The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities
Abstract
1. Introduction
2. Results
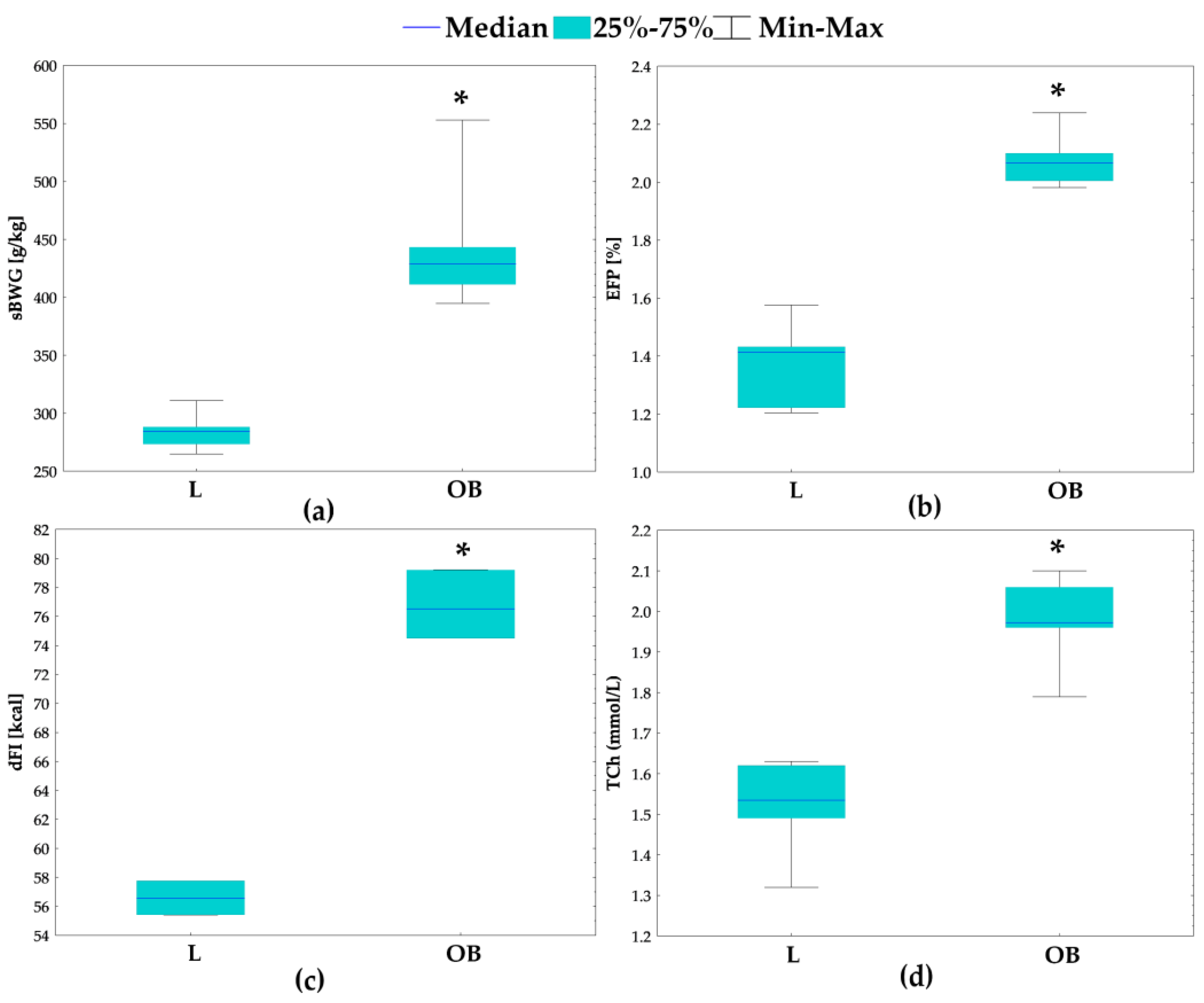
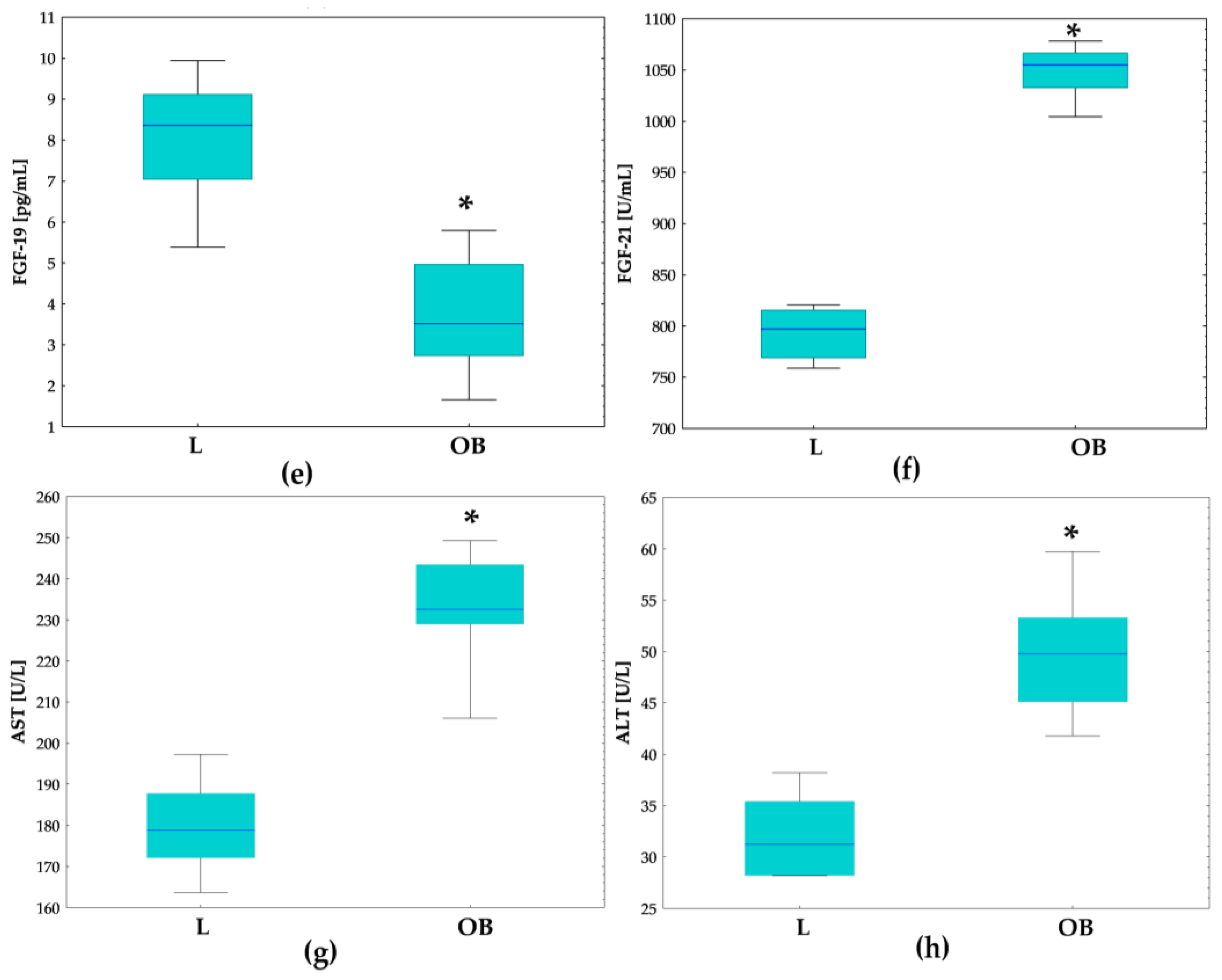
2.1. Physiological and Metabolic Parameters
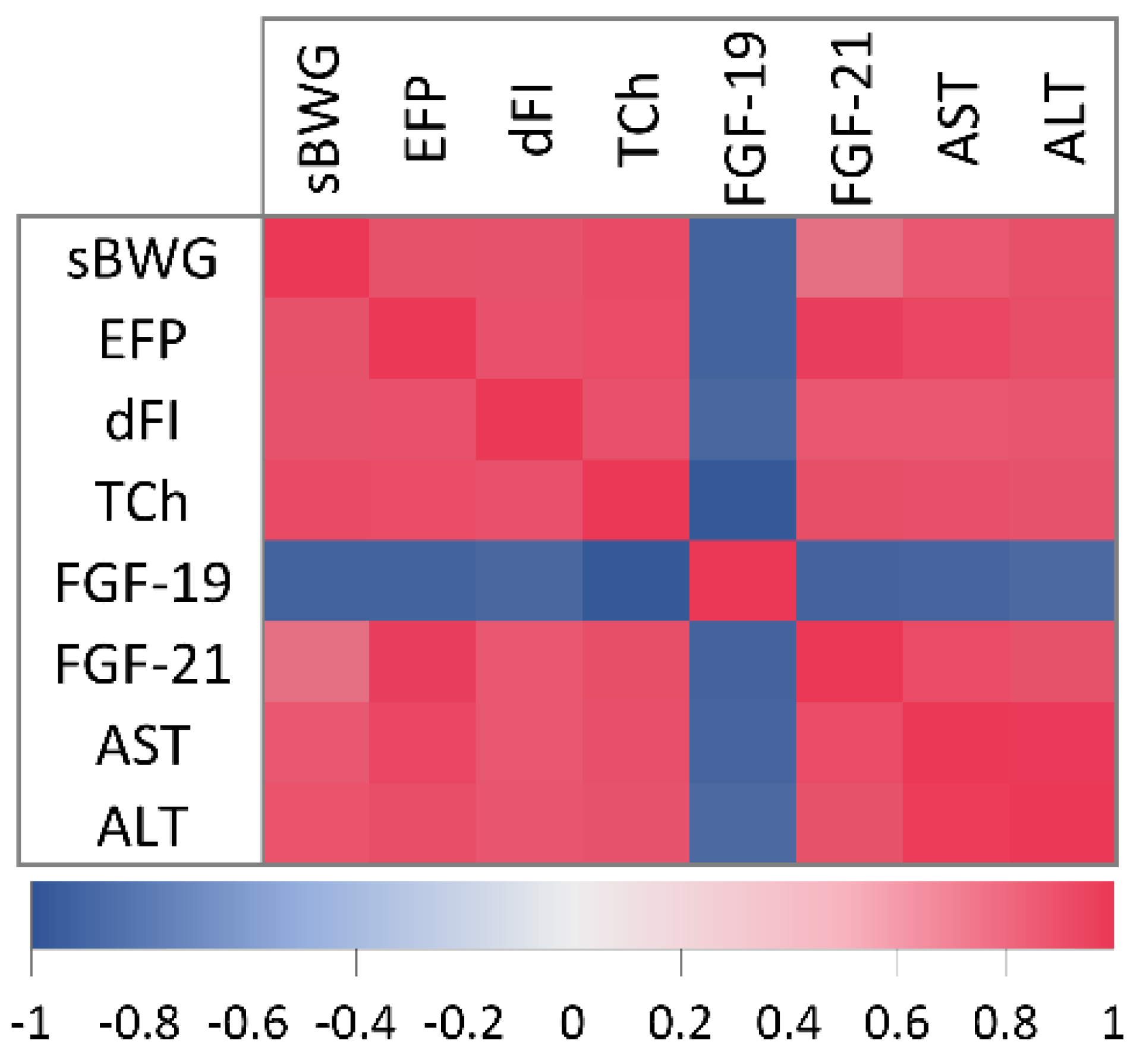
2.2. Correlation Analysis of Physiological and Metabolic Parameters
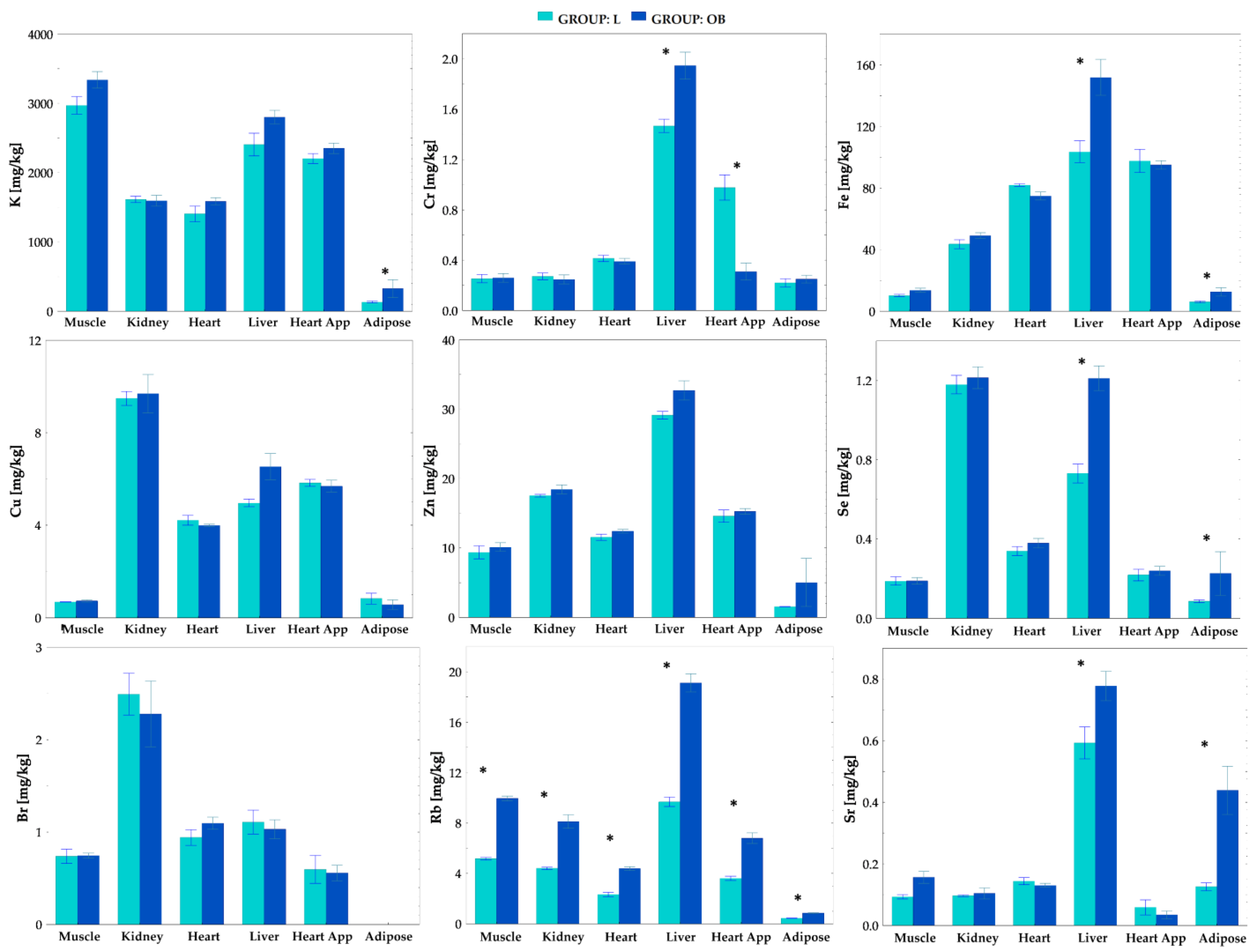
2.3. Tissue Elemental Concentrations
2.4. Intake of Elements with Food Consumption
2.5. Multivariate Discriminant Analysis—Complex Elemental Differentation of Lean and Obese Animals
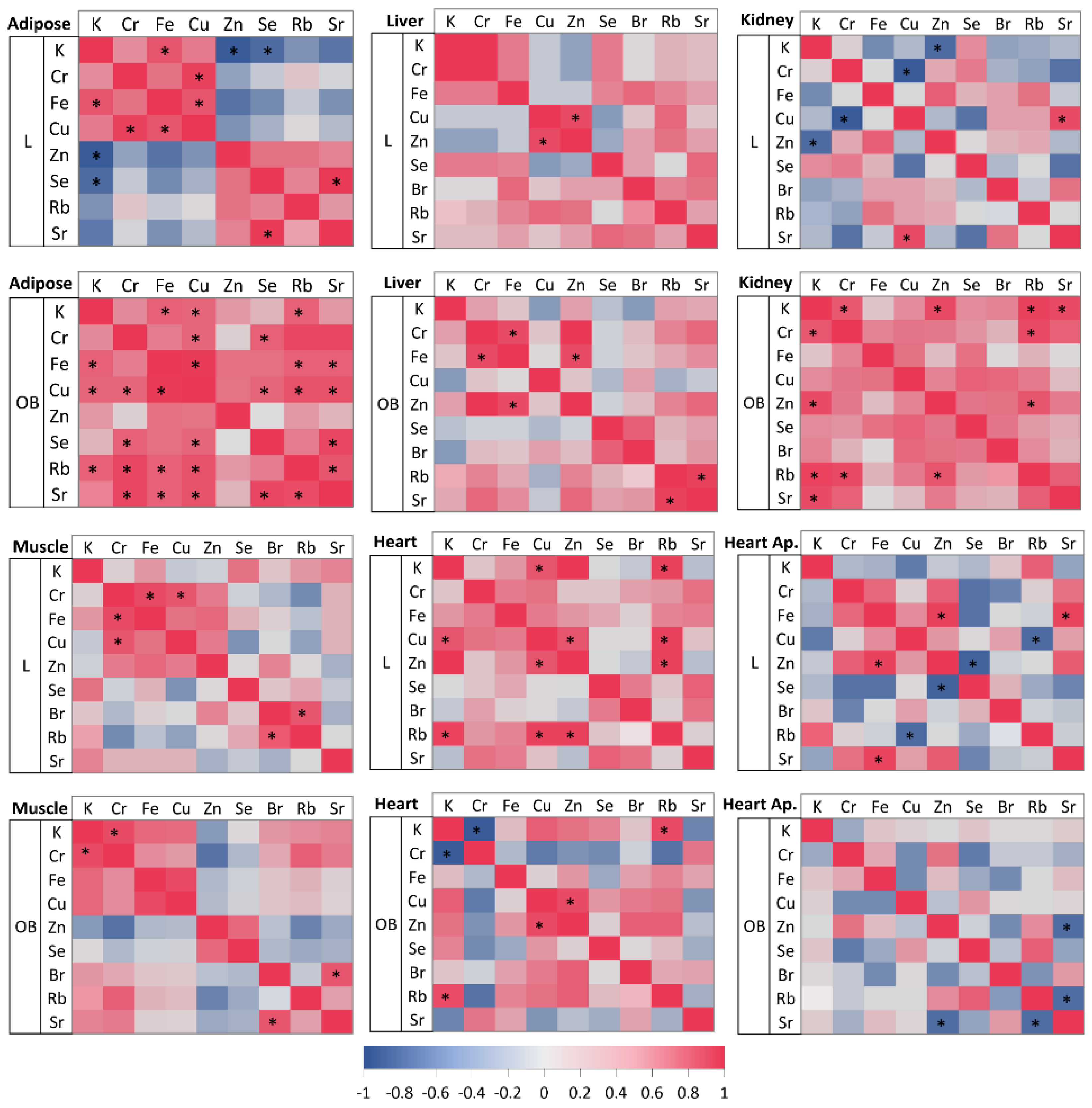
2.6. Intragroup Elemental Relationships
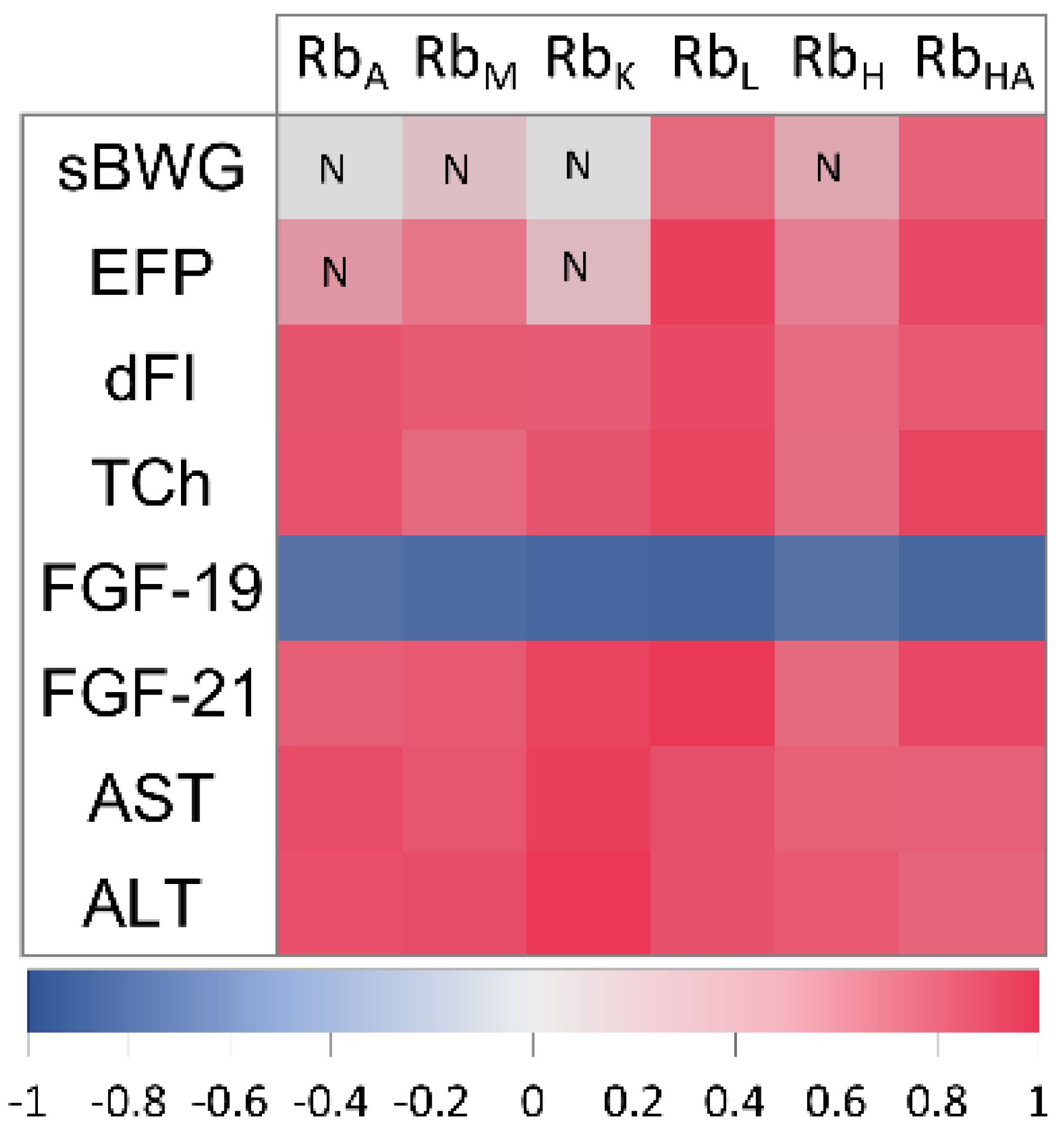
2.7. Correlation of Rb with Physiological and Metabolic Parameters
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Body Weight and Feed Intake Measurements
4.3. Biochemical Analysis of Some Blood Metabolic Parameters
4.4. Tissue Sample Preparation for the TXRF Analysis
4.5. Data Treatment and Statistical Analysis
4.6. Discriminant Analysis Based on of Tissue Elemental Concentration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Study Limitations
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Endalifer, M.L.; Diress, G. Epidemiology, Predisposing Factors, Biomarkers, and Prevention Mechanism of Obesity: A Systematic Review. J. Obes. 2020, 2020, 6134362. [Google Scholar] [CrossRef] [PubMed]
- Varda, N.M.; Medved, M.; Ojsteršek, L. The associations between some biological markers, obesity, and cardiovascular risk in Slovenian children and adolescents. BMC Pediatr. 2020, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Dong, S.Y.; Wang, M.L.; Li, J.M.; Ren, C.L.; Gao, C.Q. Obesity and novel cardiovascular markers in a population without diabetes and cardiovascular disease in China. Prev. Med. (Baltim) 2016, 91, 62–69. [Google Scholar] [CrossRef]
