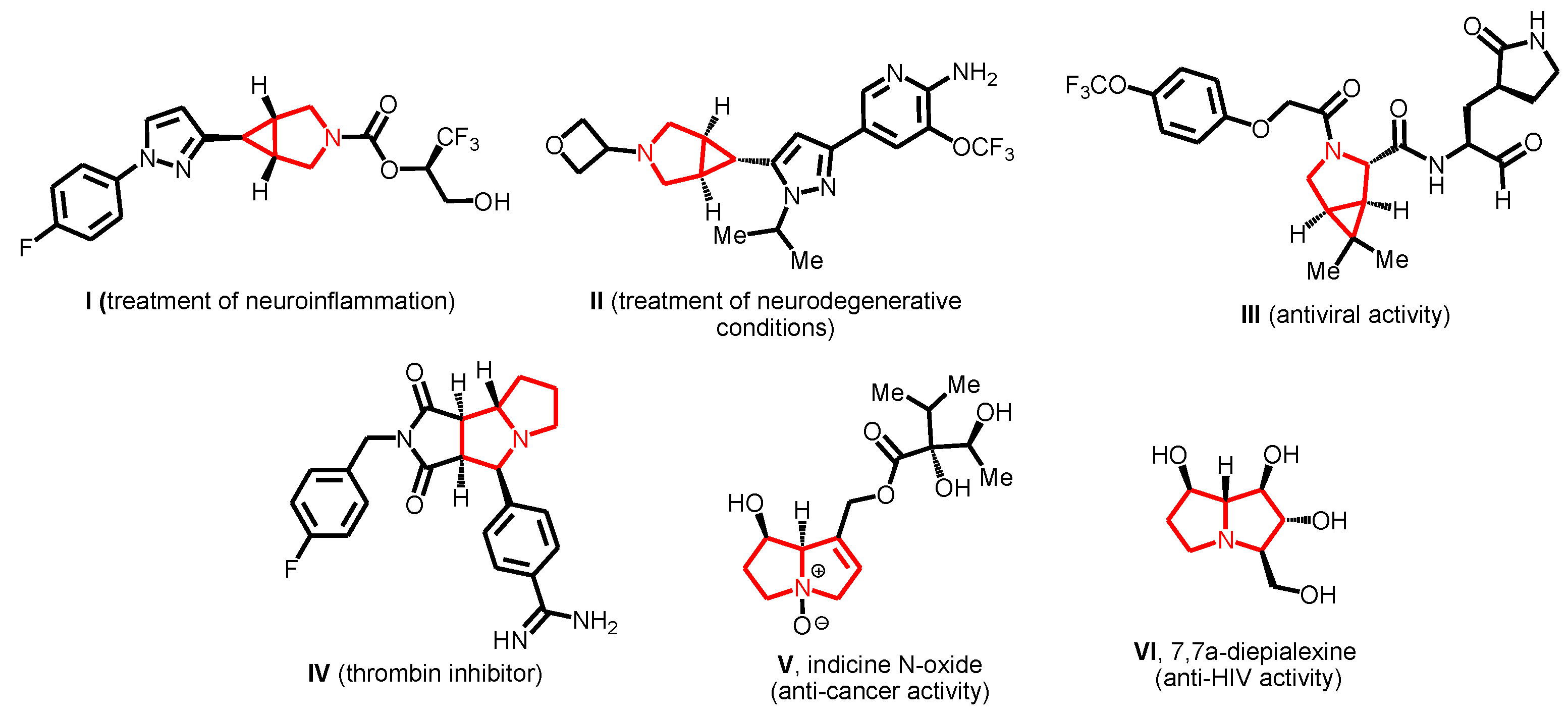
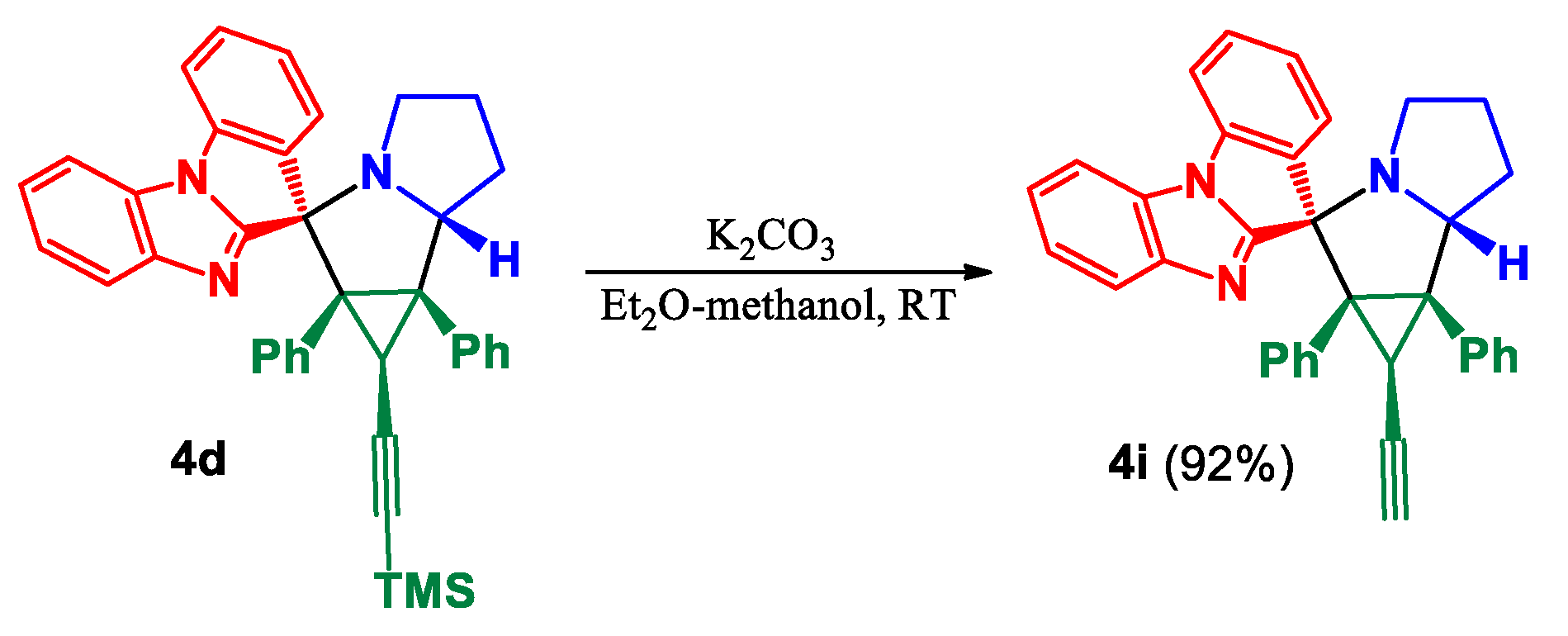
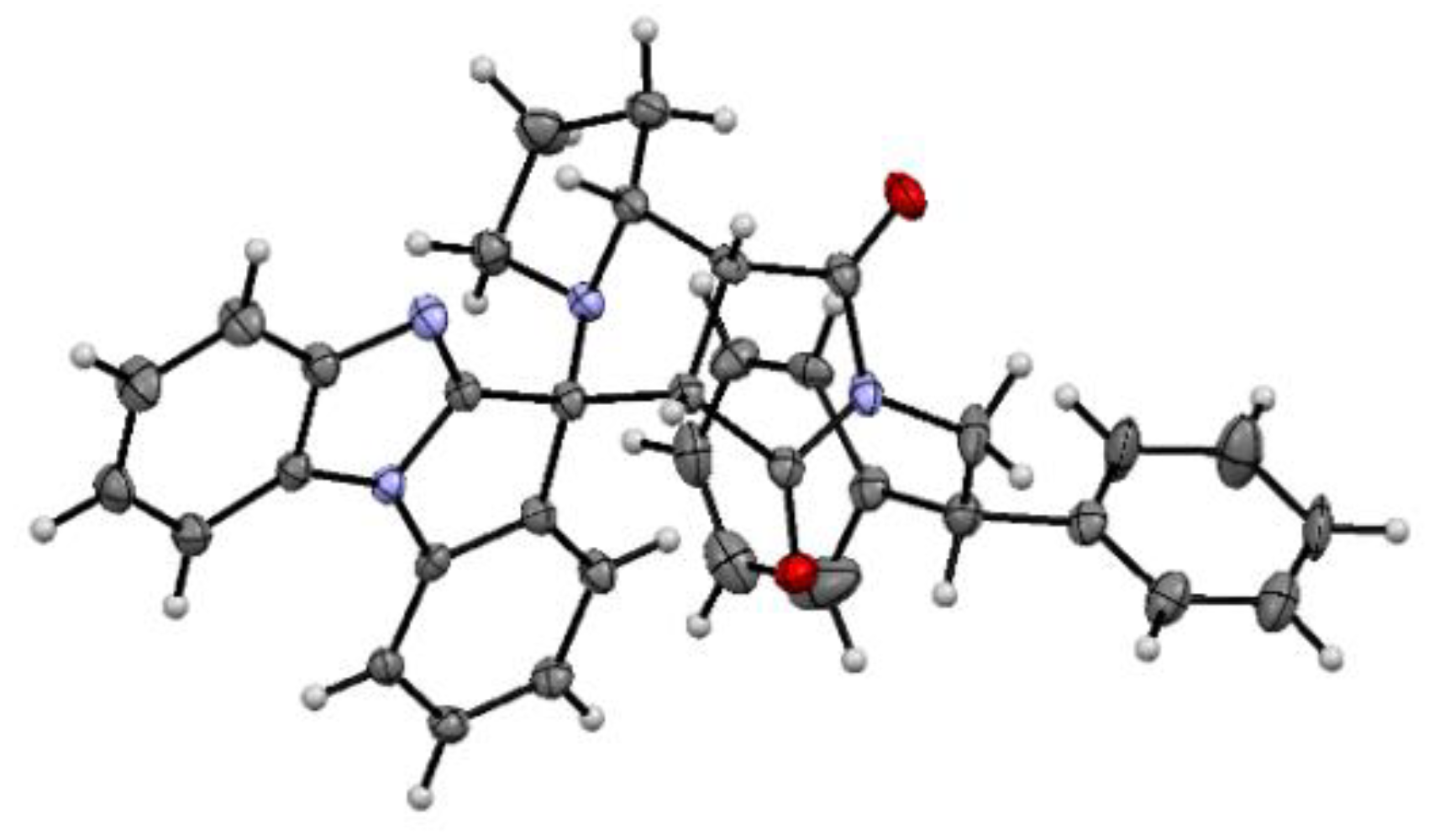
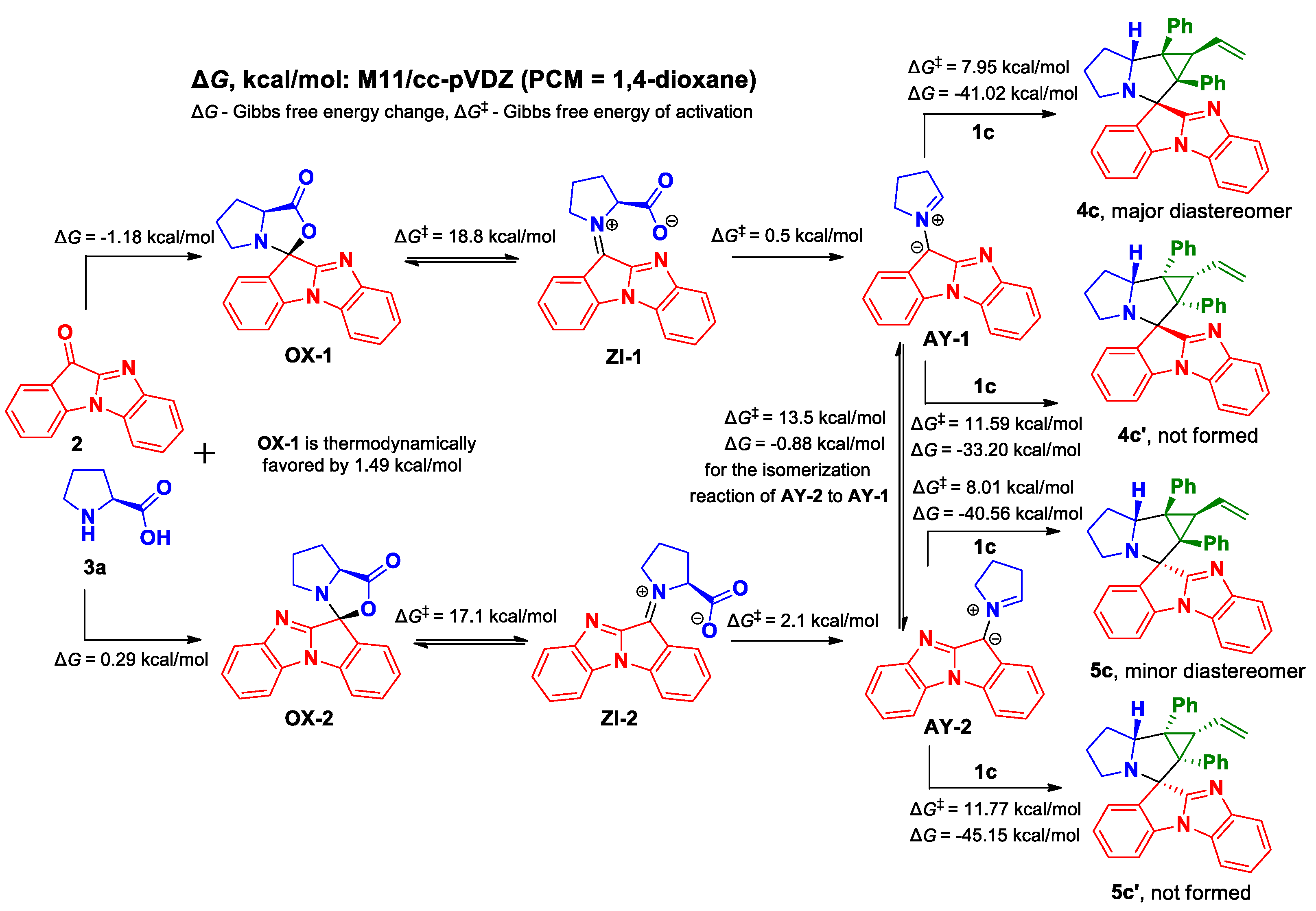
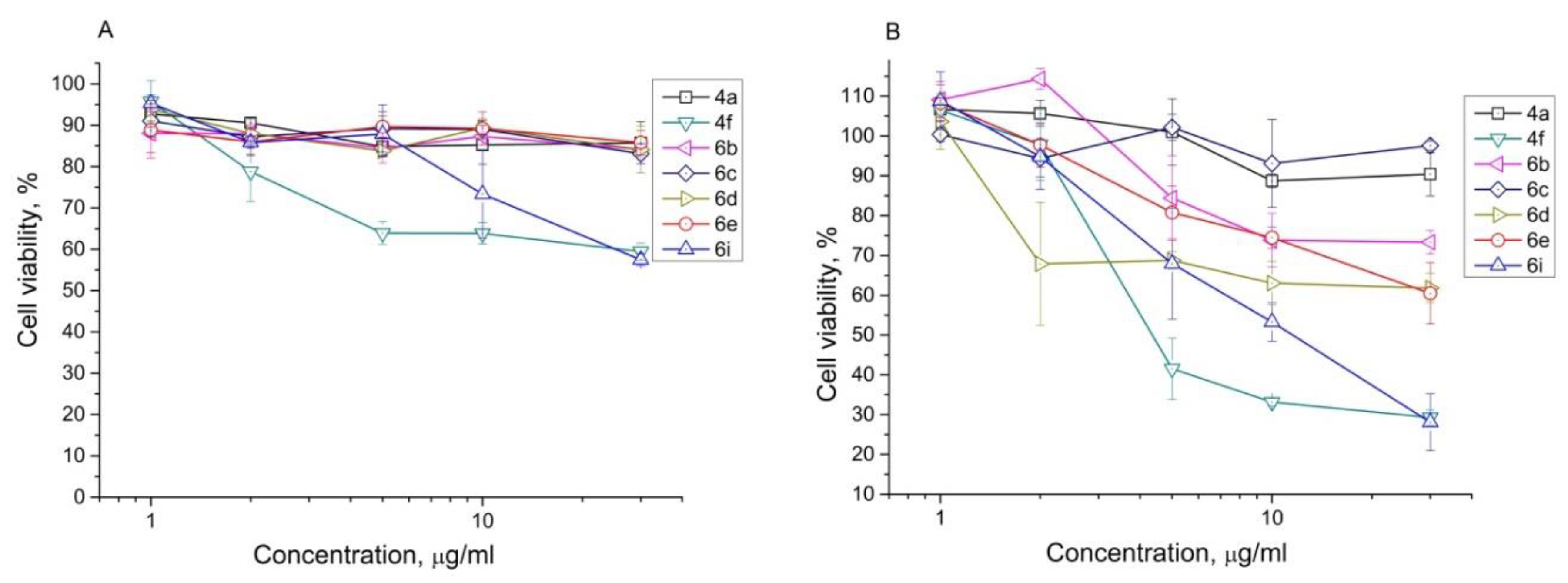
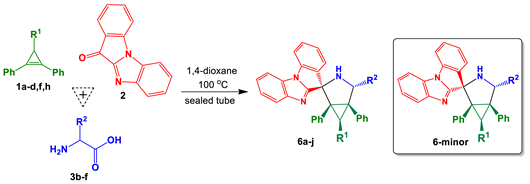
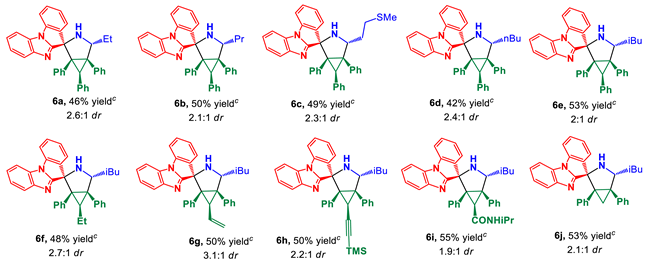
3.1. Chemistry
NMR spectra were recorded with a Bruker Avance 400 spectrometer (400.13 MHz for
1H and 100.61 MHz for
13C). Chemical shifts are reported in ppm relative to residual CHCl
3 (
1H,
δ = 7.26 ppm), CDCl
3 (
13C,
δ = 77.17), residual DMSO-
d5 (
1H, 2.50 ppm), and DMSO-
d6 (
