Ex Vivo Treatment with Allogenic Mesenchymal Stem Cells of a Healthy Donor on Peripheral Blood Mononuclear Cells of Patients with Severe Alopecia Areata: Targeting Dysregulated T Cells and the Acquisition of Immunotolerance
Abstract
1. Introduction
2. Results
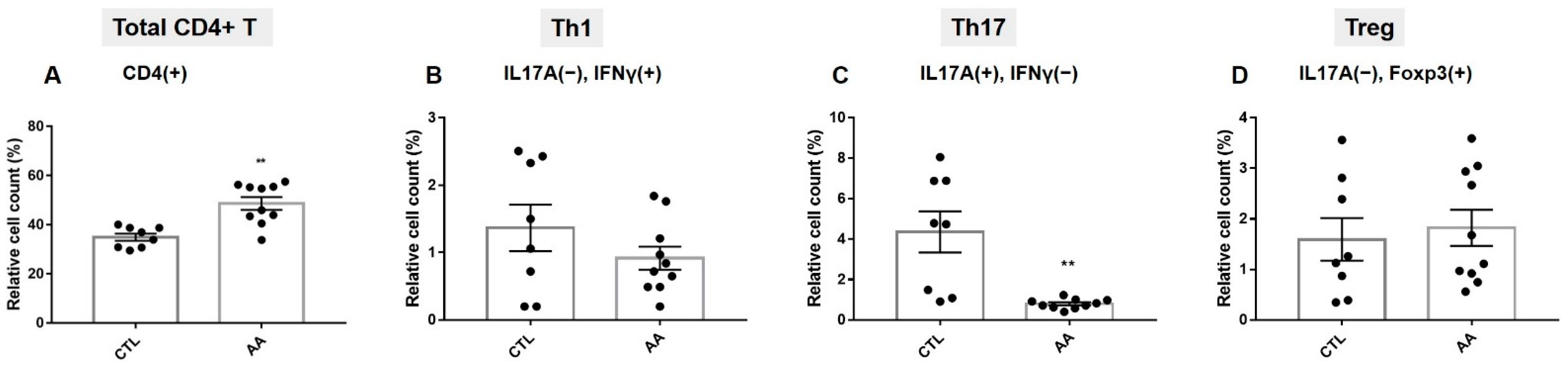
2.1. T Cell Population Characteristics in Severe AA Patients
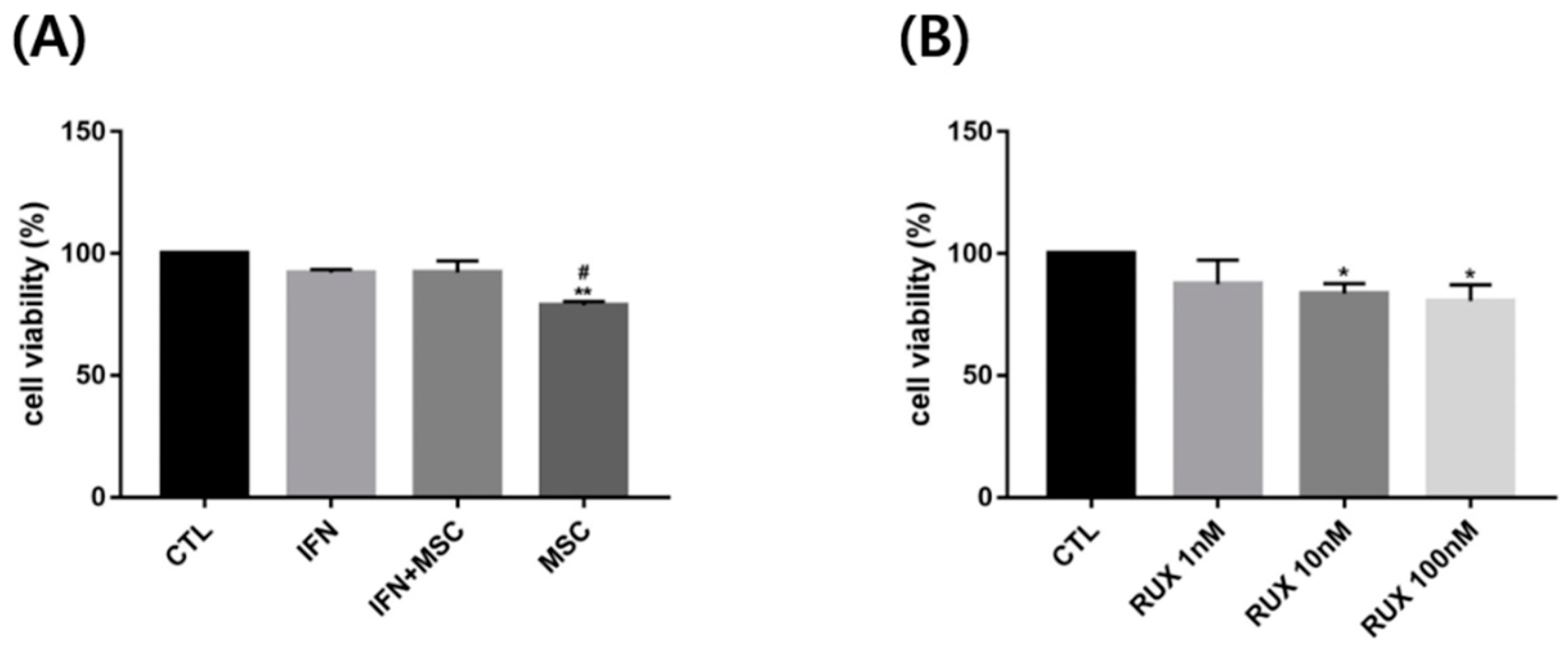
2.2. Effects of Allogenic hMSCs on T Cell Differentiation in AA Patients
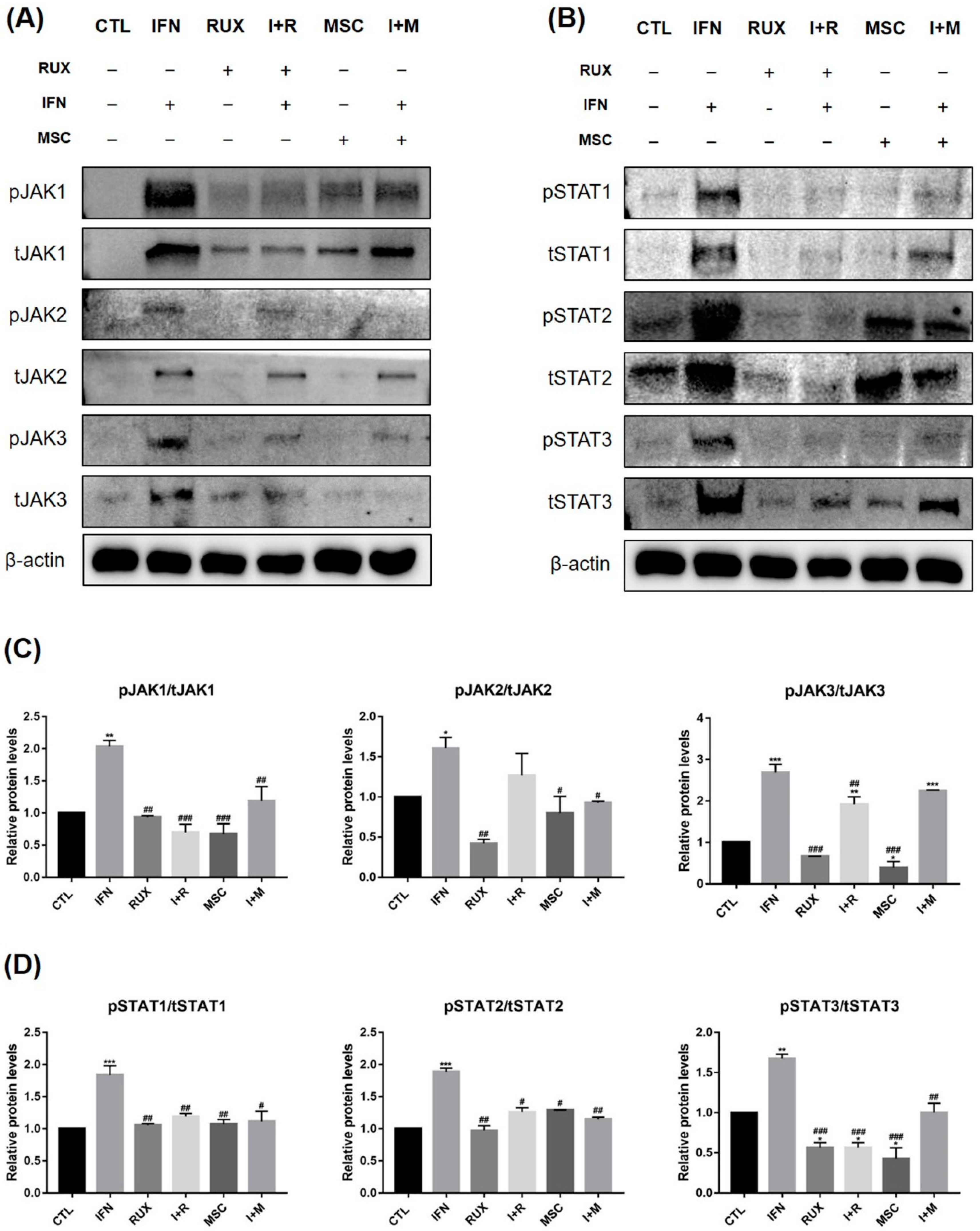
2.3. Effects of Allogenic hMSCs on the Protein Expression of JAK/STAT Pathway-Related Molecules in IFN-γ-Treated PBMCs
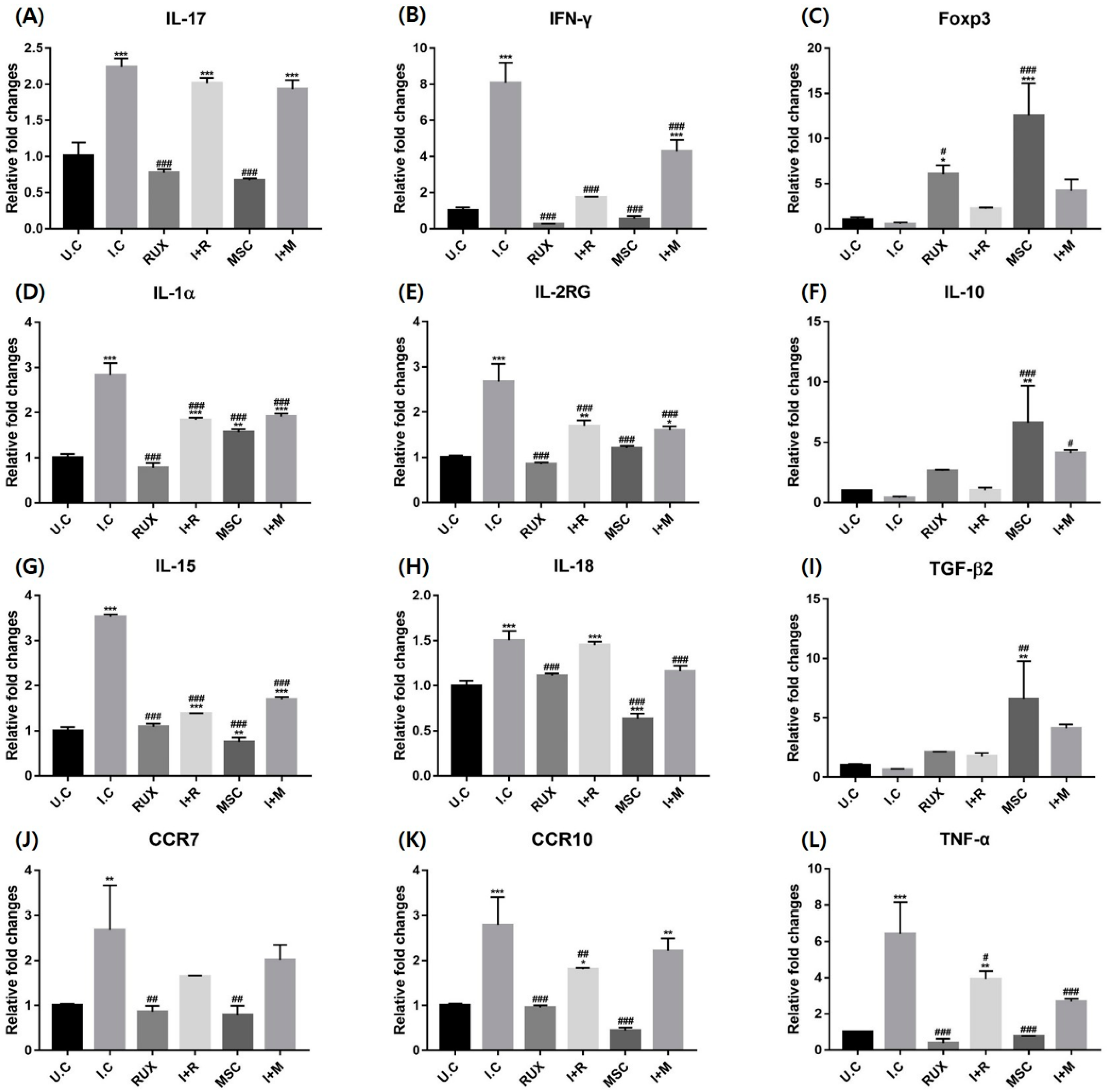
2.4. Effects of Allogenic hMSCs on the mRNA Expression of T Cell Differentiation and Inflammatory Markers in IFN-γ-Treated PBMCs
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Blood Samples
4.2. PBMC Isolation
4.3. hMSC Culture
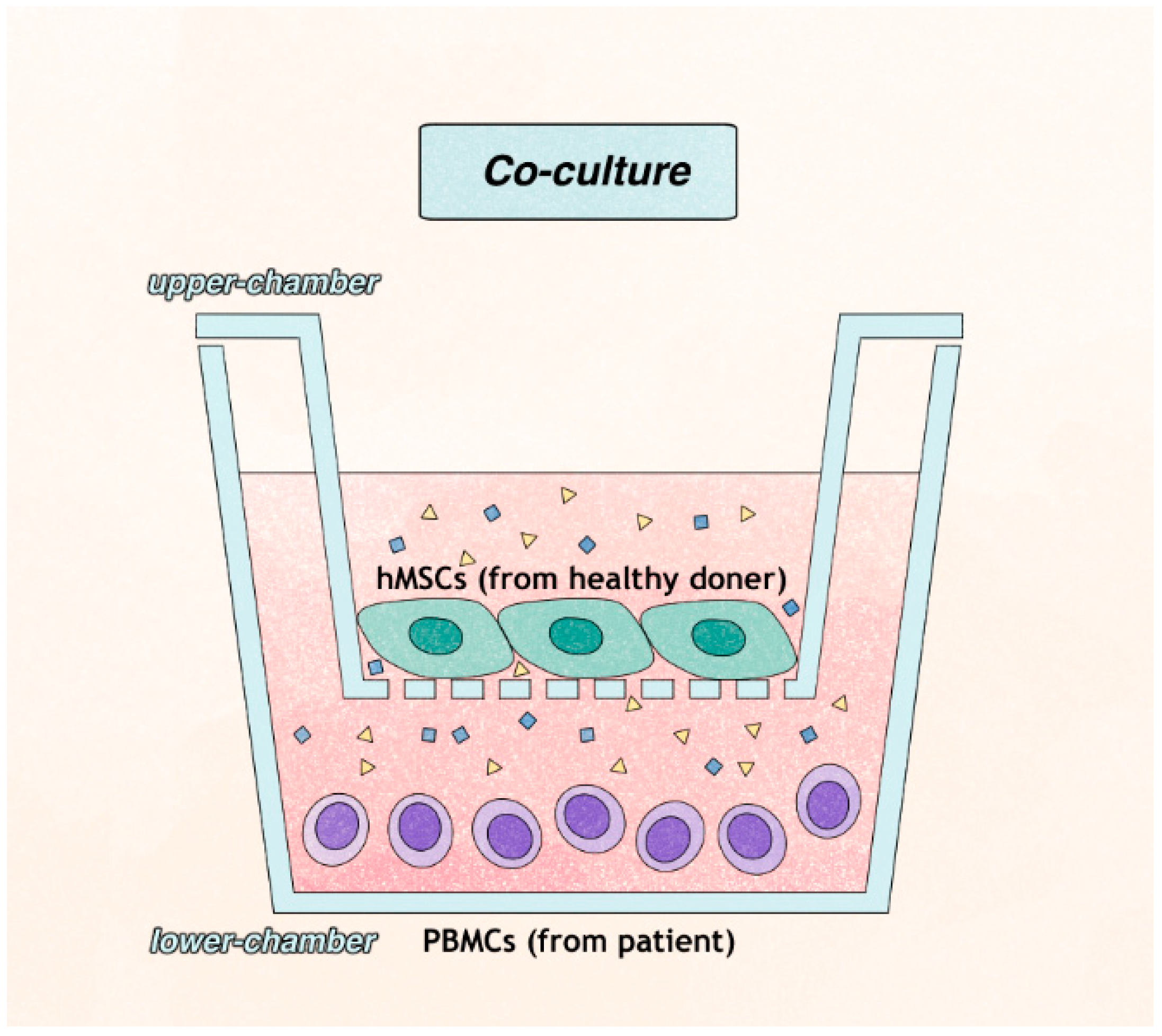
4.4. T Cell Preparation and Co-Culture with hMSCs
4.5. Flow Cytometry
4.6. In Vitro AA Modeling with PBMCs from a Healthy Donor
4.7. Cell Viability Assay
4.8. Real-Time PCR
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bertolini, M.; McElwee, K.; Gilhar, A.; Bulfone-Paus, S.; Paus, R. Hair follicle immune privilege and its collapse in alopecia areata. Exp. Dermatol. 