Abstract
This study aims to investigate the effects of CLAUDIN-6 (CLDN6) on cell apoptosis and proliferation of bovine cumulus cells (CCs). Immunofluorescence staining was used to localize CLDN6 protein in CCs. Three pairs of siRNA targeting CLDN6 and one pair of siRNA universal negative sequence as control were transfected into bovine CCs. Then, the effective siRNA was screened by real-time quantitative PCR (RT-qPCR) and Western blotting. The mRNA expression levels of apoptosis related genes (CASPASE-3, BAX and BCL-2) and proliferation related genes (PCNA, CDC42 and CCND2) were evaluated by RT-qPCR in CCs with CLDN6 knockdown. Cell proliferation, apoptosis and cell cycle were detected by flow cytometry with CCK-8 staining, Annexin V-FITC staining and propidium iodide staining, respectively. Results showed that the CLDN6 gene was expressed in bovine CCs and the protein was localized in cell membranes and cytoplasms. After CLDN6 was knocked down in CCs, the cell apoptosis rate significantly decreased and the pro-apoptotic genes BAX and CASPASE-3 were down-regulated significantly, whereas the anti-apoptotic gene BCL-2 was markedly up-regulated (p < 0.05). Additionally, CLDN6 knockdown significantly enhanced cell proliferation of CCs at 72 h after siRNA transfection. The mRNA levels of proliferation-related genes PCNA, CCND2 and CDC42 increased obviously in CCs with CLDN6 knockdown (p < 0.05). After CLDN6 was down-regulated, the percentage of CCs at S phase was significantly increased (p < 0.05). However, there was no remarkable difference in the percentages of cells at the G0/G1 phase and G2/M phase between CCs with or without CLDN6 knockdown (p > 0.05). Therefore, the expression of CLDN6 and its effects on cell proliferation, apoptosis and cell cycle of bovine CCs were first studied. CLDN6 low expression inhibited cell apoptosis, induced cell proliferation and cell cycle arrest of bovine CCs.
1. Introduction
Cumulus cells (CCs) are important somatic cells surrounding oocytes and a specific subtype of granulosa cells which provide nutrition for oocyte maturation as well as ovarian development [1,2]. CCs’ apoptosis can cause follicular atresia and their proliferation is closely related to follicular development [3,4]. This delicate balance has a crucial impact on ovarian development and oocyte maturation. Studies on the function and regulation of CCs’ growth can provide an important theoretical basis for ovarian development, oocyte maturation and subsequent embryogenesis.
Tight junctions regulate the transport of ions, macromolecules and proteins through the paracellular pathway and provide barrier function for the endothelium and epithelium [5]. The CLAUDIN (CLDN) protein family is a key tight-knit transmembrane protein and plays an important role in cell adhesion, polarity, permeability and glandular differentiation [6,7]. Currently, there are 27 members in the CLDN family that are distributed in different organs and play diverse roles. For instance, CLDN1 is mainly located in the heart, brain and lungs [8], CLDN4 is located in the ovaries, pancreas and salivary glands [9], and CLDN6 is located in embryonic stem cells, the early embryonic epithelium and fetal tissues such as the stomach, pancreas, lungs, breasts, kidneys, colon and ovaries [10,11]. CLDN6, one of the earliest proteins expressed in embryonic stem cells, is dedicated to the fate of epithelial cells [12]. As a specific surface marker of mouse and human pluripotent stem cells, CLDN6 significantly decreased its expression level during early differentiation, which was consistent with the down-regulation of pluripotency markers genes Oct4, Sox2 and Nanog [13]. However, in epidermal supra-basal layers, CLDN6 overexpression resulted in a perturbed epidermal terminal differentiation program and hyper-proliferative squamous invaginations/cysts, replacing hair follicles and a lethal barrier dysfunction [14].
Additionally, CLDN6 protein was detected in mouse embryos at all stages before preimplantation. After activity of CLDN6 was inhibited, mouse embryos had no or an immature blastocoel cavity without expansion, and blastocyst formation was remarkably reduced [15]. In Xenopus laevis, knockdown of cldn6 gene led to severe defects in pronephros tubular morphogenesis and blocked the terminal differentiation of tubule cells, which characterized by failure of apical accumulation of actin, reduced lateral expression of tight junction protein Na/K-ATPase [16].
