Advances in Intraoperative Flow Cytometry
Abstract
1. Introduction
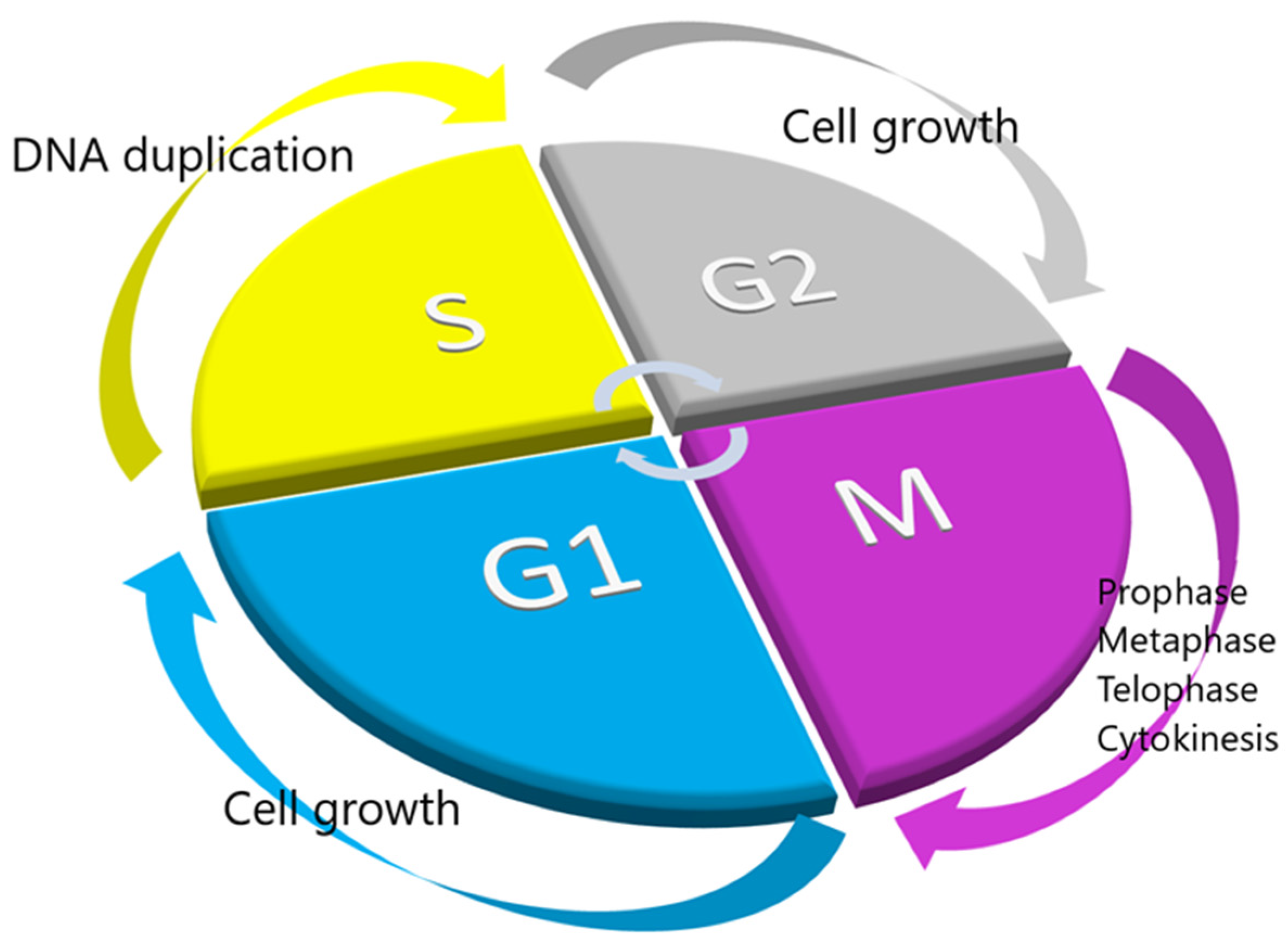
2. Intraoperative Flow Cytometry
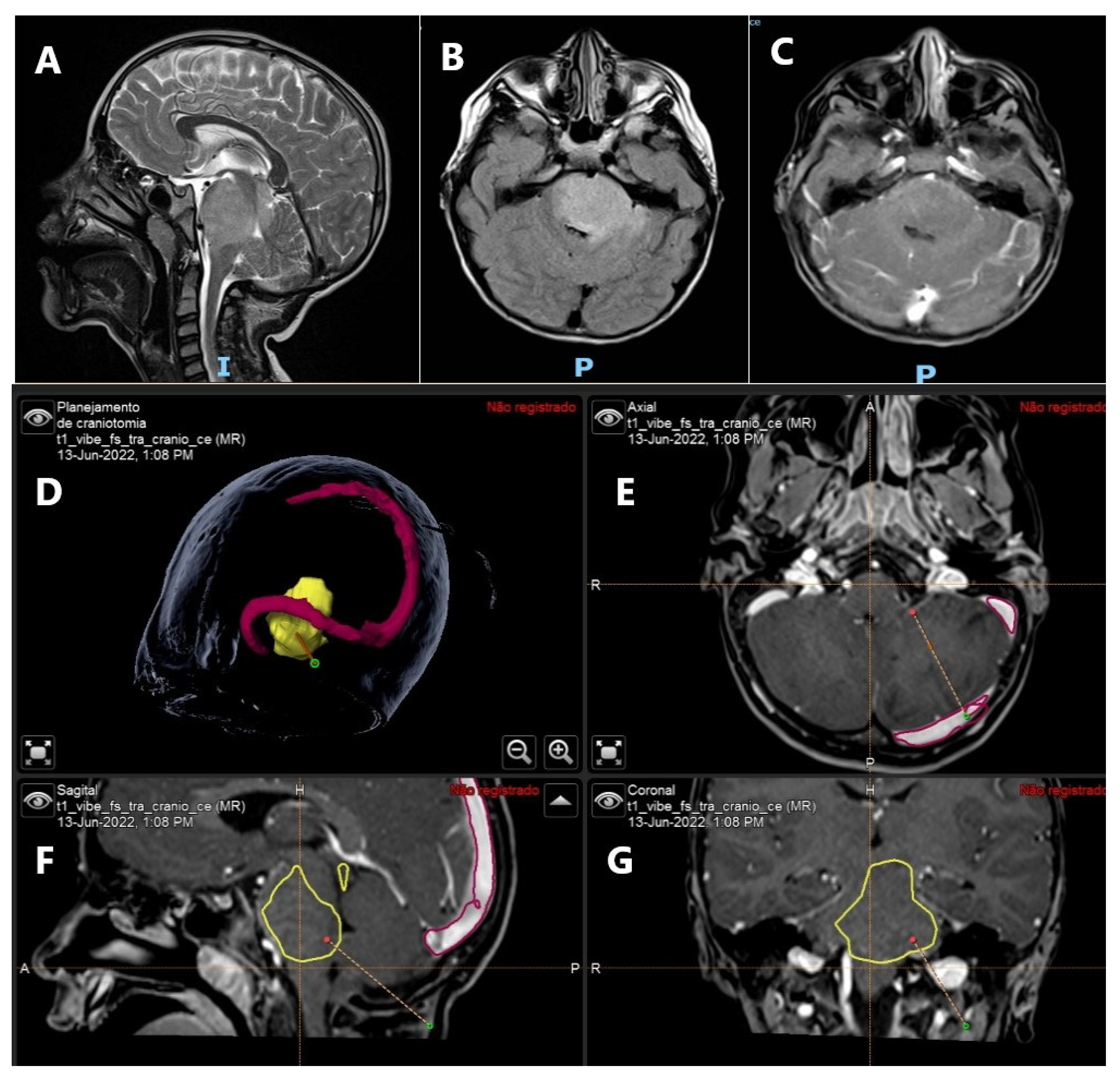
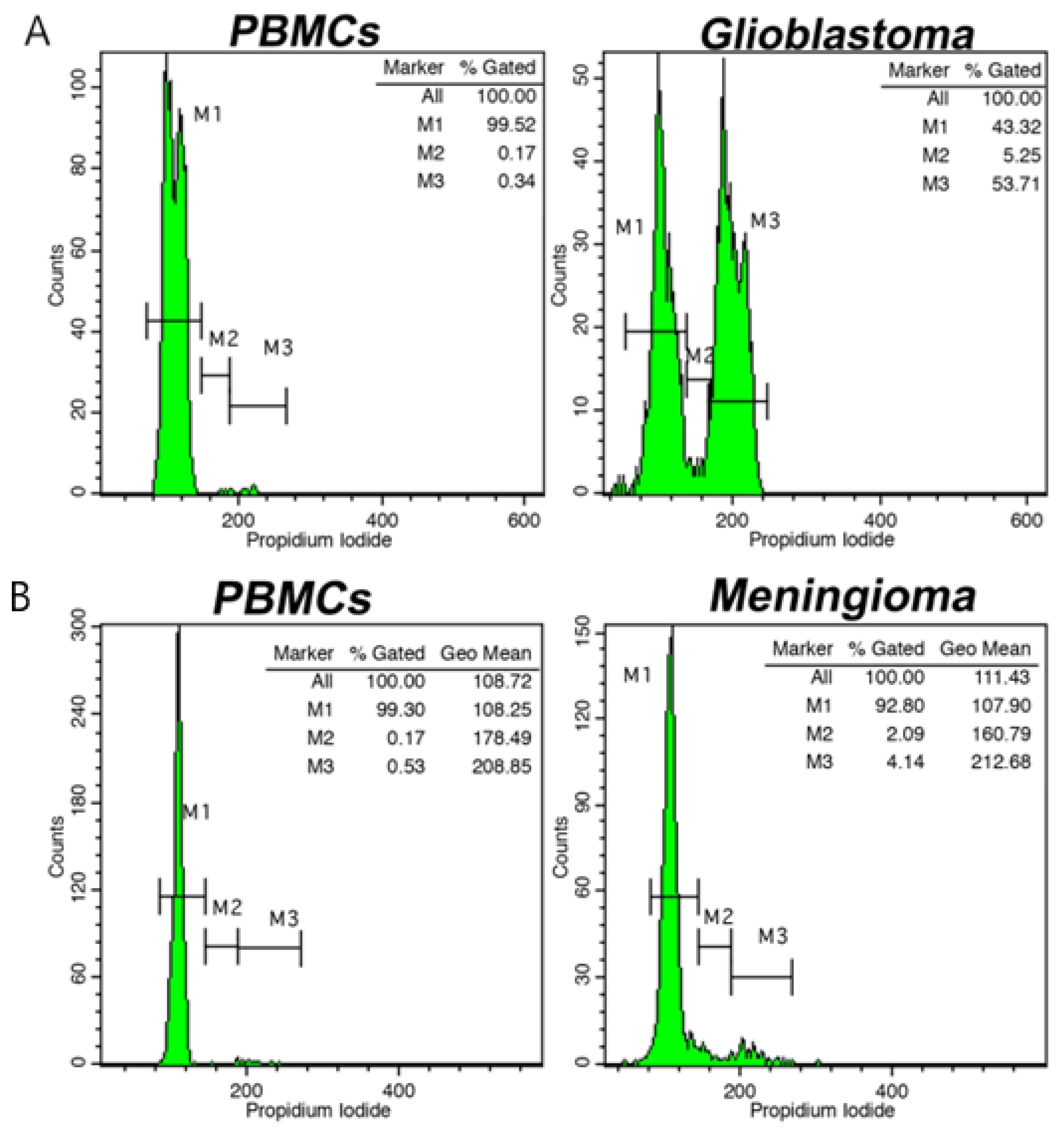
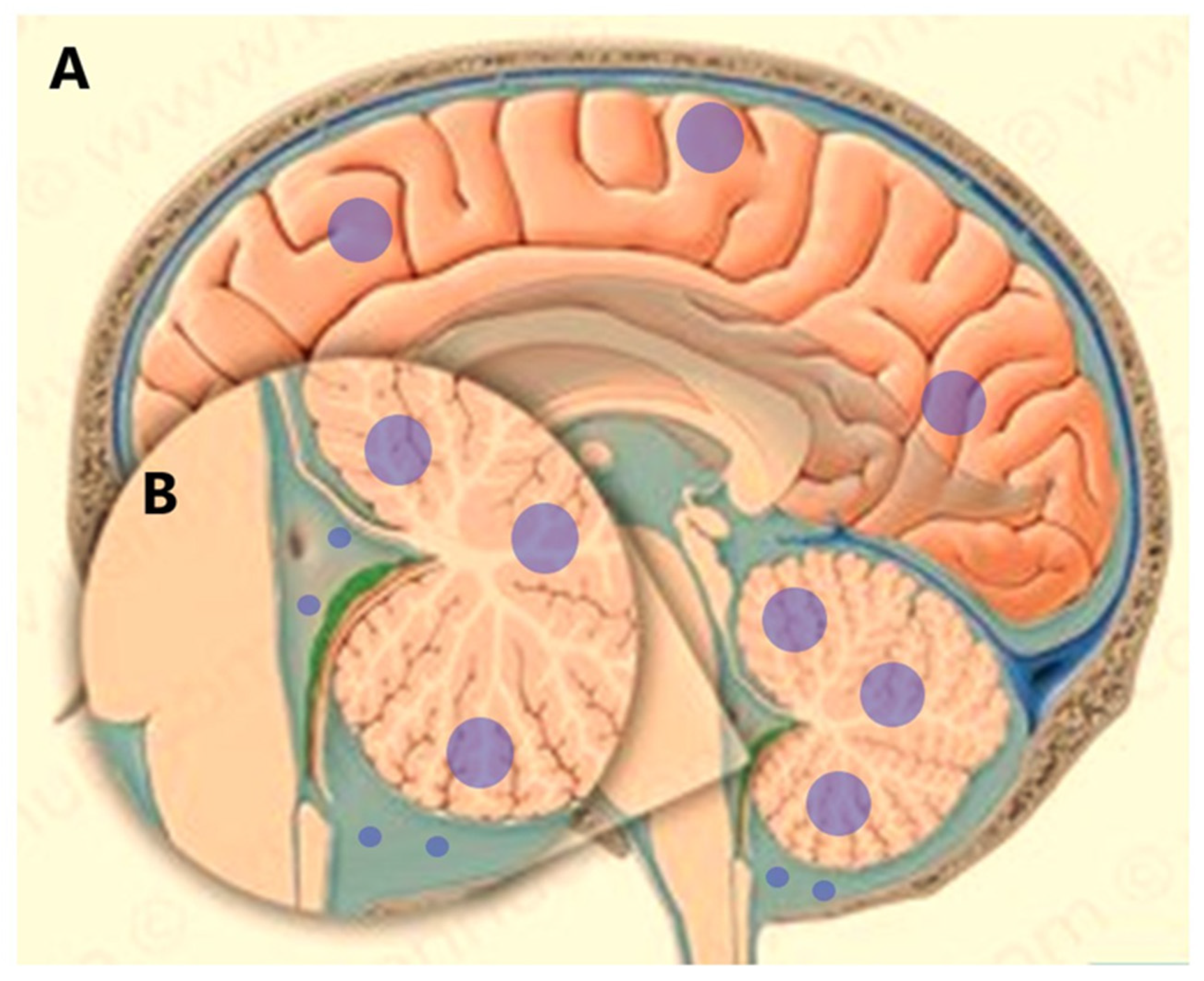
3. Brain Tumors
4. Leptomeningeal Dissemination
5. Breast Cancer
6. Head and Neck Surgery
7. Lung Cancer
8. Pancreatic Cancer
9. Liver Cancer
10. Other Cancers
11. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Betters, D.M. Use of Flow Cytometry in Clinical Practice. J. Adv. Pr. Oncol. 2015, 6, 435–440. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 5.1.1–5.1.11. [Google Scholar] [CrossRef] [PubMed]
- Audia, A.; Bannish, G.; Bunting, R.; Riveley, C. Flow cytometry and receptor occupancy in immune-oncology therapy. Expert Opin. Biol. Ther. 2021, 22, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.; Baumann, A.; Arguello, V. Recent advancements of flow cytometry: New applications in hematology and oncology. Expert Rev. Mol. Diagn. 2013, 14, 67–81. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Kobayashi, T.; Voulgaris, S.; Kyritsis, A.P. The emerging role of intraoperative flow cytometry in intracranial tumor surgery. Clin. Neurol. Neurosurg. 2020, 192, 105742. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, G.; Stefanaki, K.; Lykoudis, E.G.; Patereli, A.; Tseka, G.; Tzoufi, M.; Sfakianos, G.; Prodromou, N. The Role of Fast Cell Cycle Analysis in Pediatric Brain Tumors. Pediatr. Neurosurg. 2015, 50, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Vartholomatos, G.; Goussia, A.; Batistatou, A.; Tsamis, K.; Voulgaris, S.; Kyritsis, A.P. Fast cell cycle analysis for intraoperative characterization of brain tumor margins and malignancy. J. Clin. Neurosci. 2015, 22, 129–132. [Google Scholar] [CrossRef]
- Xia, Y.; Liao, W.; Huang, S.; Liu, Z.; Huang, X.; Yang, C.; Ye, C.; Jiang, Y.; Wang, J. Nomograms for predicting the overall and cancer-specific survival of patients with high-grade glioma: A surveillance, epidemiology, and end results study. Turk. Neurosurg. 2019, 30, 48–59. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G.; on behalf of the ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii93–iii101. [Google Scholar] [CrossRef]
- Stupp, R.; Tonn, J.C.; Brada, M.; Pentheroudakis, G.; on behalf of the ESMO Guidelines Working Group. High-grade malignant glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. S5), v190–v193. [Google Scholar] [CrossRef]