2020, 29, 703–725. [Google Scholar] [CrossRef] [PubMed]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-wide association study in alopecia areata implicates both innate and adaptive immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Gilhar, A.; Kalish, R.S. Alopecia areata: A tissue specific autoimmune disease of the hair follicle. Autoimmun. Rev. 2006, 5, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Strazzulla, L.C.; Wang, E.H.C.; Avila, L.; Lo Sicco, K.; Brinster, N.; Christiano, A.M.; Shapiro, J. Alopecia areata: An appraisal of new treatment approaches and overview of current therapies. J. Am. Acad. Dermatol. 2018, 78, 15–24. [Google Scholar] [CrossRef]
- Xing, L.; Dai, Z.; Jabbari, A.; Cerise, J.E.; Higgins, C.A.; Gong, W.; de Jong, A.; Harel, S.; DeStefano, G.M.; Rothman, L.; et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat. Med. 2014, 20, 1043–1049. [Google Scholar] [CrossRef]
- Kennedy Crispin, M.; Ko, J.M.; Craiglow, B.G.; Li, S.; Shankar, G.; Urban, J.R.; Chen, J.C.; Cerise, J.E.; Jabbari, A.; Winge, M.C.; et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight 2016, 1, e89776. [Google Scholar] [CrossRef]
- Phan, K.; Sebaratnam, D.F. JAK inhibitors for alopecia areata: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 850–856. [Google Scholar] [CrossRef]
- Gong, Y.; Zhao, Y.; Zhang, X.; Qi, S.; Li, S.; Ye, Y.; Yang, J.; Caulloo, S.; McElwee, K.J.; Zhang, X. Serum level of IL-4 predicts response to topical immunotherapy with diphenylcyclopropenone in alopecia areata. Exp. Dermatol. 2020, 29, 231–238. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, J.H.; Ban, D.H.; Byun, J.W.; Shin, J.; Choi, G.S. Can the Cytokine Analysis of the Scales on Alopecic Patch Predict the Response to Diphenylcyclopropenone Treatment in Alopecia Areata Patients? Ann. Dermatol. 2018, 30, 150–157. [Google Scholar] [CrossRef]
- Kuin, R.A.; Spuls, P.I.; Limpens, J.; van Zuuren, E.J. Diphenylcyclopropenone in patients with alopecia areata. A critically appraised topic. Br. J. Dermatol. 2015, 173, 896–909. [Google Scholar] [CrossRef]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Luque-Campos, N.; Contreras-López, R.A.; Jose Paredes-Martínez, M.; Torres, M.J.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.J.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Elmaadawi, I.H.; Mohamed, B.M.; Ibrahim, Z.A.S.; Abdou, S.M.; El Attar, Y.A.; Youssef, A.; Shamloula, M.M.; Taha, A.; Metwally, H.G.; El Afandy, M.M.; et al. Stem cell therapy as a novel therapeutic intervention for resistant cases of alopecia areata and androgenetic alopecia. J. Dermatol. Treat 2018, 29, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, B.; Wang, H.; Li, H.; Li, Q.; Zhao, D.; Chen, Y.; Zhang, Y.; Li, W.; Zhang, J.; et al. Hair regrowth in alopecia areata patients following Stem Cell Educator therapy. BMC Med. 2015, 13, 87. [Google Scholar] [CrossRef]