Recently, the role of CLDN6 in cancer has attracted extensive attention from researchers as an ideal therapeutic target. Strong evidence indicates that CLDN6 is rarely expressed in healthy adult tissues. The altered expression of CLDN6 was linked to the development of various cancers whose malignant phenotypes include proliferation and apoptosis, migration and invasion and drug resistance [17]. In cervical cancer cells, the expression of CLDN6 was down-regulated and overexpression of CLDN6 suppressed cell proliferation, colony formation in vitro and tumor growth in vivo, which were accompanied and potentially caused by the promotion of tumor cell apoptosis [18]. However, in gastric cancer tissues and cell lines, the expression of CLDN6 protein was up-regulated and an increased CLDN6 level was associated with enhanced proliferation and invasion abilities of gastric cancer [19]. Similarly, CLDN6 was up-regulated in endometrial carcinoma compared with normal tissues and cell lines. Knockdown of CLDN6 significantly inhibited cell proliferation and the colony numbers of human endometrial adenocarcinoma cells via remarkable reduction of p-AKT, p-PI3K, and mTOR expression levels [20]. In summary, there is controversy in the different expression levels of CLDN6 in different cancers. However, it was confirmed that the variety of CLDN6 expression was associated with the occurrence of cancers.
At present, studies on the function of CLDN6 is mainly related to mice and humans, whereas those concerning livestock are rarely reported. Although CLDN6 takes part in cell apoptosis and proliferation in cancers, its regulatory role in bovine CCs is unclear. In this study, we first detected the mRNA and protein expression of CLDN6 in bovine CCs. Then cell apoptosis, proliferation, cell cycle and the expression levels of related genes were determined in bovine CCs with or without CLDN6 knockdown. Our finding will provide a theoretical basis to clarify the regulation effects of CLDN6 on cumulus cell growth in livestock.
2. Results
2.1. Expression of CLDN6 Gene and Protein in Bovine CCs
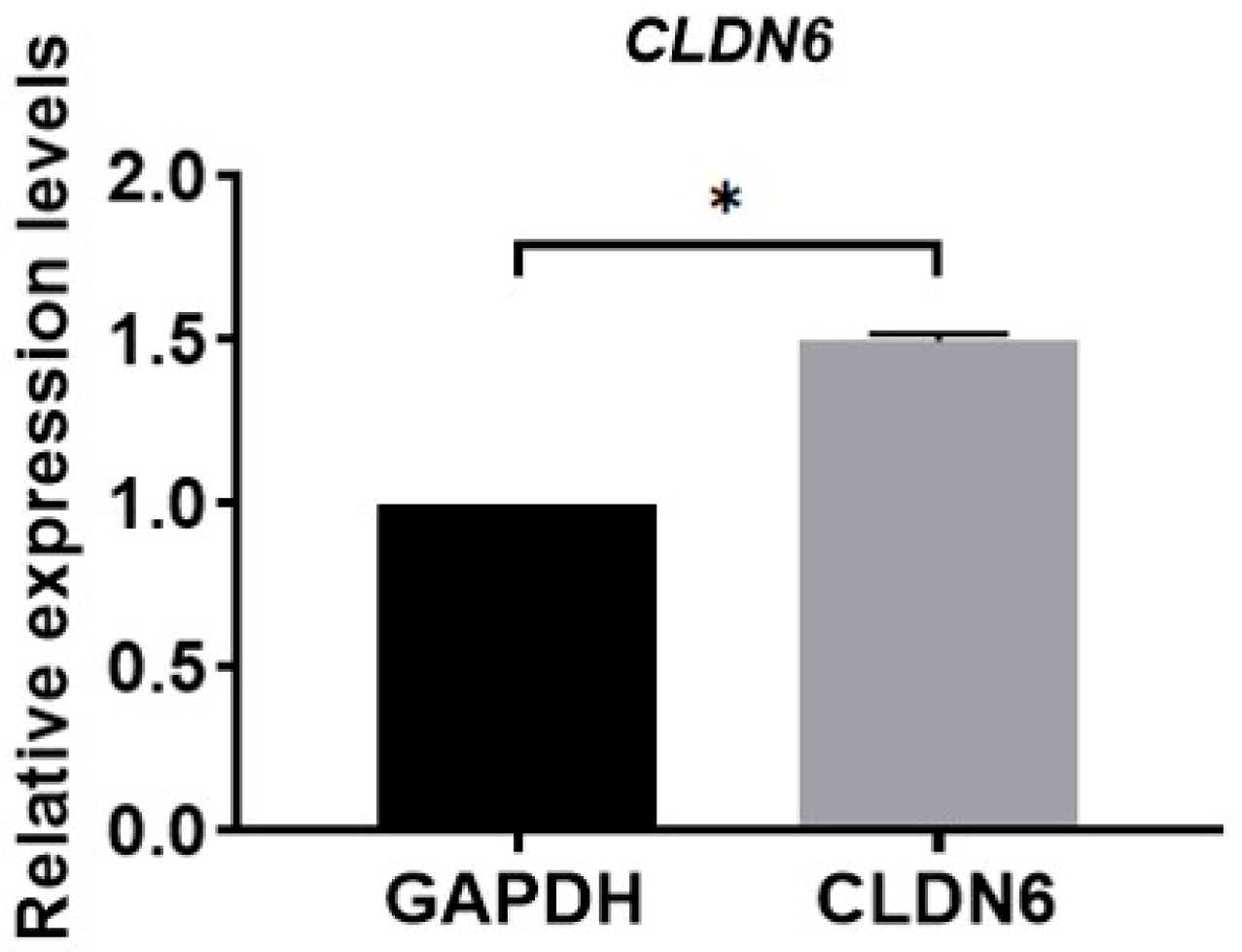
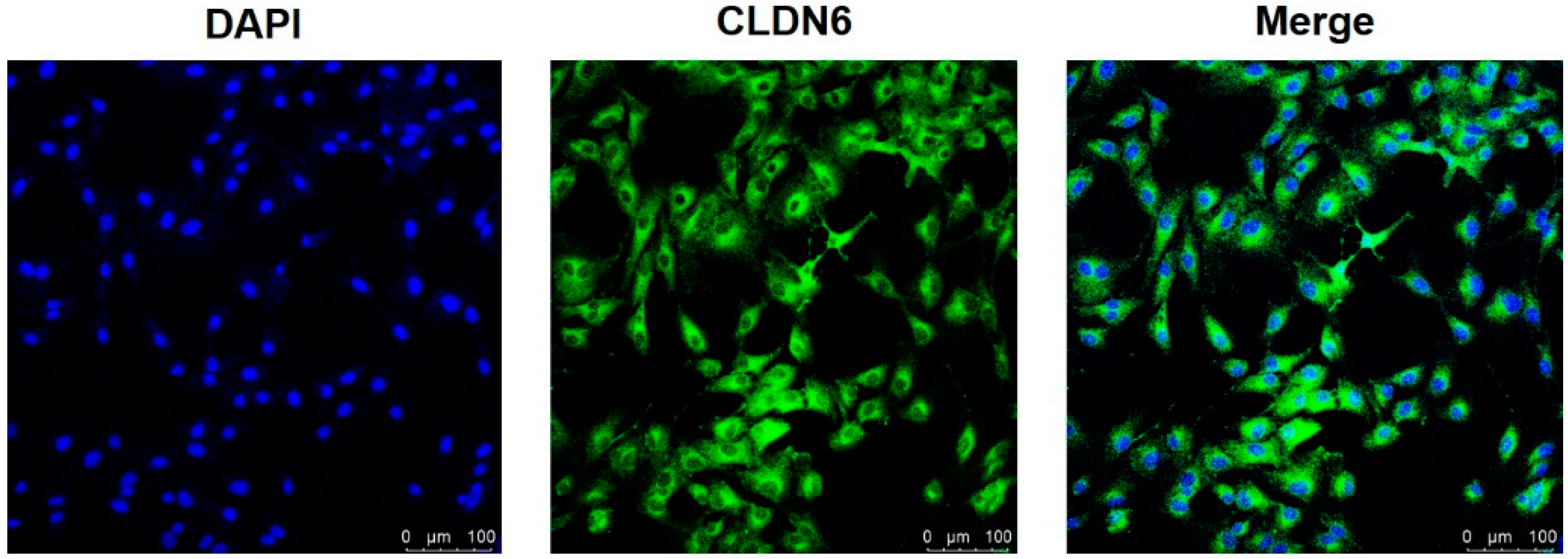
Isolated from bovine COCs matured for 22 h in vitro, cumulus cells were cultured in vitro. Under the microscope, monolayer CCs were round or oval in shape. The expression of the CLDN6 gene in bovine CCs cultured for 48 h was determined by real-time quantitative PCR (RT-qPCR) and immunofluorescence staining, respectively. Results showed that the CLDN6 gene was expressed in bovine CCs and the mRNA level was 1.5 times that of GAPDH (Figure 1). Its protein was distributed in cytoplasms and membranes (Figure 2).
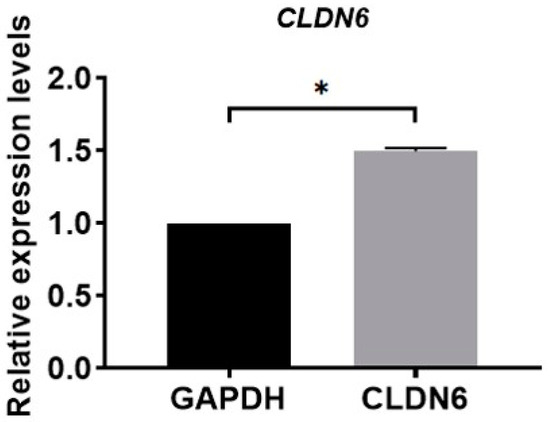
Figure 1.
Relative mRNA level of CLDN6 gene in bovine CCs was analyzed using RT-qPCR. Results were normalized to the mRNA level of GAPDH. Data are shown as means ±SD from three independent experiments. * p < 0.05.
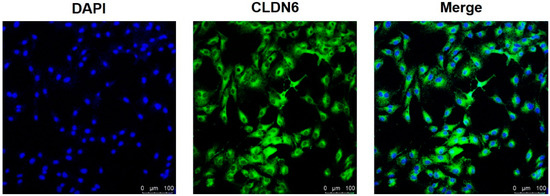
Figure 2.
CLDN6 protein in bovine CCs was localized by immunofluorescence staining. The nuclei of CCs were stained with DAPI and viewed under ultraviolet fluorescence. CLDN6 was labeled with Alexa Fluor 488 and viewed under blue fluorescence. Bar = 100 µm.
2.2. Screening of Effective siRNA Sequences Targeting Bovine CLDN6 Gene
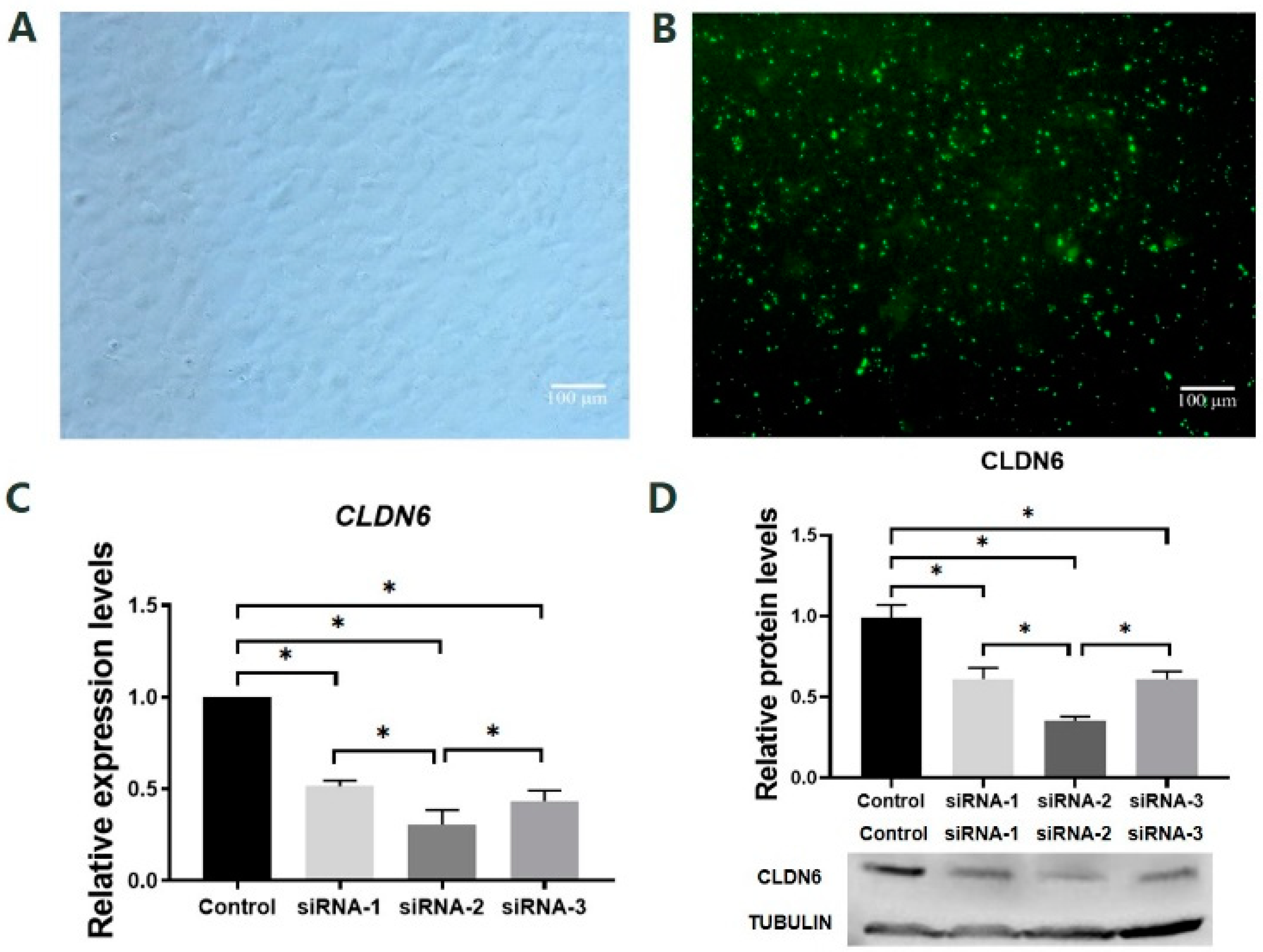
To select the most effective siRNA sequence targeting the CLDN6 gene, the mRNA and protein levels of the CLDN6 gene were examined by RT-qPCR and WB in bovine CCs cultured for 48 h after transfection of siRNA sequence targeting CLDN6 or siRNA universal negative control sequence (siNC, control group; Figure 3A,B). Results showed that all three pairs of siRNAs targeting CLDN6 significantly decreased the expression of CLDN6 at mRNA and protein levels (p < 0.05, Figure 3C,D). Among those, the largest decline in CLDN6 expression was found in bovine CCs transfected with siRNA-2 (p < 0.05). However, there was no significant difference in expression levels of CLDN6 between CCs transfected with siRNA-1 and siRNA-3. Therefore, siRNA-2 was the most efficient sequence for CLDN6 down-regulation and it could be used in subsequent experiments.