- Rykkje, A.; Li, D.; Skjøth-Rasmussen, J.; Larsen, V.; Nielsen, M.; Hansen, A.; Carlsen, J. Surgically Induced Contrast Enhancements on Intraoperative and Early Postoperative MRI Following High-Grade Glioma Surgery: A Systematic Review. Diagnostics 2021, 11, 1344. [Google Scholar] [CrossRef] [PubMed]
- Bettmann, M.A. Intraoperative MRI for Treatment of High-Grade Glioma: Is It Cost-effective? Radiology 2019, 291, 698–699. [Google Scholar] [CrossRef] [PubMed]
- Golub, D.; Hyde, J.; Dogra, S.; Nicholson, J.; Kirkwood, K.A.; Gohel, P.; Loftus, S.; Schwartz, T.H. Intraoperative MRI versus 5-ALA in high-grade glioma resection: A network meta-analysis. J. Neurosurg. 2021, 134, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Coburger, J.; Wirtz, C.R. Fluorescence guided surgery by 5-ALA and intraoperative MRI in high grade glioma: A systematic review. J. Neuro-Oncol. 2018, 141, 533–546. [Google Scholar] [CrossRef]
- Altieri, R.; Meneghini, S.; Agnoletti, A.; Tardivo, V.; Vincitorio, F.; Prino, E.; Zenga, F.; Ducati, A.; Garbossa, D. Intraoperative ultrasound and 5-ALA: The two faces of the same medal? J. Neurosurg. Sci. 2016, 63, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Pirro, V.; Alfaro, C.M.; Jarmusch, A.K.; Hattab, E.M.; Cohen-Gadol, A.A.; Cooks, R.G. Intraoperative assessment of tumor margins during glioma resection by desorption electrospray ionization-mass spectrometry. Proc. Natl. Acad. Sci. USA 2017, 114, 6700–6705. [Google Scholar] [CrossRef]
- Gholami, B.; Norton, I.; Tannenbaum, A.R.; Agar, N.Y.R. Recursive feature elimination for brain tumor classification using desorption electrospray ionization mass spectrometry imaging. Annu. Int. Conf. IEEE Eng. Med. Biol Soc. 2012, 2012, 5258–5261. [Google Scholar] [CrossRef]
- Mesiwala, A.H.; Scampavia, L.D.; Rabinovitch, P.S.; Ruzicka, J.; Rostomily, R.C. On-line Flow Cytometry for Real-time Surgical Guidance. Neurosurgery 2004, 55, 551–561. [Google Scholar] [CrossRef]
- Frederiksen, J.; Buggert, M.; Karlsson, A.C.; Lund, O. NetFCM: A semi-automated web-based method for flow cytometry data analysis. Cytom. Part A 2014, 85, 969–977. [Google Scholar] [CrossRef]
- Frederiksen, P.; Reske-Nielsen, E.; Bichel, P. Flow cytometry in tumours of the brain. Acta Neuropathol. 1978, 41, 179–183. [Google Scholar] [CrossRef]
- Kasai, H.; Kawamoto, K. Cytogenical analysis of brain tumors by FISH (fluorescence in situ hybridization) and FCM (flow cytometry). Noshuyo Byori 1995, 12, 75–82. [Google Scholar] [PubMed]