- Czarnecka, A.; Odziomek, A.; Murzyn, M.; Dubis, J.; Bagłaj-Oleszczuk, M.; Hryncewicz-Gwóźdź, A. Wharton’s jelly-derived mesenchymal stem cells in the treatment of four patients with alopecia areata. Adv. Clin. Exp. Med. 2021, 30, 211–218. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, Y.; Wang, W.; Song, A.; Mukama, O.; Huang, J.; Han, X.; Deng, S.; Lin, Z.; Habimana, J.D.D.; et al. Hair follicle-derived mesenchymal stem cells decrease alopecia areata mouse hair loss and reduce inflammation around the hair follicle. Stem Cell. Res. Ther. 2021, 12, 548. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, H.T.; Byun, J.W.; Shin, J.; Choi, G.S. Clinical efficacy of adipocyte-derived stem cells conditioned media combined with micro-injury in refractory patch of alopecia areata. Arch. Dermatol. Res. 2021, 314, 527–532. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, S.H.; Park, H.R.; Lee, Y.; Kang, H.; Kim, J.E. Mesenchymal Stem Cells Antagonize IFN-Induced Proinflammatory Changes and Growth Inhibition Effects via Wnt/β-Catenin and JAK/STAT Pathway in Human Outer Root Sheath Cells and Hair Follicles. Int. J. Mol. Sci. 2021, 22, 4581. [Google Scholar] [CrossRef]
- Kim, J.E.; Oh, J.H.; Woo, Y.J.; Jung, J.H.; Jeong, K.H.; Kang, H. Effects of mesenchymal stem cell therapy on alopecia areata in cellular and hair follicle organ culture models. Exp. Dermatol. 2020, 29, 265–272. [Google Scholar] [CrossRef]
