Self-Organization of Fullerene Derivatives in Solutions and Biological Cells Studied by Pulsed Field Gradient NMR
Abstract
1. Introduction
2. Results and Discussion
2.1. Fullerene Derivatives in Solutions
2.1.1. Nonpolar Fullerene Derivatives
2.1.2. Polar Fullerene Derivatives
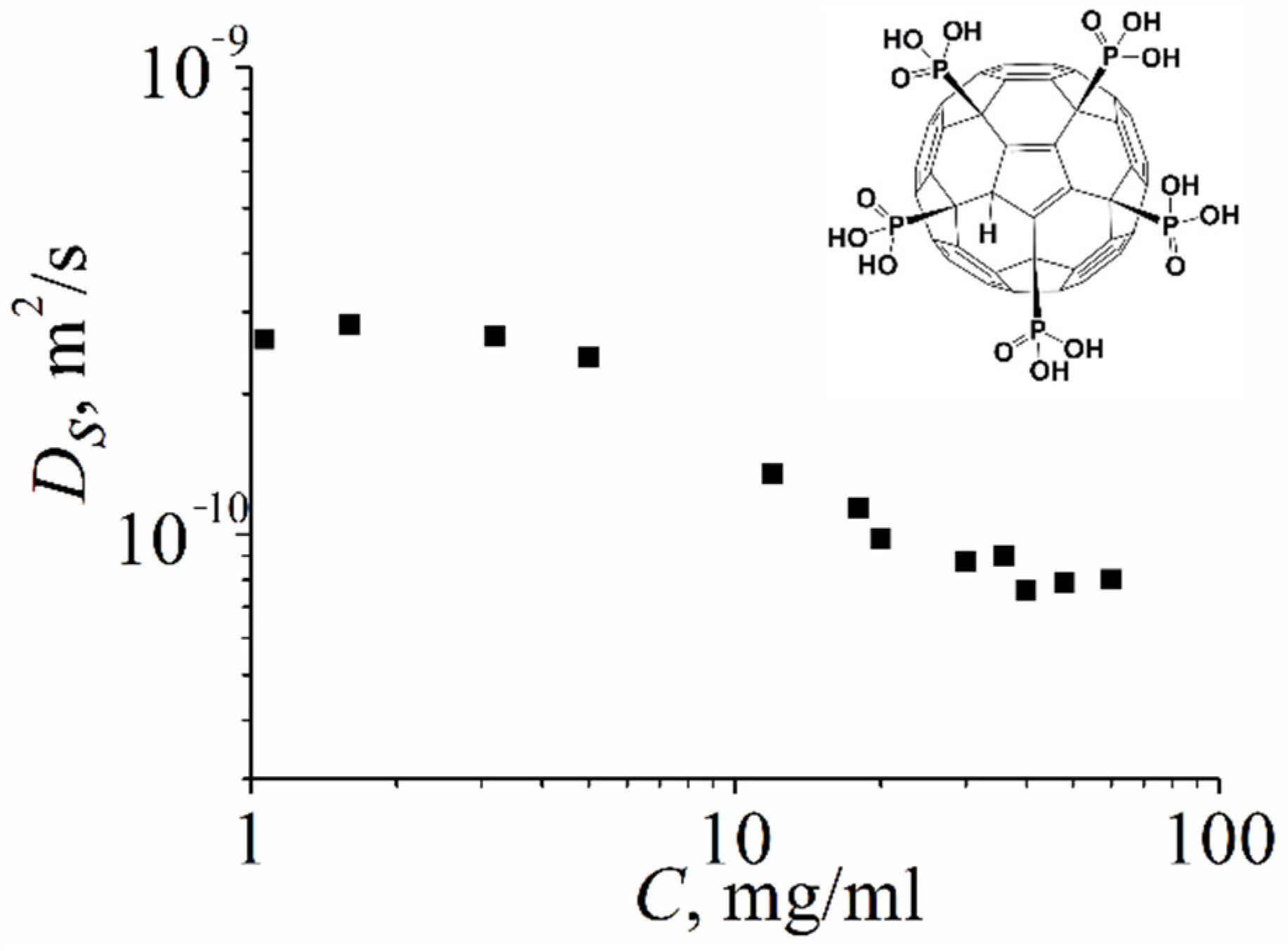
2.1.3. Water-Soluble Fullerene Derivatives
Compound VII
Compound VIII
Compound IX
Compound X
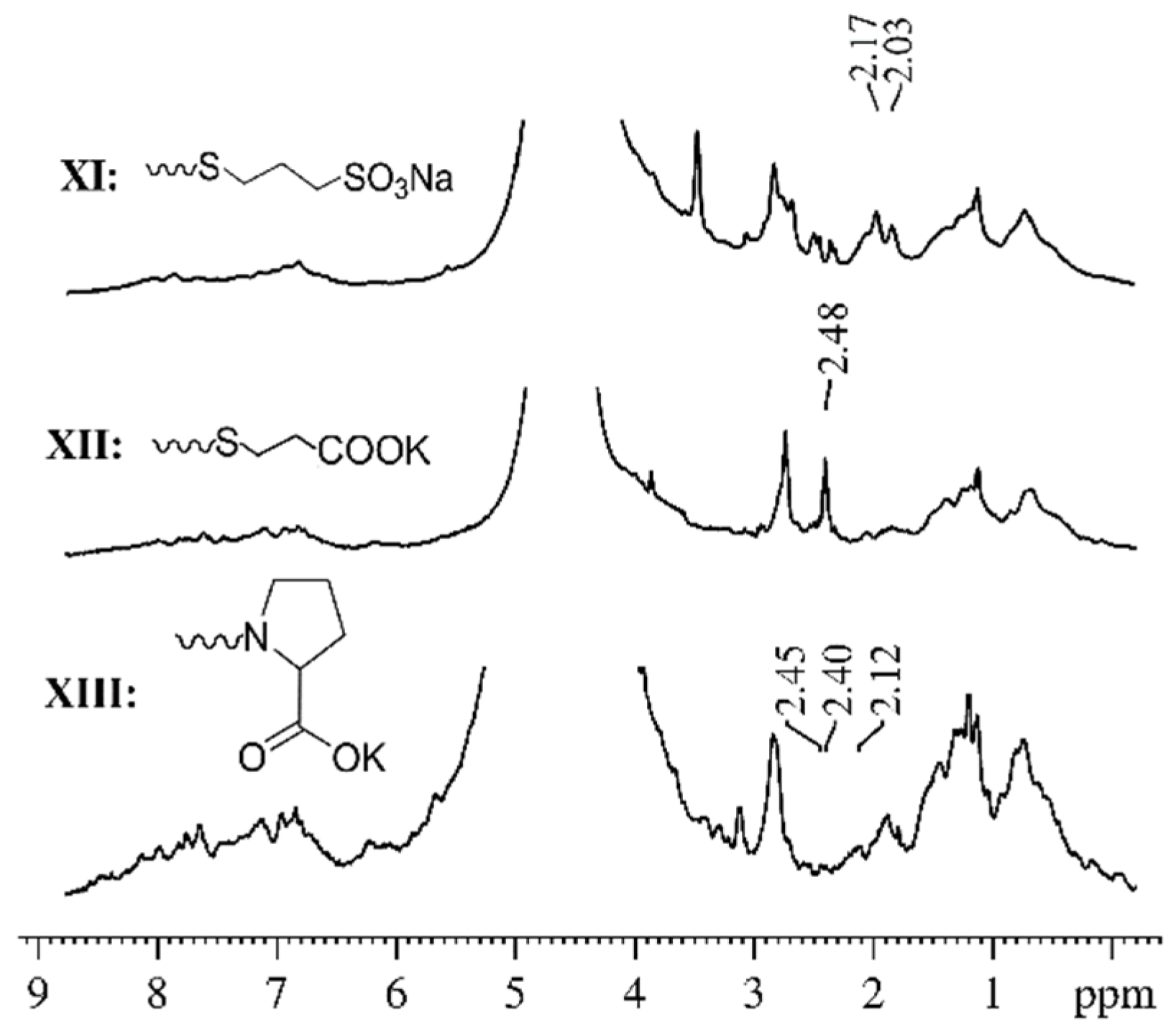
Compound XI
Compound XII
Compound XIII
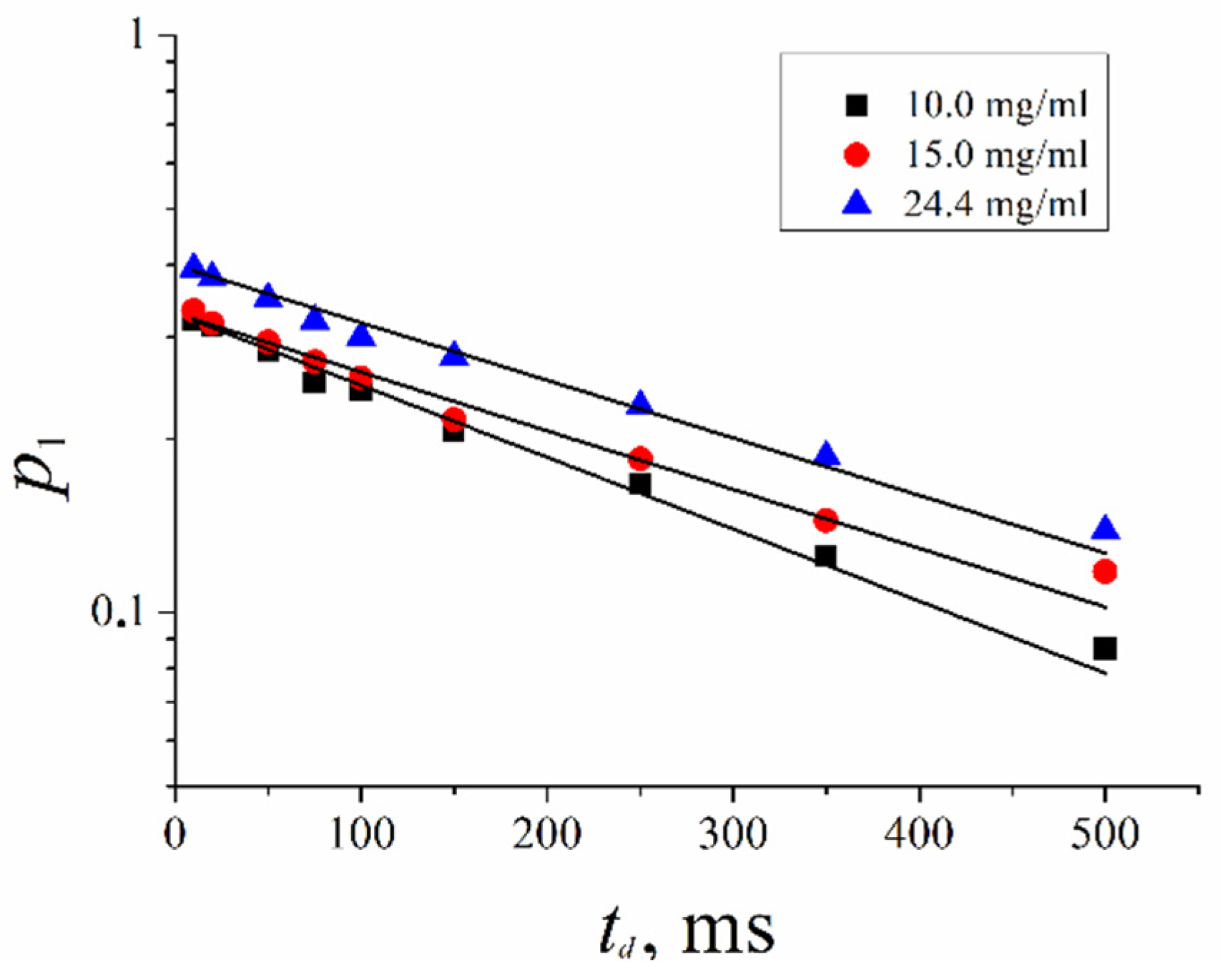
2.2. Water-Soluble Fullerene Derivatives in Red Blood Cells
3. Materials and Methods
3.1. Fullerene Derivatives
3.2. Red Blood Cells
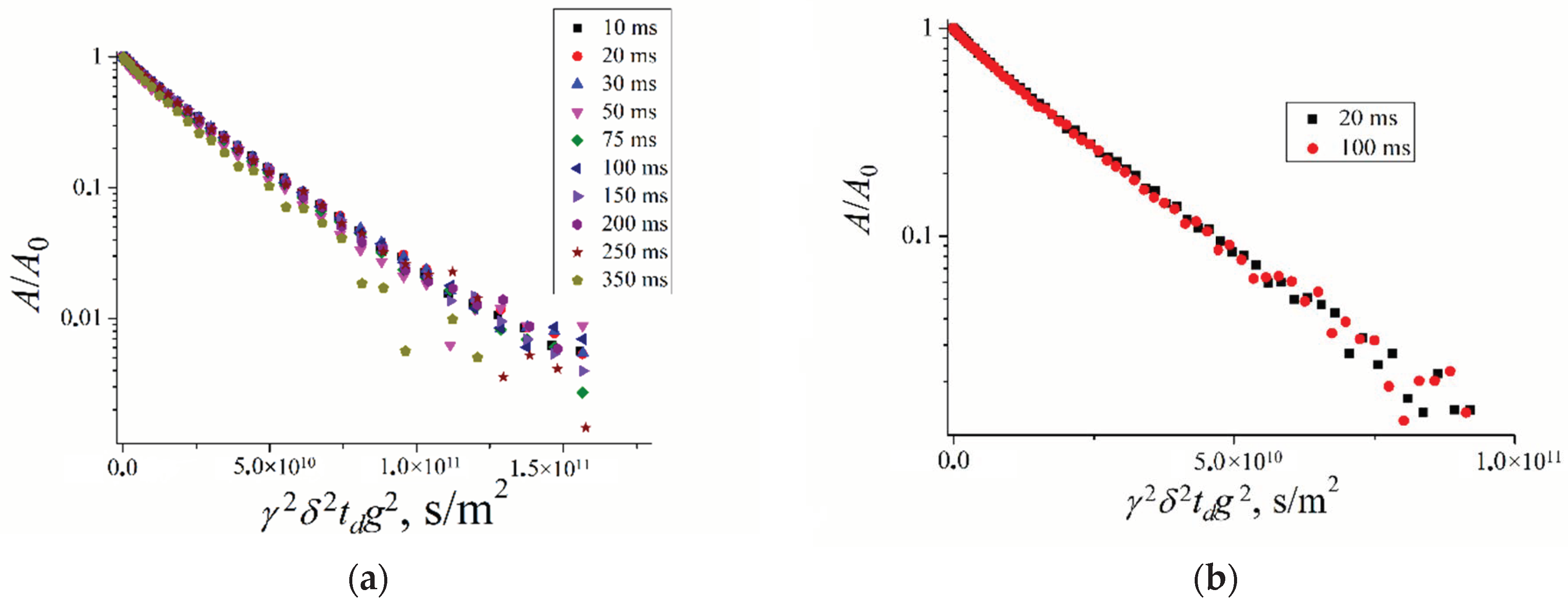
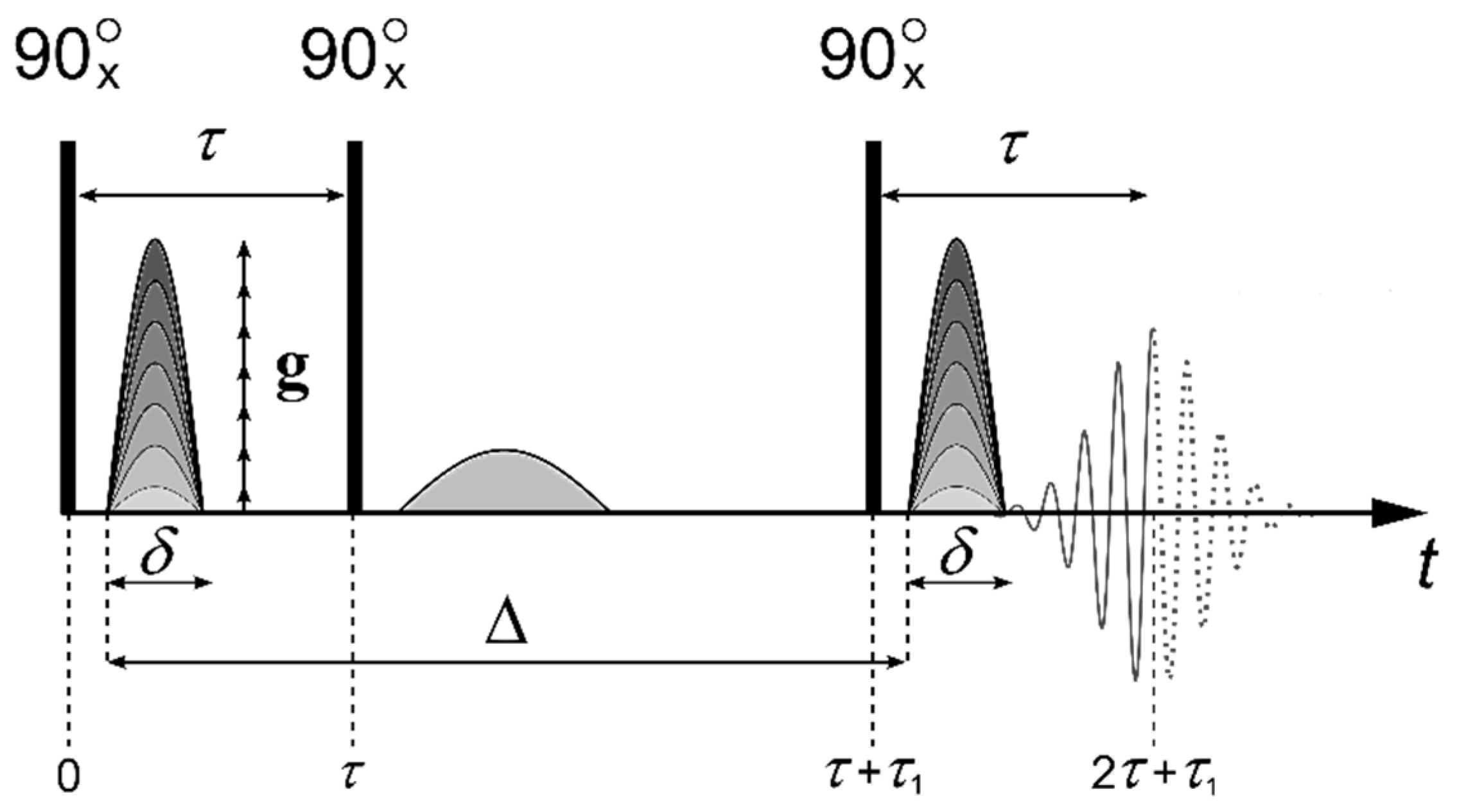
3.3. PFG NMR Technique
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langa, F.; Nierengarten, J.-F. Fullerene Principles and Applications; RCS: Cambridge, UK, 2007; 398p. [Google Scholar] [CrossRef]
- Penkova, A.V.; Acquah, S.F.A.; Piotrovskiy, L.B.; Markelov, D.A.; Semisalova, A.S.; Kroto, H.W. Fullerene derivatives as nano-additives in polymer composites. Russ. Chem. Rev. 2017, 86, 530. [Google Scholar] [CrossRef]
- Nakajima, N.; Nishi, C.; Li, F.-M.; Ikada, Y. Photo-Induced Cytotoxicity of Water-Soluble Fullerene. Fuller. Sci. Technol. 1996, 4, 1–19. [Google Scholar] [CrossRef]
- Tabata, Y.; Ishii, T.; Aoyama, T.; Oki, R.; Hirano, Y.; Ogawa, O.; Ikada, Y. Sonodynamic Effect of Polyethylene glecol-conjugated Fullerene on Tumor. In Perspectives of Fullerene Nanotechnology; Osawa, E., Ed.; Kluver Academic Publishers: Amsterdam, The Netherlands, 2001; pp. 185–199. [Google Scholar]
- Mashino, T.; Nishikawa, D.; Takanashi, K.; Usui, U.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, N.B.; Shenoy, R.U.K.; Kajampady, M.K.; DCruz, C.E.M.; Shirodkar, R.K.; Kumar, L.; Verma, R. Fullerenes for the treatment of cancer: An emerging tool. Environ. Sci. Pollut. Res. Int. 2022, 29, 58607–58627. [Google Scholar] [CrossRef]
- Friedman, S.H.; Ganapathi, P.S.; Rubin, Y.; Kenyon, G.L. Optimizing the binding of fullerene inhibitors of the HIV-1 protease through predicted increases in hydrophobic desolvation. J. Med. Chem. 1998, 41, 2424–2429. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Spalluto, G.; Balzarini, J.; Prato, M. Synthesis and anti-HIV properties of new water-soluble bis-functionalized[60]fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4437. [Google Scholar] [CrossRef]