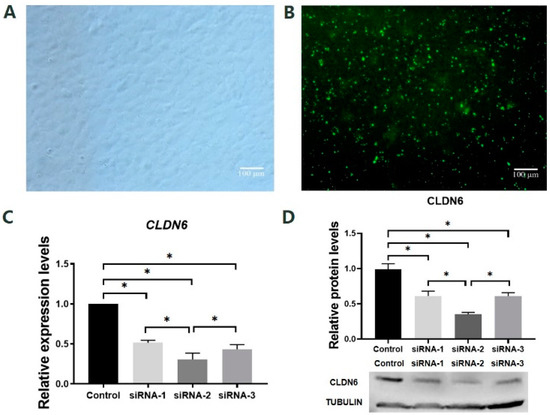
Figure 3.
Effects of siRNA targeting CLDN6 gene on its expression at mRNA and protein levels in bovine CCs. (A) CCs before siRNA transfection; (B) CCs transfected with siRNA; (C) The mRNA levels of the CLDN6 gene in cells transfected with different siRNAs; (D) CLDN6 protein levels in cells transfected with different siRNAs. Control group was cells transfected with siNC. * p < 0.05. Bar = 100 μm.
2.3. Effects of CLDN6 Knockdown on the Apoptosis of Bovine CCs
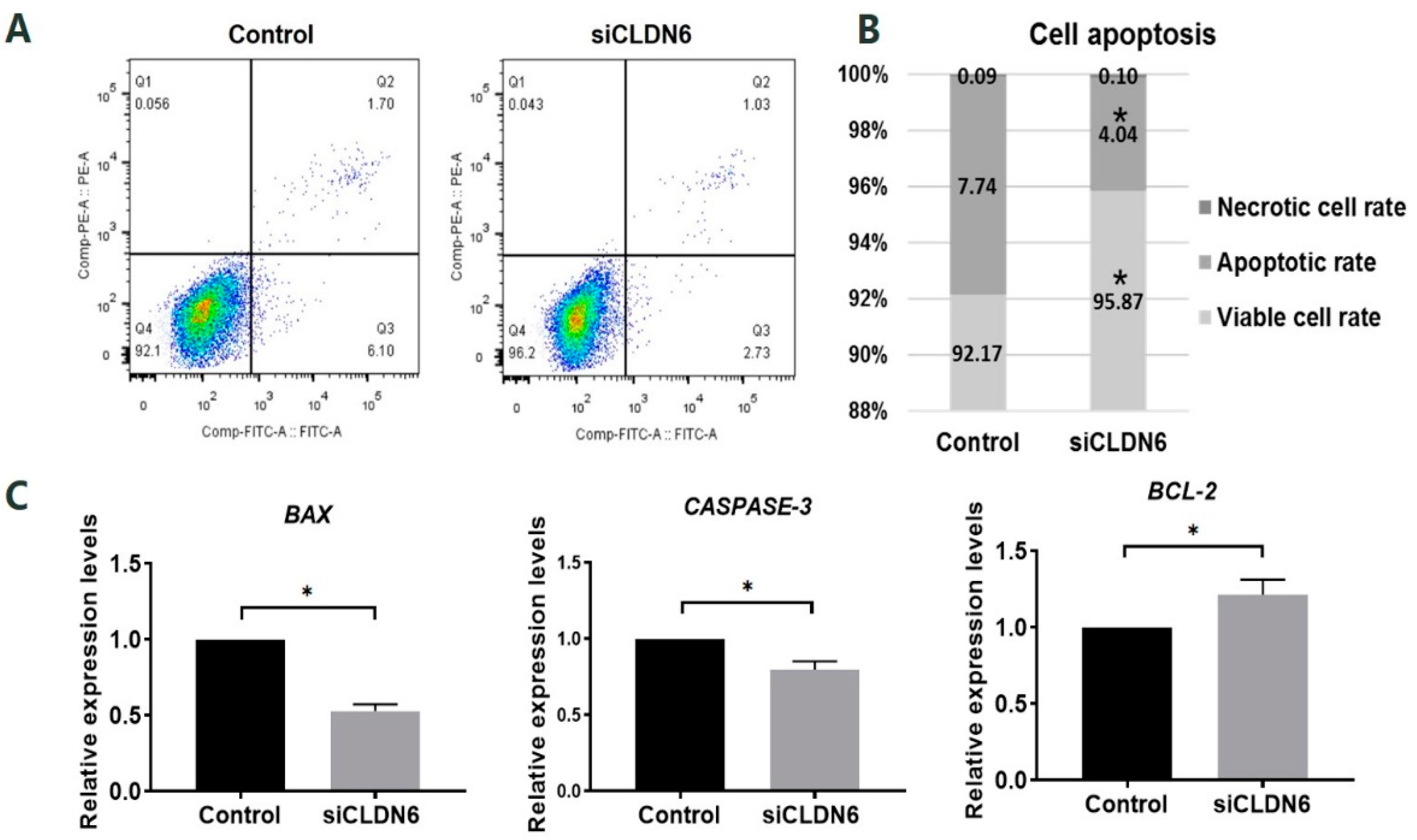
Apoptosis of bovine CCs transfected with siCLDN6 or siNC sequence (control group) were detected by flow cytometry. In Figure 4A, Q1, Q2, Q3 and Q4 refers to necrotic cells, late apoptotic cells, early apoptotic cells and viable cells, respectively. In the bovine CCs with CLDN6 knockdown, the cells’ apoptosis rate was significantly lower than that in cells of the control group (4.04 ± 0.68% vs. 7.74 ± 0.83%, p < 0.05, Figure 4B). The viable cells rate of CCs with CLDN6 knockdown was significantly higher than that in the control group (95.87 ± 0.76% vs. 92.17 ± 0.80%). The necrotic cells rates between two groups were at the same levels (0.10 ± 0.07% vs. 0.09 ± 0.03%, p > 0.05). For apoptosis-related genes, the expression of BAX, CASPASE-3 and BCL-2 were analyzed. As shown in Figure 4C, CLDN6 knockdown significantly down-regulated the mRNA levels of the pro-apoptotic genes BAX and CASPASE-3 (p < 0.05). On the contrary, the anti-apoptotic gene BCL-2 was significantly up-regulated in CCs with CLDN6 knockdown (p < 0.05).
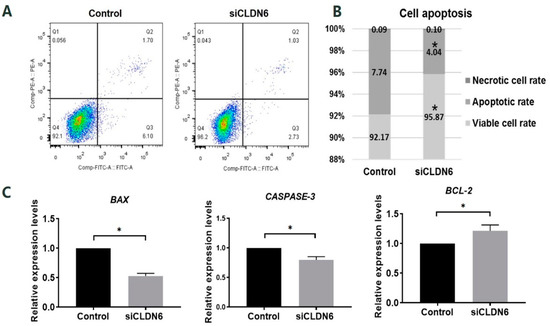
Figure 4.