- Younes, A.K.; Hammad, R.; Othman, M.; Sobhy, A. CD4, CD8 and natural killer cells are depressed in patients with alopecia areata: Their association with disease activity. BMC Immunol. 2022, 23, 13. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yamada, Y.; Fujikawa, M.; Sekiguchi, K.; Uratsuji, H.; Mori, S.; Watanabe, H.; Matsumoto, T. Altered T cell subpopulations and serum anti-TYRP2 and tyrosinase antibodies in the acute and chronic phase of alopecia areata in the C3H/HeJ mouse model. J. Dermatol. Sci. 2021, 104, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, K.; Kozłowska, M.; Kaszuba, A.; Lesiak, A.; Narbutt, J.; Zalewska-Janowska, A. Increased Serum Levels of IFN-γ, IL-1β, and IL-6 in Patients with Alopecia Areata and Nonsegmental Vitiligo. Oxid. Med. Cell. Longev. 2020, 2020, 5693572. [Google Scholar] [CrossRef] [PubMed]
- Arca, E.; Muşabak, U.; Akar, A.; Erbil, A.H.; Taştan, H.B. Interferon-gamma in alopecia areata. Eur. J. Dermatol. 2004, 14, 33–36. [Google Scholar] [PubMed]
- Jabbari, A.; Cerise, J.E.; Chen, J.C.; Mackay-Wiggan, J.; Duvic, M.; Price, V.; Hordinsky, M.; Norris, D.; Clynes, R.; Christiano, A.M. Molecular signatures define alopecia areata subtypes and transcriptional biomarkers. EBioMedicine 2016, 7, 240–247. [Google Scholar] [CrossRef]
- Gilhar, A.; Laufer-Britva, R.; Keren, A.; Paus, R. Frontiers in alopecia areata pathobiology research. J. Allergy Clin. Immunol. 2019, 144, 1478–1489. [Google Scholar] [CrossRef]
- Shin, J.M.; Choi, D.K.; Sohn, K.C.; Koh, J.W.; Lee, Y.H.; Seo, Y.J.; Kim, C.D.; Lee, J.H.; Lee, Y. Induction of alopecia areata in C3H/HeJ mice using polyinosinic-polycytidylic acid (poly[I:C]) and interferon-gamma. Sci. Rep. 2018, 8, 12518. [Google Scholar] [CrossRef]