- Alexiou, G.A.; Vartholomatos, G.; Goussia, A.; Voulgaris, S.; Kyritsis, A.P. Letter. Neurosurgery 2020, 87, E425–E426. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Kawakami, K.; Kawamura, Y.; Matsumura, H.; Ohyama, A. New sensitivity test using flow cytometry. J. Neuro-Oncol. 1988, 6, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, K.; Wada, Y.; Kumazawa, H.; Yamashita, T.; Kumazawa, T.; Matsumura, H. Experimental and clinical evaluation by flow cytometry for the mechanism of combination therapy (cisplatin and peplomycin). Cytometry 1992, 13, 307–313. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Vartholomatos, E.; Goussia, A.; Dova, L.; Karamoutsios, A.; Fotakopoulos, G.; Kyritsis, A.P.; Voulgaris, S. DNA content is associated with malignancy of intracranial neoplasms. Clin. Neurol. Neurosurg. 2013, 115, 1784–1787. [Google Scholar] [CrossRef]
- Coons, S.W.; Johnson, P.C.; Pearl, D.K.; Olafsen, A.G. Prognostic significance of flow cytometry deoxyribonucleic acid analysis of human oligodendrogliomas. Neurosurgery 1994, 34, 680–687. [Google Scholar]
- Coons, S.W.; Johnson, P.C.; Shapiro, J.R. Cytogenetic and flow cytometry DNA analysis of regional heterogeneity in a low grade human glioma. Cancer Res. 1995, 55, 1569–1577. [Google Scholar]
- Shioyama, T.; Muragaki, Y.; Maruyama, T.; Komori, T.; Iseki, H. Intraoperative flow cytometry analysis of glioma tissue for rapid determination of tumor presence and its histopathological grade: Clinical article. J. Neurosurg. 2013, 118, 1232–1238. [Google Scholar] [CrossRef]
- Vartholomatos, E.; Vartholomatos, G.; Alexiou, G.; Markopoulos, G. The Past, Present and Future of Flow Cytometry in Central Nervous System Malignancies. Methods Protoc. 2021, 4, 11. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Alexiou, G.A.; Stefanaki, K.; Lykoudis, E.G.; Tseka, G.; Tzoufi, M.; Sfakianos, G.; Prodromou, N. The value of cell cycle analysis by propidium-iodine staining of CD56+ cells in pediatric brain tumors. Clin. Neurol. Neurosurg. 2015, 133, 70–74. [Google Scholar] [CrossRef]
- Crone, K.R.; Challa, V.R.; Kute, T.E.; Moody, D.M.; Kelly, D.L., Jr. Relationship between flow cytometric features and clinical behavior of meningiomas. Neurosurgery 1988, 23, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Subirá, D.; Serrano, C.; Castañón, S.; Gonzalo, R.; Illán, J.; Pardo, J.; Martínez-García, M.; Millastre, E.; Aparisi, F.; Navarro, M.; et al. Role of flow cytometry immunophenotyping in the diagnosis of leptomeningeal carcinomatosis. Neuro-Oncol. 2011, 14, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia, F.J.V.; Carrion, N.P.; de la Cruz-Merino, L. Long-term complete response to intrathecal trastuzumab in a patient with leptomeningeal carcinomatosis due to her2- overexpressing breast cancer: Case report. Medicine 2020, 99, e18298. [Google Scholar] [CrossRef] [PubMed]