Apoptosis assay of bovine CCs with CLDN6 knockdown. (A) CCs transfected with siCLDN6 or siNC were stained with Annexin V-FITC and PI. The percentages of apoptotic cells were detected by flow cytometry. Q1 refers to necrotic cells, Q2 refers to late apoptotic cells, Q3 refers to early apoptotic cells, Q4 refers to viable cells; (B) Differences in apoptosis rate, viable cells rate and necrotic cells rate of CCs transfected with siCLDN6 or siNC were shown; (C) The mRNA levels of BAX, CASPASE-3 and BCL-2 genes in CCs transfected with siCLDN6 or siNC. Control group was the cells transfected with siNC. * p < 0.05.
2.4. Effects of CLDN6 Knockdown on the Proliferation of Bovine CCs
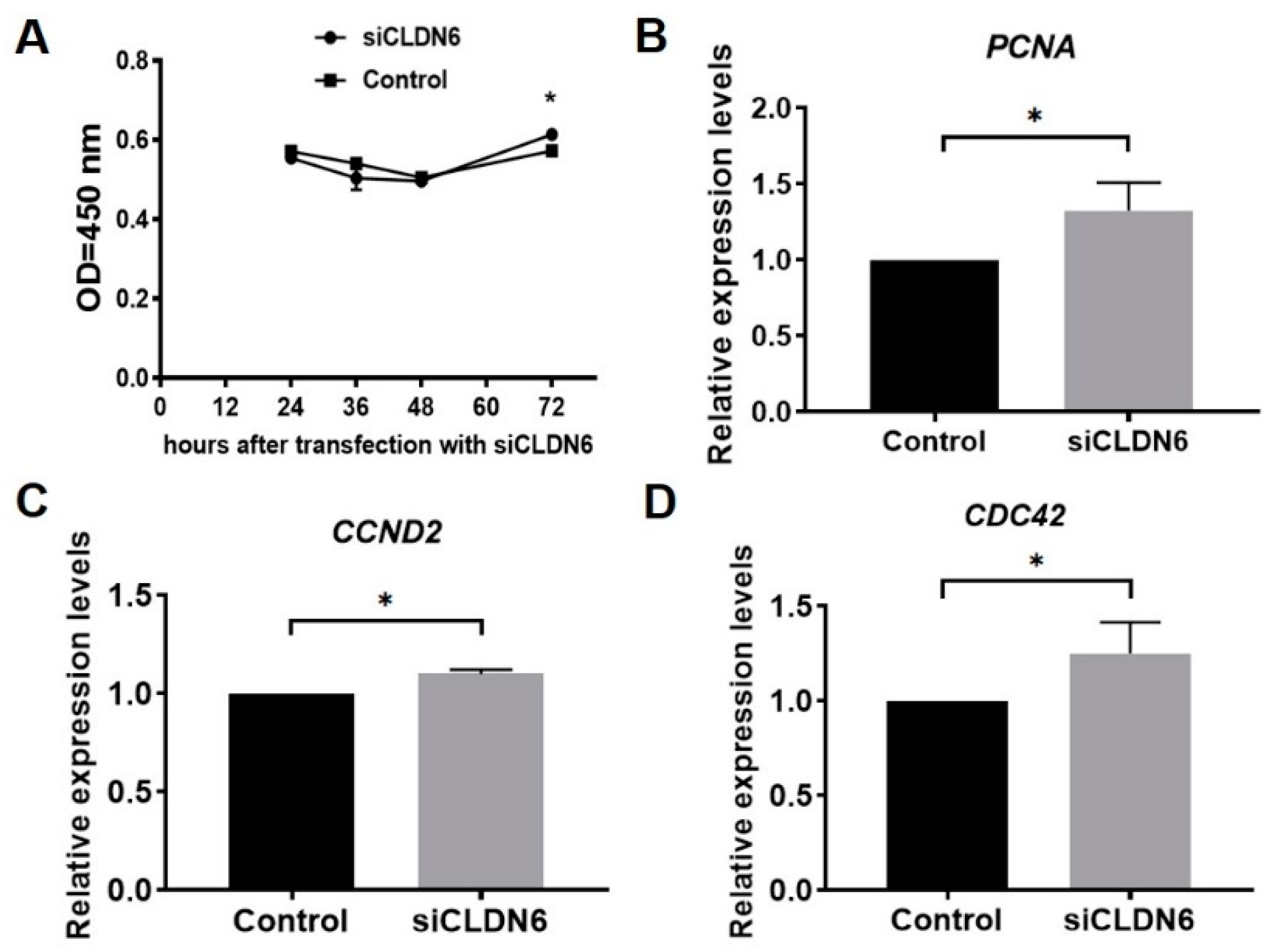
The proliferation of bovine CCs transfected with siCLDN6 or siNC sequence were detected at 24 h, 36 h, 48 h and 72 h after transfection with the CCK-8 kit. Results showed that the proliferation rates of CCs with or without CLDN6 knockdown showed the same trend, which decreased from 24 h to 48 h, then began to rise up to 72 h after siRNA transfection. Compared with the control group, CLDN6 knockdown significantly increased the CCs’ proliferation at 72 h after transfection (p < 0.05), whereas it had no influence at 24 h, 36 h and 48 h (p > 0.05, Figure 5A). Additionally, three proliferative genes, PCNA, CCND2 and CDC42, were significantly up-regulated in CCs with CLDN6 knockdown (p < 0.05, Figure 5B–D).
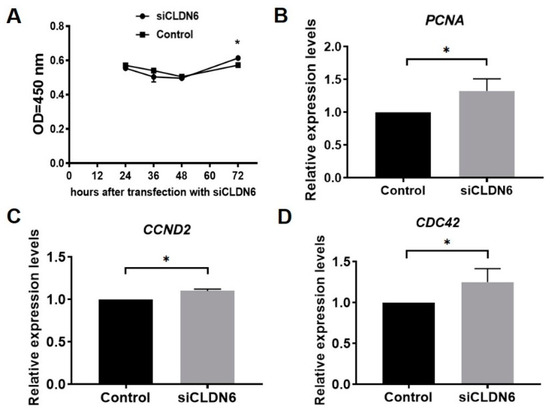
Figure 5.
Proliferation analysis of bovine CCs with CLDN6 knockdown. (A) After transfection with siCLDN6 or siNC, CCs were treated with CCK-8 and the absorbance at 450 nm was detected. * refers to significant difference (p < 0.05). (B–D) The mRNA levels of PCNA, CCND2 and CDC42 genes were measured in CCs transfected with siCLDN6 or siNC by RT-qPCR, respectively. Control group was the cells transfected with siNC. * p < 0.05.
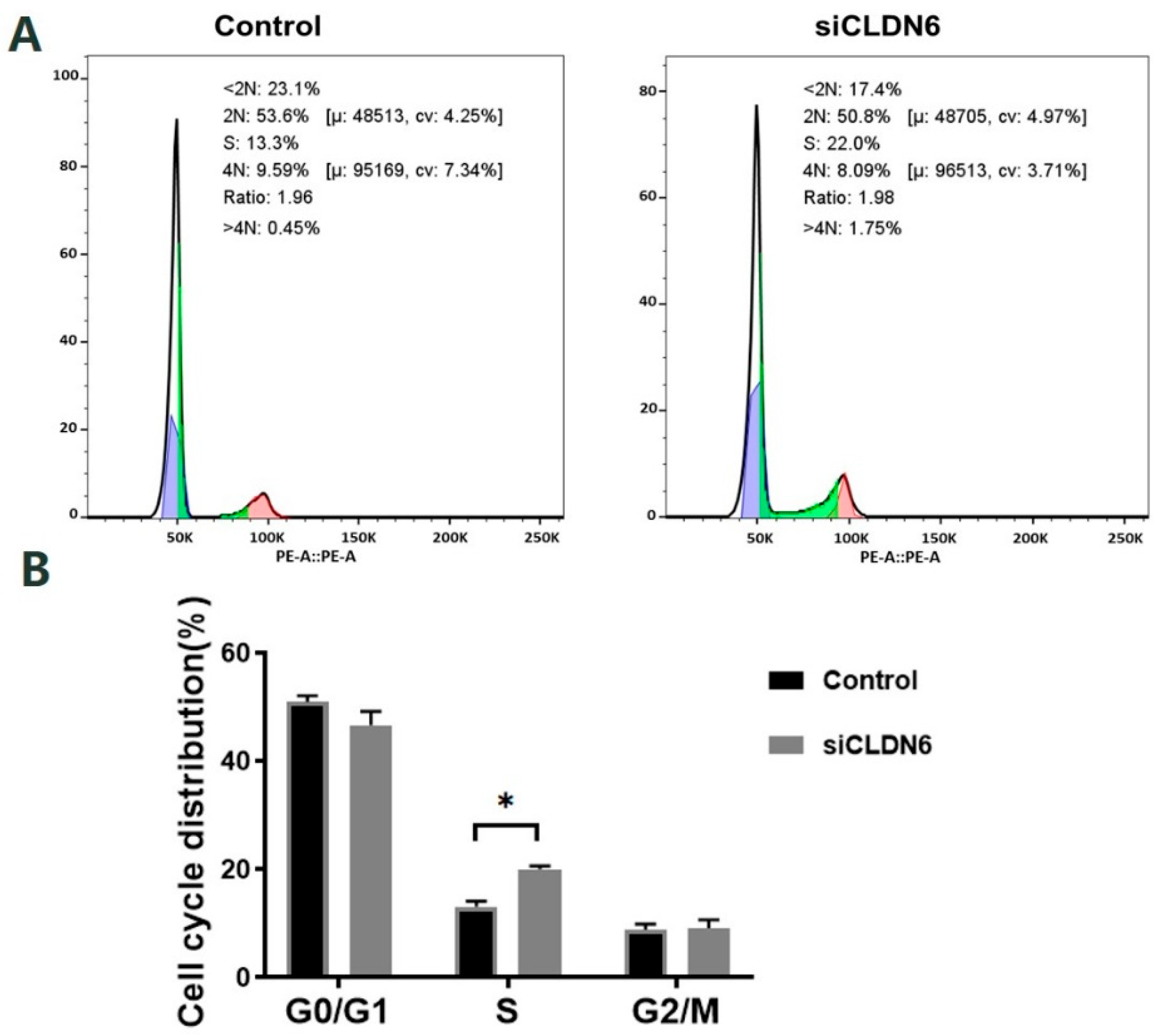
2.5. Effects of CLDN6 Knockdown on the Cell Cycle of Bovine CCs
Bovine CCs transfected with siRNA or siNC sequence were stained with PI and the cell cycle was detected by flow cytometry (Figure 6A). According to Figure 6B, CLDN6 knockdown induced the significant accumulation of cells in the S phase. However, the cell proportion in the G0/G1 phase and G2/M phase showed no significant difference in CCs with or without CLDN6 knockdown (p > 0.05).