- Han, Y.M.; Sheng, Y.Y.; Xu, F.; Qi, S.S.; Liu, X.J.; Hu, R.M.; Miao, Y.; Huang, G.Q.; Yang, Q.P. Imbalance of T-helper 17 and regulatory T cells in patients with alopecia areata. J. Dermatol. 2015, 42, 981–988. [Google Scholar] [CrossRef]
- Hamed, F.N.; Åstrand, A.; Bertolini, M.; Rossi, A.; Maleki-Dizaji, A.; Messenger, A.G.; McDonagh, A.J.G.; Tazi-Ahnini, R. Alopecia areata patients show deficiency of FOXP3+CD39+ T regulatory cells and clonotypic restriction of Treg TCRβ-chain, which highlights the immunopathological aspect of the disease. PLoS ONE 2019, 14, e0210308. [Google Scholar]
- Luz-Crawford, P.; Kurte, M.; Bravo-Alegría, J.; Contreras, R.; Nova-Lamperti, E.; Tejedor, G.; Noël, D.; Jorgensen, C.; Figueroa, F.; Djouad, F.; et al. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell. Res. Ther. 2013, 4, 65. [Google Scholar] [CrossRef]
- Dai, Z.; Chen, J.; Chang, Y.; Christiano, A.M. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI. Insight 2021, 6, e142205. [Google Scholar] [CrossRef]
- Hsu, P.J.; Liu, K.J.; Chao, Y.Y.; Sytwu, H.K.; Yen, B.L. Assessment of the Immunomodulatory Properties of Human Mesenchymal Stem Cells (MSCs). J. Vis. Exp. 2015, 106, e53265. [Google Scholar] [CrossRef] [PubMed]

| Refractory AA Group (n = 10) | Control Group (n = 8) | p-Value | |
|---|---|---|---|
| Gender | 0.138 | ||
| Female | 6 (60) | 2 (25) | |