13C, 39.52 ppm) as internal standards. Mass spectra were recorded on a HRMS-ESI-QTOF mass-analyzer, electrospray ionization, positive mode. Single-crystal X-ray diffraction experiments for compounds
4f and
8f were carried out using an Agilent Technologies «Xcalibur» diffractometer with monochromated Mo Kα radiation. Melting points were determined on a melting point apparatus and are uncorrected. 11
H-Benzo[4,5]imidazo[1,2-
a]indol-11-one was obtained from 1
H-benzo[
d]imidazole and 2-fluorobenzaldehyde, according to a known method [
37]. Cyclopropenes
1a [
41],
1b,
c [
42],
1d [
43],
1e [
44],
1f,
g [
45], and
1h [
46] were prepared according to the literature data, while α-amino acids
3 were obtained from commercial sources. Maleimides
7e [
47] and
7f [
48] were synthesized according to previously described procedures,
7a−d were obtained from commercial sources. Crystallographic data for compounds
4f (CCDC 2195042) and
8f (CCDC 2195043) have been deposited at the Cambridge Crystallographic Data Centre and can be obtained free of charge via
www.ccdc.cam.ac.uk/data_request/cif (accessed on 5 August 2022). Copies of
1H and
13C NMR spectra of compounds
4-6 and
8 are given at
Supporting Information, (Figures S1–S52). X-ray crystallographic data for compounds
4f and
8f are given at
Supporting Information, (Figures S60, S61 and Tables S1 and S2).
General Procedure A for the One-Pot Three-Component Reaction of 11H-Benzo[4,5]imidazo[1,2-a]indol-11-one, L-Proline, and Cyclopropenes
11H-Benzo[4,5]imidazo[1,2-a]indol-11-one (2, 0.3 mmol), L-proline (3a, 0.6 mmol), cyclopropene (1, 0.3 mmol), and anhydrous 1,4-dioxane (4 mL) were added to a screw-capped tube (Schuett-biotec type). The reaction vessel was closed and the reaction mixture was stirred in a preheated oil bath at 100 °C for 18 h (the reaction progress was monitored by TLC). The mixture was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was subjected to silica gel PTLC using a mixture of hexane–ethyl acetate as an eluent to give the spiro-adducts 4a−h.