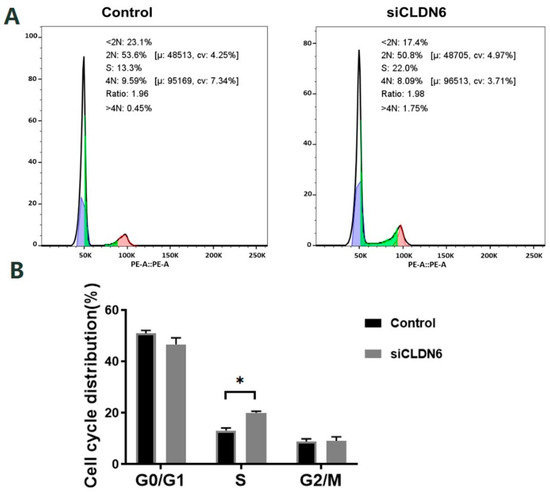
Figure 6.
Cell cycle of bovine CCs with CLDN6 knockdown. (A) After transfected with siCLDN6 or siNC, CCs were stained with PI and the DNA content of cells was detected by flow cytometry at 488 nm; (B) The distribution of CCs at different cell cycle phases. Control group was the cells transfected with siNC. * p < 0.05.
3. Discussion
CLDN6 belongs to a tight junction family and regulates cell proliferation, differentiation, invasion, metastasis, apoptosis and other processes through a variety of factors [21]. Previous studies found that CLDN6 was not expressed in healthy adult tissues but was expressed in various cancers and many types of embryonic epithelia [10,17]. Furthermore, CLDN6 was used as a surface marker of mouse pluripotent stem cells and an ideal therapeutic target in cancer [13,17]. However, little is known about the expression and function of CLDN6 in livestock adult cells. In the present study, the distribution and expression of CLDN6 in bovine CCs were first determined. Furthermore, CLDN6 knockdown had effects on apoptosis and the proliferation and cell cycle of bovine CCs.
Generally, CLDN6 is a tetraspanin transmembrane protein located within the tight junctional complex [22], in the cell membrane of primary tumors, gastric cancer cells and mouse stem cells [23,24]. In mouse neonatal kidneys, CLDN6 was highly expressed and distributed on the membrane of proximal renal tubules, thick ascending ramus, distal convoluted tubules and collecting tubules [10]. However, increasing evidence showed that beside the membrane, CLDN6 was weakly expressed in the cytoplasm of different cells such as breast cancer tissues and adjacent tissues and atypical teratoid/rhabdoid tumors [25,26,27]. The delocalization of CLDN proteins from cell membranes was common in transformed cells and ovarian cancer, which is associated with tumor cell migration and invasion [28]. Additionally, overexpression of CLDN6 with cytoplasmic tail domain deleted mis-localized CLDN6, CLDN10, CLDN11 and CLDN18 to the cytoplasm, which emphasized the importance of the CLDN tail domain in membrane targeting [29]. In addition to those mentioned, the healthy heart of a 14-year-old person showed diffuse cytoplasmic staining of cardiomyocytes and smooth muscle surrounding the vessels, without membrane staining [30]. Similarly, the CLDN6 protein was observed to be localized in the cytoplasm and membrane of normal bovine CCs by immunofluorescence staining in this study. CCs surround oocytes in COCs and regulate oocyte maturation through communication between them. Similarly, oocytes tightly control CCs to complete functions for oocyte development. Whether the reason for CLDN6’s appearance in cytoplasms of bovine CCs is related to oocyte requirements needs further research.
It is well known that the balance between cell apoptosis and proliferation is crucial to maintain the normal physiological state of multicellular organisms. Cell apoptosis is regulated by the pro-apoptotic protein BAX and anti-apoptotic protein BCL-2 with its key executioner CASPASE-3. In our study, the expression levels of BAX and CASPASE-3 were significantly down-regulated, whereas BCL-2 was significantly up-regulated in bovine CCs with CLDN6 knockdown. Furthermore, CLDN6 knockdown relieved cell apoptosis and promoted proliferation of bovine CCs, which was consistent with previous studies in human breast epithelium cell lines and cervical carcinoma [31,32]. However, CLDN6 acts as a tumor promoter to increase cell proliferation of human hepatocellular carcinoma cells and gastric cancer [19,33]. CLDN6 knockdown significantly inhibited endometrial cancer cell proliferation via the PI3K/Akt/mTOR signaling pathway [20]. In the human breast cancer cell MCF-7, for clones transfected with CLDN6, the level of apoptosis signal-regulating kinase 1 (ASK1) protein and mRNA was up-regulated, which indicates that the ASK1 signal may be associated with the pro-apoptosis effect of CLDN6 [34]. In summary, CLDN6 possesses pro- or anti-cancer effects in different cancers through its regulation of cell proliferation and apoptosis via different pathways. However, in the present study, the way in which CLDN6 modulated bovine cumulus cell growth was not involved. Whether the way is similar to those above or unique in cumulus cells is interesting since CLDN6 is lowly or not expressed in most adult tissues.
Cell proliferation is an important life activity of organisms, which is the basis of growth, development, reproduction and inheritance. According to our results, cell proliferation rates increased significantly in bovine CCs 72 h after transfection with the siRNA targeting CLDN6 gene, which was are consistent with previous studies in cervical cancer cells [18], whereas it was contrary with the results in gastric cancer cell lines and human endometrial adenocarcinoma cells [19,20]. For proliferation-related genes, CCND2 is the rate-limiting factor of cell mitosis from the G1 phase to S phase and positively accelerates proliferation during folliculogenesis [35]. It was expressed in the GCs of ovaries and its mRNA levels were promoted by FSH. Female mice with CCND2 deficiency were sterile because GCs could not proliferate and arrested the pre-antral follicle growth [36]. CDC42 is a kind of Rho-GTPase and regulates cell cycle, apoptosis and proliferation [37]. The up-regulated CDC42 inhibited cell apoptosis and promoted proliferation of porcine and chicken GCs [38,39], whereas mice lacking CDC42 in podocytes developed congenital nephropathy and died from renal failure within 2 weeks after birth [40]. CDC42 was required for tight junction formation and knockdown of CDC42 impaired junction formation in bronchial epithelial cells [41]. PCNA participated in a variety of processes of DNA metabolism, including DNA replication and repair, chromatin organization and transcription and sister chromatid cohesion [42]. PCNA underwent dynamic fluctuation during cell cycles, with a peak at the S phase, and then it was used as marker of cell proliferation [43]. In both oocytes and somatic cells during the development of fetal and neonatal mouse ovaries, the expression pattern of PCNA was closely correlated with the meiotic prophase I progression when primordial follicles started to assemble. Knockdown of PCNA in cultured mouse ovaries strikingly increased primordial follicle assembly, but contained fewer granulosa cells [44]. However, the effects of CLDN6 on the expression of PCNA, CCND2 and CDC42 genes was not estimated in other studies. In this study, CLDN6 knockdown increased the mRNA level of the above three genes in bovine CCs.