| Male | 4 (40) | 6 (75) | |
| Age, years | 43.8 ± 12.5 | 32.0 ± 4.8 | 0.018 |
| AA onset age, years | 33.4 ± 15.9 | ||
| Disease duration, months | 123.2 ± 88.4 |
| No. | Sex | Age, Years | SALT, % | AA Onset Age, Years | Disease Duration, Months | Previous Treatment for AA * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Systemic Steroid ** | Cyclosporine ** | ILI | DPCP | Oral Minoxidil | Topical Minoxidil | TCS | ||||||
| AA 1 | F | 58 | 53.7 | 52 | 72 | - | - | - | - | - | - | - |
| AA 2 | M | 36 | 59 | 15 | 252 | - | - | +,6 mo | - | - | +,6 mo | - |
| AA 3 | F | 25 | 100 | 18 | 84 | +,12 mo | +,12 mo | - | +,24 mo | - | - | - |
| AA 4 | M | 37 | 100 | 35 | 17 | - | - | +,2 mo | +,1 mo | - | - | + |
| AA 5 | F | 63 | 90 | 60 | 35 | - | +,1 mo | - | +,2 mo | - | + | - |
| AA 6 | F | 34 | 100 | 17 | 204 | - | - | - | +,84 mo | - | - | - |
| AA 7 | M | 37 | 96 | 33 | 40 | - | - | +,14 mo | - | - | - | - |
| AA 8 | M | 55 | 50 | 44 | 132 | - | - | - | - | +,24 mo | - | + |
| AA 9 | F | 53 | 63.8 | 41 | 144 | - | +,5 mo | +,2 mo | +,5 mo | - | - | + |
| AA 10 | F | 40 | 100 | 19 | 252 | - | - | - | +,12 mo | - | + | - |
| CELL SUBSET | Baseline Level | Responsiveness to Stimuli | Immunomodulation after Allogenic hMSCs |
|---|---|---|---|
| Th1 | (-) | ↑ | ↓ |
| Th17 | ↓ | (-) | ↓ |