(±)-(1’R,1a’R,2’R,6a’S,6b’S)-1’,1a’,6b’-Triphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4a) and (1’R,1a’R,2’S,6a’S,6b’S)-1’,1a’,6b’-triphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (5a)
Prepared according to the general procedure A using cyclopropene 1a (85.3 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol) and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished 4a and 5a (112 mg, 64%) as an inseparable mixture in ratio 2:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4a as a pure compound (67 mg);
Data for 4a: white solid; mp 170–172 °C; Rf 0.64 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3056, 2962, 2817, 1608, 1525, 1491, 1471, 1444, 1345, 1302, 1222, 1167, 772, 737, 706, 556, 534 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.99 (t, J = 7.7 Hz, 2 H), 7.61 (d, J = 7.7 Hz, 2 H), 7.49 (d, J = 7.7 Hz, 1 H), 7.40 (t, J = 7.7 Hz, 1 H), 7.33–7.22 (m, 7 H), 7.02 (t, J = 7.7 Hz, 1 H), 6.94 (t, J = 7.7 Hz, 2 H), 6.75 (br.s, 2 H), 6.73 (t, J = 7.7 Hz, 1 H), 6.60 (t, J = 7.7 Hz, 2 H), 6.48 (d, J = 7.7 Hz, 2 H), 4.81 (t, J = 7.0 Hz, 1 H), 3.70 (s, 1 H), 3.21 (q, J = 7.0 Hz, 1 H), 2.57(m, 1 H), 2.20 (m, 1 H), 2.03 (m, 1 H), 1.90–1.80 (m, 2 H).
13C NMR (101 MHz, CDCl3): δ = 162.3, 148.2, 138.9, 137.1, 134.7, 134.4, 132.3 (4 C), 131,3, 130.9 (2 C), 129.4, 129,1, 127.7 (2 C), 126.5 (2 C), 126.5, 126.4 (2 C), 126.4, 126.2, 125.0, 123.8, 122.7, 122.1, 120.7, 110.5, 110.2, 75.7, 71.4, 57.6, 46.5, 45.1, 31.3, 26.6, 25.0.
HRMS (ESI): m/z [M+H]+ calcd for C39H32N3: 542.2591; found: 542.2592.
(±)-(1’R,1a’R,2’R,6a’S,6b’S)-1’-Ethyl-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4b) and (±)-(1’R,1a’R,2’S,6a’S,6b’S)-1’-ethyl-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (5b)
Prepared according to the general procedure A using cyclopropene 1b (70.5 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 5:1) furnished 4b and 5b (131 mg, 83%) as an inseparable mixture in a ratio of 2.2:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4b as a pure compound (81 mg).
Data for 4b: white solid; mp 220–222 °C; Rf 0.59 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3056, 2963, 2905, 2836, 1609, 1523, 1492, 1472, 1445, 1347, 1306, 1222, 1166, 922, 748, 737, 699 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.89 (d, J = 7.5 Hz, 1 H), 7.87–7.80 (m, 1 H), 7.52–7.42 (m, 4 H), 7.36–7.27 (m, 4 H), 7.26–7.18 (m, 3 H), 6.88–6.80 (m, 3 H), 6.76 (t, J = 7.5 Hz, 2 H), 4.79 (br.m, 1 H), 3.05–2.98 (m, 1 H), 2.50–2.38 (m, 2 H), 2.33–2.11 (m, 2 H), 2.11–1.99 (m, 1 H), 1.89–1.75 (m, 1 H), 1.63–1.49 (m, 1 H), 1.47–1.34 (m, 1 H), 1.02 (t, J = 7.0 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 162.4, 148.0, 138.9, 137.7, 135.3, 132.7, 131.6 (2 C), 129.9 (2 C), 129.3, 129.1, 127.5 (2 C), 126,7 (2 C), 126.4, 126.0, 125.6, 123.7, 122.6, 122.0, 120.6, 110.5, 110.1, 71.7, 70.5, 54.4, 44.4, 41.7, 29.6, 26.8, 26.6, 19.2, 14.2.
HRMS (ESI): m/z [M+H]+ calcd for C35H32N3: 494.2591; found: 494.2597.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-1a’,6b’-Diphenyl-1’-vinyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4c) and (±)-(1’R,1a’R,2’S,6a’R,6b’S)-1a’,6b’-diphenyl-1’-vinyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (5c)
Prepared according to general procedure A using cyclopropene 1c (69.9 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 5:1) furnished 4c and 5c (101 mg, 64%) as an inseparable mixture in a ratio of 2.3:1, respectively.
Data for mixture 4c + 5c: white solid; mp 218–220 °C.