Simultaneously, CLDN6 knockdown induced cell cycle arrest in bovine CCs and cells accumulated in the S phase. In human MCF-7 cells, a high expression of CLDN6 blocked cells at the G0/G1 phase [45]. Furthermore, in breast cancer cell lines (MDA-MB-231 cells), miR-7 and miR-218 resulted in an increase of cells at the G1 phase and a decrease of cells at the S phase with reactivation of CLDN6 by means of epigenetic switches in DNA methylation and histone modification [46]. However, in gastric cancer, in cells such as AGS, MKN7 and NUGC-3, CLDN6 silencing contributes to cell cycle arrest at the G1-S checkpoint through increasing p21WAF1/Cip1 and p27Kip1 and decreasing S-phase kinase-associated protein 2 (SKP2) protein levels, which are well-known cell cycle regulators [26]. Obviously, the cell phase arrested by the variety of CLDN6 levels was distinct in different kinds of cancer.
4. Materials and Methods
All experimental protocols were conducted in accordance with the requirements of the Institutional Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences. All the chemicals used in this study were purchased from Sigma Chemicals Co. (St. Louis, MO, USA), unless otherwise indicated.
4.1. Culture of Bovine CCs
Bovine CCs were separated from the cumulus-oocyte complexes (COCs) matured in vitro. According to the procedure described previously [47], ovaries were collected from a local slaughterhouse and transported back to the laboratory within 2–3 h after washing with normal saline containing 100 IU/mL penicillin and 100 mg/mL streptomycin sulfate (1% PS) at 30 °C. COCs were aspirated from 2–6 mm follicles from clear ovaries. Only COCs with a homogeneous cytoplasm and intact cumulus cell layer were picked up for maturation in vitro in 4-well dishes (Nunc, Roskilde, Denmark) with 750 μL of M199 medium (Gibco, Life Technology, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco, Life Technology, Carlsbad, CA, USA), 0.01 IU/mL follicle-stimulating hormone (FSH), 1 IU/mL luteinizing hormone (LH) and 1 µg/mL estradiol (E2) at 38.5 °C in an atmosphere of 5% CO2 and 100% humidity.
COCs were digested with 0.1% hyaluronidase for 3 min after they were matured for 22–24 h. M199 solution containing 10% FBS was used to stop digestion. The cell suspension was recovered after centrifugation twice at 1000 rpm for 5 min with DPBS (Gibco, Life Technology, Carlsbad, CA, USA) containing 1% PS. The cells of 1 × 105/mL were cultured in a 6-well plate (Corning, Corning, NY, USA) with DMEM/Han’s F12 (DF12, Gibco, Life Technology, Carlsbad, CA, USA) medium containing 10% FBS and 1% PS at 37 °C, 5% CO2 and 100% humidity. After culturing for 48 h, CCs were trypsinized and seeded in the 6-well plate format at a density of 1 × 105/mL.
4.2. Synthesis and Transfection of Small Interfering RNA (siRNA)
Based on the sequence of the bovine CLDN6 gene (Accession No.: NC_037352.1) provided by the NCBI database, three pairs of siRNA targeting CLDN6 together with one pair of siRNA universal negative control sequence (siNC) were designed and synthesized by GenePharma Co., Ltd. (Shanghai, China). The siRNA sequences are listed in Table 1. According to the protocol described previously [48], four pairs of siRNA (100 nM) were conducted into bovine CCs at 60–70% confluency with Lipofectamine 3000 (Gibco, Life Technology, Carlsbad, CA, USA), respectively. Briefly, 3 µL of siRNA and 3 µL of Lipofectamine 3000 were respectively diluted with 100 µL serum-free medium Opti MEM (Gibco, Life Technology, Carlsbad, CA, USA) for 5 min followed by the mixing of the two solutions for 20 min. CCs were cultured with 1.5 mL serum-free Opti-MEM medium after they were washed twice with DPBS. The mixture containing Lipofectamine 3000 and siRNA was added into wells and incubated for 6 h. Then, cells were cultured with DF12 containing 10% FBS and 1% PS. The transfection efficiency was observed under fluorescence microscope (Nikon, Tokyo, Japan), and the cells were collected for subsequent experiments 48 h after transfection. The CCs transfected with siNC were the control group.

Table 1.
siRNA sequences targeting the bovine CLDN6 gene.
4.3. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
The gene expression levels between CCs with or without CLDN6 knockdown were analyzed with the CFX96TM system (Bio-RAD, Hercules, CA, USA). The CCs transfected with siRNA against bovine CLDN6 gene or siNC grew for 48 h and were harvested for RT-qPCR. A total of eight genes were selected, including bovine CLDN6 gene, apoptosis-related genes BAX, BCL-2 and CASPASE-3, proliferating cell nuclear antigen gene (PCNA), cyclin D2 gene (CCND2), cell division cycle protein 42 gene (CDC42) and reference gene GAPDH. Total RNA was isolated from CCs with RNeasy Micro Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. cDNA was synthesized using M-MLV reverse transcriptase (Promega, Madison, WI, USA). RT-qPCR volume was 15 µL, including 0.5 µL of upstream and downstream primers, 2 µL of cDNA template, 7.5 µL of TB Green Premix Ex Taq II (2×) and 4.5 µL of RNase free DD H2O. The RT-qPCR program steps were 95 °C for 30 s, 39 cycles of 95 °C for 5 s, 60 °C for 30 s. Each sample was replicated three times and data were normalized to the expression level of GAPDH in each sample. The 2–∆∆Ct value, where ∆Ct = Ct gene − Ct GAPDH and ∆∆Ct = ∆Ct siRNA − ∆Ct control, was calculated to represent the relative mRNA expression levels of genes in the control and interference groups. Primer sequences are listed in Table 2.

Table 2.
Primer sequences for RT-qPCR.
4.4. Immunofluorescence Staining
CLDN6 protein in the bovine CCs with or without CLDN6 knockdown was localized using immunofluorescence staining. Cells of 1 × 106/mL were seeded directly on cover glasses (Solarbio, Beijing, China) in a six-well plate. When the cells reached 60–80% confluency, the cover glasses were removed from the medium and washed with DPBS three times. Subsequently, the cells were fixed with 4% paraformaldehyde for 1 h and then permeated with 0.5% Triton X-100 for 40 min at room temperature. After being washed three times with DPBS containing 1% BSA (PBS-1% BSA) for 10 min, cells were incubated in PBS-1%BSA overnight at 4 °C to block the unspecific binding of antibody. Primary antibodies against CLDN6 (1:500, Merck Millipore, Darmstadt, Germany) were incubated with cells overnight at 4 °C followed by Alexa Fluor 488 goat anti-rabbit IgG (1:1000, Cell Signaling Technology, Danvers, MA, USA) at 37 °C for 1 h. Finally, 5 mg/mL DAPI (Beyotime, Shanghai, China) was used to stain cell nuclei for 5 min and the cover glasses were placed on the slides. Photographs were taken under a fluorescence confocal microscope (Leica, Wetzlar, Germany).
4.5. Western Blotting (WB)
The bulleted lists is as follows: The expression of CLDN6 protein in the bovine CCs with or without CLDN6 knockdown was detected by WB. Forty-eight hours after siRNA transfection, CCs reached 80–90% confluency and about 5 × 106 cells were collected. The total protein was extracted after cells were treated with lysis buffer. Protein concentration was quantified with a BCA kit (Beyotime, Shanghai, China). Protein samples were resolved by SDS-PAGE (12% acrylamide gel containing 0.1% SDS) and transferred by electrophoresis to a nitrate cellulose membrane (BioTraceNT, Pall, Port Washington, NY, USA). After blocking with 5% skim milk for 1 h, the membranes were incubated with rabbit antibody against CLDN6 (1:500; Merck Millipore, Darmstadt, Germany) or mouse antibody against β-TUBULIN (1:2000; Proteintech Group, Rosemont, IL USA) overnight at 4 °C. Goat anti-rabbit IgG-HRP (1:5000; Gene-protein Link, Beijing, China) and goat anti-rat IgG-HRP (1:5000; Gene-protein Link, Beijing, China) were incubated as secondary antibodies at 37 °C for 1 h. Enhanced chemiluminescence (ECL) reagent (Mei5 Biotechnology, Beijing, China) was used to detect protein. Exposed in a chemiluminescence instrument, the images of protein bands were quantified with ImageJ software (1.8.0, NIH, Bethesda, ML, USA).