- Zagouri, F.; Zoumpourlis, P.; Le Rhun, E.; Bartsch, R.; Zografos, E.; Apostolidou, K.; Dimopoulos, M.-A.; Preusser, M. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: A metanalysis with meta-regression. Cancer Treat. Rev. 2020, 88, 102046. [Google Scholar] [CrossRef]
- Abe, M.; Osoegawa, A.; Karashima, T.; Takumi, Y.; Kobayashi, R.; Hashimoto, T.; Miyawaki, M.; Takeuchi, H.; Okamoto, T.; Sugio, K. Erlotinib and bevacizumab combination therapy for afatinib-refractory leptomeningeal carcinomatosis from EGFR-mutated lung cancer. Int. Cancer Conf. J. 2019, 8, 81–85. [Google Scholar] [CrossRef]
- Su, Y.-H.; Chiang, C.-L.; Yang, H.-C.; Hu, Y.-S.; Chen, Y.-W.; Luo, Y.-H.; Chen, C.-J.; Wu, H.-M.; Lin, C.-J.; Lee, C.-C. Cerebrospinal fluid diversion and outcomes for lung cancer patients with leptomeningeal carcinomatosis. Acta Neurochir. 2021, 164, 459–467. [Google Scholar] [CrossRef]
- Li, M.; Ren, X.; Jiang, H.; Yang, K.; Huang, W.; Yu, K.; Chen, H.; Dong, G.; Cui, Y.; Lin, S. Supratentorial high-grade astrocytoma with leptomeningeal spread to the fourth ventricle: A lethal dissemination with dismal prognosis. J. Neuro-Oncology 2019, 142, 253–261. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, G.; He, H.; Yuan, T.; Wang, Y.; Li, Y.; Shi, W.; Gao, P.; Dong, L.; Zhao, G. Leptomeningeal metastasis from solid tumors: Clinical features and its diagnostic implication. Sci. Rep. 2018, 8, 10445. [Google Scholar] [CrossRef]
- Pellerino, A.; Brastianos, P.; Rudà, R.; Soffietti, R. Leptomeningeal Metastases from Solid Tumors: Recent Advances in Diagnosis and Molecular Approaches. Cancers 2021, 13, 2888. [Google Scholar] [CrossRef]
- Milojkovic Kerklaan, B.; Pluim, D.; Bol, M.; Hofland, I.; Westerga, J.; van Tinteren, H.; Brandsma, D. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro-oncology 2016, 18, 855–862. [Google Scholar] [CrossRef]