- Mashino, T.; Shimotohno, K.; Ikegami, N.; Nishikawa, D.; Okuda, K.; Takanashi, K.; Nakamura, S.; Mochizuki, M. Human immunodeficiency virus-reverse transcriptase inhibition and hepatitis C virus RNA-dependent RNA polymerase inhibition activities of fullerene derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 1107. [Google Scholar] [CrossRef]
- Castro, E.; Garcia, A.H.; Zavalaa, G.; Echegoyen, L. Fullerenes in biology and medicine. J. Mater. Chem. B 2017, 5, 6523. [Google Scholar] [CrossRef]
- Khalikov, S.K.; Sharipova, D.; Zafarov, S.Z.; Umarkhon, M.; Alieva, S.V. Synthesis and Characterization of Fullero-C60 a-Amino Acids with Antiviral Properties. Chem. Nat. Compd. 2017, 53, 121–127. [Google Scholar] [CrossRef]
- Bosi, S.; Da Ros, T.; Castellano, S.; Bafni, E.; Prato, M. Antimycobacterial activity of ionic fullerene derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef]
- Su, Y.; Ding, M.; Dong, H.; Hu, Y.; Yang, D.; Shao, J.; Huang, B. Recent advances in nanozymes for combating bacterial infection. Mater. Chem. Front. 2022, 6, 2596–2609. [Google Scholar] [CrossRef]
- Belik, A.Y.; Rybkin, A.Y.; Voronov, I.V.; Goryachev, N.S.; Volyniuk, D.; Grasulevicius, J.V.; Troshin, P.A.; Kotelnikov, A.I. Non-covalent complexes of polycationic fullerene C60 derivative with xanthene dyes—Spectral and photochemical properties in water and in liposomes. Dyes Pigments 2017, 139, 65–72. [Google Scholar] [CrossRef]
- Mikhailov, P.A.; Kraevaya, O.A.; Belik, A.Y.; Rybkin, A.Y.; Khakina, E.A.; Goryachev, N.S.; Usol’tseva, L.I.; Romanenko, Y.V.; Koifman, O.I.; Gushchina, O.I.; et al. Synthesis, photophysical properties, and photochemical activity of the water-soluble dyad based on fullerene C60 and chlorin e6 derivatives. Dokl. Phys. Chem. 2017, 477, 226–230. [Google Scholar] [CrossRef]
- Belik, A.Y.; Rybkin, A.Y.; Goryachev, N.S.; Sadkov, A.P.; Filatova, N.V.; Buyanovskaya, A.G.; Talanova, V.N.; Klemenkova, Z.S.; Romanova, V.S.; Koifman, M.O.; et al. Nanoparticles of water-soluble dyads based on amino acid fullerene C60 derivatives and pyropheophorbide: Synthesis, photophysical properties, and photodynamic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 260, 119885. [Google Scholar] [CrossRef]
- Yurkova, A.A.; Khakina, E.A.; Troyanov, S.I.; Chernyak, A.V.; Shmygleva, L.; Peregudov, A.A.; Martynenko, V.M.; Dobrovolskiy, Y.A.; Troshin, P.A. Arbuzov chemistry with chlorofullerene C60Cl6: A powerful method for selective synthesis of highly functionalized [60]fullerene derivatives. Chem. Commun. 2012, 48, 8916. [Google Scholar] [CrossRef]