- Dolegowska, K.; Marchelek-Mysliwiec, M.; Nowosiad-Magda, M.; Slawinski, M.; Dolegowska, B. FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 2019, 75, 229–240. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, L.; Lin, X.; Cheng, P.; He, L.; Li, X.; Lu, X.; Tan, Y.; Yang, H.; Cai, L.; et al. Minireview: Roles of fibroblast growth factors 19 and 21 in metabolic regulation and chronic diseases. Mol. Endocrinol. 2015, 29, 1400–1413. [Google Scholar] [CrossRef]
- Davis, D.; Garg, C.; Lahiri, S.S. Therapeutic potential of FGF21 in diabetes. J. Med. Plants Stud. 2017, 5, 364–372. [Google Scholar]
- Tinkov, A.A.; Bogdański, P.; Skrypnik, D.; Skrypnik, K.; Skalny, A.V.; Aaseth, J.; Skalnaya, M.G.; Suliburska, J. Trace element and mineral levels in serum, hair, and urine of obese women in relation to body composition, blood pressure, lipid profile, and insulin resistance. Biomolecules 2021, 11, 689. [Google Scholar] [CrossRef]
- Tinkov, A.; Tinkov, A.; Skalny, A.; Skalnaya, M.; Demidov, V.; Serebryansky, E.; Nikonorov, A. Hair ultra-trace elements in relation to age and body mass index in adult women. J. Elem. 2015, 21, 211–220. [Google Scholar] [CrossRef]
- Aiginger, H.; Wobrauschek, P.; Streli, C. Principles and Development of Total Reflection X-ray Fluorescence Analysis. Anal. Sci. 1995, 11, 471–475. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. J. Am. Med. Assoc. 2003, 289, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. Risk factors for coronary artery disease: Historical perspectives. Heart Views 2017, 18, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Singla, P. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Battisti, W.P.; Palmisano, J.; Keane, W.F. Dyslipidemia in patients with type 2 diabetes. Relationships between lipids, kidney disease and cardiovascular disease. Clin. Chem. Lab. Med. 2003, 41, 1174–1181. [Google Scholar] [CrossRef]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest. Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Ziomber, A.; Surowka, A.D.; Antkiewicz-Michaluk, L.; Romanska, I.; Wrobel, P.; Szczerbowska-Boruchowska, M. Combined brain Fe, Cu, Zn and neurometabolite analysis—A new methodology for unraveling the efficacy of transcranial direct current stimulation (tDCS) in appetite control. Metallomics 2018, 10, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Surowka, A.D.; Ziomber, A.; Czyzycki, M.; Migliori, A.; Kasper, K.; Szczerbowska-Boruchowska, M. Molecular and elemental effects underlying the biochemical action of transcranial direct current stimulation (tDCS) in appetite control. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Ziomber, A.; Rokita, E.; Kaszuba-Zwoinska, J.; Romańska, I.; Michaluk, J.; Antkiewicz-Michaluk, L. Repeated transcranial direct current stimulation induces behavioral, metabolic and neurochemical effects in rats on high-calorie diet. Front. Behav. Neurosci. 2018, 11, 262. [Google Scholar] [CrossRef]
- Ziomber, A.; Juszczak, K.; Kaszuba-Zwoinska, J.; Machowska, A.; Zaraska, K.; Gil, K.; Thor, P. Magnetically induced vagus nerve stimulation and feeding behavior in rats. J. Physiol. Pharmacol. 2009, 60, 71. [Google Scholar]
- Maric, I.; Krieger, J.P.; van der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Wernstedt Asterholm, I.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 141. [Google Scholar] [CrossRef]