- Subirá, D.; Simó, M.; Illán, J.; Serrano, C.; Castañón, S.; Gonzalo, R.; Granizo, J.J.; Martínez-García, M.; Navarro, M.; Pardo, J.; et al. Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin. Exp. Metastasis 2015, 32, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.E.; Shah, K.; Yang, Q.; Haffty, B.G. Prognosis for Survival of Young Women with Breast Cancer by Quantitative p53 Immunohistochemistry. Cancer Clin. Oncol. 2012, 1, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Williams, F. Assessment of Breast Cancer Treatment Delay Impact on Prognosis and Survival: A Look at the Evidence from Systematic Analysis of the Literature. J. Cancer Biol. Res. 2015, 3, 1071. [Google Scholar]
- Coldman, A.J.; Phillips, N. Breast cancer survival and prognosis by screening history. Br. J. Cancer 2014, 110, 556–559. [Google Scholar] [CrossRef][Green Version]
- Tiainen, S.; Masarwah, A.; Oikari, S.; Rilla, K.; Hämäläinen, K.; Sudah, M.; Sutela, A.; Vanninen, R.; Ikonen, J.; Tammi, R.; et al. Tumor microenvironment and breast cancer survival: Combined effects of breast fat, M2 macrophages and hyaluronan create a dismal prognosis. Breast Cancer Res. Treat. 2019, 179, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Moureau-Zabotto, L.; Bouchet, C.; Cesari, D.; Uzan, S.; Lefranc, J.P.; Antoine, M.; Fleury-Feith, J. Combined flow cytometry determination of S-phase fraction and DNA ploidy is an independent prognostic factor in node-negative invasive breast carcinoma: Review of a series of 271 patients with stage I and II breast cancer. Cancer Radiother. 2005, 9, 575–586. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Harissis, H.; Andreou, M.; Tatsi, V.; Pappa, L.; Kamina, S.; Batistatou, A.; Markopoulos, G.S.; Alexiou, G.A. Rapid Assessment of Resection Margins During Breast Conserving Surgery Using Intraoperative Flow Cytometry. Clin. Breast Cancer 2021, 21, e602–e610. [Google Scholar] [CrossRef]
- Pinto, A.E.; Pereira, T.; Silva, G.L.; André, S. Prognostic relevance of DNA flow cytometry in breast cancer revisited: The 25-year experience of the Portuguese Institute of Oncology of Lisbon. Oncol. Lett. 2017, 13, 2027–2033. [Google Scholar] [CrossRef][Green Version]
- Andreou, M.; Vartholomatos, E.; Harissis, H.; Markopoulos, G.S.; Alexiou, G.A. Past, Present and Future of Flow Cytometry in Breast Cancer—A Systematic Review. EJIFCC 2019, 30, 423–437. [Google Scholar]
- Hu, Y.; Fan, L.; Zheng, J.; Cui, R.; Liu, W.; He, Y.; Li, X.; Huang, S. Detection of circulating tumor cells in breast cancer patients utilizing multiparameter flow cytometry and assessment of the prognosis of patients in different CTCs levels. Cytom. Part A 2010, 77A, 213–219. [Google Scholar] [CrossRef]
- Cohen, O.; Brauer, P.R.; Judson, B.L.; Burtness, B.A.; Earles, J.; Mehra, S. Guideline - Adherence in advanced stage head and neck cancer is associated with improved survival—A National study. Oral Oncol. 2021, 125, 105694. [Google Scholar] [CrossRef] [PubMed]
