Therapeutic Potential of Targeting the Oxytocinergic System
Author Contributions
Funding
Conflicts of Interest
References
- Grinevich, V.; Knobloch-Bollmann, H.S.; Eliava, M.; Busnelli, M.; Chini, B. Assembling the Puzzle: Pathways of Oxytocin Signaling in the Brain. Biol. Psychiatry 2016, 79, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Althammer, F.; Grinevich, V. Diversity of oxytocin neurons: Beyond magno- and parvocellular cell types? J. Neuroendocrinol. 2017, 30, e12549. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef]
- Friuli, M.; Eramo, B.; Valenza, M.; Scuderi, C.; Provensi, G.; Romano, A. Targeting the Oxytocinergic System: A Possible Pharmacological Strategy for the Treatment of Inflammation Occurring in Different Chronic Diseases. Int. J. Mol. Sci. 2021, 22, 10250. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Escobar, J.B.; Mendelowitz, D. Sex Differences in the Hypothalamic Oxytocin Pathway to Locus Coeruleus and Augmented Attention with Chemogenetic Activation of Hypothalamic Oxytocin Neurons. Int. J. Mol. Sci. 2021, 22, 8510. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Pérez, C.; Reguilón, M.D.; Miñarro, J.; Rodríguez-Arias, M. Oxytocin Signaling as a Target to Block Social Defeat-Induced Increases in Drug Abuse Reward. Int. J. Mol. Sci. 2021, 22, 2372. [Google Scholar] [CrossRef] [PubMed]
- Sundar, M.; Patel, D.; Young, Z.; Leong, K.C. Oxytocin and Addiction: Potential Glutamatergic Mechanisms. Int. J. Mol. Sci. 2021, 22, 2405. [Google Scholar] [CrossRef] [PubMed]
- Laviola, G.; Busdraghi, L.M.; Meschino, N.; Petrella, C.; Fiore, M. Aberrant Early in Life Stimulation of the Stress-Response System Affects Emotional Contagion and Oxytocin Regulation in Adult Male Mice. Int. J. Mol. Sci. 2021, 22, 5039. [Google Scholar] [CrossRef] [PubMed]
- Baldi, E.; Costa, A.; Rani, B.; Passani, M.B.; Blandina, P.; Romano, A.; Provensi, G. Oxytocin and Fear Memory Extinction: Possible Implications for the Therapy of Fear Disorders? Int. J. Mol. Sci. 2021, 22, 10000. [Google Scholar] [CrossRef]
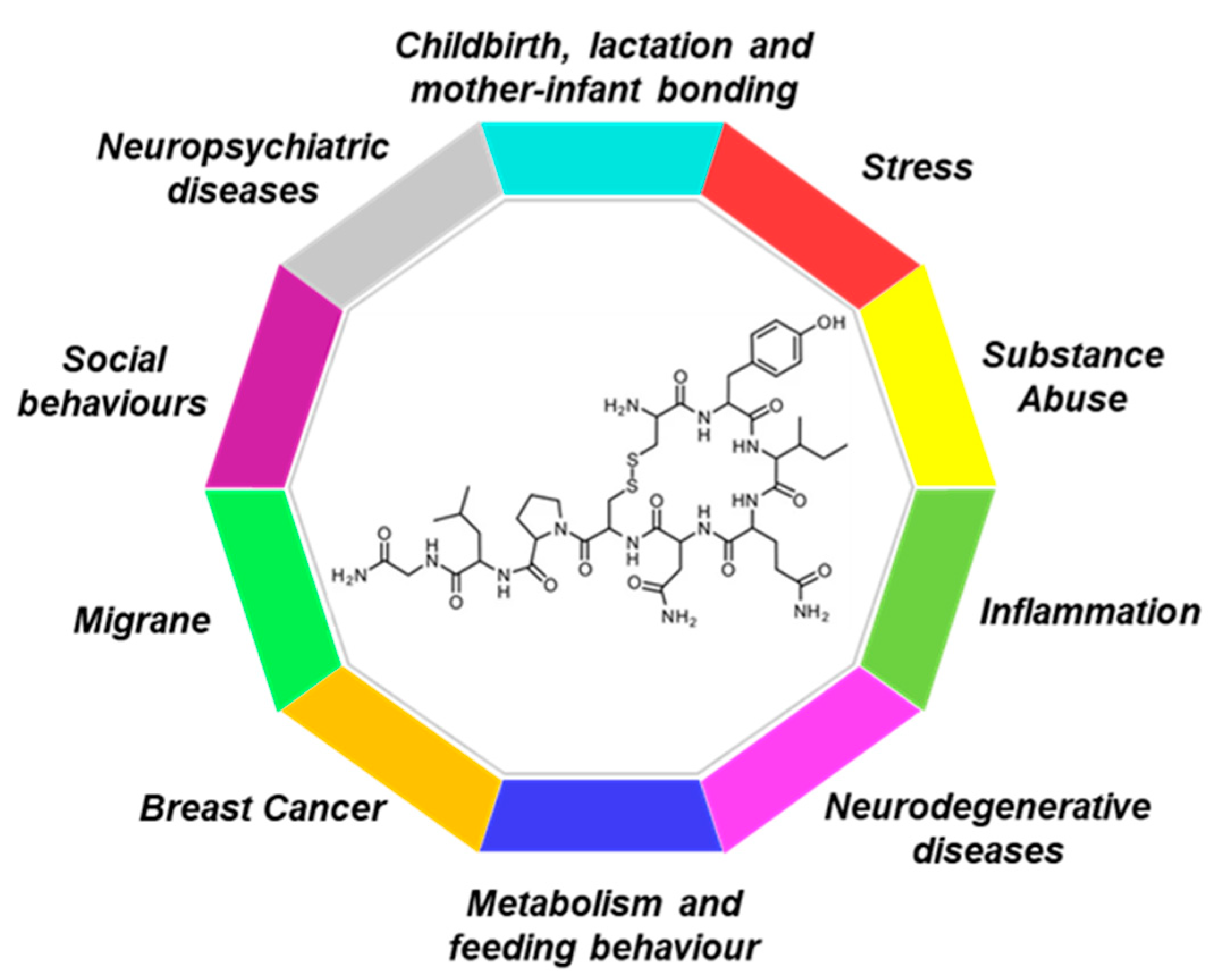
| Condition | Sientific Evidence | Preclinical | Clinical |
|---|---|---|---|
| Breast Cancer | Potential protective role in breast cancer | X | X |
| Childbirth, lactation and mother-infant bonding | Induction of labour | X | |
| Involution of the post-partum uterus through tonal contractility of the uterine muscles | X | X | |
| Stimulation of milk release from the mammary glands through the lactiferous ducts | X | X | |
| Facilitation of maternal care behaviors | X | X | |
| Potential role in preventing mother’s PTSD following childbirth | X | X | |
| Inflammation | Alleviation of oxidative stress and inflammation murine model of ASD | X | |
| Improvement of inflammation on ovarian tumor cells and after I/R injury | X | ||
| Control of peripheral and central inflammatory response occurring in chronic diseases | X | X | |
| Metabolism and feeding behaviour | Increase in energy expenditure and lipolysis and improvement of glucose homeostasis | X | |