- Ziomber-Lisiak, A.; Talaga-Ćwiertnia, K.; Sroka-Oleksiak, A.; Surówka, A.D.; Juszczak, K.; Szczerbowska-Boruchowska, M. Repetitive transcranial direct current stimulation modulates the braingutmicrobiome axis in obese rodents. Pharmacol. Rep. 2022, 74, 871–889. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Escuredo, J.M.; Gómez-Ambrosi, J.; Catalan, V.; Domingo, P.; Giralt, M.; Frühbeck, G.; Villarroya, F. Opposite alterations in FGF21 and FGF19 levels and disturbed expression of the receptor machinery for endocrine FGFs in obese patients. Int. J. Obes. 2015, 39, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; John, L.M.; Adams, S.H.; Yu, X.X.; Tomlinson, E.; Renz, M.; Williams, P.M.; Soriano, R.; Corpuz, R.; Moffat, B.; et al. Fibroblast growth factor 19 increases metabolic rate and reverses dietary and leptin-deficient diabetes. Endocrinology 2004, 145, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Janus, D.; Dolezal-Oltarzewska, K.; Kalicka-Kasperczyk, A.; Poplawska, K.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. A decrease in fasting FGF19 levels is associated with the development of non-alcoholic fatty liver disease in obese adolescents. J. Pediatr. Endocrinol. Metab. 2012, 25, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic mice expressing human fibroblast growth factor-19 display increased metabolic rate and decreased adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- Morrice, N.; McIlroy, G.D.; Tammireddy, S.R.; Reekie, J.; Shearer, K.D.; Doherty, M.K.; Delibegović, M.; Whitfield, P.D.; Mody, N. Elevated Fibroblast growth factor 21 (FGF21) in obese, insulin resistant states is normalised by the synthetic retinoid Fenretinide in mice. Sci. Rep. 2017, 7, srep43782. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delfín, J.; Hondares, E.; Iglesias, R.; Giralt, M.; Caelles, C.; Villarroya, F. TNF-α represses β-klotho expression and impairs FGF21 action in adipose cells: Involvement of JNK1 in the FGF21 pathway. Endocrinology 2012, 153, 4238–4245. [Google Scholar] [CrossRef]