- Kutz, L.M.; Abel, J.; Schweizer, D.; Tribius, S.; Krüll, A.; Petersen, C.; Löser, A. Quality of life, HPV-status and phase angle predict survival in head and neck cancer patients under (chemo)radiotherapy undergoing nutritional intervention: Results from the prospective randomized HEADNUT-trial. Radiother. Oncol. 2021, 166, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Korpics, M.C.; Turchan, W.T.; Koshy, M.; Spiotto, M.T. Decreased overall survival in patients with locally advanced head and neck cancer receiving definitive radiotherapy and concurrent cetuximab: National Cancer Database analysis. Head Neck 2022, 44, 1528–1544. [Google Scholar] [CrossRef] [PubMed]
- Atallah, I.; Milet, C.; Coll, J.-L.; Reyt, E.; Righini, C.A.; Hurbin, A. Role of near-infrared fluorescence imaging in head and neck cancer surgery: From animal models to humans. Eur. Arch. Oto-Rhino-Laryngol. 2014, 272, 2593–2600. [Google Scholar] [CrossRef]
- Prince, A.; Moore, L.S.; Tipirneni, K.E.; Ramesh, T.; Limdi, M.A.; Bevans, S.L.; Walsh, E.M.; Greene, B.; Rosenthal, E.L.; Warram, J.M. Evaluation of optical imaging agents in a fluorescence-guided surgical model of head and neck cancer. Surg. Oncol. 2018, 27, 225–230. [Google Scholar] [CrossRef]
- Wedman, J.; Pruim, J.; Roodenburg, J.L.N.; Halmos, G.B.; Langedijk, J.A.; Dierckx, R.A.J.O.; Van Der Laan, B.F.A.M. Alternative PET tracers in head and neck cancer. A review. Eur. Arch. Oto-Rhino-Laryngol. 2012, 270, 2595–2601. [Google Scholar] [CrossRef]
- Vartholomatos, G.; Basiari, L.; Exarchakos, G.; Kastanioudakis, I.; Komnos, I.; Michali, M.; Markopoulos, G.S.; Batistatou, A.; Papoudou-Bai, A.; Alexiou, G.A. Intraoperative flow cytometry for head and neck lesions. Assessment of malignancy and tumour-free resection margins. Oral Oncol. 2019, 99, 104344. [Google Scholar] [CrossRef]
- Epp, R.A.; Justice, W.M.; Garcia, F.U.; McGregor, D.H.; Giri, S.P.; Kimler, B.F. Retrospective DNA Ploidy Analysis by Image and Flow Cytometry in Head and Neck Cancer. Laryngoscope 1996, 106, 1306–1313. [Google Scholar] [CrossRef]
- Joensuu, H. DNA flow cytometry in the prediction of survival and response to radiotherapy in head and neck cancer. A review. Acta Oncol. 1990, 29, 513–516. [Google Scholar] [CrossRef]
- Goldsmith, M.M.; Cresson, D.H.; Arnold, L.A.; Postma, D.S.; Askin, F.B.; Pillsbury, H.C. Part I. DNA Flow Cytometry as a Prognostic Indicator in Head and Neck Cancer. Otolaryngol. Neck Surg. 1987, 96, 307–318. [Google Scholar] [CrossRef]
