.M.; Fulop, T. The Role of Elastin-Derived Peptides in Human Physiology and Diseases. Matrix Biol. 2019, 84, 81–96. [Google Scholar] [CrossRef]
- Karnik, S.K.; Wythe, J.D.; Sorensen, L.; Brooke, B.S.; Urness, L.D.; Li, D.Y. Elastin Induces Myofibrillogenesis via a Specific Domain, VGVAPG. Matrix Biol. 2003, 22, 409–425. [Google Scholar] [CrossRef]
- Owens, E.A.; Jie, L.; Reyes, B.A.S.; Van Bockstaele, E.J.; Osei-Owusu, P. Elastin Insufficiency Causes Hypertension, Structural Defects and Abnormal Remodeling of Renal Vascular Signaling. Kidney Int. 2017, 92, 1100–1118. [Google Scholar] [CrossRef]
- Faury, G.; Ristori, M.T.; Verdetti, J.; Jacob, M.P.; Robert, L. Role of the elastin-laminin receptor in the vasoregulation. C. R. Acad. Sci. III 1994, 317, 807–811. [Google Scholar] [PubMed]
- Faury, G.; Ristori, M.T.; Verdetti, J.; Jacob, M.P.; Robert, L. Effect of Elastin Peptides on Vascular Tone. J. Vasc. Res. 1995, 32, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Faury, G.; Chabaud, A.; Ristori, M.T.; Robert, L.; Verdetti, J. Effect of Age on the Vasodilatory Action of Elastin Peptides. Mech. Ageing Dev. 1997, 95, 31–42. [Google Scholar] [CrossRef]
- Faury, G.; Garnier, S.; Weiss, A.S.; Wallach, J.; Fülöp, T.; Jacob, M.P.; Mecham, R.P.; Robert, L.; Verdetti, J. Action of Tropoelastin and Synthetic Elastin Sequences on Vascular Tone and on Free Ca2+ Level in Human Vascular Endothelial Cells. Circ. Res. 1998, 82, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Chilkoti, A. Purification of Recombinant Proteins by Fusion with Thermally-Responsive Polypeptides. Nat. Biotechnol. 1999, 17, 1112–1115. [Google Scholar] [CrossRef]
- Faury, G.; Maher, G.M.; Li, D.Y.; Keating, M.T.; Mecham, R.P.; Boyle, W.A. Relation between Outer and Luminal Diameter in Cannulated Arteries. Am. J. Physiol. 1999, 277, H1745–H1753. [Google Scholar] [CrossRef]
- Gibbons, C.A.; Shadwick, R.E. Functional Similarities in the Mechanical Design of the Aorta in Lower Vertebrates and Mammals. Experientia 1989, 45, 1083–1088. [Google Scholar] [CrossRef]
- Liu, K.L.; Canaple, L.; Del Carmine, P.; Gauthier, K.; Beylot, M.; Lo, M. Thyroid Hormone Receptor-α Deletion Decreases Heart Function and Exercise Performance in Apolipoprotein E-Deficient Mice. Physiol. Genom. 2016, 48, 73–81. [Google Scholar] [CrossRef]
- Shikata, T.; Uzawa, T.; Yoshiwara, N.; Akatsuka, T.; Yamazaki, S. Staining Methods of Australia Antigen in Paraffin Section—Detection of Cytoplasmic Inclusion Bodies. Jpn. J. Exp. Med. 1974, 44, 25–36. [Google Scholar]
- Henwood, T. Shikata’s Orcein Stain—A Routine Stain for Liver Biopsies. Aust. J. Med. Lab. Sci. 1983, 4, 76–80. [Google Scholar]
- Bauman, T.M.; Nicholson, T.M.; Abler, L.L.; Eliceiri, K.W.; Huang, W.; Vezina, C.M.; Ricke, W.A. Characterization of Fibrillar Collagens and Extracellular Matrix of Glandular Benign Prostatic Hyperplasia Nodules. PLoS ONE 2014, 9, e109102. [Google Scholar] [CrossRef]
- Bredfeldt, J.S.; Liu, Y.; Pehlke, C.A.; Conklin, M.W.; Szulczewski, J.M.; Inman, D.R.; Keely, P.J.; Nowak, R.D.; Mackie, T.R.; Eliceiri, K.W. Computational Segmentation of Collagen Fibers from Second-Harmonic Generation Images of Breast Cancer. J. Biomed. Opt. 2014, 19, 016007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Keikhosravi, A.; Mehta, G.S.; Drifka, C.R.; Eliceiri, K.W. Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol. Biol. 2017, 1627, 429–451. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Keikhosravi, A.; Pehlke, C.A.; Bredfeldt, J.S.; Dutson, M.; Liu, H.; Mehta, G.S.; Claus, R.; Patel, A.J.; Conklin, M.W.; et al. Fibrillar Collagen Quantification With Curvelet Transform Based Computational Methods. Front. Bioeng. Biotechnol. 2020, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- CT-FIRE. Available online: https://eliceirilab.org/software/ctfire/ (accessed on 17 March 2022).