- Reinehr, T.; Woelfle, J.; Wunsch, R.; Roth, C.L. Fibroblast Growth Factor 21 (FGF-21) and its relation to obesity, metabolic syndrome, and nonalcoholic fatty liver in children: A longitudinal analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2143–2150. [Google Scholar] [CrossRef]
- Miyata, M.; Sakaida, Y.; Matsuzawa, H.; Yoshinari, K.; Yamazoe, Y. Fibroblast growth factor 19 treatment ameliorates disruption of hepatic lipid metabolism in farnesoid X receptor (Fxr)-null mice. Biol. Pharm. Bull. 2011, 34, 1885–1889. [Google Scholar] [CrossRef]
- Wu, X.; Ge, H.; Baribault, H.; Gupte, J.; Weiszmann, J.; Lemon, B.; Gardner, J.; Fordstrom, P.; Tang, J.; Zhou, M.; et al. Dual actions of fibroblast growth factor 19 on lipid metabolism. J. Lipid Res. 2013, 54, 325–332. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Boney-Montoya, J.; Choi, M.; He, T.; Sunny, N.E.; Satapati, S.; Suino-Powell, K.; Xu, H.E.; Gerard, R.D.; Finck, B.N.; et al. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway. Cell Metab. 2011, 13, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, C.; Luo, Y.; Jin, C.; Wang, F.; McKeehan, W.L. FGFR4 prevents hyperlipidemia and insulin resistance but underlies high-fat diet-induced fatty liver. Diabetes 2007, 56, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Eren, F.; Yonal, O.; Kurt, R.; Aktas, B.; Celikel, C.A.; Ozdogan, O.; Imeryuz, N.; Kalayci, C.; Avsar, E. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur. J. Clin. Investig. 2010, 40, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yeung, D.C.Y.; Karpisek, M.; Stejskal, D.; Zhou, Z.G.; Liu, F.; Wong, R.L.C.; Chow, W.S.; Tso, A.W.K.; Lam, K.S.L.; et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008, 57, 1246–1253. [Google Scholar] [CrossRef]