- Zhang, L.; Hsieh, M.-C.; Rennert, L.; Neroda, P.; Wu, X.-C.; Hicks, C.; Wu, J.; Gimbel, R. Diagnosis-to-surgery interval and survival for different histologies of stage I–IIA lung cancer. Transl. Lung Cancer Res. 2021, 10, 3043–3058. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, J.L.; Wang, H.; Yu, W.; Xu, T. Surgery and Surgery Approach Affect Survival of Patients With Stage I-IIA Small-Cell Lung Cancer: A Study Based SEER Database by Propensity Score Matching Analysis. Front. Surg. 2022, 9, 735102. [Google Scholar] [CrossRef] [PubMed]
- Rami-Porta, R.; Wittekind, C.; Goldstraw, P.; International Association for the Study of Lung Cancer Staging Committee. Complete resection in lung cancer surgery: Proposed definition. Lung Cancer 2005, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ge, M.J.; Huang, H.B. The Effects of Different Surgical Approaches on the Perioperative Level of Circulating Tumor Cells in Patients with Non-Small Cell Lung Cancer. Thorac. Cardiovasc. Surg. 2015, 64, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.H.J.; Zhou, Y.; Li, L.; Bao, G.; Feng, J.; Sha, H. Hematogenous dissemination of lung cancer cells during surgery: Quantitative detection by flow cytometry and prognostic significance. Lung Cancer 2002, 37, 293–301. [Google Scholar] [CrossRef]
- Bai, J.; Huang, L.; Gao, Z.; Lu, Q.; Wang, J.; Zhao, Q. Soil seed banks and their germination responses to cadmium and salinity stresses in coastal wetlands affected by reclamation and urbanization based on indoor and outdoor experiments. J. Hazard. Mater. 2014, 280, 295–303. [Google Scholar] [CrossRef]
- Luo, W.; Tao, J.; Zheng, L.; Zhang, T. Current epidemiology of pancreatic cancer: Challenges and opportunities. Chin. J. Cancer Res. 2020, 32, 705–719. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Khalaf, N.; El-Serag, H.B.; Abrams, H.R.; Thrift, A.P. Burden of Pancreatic Cancer: From Epidemiology to Practice. Clin. Gastroenterol. Hepatol. 2020, 19, 876–884. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Goussia, A.; Bali, C.D.; Messinis, T.; Alexiou, G.; Vartholomatos, G. Resection Margins Assessment by Intraoperative Flow Cytometry in Pancreatic Cancer. Ann. Surg. Oncol. 2022, 29, 4643–4645. [Google Scholar] [CrossRef]