- Khakina, E.A.; Kraevaya, O.A.; Popova, M.L.; Peregudov, A.S.; Troyanov, S.I.; Chernyak, A.V.; Martynenko, V.M.; Kulikov, A.V.; Scholse, D.; Troshin, P.A. Synthesis of different types of alkoxy fullerene derivatives from chlorofullerene C60Cl6. Org. Biomol. Chem. 2017, 15, 773–777. [Google Scholar] [CrossRef]
- Kraevaya, O.A.; Peregudov, A.S.; Godovikov, I.A.; Shchurik, E.V.; Martynenko, V.M.; Shestakov, A.F.; Balzarini, J.; Schols, D.; Troshin, P.A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: A synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem. Commun. 2020, 56, 1179–1182. [Google Scholar] [CrossRef]
- Ros, T.D.; Prato, M. Medicinal chemistry with fullerenes and fullerene derivatives. Chem. Commun. 1999, I.8, 663–669. [Google Scholar] [CrossRef]
- Rokitskaya, T.I.; Antonenko, Y.N. Fullerenol C60(OH)24 increases ion permeability of lipid membranes in a pH-dependent manner. Biochim. Biophys. Acta 2016, 1858, 1165–1174. [Google Scholar] [CrossRef]
- Wattraint, O.; Sarazin, C. Diffusion measurements of water, ubiquinone and lipid bilayer inside a cylindrical nanoporous support: A stimulated echo pulsed-field gradient MAS-NMR investigation. Biochim. Biophys. Acta 2005, 1713, 65–72. [Google Scholar] [CrossRef][Green Version]
- Leal, C.; Sandström, D.; Nevsten, P.; Topgaard, D. Local and translational dynamics in DNA–lipid assemblies monitored by solid-state and diffusion NMR. Biochim. Biophys. Acta 2008, 1778, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Tardy-Laporte, C.; Arnold, A.A.; Genard, B.; Gastineau, R.; Morançais, M.; Mouget, J.-L.; Tremblay, R.; Marcotte, I. A 2H solid-state NMR study of the effect of antimicrobial agents on intact Escherichia coli without mutating. Biochim. Biophys. Acta 2013, 1828, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Krokosz, A.; Puchala, M. Fullerenol C60(OH)36 could associate to band 3 protein of human erythrocyte membranes. Biochim. Biophys. Acta 2013, 1828, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Krokosz, A.; Puchala, M. Membrane fluidity and activity of membrane ATPases in human erythrocytes under the influence of polyhydroxylated fullerene. Biochim. Biophys. Acta 2013, 1828, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhou, S.; Guo, L.; Wang, L.; Yao, F.; Hu, Y.; Li, H.; Hao, J. Aggregation Behavior and Antioxidant Properties of Amphiphilic Fullerene C60 Derivatives Cofunctionalized with Cationic and Nonionic Hydrophilic Groups. Langmuir 2019, 35, 6939–6949. [Google Scholar] [CrossRef]