- Mai, K.; Andres, J.; Biedasek, K.; Weicht, J.; Bobbert, T.; Sabath, M.; Meinus, S.; Reinecke, F.; Möhlig, M.; Weickert, M.O.; et al. Free fatty acids link metabolism and regulation of the insulin-sensitizing fibroblast growth factor-21. Diabetes 2009, 58, 1532–1538. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Aguilar-Salinas, C.; Cuevas-Ramos, G.; Cuevas-Sosa, A.; Gomez-Perez, F. The Role of Fibroblast Growth Factor 21 (FGF21) on Energy Balance, Glucose and Lipid Metabolism. Curr. Diabetes Rev. 2009, 5, 216–220. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Dutchak, P.A.; Katafuchi, T.; Bookout, A.L.; Choi, J.H.; Yu, R.T.; Mangelsdorf, D.J.; Kliewer, S.A. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 2012, 148, 556–567. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef]
- Ceja-galicia, Z.A.; Daniel, A.; María, A.; Pablo, P.; Ostrosky-wegman, P.; Díaz-Villasenor, A. Molecular and Cellular Endocrinology Effects of arsenic on adipocyte metabolism: Is arsenic an obesogen? Mol. Cell. Endocrinol. 2017, 5, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Tinkov, A.A.; Filippini, T.; Ajsuvakova, O.P.; Aaseth, J.; Gluhcheva, Y.G.; Ivanova, J.M.; Bjørklund, G.; Skalnaya, M.G.; Gatiatulina, E.R.; Popova, E.V.; et al. The role of cadmium in obesity and diabetes. Sci. Total Environ. 2017, 601–602, 741–755. [Google Scholar] [CrossRef] [PubMed]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Iavicoli, I.; Fontana, L.; Bergamaschi, A. The effects of metals as endocrine disruptors. J. Toxicol. Environ. Health Part B Crit. Rev. 2009, 12, 206–223. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Molecular, Clinical and Environmental Toxicology. Mol. Clin. Environ. Toxicol. 2012, 101, 133–164. [Google Scholar]