- Liu, D.; Song, T. Changes in and challenges regarding the surgical treatment of hepatocellular carcinoma in China. Biosci. Trends 2021, 15, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Hibi, T. Surgical treatment of hepatocellular carcinoma. Biosci. Trends. 2021, 15, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.T.; Yang, Z.F.; Ho, D.W.; Ng, M.N.; Yu, W.C.; Wong, J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: A prospective study. Ann. Surg. 2011, 254, 569–576. [Google Scholar] [CrossRef]
- Markopoulos, G.S.; Glantzounis, G.K.; Goussia, A.C.; Lianos, G.D.; Karampa, A.; Alexiou, G.A.; Vartholomatos, G. Touch Imprint Intraoperative Flow Cytometry as a Complementary Tool for Detailed Assessment of Resection Margins and Tumor Biology in Liver Surgery for Primary and Metastatic Liver Neoplasms. Methods Protoc. 2021, 4, 66. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, W.; Zhang, D.; Pang, Y.; Shi, J.; Wan, S.; Cheng, S. Circulating tumor cell detection in hepatocellular carcinoma based on karyoplasmic ratios using imaging flow cytometry. Sci. Rep. 2016, 6, 39808. [Google Scholar] [CrossRef] [PubMed]
- Georvasili, V.K.; Markopoulos, G.S.; Batistatou, A.; Mitsis, M.; Messinis, T.; Lianos, G.D.; Alexiou, G.; Vartholomatos, G.; Bali, C.D. Detection of cancer cells and tumor margins during colorectal cancer surgery by intraoperative flow cytometry. Int. J. Surg. 2022, 104, 106717. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadi, Z.; Mantziou, S.; Akrivis, C.; Paschopoulos, M.; Balasi, E.; Lianos, G.D.; Alexiou, G.A.; Mitsis, M.; Vartholomatos, G.; Markopoulos, G.S. Intraoperative Flow Cytometry for the Characterization of Gynecological Malignancies. Biology 2022, 11, 1339. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato Figueiredo, M.V.; Alexiou, G.A.; Vartholomatos, G.; Rehder, R. Advances in Intraoperative Flow Cytometry. Int. J. Mol. Sci. 2022, 23, 13430. https://doi.org/10.3390/ijms232113430
D’Amato Figueiredo MV, Alexiou GA, Vartholomatos G, Rehder R. Advances in Intraoperative Flow Cytometry. International Journal of Molecular Sciences. 2022; 23(21):13430. https://doi.org/10.3390/ijms232113430
Chicago/Turabian StyleD’Amato Figueiredo, Marcos V., George A. Alexiou, George Vartholomatos, and Roberta Rehder. 2022. "Advances in Intraoperative Flow Cytometry" International Journal of Molecular Sciences 23, no. 21: 13430. https://doi.org/10.3390/ijms232113430
APA StyleD’Amato Figueiredo, M. V., Alexiou, G. A., Vartholomatos, G., & Rehder, R. (2022). Advances in Intraoperative Flow Cytometry. International Journal of Molecular Sciences, 23(21), 13430. https://doi.org/10.3390/ijms232113430