- Harano, K.; Nakamura, E. Interfacial Chemistry of Conical Fullerene Amphiphiles in Water. Acc. Chem. Res. 2019, 52, 2090–2100. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Zhou, S.; Li, H.; Hao, J. Self-assembly of fullerene C60-based amphiphiles in solutions. Chem. Soc. Rev. 2022, 51, 3226–3242. [Google Scholar] [CrossRef]
- Nakamura, E.; Isobe, H. Functionalized Fullerenes in Water. The First 10 Years of Their Chemistry, Biology, and Nanoscience. Acc. Chem. Res. 2003, 36, 807. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Liang, D.; Gan, L.; Li, Y. Facile Synthesis of Isomerically Pure Fullerenols and Formation of Spherical Aggregates from C60(OH)8. Angew. Chem. Int. Ed. 2010, 49, 5293. [Google Scholar] [CrossRef]
- E, Y.; Bai, L.; Fan, L.; Han, M.; Zhang, X.; Yang, S. Electrochemically generated fluorescent fullerene[60] nanoparticles as a new and viable bioimaging platform. J. Mater. Chem. 2011, 21, 819–823. [Google Scholar] [CrossRef]
- Wang, G.; Zhao, G.; Yan, L. Investigation on the aggregation properties of cationic [60]fullerene derivative. Chin. Sci. Bull. 2004, 49, 1441. [Google Scholar] [CrossRef]
- Liao, Q.; Qu, X.; Chen, L.; Jin, X. Aggregates of Polymer-Substituted Fullerenes. J. Phys. Chem. B 2006, 110, 7153. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Böttcher, C.; Hirsch, A. Sugar Balls: Synthesis and Supramolecular Assembly of [60]Fullerene Glycoconjugates. Eur. J. Org. Chem. 2007, 2007, 2659–2666. [Google Scholar] [CrossRef]
- Braun, M.; Hartnagel, U.; Ravanelli, E.; Schade, B.; Böttcher, C.; Vostrowsky, O.; Hirsch, A. Amphiphilic [5:1]- and [3:3]-Hexakisadducts of C60. Eur. J. Org. Chem. 2004, 2004, 1983–2001. [Google Scholar] [CrossRef]
- Avilova, I.A.; Chernyak, A.V.; Zhilenkov, A.V.; Troshin, P.A.; Volkov, V.I. The self-organization of water soluble fullerene derivatives studied by pulsed field gradient NMR. Mendeleev Commun. 2016, 26, 146. [Google Scholar] [CrossRef]
- Hong, Y.S.; Kim, K.C.; Volkov, V.I.; Skirda, V.D.; Lee, C.-H. Structural and dynamic properties of polyoxyethylene sorbitan monooleate micelle in water dispersion studied by pulsed field gradient NMR. Appl. Magn. Reson. 2005, 29, 351. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Li, C.; Li, G.-Y.; Jiang, X.-K.; Li, Z.-T. Hydrogen-Bonded Aryl Amide Macrocycles: Synthesis, Single-Crystal Structures, and Stacking Interactions with Fullerenes and Coronene. J. Org. Chem. 2008, 73, 1745–1751. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Bichan, N.G.; Khodov, I.A.; Antina, E.V.; Berezin, M.B.; Vyugin, A.I. Novel non-covalent supramolecular systems based on zinc(II) bis(dipyrromethenate)s with fullerenes. J. Mol. Liq. 2018, 269, 327–334. [Google Scholar] [CrossRef]