4.6. Apoptosis Analysis
Apoptosis rates of the bovine CCs with or without CLDN6 knockdown were detected using an Annexin V-FITC (AVF)/propidium iodide (PI) double-staining apoptosis kit (Beyotime, Shanghai, China). The CCs transfected with siRNA or siNC were cultured for 48 h, digested with 0.25% trypsin and collected. After being centrifuged twice at 1000 rpm for 5 min with DPBS, cells were stained for 10 min with 5 µL AVF and 50 µg/mL PI at room temperature. Cells at a density of 1 × 106/mL were analyzed within 1 h by FACS Verse flow cytometry (Becton Dickinson, Franklin Lakes, NJ, USA). Viable cells were AVF and PI negative, cells in early apoptosis were AVF positive and PI negative, cells in late apoptosis were AVF and PI positive, and necrotic cells were AVF negative and PI positive. The rates of apoptotic cells were presented as the sum of early and late apoptosis rates.
4.7. Cell Proliferation Assay
A cell counting kit-8 (CCK-8, Dojindo, Kumamoto, Japan) was used to detect the proliferation of bovine CCs with or without CLDN6 knockdown. According to the manual, WST-8 is a tetrazolium salt and functions as the chromogenic redox indicator. It can form an orange color and water-soluble formazan upon reduction by cellular dehydrogenases. The intensity of the color is proportional to the number of living cells. Bovine CCs were digested with 0.25% trypsin and seeded at a density of 2 × 103 cells/well in 96-well plates (Corning, Corning, NY, USA). After 24 h, cells were transfected with siRNA against the CLDN6 gene or siNC. Then, 10 μL CCK-8 solution was added to each well at 24 h, 36 h, 48 h and 72 h after transfection and retained for 1 h at 37 °C without light. The absorbance was detected at 450 nm by an enzyme labeling instrument (Tecan, Zürich, Switzerland).
4.8. Cell Cycle Analysis
A cell cycle and apoptosis analysis kit (Beyotime, Shanghai, China) was used to detect the percentage of CCs at different phases of cell cycle. CCs transfected with siRNA against CLDN6 gene or siNC were cultured for 48 h and were harvested as described in apoptosis analysis. After twice washing with DPBS, cells were incubated with precooled 70% ethanol overnight at 4 °C. Following removal of 70% ethanol by centrifugation, cells were stained with PI for 30 min at 37 °C. DNA content was detected by Flow cytometry at 488 nm. The data were analyzed by Flow Jo (V10, TreeStar, Ashland, OR, USA) and the percentage of cells in the G1, S, M and G2 phase was calculated.
4.9. Statistical Analysis
All experiments were repeated at least three times. Data in each experiment are expressed as the mean ±SD. Statistical analyses were performed using one-way ANOVA with Duncan’s test for post hoc analysis using SAS version 9 (SAS Institute Inc., Cary, NC, USA). p < 0.05 was considered statistically significant.
5. Conclusions
This study observed for the first time the expression of CLDN6 in bovine cumulus cells cultured in vitro. Furthermore, CLDN6 knockdown significantly inhibited apoptosis of bovine CCs and induced S phase arrest and cell proliferation. These findings provide a reference for revealing the function and regulation mechanism of CLDN6 in cumulus cell growth and oocyte and embryo development of livestock.
Author Contributions
Conceptualization, W.D.; Methodology, W.W. and Y.T.; Software, X.Z. and N.C.; Validation. H.Z. (Huiying Zou) and W.D.; Formal analysis, H.H.; Investigation, Y.P.; Resources, H.Z. (Huabin Zhu) and D.Y.; Data curation, W.W. and N.C.; Writing—original draft. W.W. and N.C.; Writing—review and editing, W.D. and W.W.; Supervision, Project administration and Funding acquisition, W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 31902163 and the Agricultural Science and Technology Innovation Program, grant numbers ASTIP-IAS06, ASTIP-IAS04-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Diaz, F.J.; O’Brien, M.J.; Wigglesworth, K.; Eppig, J.J. The preantral granulosa cell to cumulus cell transition in the mouse ovary: Development of competence to undergo expansion. Dev. Biol. 2006, 299, 91–104. [Google Scholar] [CrossRef]
- Auclair, S.; Uzbekov, R.; Elis, S.; Sanchez, L.; Kireev, I.; Lardic, L.; Dalbies-Tran, R.; Uzbekova, S. Absence of cumulus cells during in vitro maturation affects lipid metabolism in bovine oocytes. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E599–E613. [Google Scholar] [CrossRef] [PubMed]
- Hughes, F.M., Jr.; Gorospe, W.C. Biochemical identification of apoptosis (programmed cell death) in granulosa cells: Evidence for a potential mechanism underlying follicular atresia. Endocrinology 1991, 129, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Elgebaly, M.M.; Hazaa, A.B.M.; Amer, H.A.; Mesalam, A. L-Cysteine improves bovine oocyte developmental competence in vitro via activation of oocyte-derived growth factors BMP-15 and GDF-9. Reprod. Domest. Anim. 2022, 57, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Schutz, L.F.; Robinson, C.L.; Totty, M.L.; Spicer, L.J. Evidence that gene expression of ovarian follicular tight junction proteins is regulated in vivo and in vitro in cattle. J. Anim. Sci. 2017, 95, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.L.; Singh, K.; Mangray, S.; Tavares, R.; Noble, L.; Resnick, M.B.; Yakirevich, E. Claudin expression in high-grade invasive ductal carcinoma of the breast: Correlation with the molecular subtype. Mod. Pathol. 2013, 26, 485–495. [Google Scholar] [CrossRef]
- Gunzel, D.; Yu, A.S. Claudins and the modulation of tight junction permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef]
- Wang, H.F.; Zheng, S.F.; Chen, Y.; Zhou, Z.Y.; Xu, J. Correlations between claudin-1 and PIGF expressions in retinoblastoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4196–4203. [Google Scholar] [PubMed]
- Schaefer, I.M.; Agaimy, A.; Fletcher, C.D.; Hornick, J.L. Claudin-4 expression distinguishes SWI/SNF complex-deficient undifferentiated carcinomas from sarcomas. Mod. Pathol. 2017, 30, 539–548. [Google Scholar] [CrossRef]
- Abuazza, G.; Becker, A.; Williams, S.S.; Chakravarty, S.; Truong, H.T.; Lin, F.; Baum, M. Claudins 6, 9, and 13 are developmentally expressed renal tight junction proteins. Am. J. Physiol. Renal Physiol. 2006, 291, F1132–F1141. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, T.; Sherman-Baust, C.A.; Poosala, S.; Mullin, J.M.; Morin, P.J. Age-related changes of claudin expression in mouse liver, kidney, and pancreas. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1146–1153. [Google Scholar] [CrossRef]
- Ben-David, U.; Nudel, N.; Benvenisty, N. Immunologic and chemical targeting of the tight-junction protein Claudin-6 eliminates tumorigenic human pluripotent stem cells. Nat. Commun. 2013, 4, 1992. [Google Scholar] [CrossRef]
- Wang, L.L.; Xue, Y.; Shen, Y.H.; Li, W.; Cheng, Y.; Yan, X.S.; Shi, W.S.; Wang, J.; Gong, Z.J.; Yang, G.H.; et al. Claudin 6: A novel surface marker for characterizing mouse pluripotent stem cells. Cell Res. 2012, 22, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Troy, T.C.; Turksen, K. The targeted overexpression of a Claudin mutant in the epidermis of transgenic mice elicits striking epidermal and hair follicle abnormalities. Mol. Biotechnol. 2007, 36, 166–174. [Google Scholar] [CrossRef]
- Moriwaki, K.; Tsukita, S.; Furuse, M. Tight junctions containing claudin 4 and 6 are essential for blastocyst formation in preimplantation mouse embryos. Dev. Biol. 2007, 312, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, X.L.; Li, C.C.; Mao, B.Y. Xenopus Claudin-6 is required for embryonic pronephros morphogenesis and terminal differentiation. Biochem. Biophys. Res. Commun. 2015, 462, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, X.Y.; Fan, J.J.; Du, X.B. Claudin 6: Therapeutic prospects for tumours, and mechanisms of expression and regulation (Review). Mol. Med. Rep. 2021, 24, 677. [Google Scholar] [CrossRef]