| Treg | ↓ | ↑ | ↑↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Lee, Y.-J.; Lee, K.-J.; Park, S.-H.; Kang, H. Ex Vivo Treatment with Allogenic Mesenchymal Stem Cells of a Healthy Donor on Peripheral Blood Mononuclear Cells of Patients with Severe Alopecia Areata: Targeting Dysregulated T Cells and the Acquisition of Immunotolerance. Int. J. Mol. Sci. 2022, 23, 13228. https://doi.org/10.3390/ijms232113228
Kim J-E, Lee Y-J, Lee K-J, Park S-H, Kang H. Ex Vivo Treatment with Allogenic Mesenchymal Stem Cells of a Healthy Donor on Peripheral Blood Mononuclear Cells of Patients with Severe Alopecia Areata: Targeting Dysregulated T Cells and the Acquisition of Immunotolerance. International Journal of Molecular Sciences. 2022; 23(21):13228. https://doi.org/10.3390/ijms232113228
Chicago/Turabian StyleKim, Jung-Eun, Yu-Jin Lee, Kyung-Jae Lee, Song-Hee Park, and Hoon Kang. 2022. "Ex Vivo Treatment with Allogenic Mesenchymal Stem Cells of a Healthy Donor on Peripheral Blood Mononuclear Cells of Patients with Severe Alopecia Areata: Targeting Dysregulated T Cells and the Acquisition of Immunotolerance" International Journal of Molecular Sciences 23, no. 21: 13228. https://doi.org/10.3390/ijms232113228
APA StyleKim, J.-E., Lee, Y.-J., Lee, K.-J., Park, S.-H., & Kang, H. (2022). Ex Vivo Treatment with Allogenic Mesenchymal Stem Cells of a Healthy Donor on Peripheral Blood Mononuclear Cells of Patients with Severe Alopecia Areata: Targeting Dysregulated T Cells and the Acquisition of Immunotolerance. International Journal of Molecular Sciences, 23(21), 13228. https://doi.org/10.3390/ijms232113228