- Ksenofontov, A.A.; Lukanov, M.M.; Bichan, N.G.; Khodov, I.A.; Kudryakova, N.O.; Ksenofontova, K.V.; Antina, E.V. Non-covalent supramolecular systems with photoinduced electron transfer based on zinc bis(dipyrromethenate)s and C60. Dyes Pigments 2021, 185, 108918. [Google Scholar] [CrossRef]
- Hirao, T.; Fujii, N.; Iwabe, Y.; Haino, T. Self-sorting behavior in supramolecular fullerene polymerization directed by host–guest complexation between calix[5]arene and C60. Chem. Commun. 2021, 57, 11831–11834. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Lu, M.; Zhang, X.; Li, H.; Zhang, X.; Yang, X.; Li, X.; Tu, Y.; Li, C.Y. Incorporating Pendent Fullerenes with High Refractive Index Backbones: A Conjunction Effect Method for High Refractive Index Polymers. Macromolecules 2015, 48, 8480–8488. [Google Scholar] [CrossRef]
- Volkov, V.I.; Chernyak, A.V.; Avilova, I.A.; Slesarenko, N.A.; Melnikova, D.L.; Skirda, V.D. Molecular and Ionic Diffusion in Ion Exchange Membranes and Biological Systems (Cells and Proteins) Studied by NMR. Membranes 2021, 11, 385. [Google Scholar] [CrossRef]
- Gianolio, E.; Ferrauto, G.; Di Gregorio, E.; Aime, S. Re-evaluation of the water exchange lifetime value across red blood cell membrane. Biochim. Biophys. Acta 2016, 1858, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, G.; Orädd, G. Lipid lateral diffusion and membrane heterogeneity. Biochim. Biophys. Acta 2009, 1788, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Waldeck, A.R.; Kuchel, P.W.; Lennon, A.J.; Chapman, B.E. NMR diffusion measurements to characterise membrane transport and solute binding. Prog. Nucl. Magn. Reson. Spectrosc. 1997, 30, 39–68. [Google Scholar] [CrossRef]
- Anselmi, C.; Bernardi, F.; Centini, M.; Gaggelli, E.; Gaggelli, N.; Valensin, D.; Valensin, G. Interaction of ferulic acid derivatives with human erythrocytes monitored by pulsed field gradient NMR diffusion and NMR relaxations studies. Chem. Phys. Lipids 2005, 134, 109–117. [Google Scholar] [CrossRef]
- Cho, C.-H.; Hong, Y.-S.; Kang, K.; Volkov, V.I.; Skirda, V.D.; Lee, C.-Y.J.; Lee, C.-H. Water self-diffusion in Chlorella sp. studied by pulse field gradient NMR. Magn. Reson. Imaging 2003, 21, 1009–1017. [Google Scholar] [CrossRef]
- Suh, K.-J.; Hong, Y.-S.; Skirda, V.D.; Volkov, V.I.; Lee, C.-Y.; Lee, C.-H. Water self-diffusion behavior in yeast cells studied by pulsed field gradient NMR. Biophys. Chem. 2003, 104, 121–130. [Google Scholar] [CrossRef]