IR (KBr): 3055, 2967, 2910, 2869, 2809, 1610, 1526, 1493, 1473, 1446, 1348, 1223, 1168, 998, 902, 740, 699, 443 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.5 Hz, 1 H(5c)), 7.97 (d, J = 7.5 Hz, 1 H(4c)), 7.90 (d, J = 7.5 Hz, 1 H(4c)),7.86 (d, J = 7.5 Hz, 2 H(4c)), 7.85 (d, J = 7.5 Hz, 1 H(5c)), 7.65 (d, J = 7.5 Hz, 2 H(5c)), 7.59 (d, J = 7.5 Hz, 1 H(5c)), 7.49 (d, J = 7.5 Hz, 1 H(4c)), 7.45–7.21 (m, 8 H(4c +5c)), 7.17 (d, J = 7.5 Hz, 1 H(5c)), 6.97 (d, J = 7.5 Hz, 2 H(4c)), 6.82–6.76 (m, 1 H(4c) + 1 H(5c)), 6.75–6.65 (m, 2 H(4c) + 4 H(5c)), 5.62 (dd, J = 17.0, 2.5 Hz, 1 H(5c)), 5.49 (dd, J = 17.0, 2.5 Hz, 1 H(4c)), 5.16 (dt, J = 17.0, 10.3 Hz, 1 H(4c)), 5.05 (dd, J = 10.3, 2.5 Hz, 1 H(4c)), 5.01 (dd, J = 10.3, 2.5 Hz, 1 H(5c)), 4.95 (dt, J = 17.0, 10.3 Hz, 1 H(5c)), 4.76 (dd, J = 8.5, 6.0 Hz, 1 H(4c + 5c)), 4.12 (br.q, J = 7.2 Hz, 1 H(5c)), 3.30 (d, J = 9.6 Hz, 1 H(5c)), 3.25 (d, J = 10.6 Hz, 1 H(4c)), 3.10 (q, J = 7.2 Hz, 1 H(4c)), 2.80 (br.t, J = 8.0 Hz, 1 H(5c)), 2.50 (td, J = 8.0, 4.0 Hz, 1 H(4c)), 2.27–1.91 (m, 3 H(4c + 5c)), 1.91–1.73 (m, 2 H(4c + 5c)).
13C NMR (101 MHz, CDCl3): δ = 162.2 (4c), 148.2 (4c), 138.9 (4c), 138.0 (4c), 136.6 (4c), 134.6 (4c), 132.6, 132.1 (2 C (4c)), 131.4 (2 C(4c)), 131.1 (4c), 129.4 (4c), 129.2 (4c), 128.1 (4c), 127.9 (2 C (4c)), 126.8 (4c), 126.7 (2 C), 126.4, 126.3 (4c), 124.3 (5c), 123.8 (4c), 123.3 (5c), 122.7 (4c), 122.4 (5c), 122.1 (4c), 120.8 (5c), 120.7 (4c), 114.1 (5c), 113.3 (4c), 110.5 (4c), 110.4 (5c), 110.2 (4c), 110.1 (5c), 74.3 (4c), 70.9 (4c), 55.2 (4c), 49.4 (4c), 45.0 (4c), 44.9 (4c), 32.2 (5c), 30.7 (4c), 27.8 (5c), 27.2 (5c), 26.6 (4c), 25.1 (4c).
HRMS (ESI): m/z [M+H]+ calcd for C35H30N3: 492.2434; found: 492.2439.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-1a’,6b’-Diphenyl-1’-((trimethylsilyl)ethynyl)-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4d) and (±)-(1’R,1a’R,2’S,6a’R,6b’S)-1a’,6b’-diphenyl-1’-((trimethylsilyl)ethynyl)-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (5d)
Prepared according to general procedure A using cyclopropene 1d (92.3 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished 4d and 5d as separated diastereomers.
Data for major diastereomer 4d: yield 101 mg (56%); white solid; mp 189–191 °C; Rf0.60 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3057, 2969, 2835, 2152, 1609, 1526, 1492, 1471, 1446, 1344, 1250, 1223, 849, 840, 735, 697 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.91–7.84 (m, 2 H), 7.65 (d, J = 7.5 Hz, 2 H), 7.52–7.47 (m, 1 H), 7.44 (t, J = 7.5 Hz, 1 H), 7.37–7.20 (m, 7 H), 6.95 (d, J = 7.5 Hz, 2 H), 6.86 (t, J = 7.5 Hz, 1 H), 6.76 (t, J = 7.5 Hz, 2 H), 4.85 (dd, J = 5.0, 9.0 Hz, 1 H), 3.42 (s, 1 H), 2.92–2.82 (m, 1 H), 2.44–2.33 (m, 1 H), 2.22–1.98 (m, 3 H), 1.85–1.70 (m, 1 H), −0.05 (s, 9 H).
13C NMR (101 MHz, CDCl3): δ = 160.9, 147.9, 138.7, 135.2, 134.0, 132.3 (2 C), 130.6, 130.1 (2 C), 129.5, 129.2, 127.1 (2 C), 126.7, 126,5 (2 C), 126.2, 125.9, 124.1, 122.8, 122.2, 120.6, 110.5, 110.2, 104.2, 92.0, 70.7, 69.6, 56.5, 43.6, 43.0, 26.5, 25.3, 19.0, −0.5 (3 C).
HRMS (ESI): m/z [M+H]+ calcd for C38H36N3Si: 562.2673; found: 562.2679.