- Ito, Y.; Takasawa, A.; Takasawa, K.; Murakami, T.; Akimoto, T.; Kyuno, D.; Kawata, Y.; Shano, K.; Kirisawa, K.; Ota, M.; et al. Aberrant expression of claudin-6 contributes to malignant potentials and drug resistance of cervical adenocarcinoma. Cancer Sci. 2022, 113, 1519–1530. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.T.; Zhang, Y.Q.; Li, Q.L.; Zhang, Z.Z.; Zhao, G.; Xu, J. CLDN6 promotes tumor progression through the YAP1-snail1 axis in gastric cancer. Cell Death Dis. 2019, 10, 949. [Google Scholar] [CrossRef]
- Cao, X.; He, G.Z. Knockdown of CLDN6 inhibits cell proliferation and migration via PI3K/AKT/mTOR signaling pathway in endometrial carcinoma cell line HEC-1-B. Onco Targets Ther. 2018, 11, 6351–6360. [Google Scholar] [CrossRef]
- Kwon, M.J. Emerging roles of claudins in human cancer. Int. J. Mol. Sci. 2013, 14, 18148–18180. [Google Scholar] [CrossRef]
- Lewis, J.B.; Milner, D.C.; Lewis, A.L.; Dunaway, T.M.; Egbert, K.M.; Albright, S.C.; Merrell, B.J.; Monson, T.D.; Broberg, D.S.; Gassman, J.R.; et al. Up-regulation of Claudin-6 in the distal lung impacts secondhand smoke-induced inflammation. Int. J. Environ. Res. Public Health 2016, 13, 1018. [Google Scholar] [CrossRef]
- Sugimoto, K.; Ichikawa-Tomikawa, N.; Satohisa, S.; Akashi, Y.; Kanai, R.; Saito, T.; Sawada, N.; Chiba, H. The tight-junction protein claudin-6 induces epithelial differentiation from mouse F9 and embryonic stem cells. PLoS ONE 2013, 8, e75106. [Google Scholar] [CrossRef]
- Kohmoto, T.; Masuda, K.; Shoda, K.; Takahashi, R.; Ujiro, S.; Tange, S.; Ichikawa, D.; Otsuji, E.; Imoto, I. Claudin-6 is a single prognostic marker and functions as a tumor-promoting gene in a subgroup of intestinal type gastric cancer. Gastric Cancer 2020, 23, 403–417. [Google Scholar] [CrossRef]
- Song, P.Y.; Li, Y.R.; Dong, Y.; Liang, Y.Y.; Qu, H.N.; Qi, D.; Lu, Y.; Jin, X.S.; Guo, Y.T.; Jia, Y.Y.; et al. Estrogen receptor beta inhibits breast cancer cells migration and invasion through CLDN6-mediated autophagy. J. Exp. Clin. Cancer Res. 2019, 38, 354. [Google Scholar] [CrossRef]
- Birks, D.K.; Kleinschmidt-DeMasters, B.K.; Donson, A.M.; Barton, V.N.; McNatt, S.A.; Foreman, N.K.; Handler, M.H. Claudin 6 is a positive marker for atypical teratoid/rhabdoid tumors. Brain Pathol. 2010, 20, 140–150. [Google Scholar] [CrossRef]
- Antonelli, M.; Hasselblatt, M.; Haberler, C.; Di Giannatale, A.; Garre, M.L.; Donofrio, V.; Lauriola, L.; Ridola, V.; Arcella, A.; Fruhwald, M.; et al. Claudin-6 is of limited sensitivity and specificity for the diagnosis of atypical teratoid/rhabdoid tumors. Brain Pathol. 2011, 21, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.B.; Sharma, A.; Dhawan, P. Claudin family of proteins and cancer: An overview. J. Oncol. 2010, 2010, 541957. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Troy, T.C.; Turksen, K. Role of the Cldn6 cytoplasmic tail domain in membrane targeting and epidermal differentiation in vivo. Mol. Cell Biol. 2006, 26, 5876–5887. [Google Scholar] [CrossRef]
- Sullivan, L.M.; Yankovich, T.; Le, P.; Martinez, D.; Santi, M.; Biegel, J.A.; Pawel, B.R.; Judkins, A.R. Claudin-6 is a nonspecific marker for malignant rhabdoid and other pediatric tumors. Am. J. Surg. Pathol. 2012, 36, 73–80. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, Q.; Liu, Y.F.; Xu, X.M.; Quan, C.S. Gene silencing of claudin-6 enhances cell proliferation and migration accompanied with increased MMP-2 activity via p38 MAPK signaling pathway in human breast epithelium cell line HBL-100. Mol. Med. Rep. 2013, 8, 1505–1510. [Google Scholar] [CrossRef]
- Zhang, X.W.; Ruan, Y.; Li, Y.R.; Lin, D.J.; Liu, Z.J.; Quan, C.S. Expression of apoptosis signal-regulating kinase 1 is associated with tight junction protein claudin-6 in cervical carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 5535–5541. [Google Scholar]
- Lu, Y.; Dang, Q.H.; Bo, Y.; Su, X.J.; Wang, L.P.; Sun, J.Q.; Wei, J.Y.; Quan, C.S.; Li, Y.R. The expression of CLDN6 in hepatocellular carcinoma tissue and the effects of CLDN6 on biological phenotypes of hepatocellular carcinoma cells. J. Cancer 2021, 12, 5454–5463. [Google Scholar] [CrossRef]
- Guo, Y.X.; Xu, X.M.; Liu, Z.J.; Zhang, T.; Zhang, X.W.; Wang, L.P.; Wang, M.; Liu, Y.Y.; Lu, Y.; Liu, Y.P.; et al. Apoptosis signal-regulating kinase 1 is associated with the effect of claudin-6 in breast cancer. Diagn. Pathol. 2012, 7, 111. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.R.; Wu, S.; Tang, J.H.; Chen, G.G.; Li, F.G. miR-135a suppresses granulosa cell growth by targeting Tgfbr1 and Ccnd2 during folliculogenesis in mice. Cells 2021, 10, 2104. [Google Scholar] [CrossRef]
- Robker, R.L.; Richards, J.S. Hormone-induced proliferation and differentiation of granulosa cells: A coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol. Endocrinol. 1998, 12, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.J.; Li, H.Y.; Liu, Y.; Wang, X.F.; Xiang, W.P. Advances in the study of CDC42 in the female reproductive system. J. Cell Mol. Med. 2022, 26, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Yang, Y.F.; Wang, Y.L.; Li, Y.; He, Y.M.; Duan, J.X.; Xu, D.J.; Pei, Y.F.; Cheng, J.Y.; Yang, L.; et al. Phospholipase C inhibits apoptosis of porcine primary granulosa cells cultured in vitro. J. Ovarian Res. 2019, 12, 90. [Google Scholar] [CrossRef]
- Xu, R.F.; Qin, N.; Xu, X.X.; Sun, X.; Chen, X.X.; Zhao, J.H. Inhibitory effect of SLIT2 on granulosa cell proliferation mediated by the CDC42-PAKs-ERK1/2 MAPK pathway in the prehierarchical follicles of the chicken ovary. Sci. Rep. 2018, 8, 9168. [Google Scholar] [CrossRef]
- Scott, R.P.; Hawley, S.P.; Ruston, J.; Du, J.M.; Brakebusch, C.; Jones, N.; Pawson, T. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 2012, 23, 1149–1154. [Google Scholar] [CrossRef]
- Wallace, S.W.; Durgan, J.; Jin, D.; Hall, A. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol. Biol. Cell 2010, 21, 2996–3006. [Google Scholar] [CrossRef]
- Cardano, M.; Tribioli, C.; Prosperi, E. Targeting proliferating cell nuclear antigen (PCNA) as an effective strategy to inhibit tumor cell proliferation. Curr. Cancer Drug Targets 2020, 20, 240–252. [Google Scholar] [CrossRef]
- Celis, J.E.; Celis, A. Cell cycle-dependent variations in the distribution of the nuclear protein cyclin proliferating cell nuclear antigen in cultured cells: Subdivision of S phase. Proc. Natl. Acad. Sci. USA 1985, 82, 3262–3266. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hua, J.; Zhang, Y.W.; Jiang, X.H.; Zhang, H.; Ma, T.L.; Zheng, W.; Sun, R.; Shen, W.; Sha, J.H.; et al. Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS ONE 2011, 6, e16046. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, Y.F.; Ren, Y.; Xu, X.M.; Yu, L.N.; Li, Y.L.; Quan, C.S. Tight junction protein, claudin-6, downregulates the malignant phenotype of breast carcinoma. Eur. J. Cancer Prev. 2010, 19, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhu, F.F.; Chen, P.X. miR-7 and miR-218 epigenetically control tumor suppressor genes RASSF1A and Claudin-6 by targeting HoxB3 in breast cancer. Biochem. Biophys. Res. Commun. 2012, 424, 28–33. [Google Scholar] [CrossRef]
- Sun, W.J.; Pang, Y.W.; Liu, Y.; Hao, H.S.; Zhao, X.M.; Qin, T.; Zhu, H.B.; Du, W.H. Exogenous glutathione supplementation in culture medium improves the bovine embryo development after in vitro fertilization. Theriogenology 2015, 84, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Li, X.L.; Wang, W.J.; Hao, H.S.; Zou, H.Y.; Pang, Y.W.; Zhao, X.M.; Zhu, H.B.; Du, W.H. Knockdown of bone morphogenetic protein 4 gene induces apoptosis and inhibits proliferation of bovine cumulus cells. Theriogenology 2022, 188, 28–36. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).