- Avilova, I.A.; Vasil’ev, S.G.; Rimareva, L.V.; Serba, E.M.; Volkova, L.D.; Volkov, V.I. Water metabolism in cells of Saccharomyces cerevisiae of races Y-3137 and Y-3327, according to pulsed-field gradient NMR data. Russ. J. Phys. Chem. A 2015, 89, 710. [Google Scholar] [CrossRef]
- Avilova, I.A.; Smolina, A.V.; Kotelnikov, A.I.; Kotelnikova, R.A.; Loskutov, V.V.; Volkov, V.I. Self-diffusion of water and blood lipids in mouse erythrocytes. Appl. Magn. Reson. 2016, 47, 335–347. [Google Scholar] [CrossRef]
- Avilova, I.A.; Khakina, E.A.; Kraevaya, O.A.; Kotelnikov, A.I.; Kotelnikova, R.A.; Troshin, P.A.; Volkov, V.I. Self-diffusion of water-soluble fullerene derivatives in mouse erythrocytes. Biochim. Biophys. Acta BBA-Biomembr. 2018, 1860, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Spernol, A.; Wirtz, K. Zur Mikroreibung in Flüssigkeiten. Z. Nat. A 1953, 8, 522–532. [Google Scholar] [CrossRef]
- Gierer, A.; Wirtz, K. Molekulare Theorie der Mikroreibung. Z. Nat. A 1953, 8, 532–538. [Google Scholar] [CrossRef]
- Perrin, F. Mouvement brownien d’un ellipsoide—I. Dispersion diélectrique pour des molécules ellipsoidales. J. Phys. Radium 1936, 7, 497–511. [Google Scholar] [CrossRef]
- Edward, J.T. Molecular volumes and the Stokes-Einstein equation. J. Chem. Educ. 1970, 47, 261. [Google Scholar] [CrossRef]
- Kawasaki, S.; Aketa, T.; Touhara, H.; Okino, F.; Boltalina, O.V.; Gol’dt, I.V.; Troyanov, S.I.; Taylor, R. Crystal Structures of the Fluorinated Fullerenes C60F36 and C60F48. J. Phys. Chem. B 1999, 103, 1223. [Google Scholar] [CrossRef]
- Chernyak, A.V.; Avilova, I.A.; Khakina, E.A.; Mumyatov, A.V.; Zabrodin, V.A.; Troshin, P.A.; Volkov, V.I. Supramolecular Self-Organization of Fullerene Derivatives in Solutions Studied by Pulsed Field Gradient NMR Technique. Appl. Magn. Reson. 2016, 47, 859–868. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Susarova, D.K.; Mukhacheva, O.A.; Troshin, P.A.; Razumov, V.F. Fullerene Derivatives with Reduced Electron Affinity and a Photovoltaic Cell Using the Same. European Patent Application No. EP 12180839.8A, 17 August 2012. [Google Scholar]
- Mumyatov, A.V.; Goryachev, A.E.; Prudnov, F.A.; Mukhacheva, O.A.; Sagdullina, D.K.; Chernyak, A.V.; Troyanov, S.I.; Troshin, P.A. Monocyclopropanated fullerene derivatives with decreased electron affinity as promising electron acceptor materials for organic solar cells. Synth. Met. 2020, 270, 116565. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Knight, B.W.; LePeq, F.; Wudl, F.; Yao, J.; Wilkins, C.L. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60, 532–538. [Google Scholar] [CrossRef]