- Kawakami, T.; Nishiyama, K.; Kadota, Y.; Sato, M.; Inoue, M.; Suzuki, S. Cadmium modulates adipocyte functions in metallothionein-null mice. Toxicol. Appl. Pharmacol. 2013, 272, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk, P.; Wróbel, P.M.; Dudała, J.; Geraki, K.; Szczerbowska-Boruchowska, M.; Radwańska, E.; Krzyżewski, R.M.; Adamek, D.; Lankosz, M. Elemental Composition of Skeletal Muscle Fibres Studied with Synchrotron Radiation X-ray Fluorescence (SR-XRF). Int. J. Mol. Sci. 2022, 23, 7931. [Google Scholar] [CrossRef]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Fieve, R.R.; Meltzer, H.; Dunner, D.L.; Levitt, M.; Mendlewicz, J.; Thomas, A. Rubidium: Biochemical, behavioral, and metabolic studies in humans. Am. J. Psychiatry 1973, 130, 55–61. [Google Scholar] [CrossRef]
- Meltzer, H.L.; Taylor, R.M.; Platman, S.R.; Fieve, R.R. Rubidium: A potential modifier of affect and behaviour. Nature 1969, 223, 321–322. [Google Scholar] [CrossRef]
- Stolk, J.M.; Nowack, W.J.; Barchas, J.D.; Platman, S.R. Brain norepinephrine: Enhanced turnover after rubidium treatment. Science 1970, 168, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.H.; Maturen, A.; Sky-Peck, H.H. Pharmacologic role of rubidium in psychiatric research. Compr. Ther. 1987, 13, 46–54. [Google Scholar] [PubMed]
- Tapdiqov, S.Z. Synthesis and investigation of chemical structure of N-methyl N-benzyl chitosan by the co-alkylation method. Asian J. Biomed. Pharm. Sci. 2018, 8, 18. [Google Scholar] [CrossRef]
- Roberts, B.R.; Doecke, J.D.; Rembach, A.; Yévenes, L.F.; Fowler, C.J.; McLean, C.A.; Lind, M.; Volitakis, I.; Masters, C.L.; Bush, A.I.; et al. Rubidium and potassium levels are altered in Alzheimer’s disease brain and blood but not in cerebrospinal fluid. Acta Neuropathol. Commun. 2016, 4, 119. [Google Scholar] [CrossRef]

| Element | PWLA | PWLM | PWLK | PWLL | PWLH | PWLHA |