| Decrease in the intake of palatable and sweet foods | X | ||
| Anabolic effect on lean mass and bone mineral density | X | ||
| Reduction in caloric intake, increase in fat oxidation, improvement of insulin sensitivity | X | X | |
| Modulation of both homeostatic and reward-driven food intake | X | X | |
| Migraine | Reduction in pain, decrease in the frequency of headaches in both chronic and high frequency episodic migraineurs | X | |
| Neuropsychiatric diseases | Improvement of the symptoms associated with depression (sleep disorders and anhedonia) | X | |
| Increase in social reward and working memory in PTSD patients | X | ||
| Improvement of the social function of autistic patients (processing of social information, empathy and social communication ability) | X | ||
| Amelioration of both the positive and negative symptoms and restoration of social cognitive deficits in patients with schizophrenia | X | ||
| Anxiolytic and antidepressant effects | X | X | |
| Neurodegenerative diseases | Neuroprotective potential in Alzheimer’s disease by restoring cognition and suppressing β-amyloid, Tau accumulation, and neuronal death | X | |
| Amelioration of the locomotor disabilities and anxiety-like behaviors and improvement of oxidative stress in MPTP mice | X | ||
| Social behaviours | Decrease hyper aggressive behaviour, accompanied by an increase in social contact | X | |
| Potential inhibition of aggression and violent behavior | X | ||
| Pro-social effect, Modulation of fear mmemory extinction | X | X | |
| Substance Abuse | Decrease in locomotor hyperactivity and stereotyped behaviours after exposure to psychostimulants such as cocaine and methamphetamine | X | |
| Anticraving properties in drug addiction (including alcohol and opioids) | X | X | |
| Promoting of positive social interactions and social reward in drug addiction | X | X | |
| Stress | Promotion of neuronal regeneration processes, rescuing the suppression of neurogenesis processes induced by prolonged exposure to stress episodes and glucocorticoids; | X | |
| Modulation of stress responses and facilitation of adaptation to stress | X | X |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Provensi, G. Therapeutic Potential of Targeting the Oxytocinergic System. Int. J. Mol. Sci. 2022, 23, 13295. https://doi.org/10.3390/ijms232113295
Romano A, Provensi G. Therapeutic Potential of Targeting the Oxytocinergic System. International Journal of Molecular Sciences. 2022; 23(21):13295. https://doi.org/10.3390/ijms232113295
Chicago/Turabian StyleRomano, Adele, and Gustavo Provensi. 2022. "Therapeutic Potential of Targeting the Oxytocinergic System" International Journal of Molecular Sciences 23, no. 21: 13295. https://doi.org/10.3390/ijms232113295
APA StyleRomano, A., & Provensi, G. (2022). Therapeutic Potential of Targeting the Oxytocinergic System. International Journal of Molecular Sciences, 23(21), 13295. https://doi.org/10.3390/ijms232113295