Data for minor diastereomer 5d: yield 11 mg (6%); white solid; mp 242–245 °C; Rf 0.48 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3057, 2969, 2954, 2835, 2154, 1609, 1492, 1471, 1344, 1223, 850, 735, 697 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.06–7.99 (m, 1 H), 7.66–7.56 (m, 2 H), 7.50 (d, J = 7.5 Hz, 2 H), 7.43–7.35 (m, 2 H), 7.35–7.25 (m, 3 H), 7.24–7.13 (m, 3 H), 6.87 (t, J = 7.5 Hz, 1 H), 6.75 (t, J = 7.5 Hz, 2 H), 6.68 (d, J = 7.5 Hz, 2 H), 4.97 (br.s, 1 H), 3.89 (br.s, 1 H), 3.29 (s, 1 H), 2.73 (br.s, 1 H), 2.31 (br.s, 1 H), 2.22 (br.s, 1 H), 2.07 (br.s, 2 H), −0.13 (s, 9 H).
13C NMR (101 MHz, CDCl3): δ = 158.0, 147.8, 141.0, 136.6, 135.1, 132.1 (2 C), 131.0, 129.9 (2 C), 129.3, 128.5, 127.3 (2 C), 126.5 (4 C), 125.6, 124.1, 123.4, 122.5, 120.9, 110.5, 110.2, 103.4, 92.1, 73.0, 72.9, 57.6, 48.6, 48.3, 28.5, 27.2, 21.8, −0.6 (3 C).
HRMS (ESI): m/z [M+H]+ calcd for C38H36N3Si: 562.2673; found: 562.2672.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-Methyl 1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carboxylate (4e) and (±)-(1’R,1a’R,2’S,6a’R,6b’S)-methyl 1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo [4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carboxylate (5e)
Prepared according to the general procedure A using cyclopropene 1e (80.1 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished 4e and 5e (112 mg, 67%) as an inseparable mixture in a ratio of 3:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4e as a pure compound (71 mg).
Data for 4e: white solid; mp 242–245 °C; Rf 0.36 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3056, 2949, 2843, 1740, 1609, 1539, 1492, 1472, 1447, 1357, 1223, 1169, 922, 741, 695, 618 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00–7.90 (m, 2 H), 7.71 (d, J = 7.7 Hz, 2 H), 7.55–7.44 (m, 2 H), 7.42–7.25 (m, 7 H), 6.88 (d, J = 7.7 Hz, 2 H), 6.79 (t, J = 7.7 Hz, 1 H), 6.70 (t, J = 7.7 Hz, 2 H), 4.75 (br.s, 1 H), 3.38 (s, 3 H), 3.37 (s, 1 H), 3.08 (m, 1 H), 2.51 (br.s, 1 H), 2.14 (m, 1 H), 1.98 (m, 1 H), 1.87–1.75 (m, 2 H).
13C NMR (101 MHz, CDCl3): δ = 170.0, 161.3, 148.0, 138.9, 134.4, 133.5, 131.6, 130.7 (2 C), 130.5 (2 C), 129.8, 128.9, 127.8 (2 C), 126.8, 126.7, 126.6 (2 C), 126.3, 124.1, 123.1, 122.4, 120.7, 110.8, 110,3, 74.7, 71.1, 58.2, 51.1, 48.3, 45.0, 28.8, 26.5, 25.2.
HRMS (ESI): m/z [M+H]+ calcd for C35H30N3O2: 524.2333; found: 524.2333.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-N-Isopropyl-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carboxamide (4f) and (±)-(1’R,1a’R,2’S,6a’R,6b’S)-N-isopropyl-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carboxamide (5f)
Prepared according to the general procedure A using cyclopropene 1f (88.8 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 1:2) furnished 4f and 5f (141 mg, 80%) as an inseparable mixture in a ratio of 5:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4f as a pure compound (105 mg).
Data for 4f: white solid; mp 242–244 °C; Rf 0.69 (SiO2, hexane–ethyl acetate, 1:2).
IR (KBr): 3423, 3048, 2965, 2844, 1644, 1609, 1532, 1493, 1472, 1446, 1352, 1222, 1168, 930, 764, 740, 716, 699, 668, 496 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.05 (d, J = 7.7 Hz, 1 H), 7.94 (d, J = 7.7 Hz, 1 H), 7.88 (d, J = 7.7 Hz, 2 H), 7.50–7.40 (m, 4 H), 7.38–7.22 (m, 6 H), 7.03 (d, J = 7.7 Hz, 2 H), 6.84 (t, J = 7.7 Hz, 1 H), 6.75 (t, J = 7.7 Hz, 2 H), 4.72 (t, J = 6.0 Hz, 1 H), 3.69 (m, 1 H), 3.49 (d, J = 6.0 Hz, 1 H), 3.26 (s, 1 H), 3.19 (m, 1 H), 2.52 (m, 1 H), 2.11(m, 1 H), 2.00–1.80 (m, 3 H), 0.55 (d, J = 6.5 Hz, 3 H), 0.27 (d, J = 6.5 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 168.0, 161.5, 138.9, 133.7, 133.3, 131.9 (2 C), 131.5 (2 C), 131.0, 129.8 (2 C), 128.9 (2 C), 128.2 (2 C), 127.5, 127.4, 127.3 (2 C), 126.9, 124.2, 122.9, 122.3, 120.7, 110.6, 110.2, 75.2, 56.0, 47.2, 45.9, 40.7, 31.6, 26.5, 25.4, 21.6, 21.3.