- Voronov, I.I.; Martynenko, V.M.; Chernyak, A.V.; Balzarini, J.; Schols, D.; Troshin, P.A. Synthesis and Antiviral Activity of Water-Soluble Polycarboxylic Derivatives of [60]Fullerene Loaded with 3,4-Dichlorophenyl Units. Chem. Biodivers. 2018, 15, e1800293. [Google Scholar] [CrossRef]
- Hsieh, F.-Y.; Zhilenkov, A.V.; Voronov, I.I.; Khakina, E.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.-h. Water-soluble fullerene derivatives as brain drugs: Surface chemistry determines neuroprotective and antitumor effects. ACS Appl. Mater. Interfaces 2017, 9, 11482–11492. [Google Scholar] [CrossRef] [PubMed]
- Khakina, E.A.; Yurkova, A.A.; Peregudov, A.S.; Troyanov, S.I.; Trush, V.; Vovk, A.I.; Mumyatov, A.V.; Martynenko, V.M.; Balzarini, J.; Troshin, P.A. Highly selective reactions of C60Cl6 with thiols for synthesis of functionalized [60]fullerene derivatives. Chem. Commun. 2012, 48, 7158–7160. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.-W.; Zhilenkov, A.V.; Kraevaya, O.A.; Mischenko, D.V.; Troshin, P.A.; Hsu, S.-h. Toward Understanding the Antitumor Effects of Water-Soluble Fullerene Derivatives on Lung Cancer Cells: Apoptosis or Autophagy Pathways? J. Med. Chem. 2019, 62, 7111–7125. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, A. Gradient NMR Method for Studies of Water Translational Diffusion in Plants. Membranes 2021, 11, 487. [Google Scholar] [CrossRef] [PubMed]
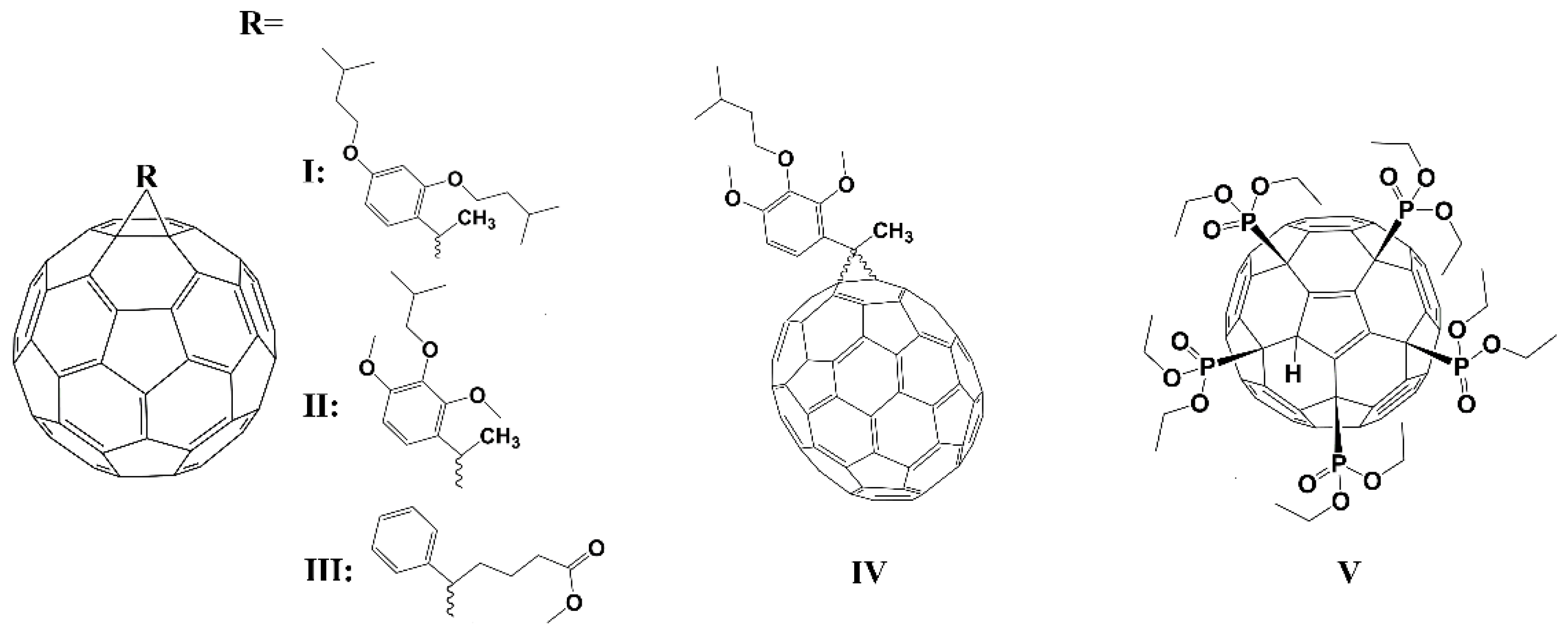
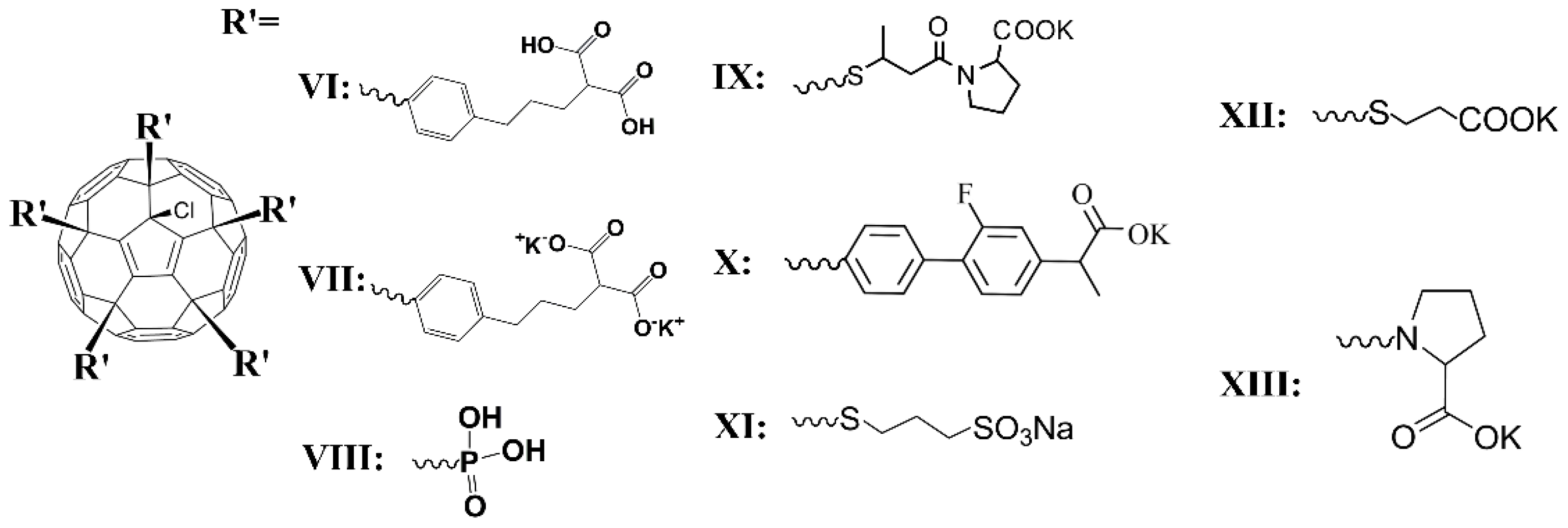
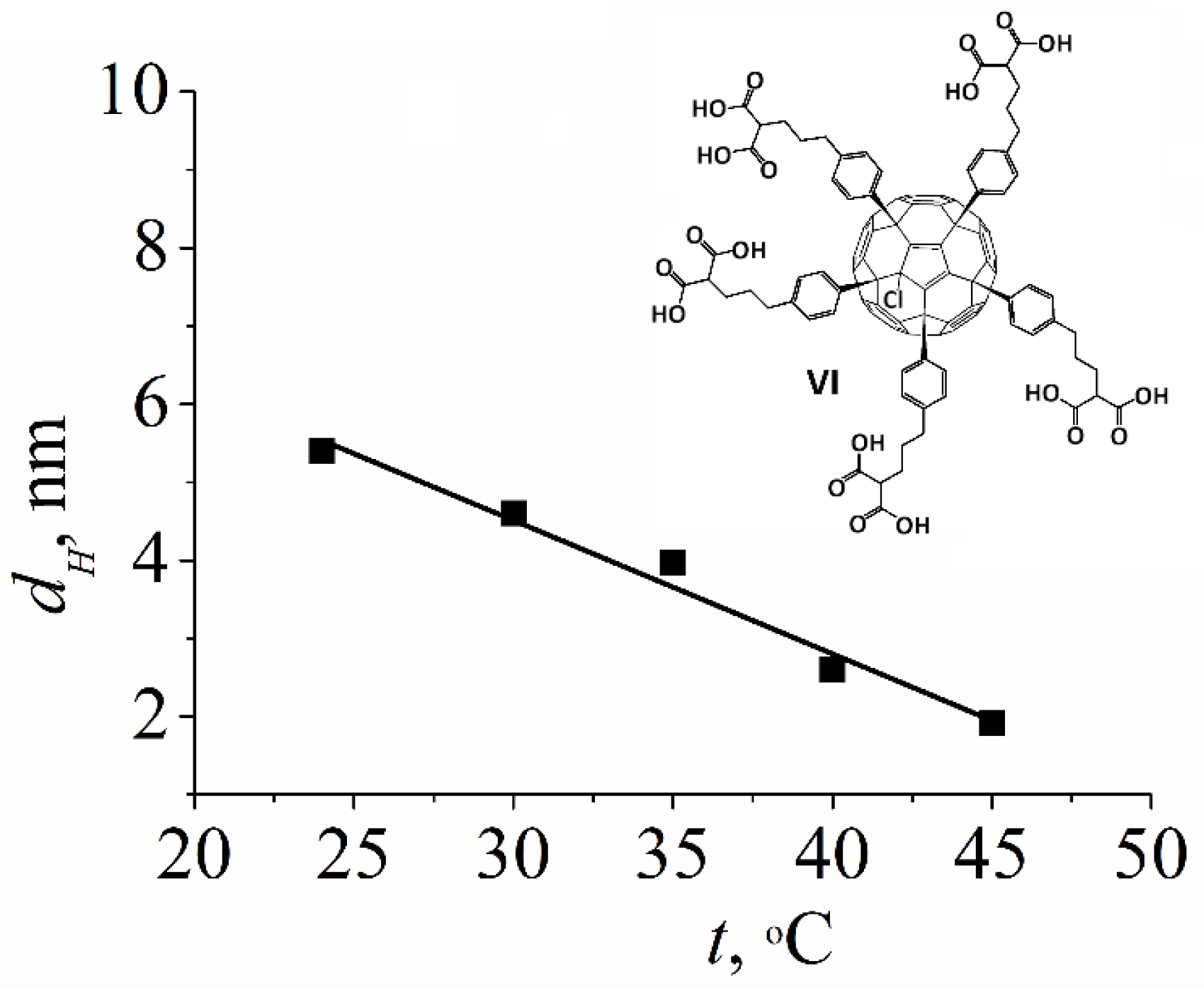
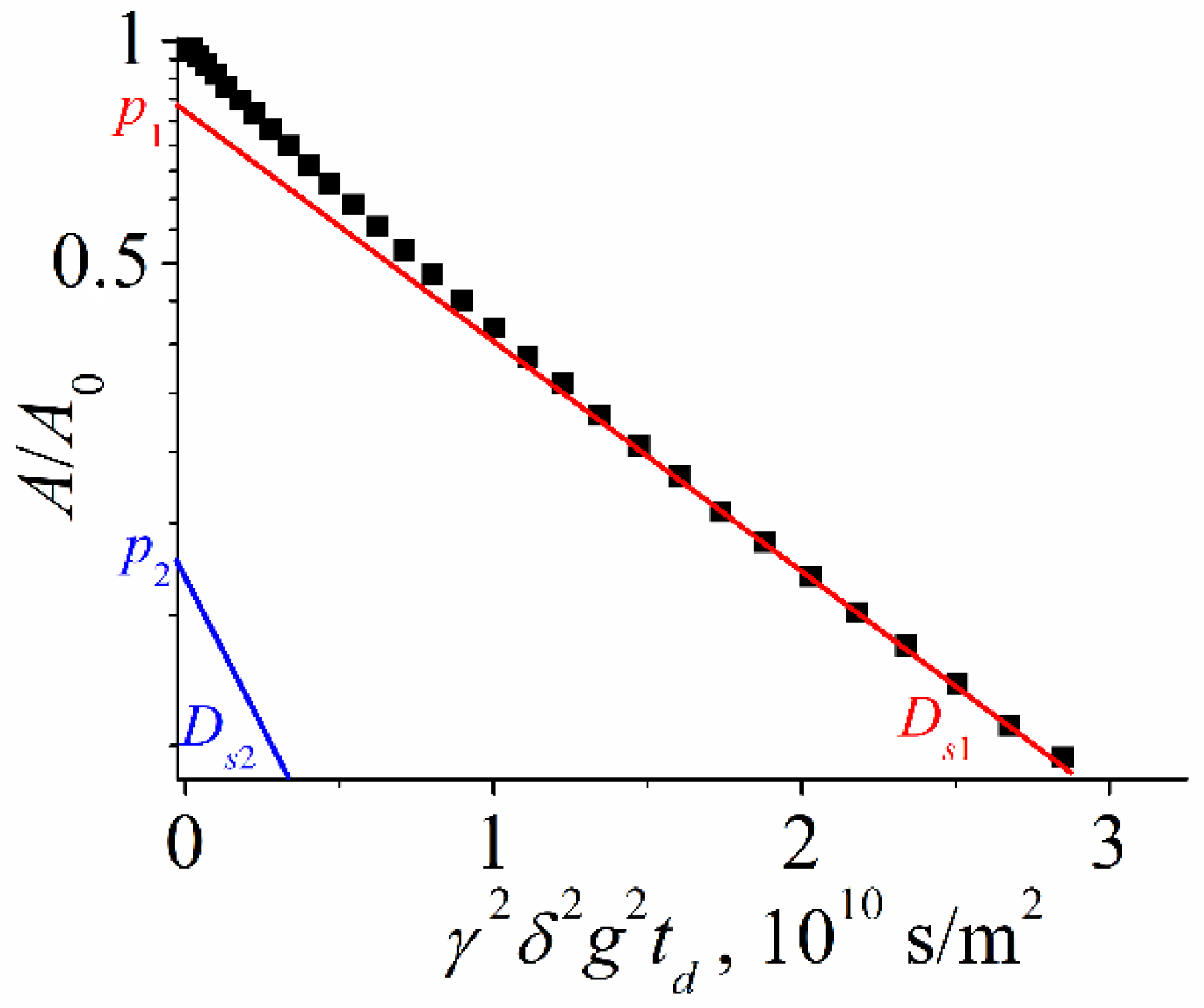



| Compound | Solvent | Ds, m2/s | dH, nm |
|---|---|---|---|
| I | CDCl3 | 5.9·10−10 | 1.3 ± 0.1 |
| CS2 | 1.0·10−9 | 1.2 ± 0.1 | |
| II | CDCl3 | 6.3·10−10 | 1.3 ± 0.1 |
| CS2 | 1.1·10−9 | 1.2 ± 0.1 | |
| C6D5CD3 | 6.6·10−10 | 1.2 ± 0.1 | |
| III | CDCl3 | 6.9·10−10 | 1.2 ± 0.1 |
| IV | C6D5CD3 | 6.1·10−10 | 1.2 ± 0.1 |
| V | CDCl3 | 5.8·10−10 | 1.4 ± 0.1 |
| Solvent | Ds, m2/s | dH, nm |
|---|---|---|
| acetone-d6 | 5.7·10−10 | 2.4 ± 0.2 |
| DMSO | 8.5·10−11 | 2.3 ± 0.2 |
| The molar ratio DMSO:D2O = 3:1 | 2.1·10−11 | 5.4 ± 0.5 |
| The molar ratio DMSO:D2O = 1:1 | 2.3·10−11 | 6.3 ± 0.6 |
| The molar ratio DMSO:D2O = 1:3 | 2.3·10−11 | 9.6 ± 0.9 |
| C, mg/mL | Ds, m2/s | p | dH, nm | |||
|---|---|---|---|---|---|---|
| Ds1 | Ds2 | p1 | p2 | dH1 | dH2 | |
| 6 | 7.1·10−11 | 2.1·10−10 | 0.60 | 0.40 | 5.5 ± 0.5 | 1.8 ± 0.2 |
| 12 | 6.6·10−11 | 1.7·10−10 | 0.70 | 0.30 | 5.9 ± 0.6 | 2.3 ± 0.2 |
| 14 | 7.1·10−11 | 1.7·10−10 | 0.80 | 0.20 | 5.5 ± 0.5 | 2.3 ± 0.2 |
| 24 | 6.9·10−11 | 2.0·10−10 | 0.87 | 0.13 | 5.7 ± 0.6 | 1.9 ± 0.2 |
| C, mg/mL | 1H | 19F | ||