|---|---|---|---|---|---|---|
| K | - 1 | 0.68 | - | - | 0.11 | 0.78 |
| Cr | 0.76 2 | - | - | 0.70 | - | 0.31 |
| Fe | - | - | - | - | 0.65 | - |
| Cu | - | - | - | - | - | - |
| Zn | - | 0.53 | 0.54 | 0.36 | - | - |
| Se | - | - | 0.78 | - | - | 0.51 |
| Br | - | 0.49 | - | - | - | - |
| Rb | 0.0643 | 0.013 | 0.037 | 0.087 | 0.023 | 0.33 |
| Sr | - | 0.86 | 0.47 | data | data | data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziomber-Lisiak, A.; Piana, K.; Ostachowicz, B.; Wróbel, P.; Kasprzyk, P.; Kaszuba-Zwoińska, J.; Baranowska-Chowaniec, A.; Juszczak, K.; Szczerbowska-Boruchowska, M. The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities. Int. J. Mol. Sci. 2022, 23, 13437. https://doi.org/10.3390/ijms232113437
Ziomber-Lisiak A, Piana K, Ostachowicz B, Wróbel P, Kasprzyk P, Kaszuba-Zwoińska J, Baranowska-Chowaniec A, Juszczak K, Szczerbowska-Boruchowska M. The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities. International Journal of Molecular Sciences. 2022; 23(21):13437. https://doi.org/10.3390/ijms232113437
Chicago/Turabian StyleZiomber-Lisiak, Agata, Kaja Piana, Beata Ostachowicz, Paweł Wróbel, Paula Kasprzyk, Jolanta Kaszuba-Zwoińska, Agnieszka Baranowska-Chowaniec, Kajetan Juszczak, and Magdalena Szczerbowska-Boruchowska. 2022. "The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities" International Journal of Molecular Sciences 23, no. 21: 13437. https://doi.org/10.3390/ijms232113437
APA StyleZiomber-Lisiak, A., Piana, K., Ostachowicz, B., Wróbel, P., Kasprzyk, P., Kaszuba-Zwoińska, J., Baranowska-Chowaniec, A., Juszczak, K., & Szczerbowska-Boruchowska, M. (2022). The New Markers of Early Obesity-Related Organ and Metabolic Abnormalities. International Journal of Molecular Sciences, 23(21), 13437. https://doi.org/10.3390/ijms232113437