- Smith, J.J.; Kampine, J.P. Circulatory Physiology: The Essentials, 3rd ed.; Williams & Wilkins: Baltimore, MD, USA, 1990; ISBN 978-0-683-07775-9. [Google Scholar]



| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Eln+/+ | Eln+/- | Eln+/+ | Eln+/- | |||||
| NaCl | SEP | NaCl | SEP | NaCl | SEP | NaCl | SEP | |
| BW (g) | 34.3 ± 1.2 | 34.7 ± 1.1 | 36.2 ± 1.6 | 34.1 ± 1.0 | 26.6 ± 0.8 | 25.7 ± 0.7 | 27.5 ± 1.2 | 27.3 ± 1.5 |
| Hematocrit (%) & | 37.8 ± 1.4 | 38.7 ± 0.8 | 39.4 ± 1.3 | 40.7 ± 1.4 | 39.9 ± 1.0 | 37.9 ± 1.8 | 40.0 ± 1.8 | 35.7 ± 1.3 |
| HW/BW (%) $ | 0.46 ± 0.01 | 0.48 ± 0.01 | 0.48 ± 0.02 | 0.52 ± 0.01 | 0.47 ± 0.02 | 0.45 ± 0.01 | 0.49 ± 0.02 | 0.47 ± 0.02 |
| LV+S/BW (%) $ | 0.35 ± 0.01 | 0.37 ± 0.01 | 0.36 ± 0.02 | 0.41 ± 0.01 | 0.35 ± 0.01 | 0.32 ± 0.01 | 0.37± 0.01 | 0.35 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boëté, Q.; Lo, M.; Liu, K.-L.; Vial, G.; Lemarié, E.; Rougelot, M.; Steuckardt, I.; Harki, O.; Couturier, A.; Gaucher, J.; et al. Physiological Impact of a Synthetic Elastic Protein in Arterial Diseases Related to Alterations of Elastic Fibers: Effect on the Aorta of Elastin-Haploinsufficient Male and Female Mice. Int. J. Mol. Sci. 2022, 23, 13464. https://doi.org/10.3390/ijms232113464
Boëté Q, Lo M, Liu K-L, Vial G, Lemarié E, Rougelot M, Steuckardt I, Harki O, Couturier A, Gaucher J, et al. Physiological Impact of a Synthetic Elastic Protein in Arterial Diseases Related to Alterations of Elastic Fibers: Effect on the Aorta of Elastin-Haploinsufficient Male and Female Mice. International Journal of Molecular Sciences. 2022; 23(21):13464. https://doi.org/10.3390/ijms232113464
Chicago/Turabian StyleBoëté, Quentin, Ming Lo, Kiao-Ling Liu, Guillaume Vial, Emeline Lemarié, Maxime Rougelot, Iris Steuckardt, Olfa Harki, Axel Couturier, Jonathan Gaucher, and et al. 2022. "Physiological Impact of a Synthetic Elastic Protein in Arterial Diseases Related to Alterations of Elastic Fibers: Effect on the Aorta of Elastin-Haploinsufficient Male and Female Mice" International Journal of Molecular Sciences 23, no. 21: 13464. https://doi.org/10.3390/ijms232113464
APA StyleBoëté, Q., Lo, M., Liu, K.-L., Vial, G., Lemarié, E., Rougelot, M., Steuckardt, I., Harki, O., Couturier, A., Gaucher, J., Bouyon, S., Demory, A., Boutin-Paradis, A., El Kholti, N., Berthier, A., Pépin, J.-L., Briançon-Marjollet, A., Lambert, E., Debret, R., & Faury, G. (2022). Physiological Impact of a Synthetic Elastic Protein in Arterial Diseases Related to Alterations of Elastic Fibers: Effect on the Aorta of Elastin-Haploinsufficient Male and Female Mice. International Journal of Molecular Sciences, 23(21), 13464. https://doi.org/10.3390/ijms232113464