|---|---|---|---|---|
| dH1, nm | dH2, nm | dH1, nm | dH2, nm | |
| 6 | 7.6 ± 0.4 | 0.8 ± 0.1 | 7.6 ± 0.4 | 1.0 ± 0.1 |
| 8 | 7.6 ± 0.4 | 1.0 ± 0.1 | 7.8 ± 0.4 | 1.0 ± 0.1 |
| 12 | 7.4 ± 0.4 | 1.1 ± 0.1 | 8.2 ± 0.4 | 1.1 ± 0.1 |
| 16 | 7.6 ± 0.4 | 1.1 ± 0.1 | 8.0 ± 0.4 | 1.1 ± 0.1 |
| 24 | 8.0 ± 0.4 | 1.3 ± 0.1 | 8.4 ± 0.4 | 1.6 ± 0.1 |
| 30 | 7.9 ± 0.4 | 1.5 ± 0.1 | 8.8 ± 0.4 | 1.9 ± 0.1 |
| 50 | 9.8 ± 0.4 | 2.4 ± 0.1 | 10.6 ± 0.4 | 2.4 ± 0.1 |
| Compound | Aqueous Solution | RBCs Suspension | |||
|---|---|---|---|---|---|
| Ds1w·1011, m2/s | Ds2w·1010, m2/s | Ds1s·1012, m2/s | Ds2s·1011, m2/s | Ds3s·1010, m2/s | |
| XI | 7.4 ± 0.7 | 4.1 ± 0.4 | 5.5 ± 0.8 | 3.9 ± 0.6 | 5.5 ± 0.8 |
| XII | 7.5 ± 1.5 | 4.3 ± 0.8 | 5.0 ± 1.0 | 4.4 ± 0.9 | 7.1 ± 1.4 |
| XII | 4.9 ± 0.5 | 1.2 ± 0.1 | 6.0 ± 1.0 | 3.8 ± 0.6 | 8.0 ± 1.0 |
| Compound | Ds1s·1012, m2/s | p1(0) | τ, ms |
|---|---|---|---|
| XI | 5.5 ± 0.8 | 0.33 | 440 ± 70 |
| XII | 5.0 ± 1.0 | 0.13 | 470 ± 70 |
| XIII | 6.0 ± 1.0 | 0.06 | 1200 ± 300 |
| C, M | τ1, ms |
|---|---|
| 1.99·10−3 | 280 ± 15 |
| 9.12·10−3 | 300 ± 20 |
| 1.19·10−2 | 440 ± 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avilova, I.A.; Chernyak, A.V.; Soldatova, Y.V.; Mumyatov, A.V.; Kraevaya, O.A.; Khakina, E.A.; Troshin, P.A.; Volkov, V.I. Self-Organization of Fullerene Derivatives in Solutions and Biological Cells Studied by Pulsed Field Gradient NMR. Int. J. Mol. Sci. 2022, 23, 13344. https://doi.org/10.3390/ijms232113344
Avilova IA, Chernyak AV, Soldatova YV, Mumyatov AV, Kraevaya OA, Khakina EA, Troshin PA, Volkov VI. Self-Organization of Fullerene Derivatives in Solutions and Biological Cells Studied by Pulsed Field Gradient NMR. International Journal of Molecular Sciences. 2022; 23(21):13344. https://doi.org/10.3390/ijms232113344
Chicago/Turabian StyleAvilova, Irina A., Alexander V. Chernyak, Yuliya V. Soldatova, Alexander V. Mumyatov, Olga A. Kraevaya, Ekaterina A. Khakina, Pavel A. Troshin, and Vitaliy I. Volkov. 2022. "Self-Organization of Fullerene Derivatives in Solutions and Biological Cells Studied by Pulsed Field Gradient NMR" International Journal of Molecular Sciences 23, no. 21: 13344. https://doi.org/10.3390/ijms232113344
APA StyleAvilova, I. A., Chernyak, A. V., Soldatova, Y. V., Mumyatov, A. V., Kraevaya, O. A., Khakina, E. A., Troshin, P. A., & Volkov, V. I. (2022). Self-Organization of Fullerene Derivatives in Solutions and Biological Cells Studied by Pulsed Field Gradient NMR. International Journal of Molecular Sciences, 23(21), 13344. https://doi.org/10.3390/ijms232113344