HRMS (ESI): m/z [M+H]+ calcd for C37H35N4O: 551.2805; found: 551.2805.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-1a’,6b’-Diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carbonitrile (4g) and (±)-(1’R,1a’R,2’S,6a’R,6b’S)-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine]-1’-carbonitrile (5g)
Prepared according to the general procedure A using cyclopropene 1g (69.5 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 2:1) furnished 4g and 5g (91 mg, 58%) as an inseparable mixture in a ratio of 4:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4g as a pure compound (35 mg).
Data for 4g: beige solid; mp 247–250 °C; Rf 0.39 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3067, 2960, 2813, 2234, 1611, 1521, 1496, 1474, 1446, 1353, 1308, 1223, 1169, 924, 843, 735, 702, 478 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.73 (br.d, J = 7.7 Hz, 1 H), 7.39 (d, J = 7.7 Hz, 1 H), 7.33 (d, J = 7.7 Hz, 2 H), 7.55–7.45 (m, 2 H), 7.40(t, J = 7.7 Hz, 2 H), 7.35–7.25 (m, 5 H), 6.98 (d, J = 7.7 Hz, 2 H), 6.92 (t, J = 7.7 Hz, 1 H), 6.82 (t, J = 7.7 Hz, 2 H), 4.81 (br.s, 1 H), 3.52 (s, 1 H), 2.77 (m, 1 H), 2.35 (m, 1 H), 2.19 (m, 1 H), 2.10–1.90(m, 2 H), 1.67 (m, 1 H).
13C NMR (101 MHz, CDCl3): δ = 159.2, 147.4, 138.6, 133.0, 132.9, 131.3 (3 C), 130.2, 129.7 (2 C), 129.1, 128.2 (2 C), 127.9, 127.5, 127.4 (2 C), 125.3, 124.4, 123.4, 122.6, 120.6, 118.4, 110.9, 110.4, 70.9, 68.8, 56.4, 43.6, 41.8, 26.2, 24.5, 14.4.
HRMS (ESI): m/z [M+H]+ calcd for C34H27N4: 491.2230; found: 491.2230.
(±)-(1a’R,2’R,6a’R,6b’S)-1a’,6b’-Diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4h) and (±)-(1a’R,2’S,6a’R,6b’S)-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo [4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (5h)
Prepared according to the general procedure A using cyclopropene 1h (61.5 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-proline (3a) (73.2 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished 4h and 5h (91 mg, 61%) as an inseparable mixture in a ratio of 3:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 4h as a pure compound (32 mg).
Data for 4h: white solid; mp 230–231 °C; Rf 0.54 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3055, 2968, 2869, 2819, 1609, 1519, 1495, 1471, 1446, 1341, 1223, 1169, 1144, 1057, 1023, 926,775, 740, 701,619 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.93 (d, J = 7.5 Hz, 1 H), 7.88 (d, J = 7.5 Hz, 1 H), 7.51 (d, J = 7.5 Hz, 1 H), 7.46 (d, J = 7.5 Hz, 2 H), 7.40 (t, J = 7.5 Hz, 1 H), 7.33–7.22 (m, 6 H), 7.18 (t, J = 7.5 Hz, 1 H), 6.90 (d, J = 7.5 Hz, 2 H), 6.80 (t, J = 7.5 Hz, 1 H), 6.73 (t, J = 7.5 Hz, 2 H), 4.98 (dd, J = 5.0, 9.0 Hz, 1 H), 2.97–2.90 (m, 1 H), 2.48 (d, J = 5.0 Hz, 1 H), 2.46–2.39 (m, 1 H), 2.22–1.97 (m, 3 H), 1.76–1.63 (m, 1 H), 1.59 (d, J = 5.0 Hz, 1 H).
13C NMR (101 MHz, CDCl3): δ = 161.8, 148.1, 138.7, 138.4, 135.0, 131.5 (2 C), 129.3, 129.2, 128.6 (3 C), 127.8 (2 C), 126.9 (2 C), 126.4, 126.0, 125.9, 123.7, 122.7, 122.1, 120.7, 110.5, 110.2, 69.8, 69.7, 53.1, 43.9, 39.1, 26.8, 25.2, 18.0.
HRMS (ESI): m/z [M+H]+ calcd for C33H28N3: 466.2278; found: 466.2285.
(±)-(1’R,1a’R,2’R,6a’R,6b’S)-1’-Ethynyl-1a’,6b’-diphenyl-1a’,4’,5’,6’,6a’,6b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-cyclopropa[a]pyrrolizine] (4i)
Cycloadduct 4d (80 mg, 0.142 mmol) was dissolved in ether (1 mL) and methanol (3 mL). Anhydrous K2CO3 (20.9 mg; 0.151 mmol) was added and the resulting suspension was stirred at ambient temperature for 42 h in a sealed tube. The precipitate that formed was filtered off and washed with methanol and hexane to afford pure product 4i.
Yield: 64.9 мг (92%); white solid; mp 259–262 °C; Rf 0.43 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3296, 3056, 2971, 2911, 2814, 1611, 1528, 1494, 1473, 1447, 1349, 1224, 1169, 926, 742, 704, 644, 624, 550, 442 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.92–7.86 (m, 2 H), 7.74 (d, J = 7.8 Hz, 2 H), 7.52–7.42 (m, 2 H), 7.40–7.20 (m, 7 H), 6.96 (d, J = 7.8 Hz, 2 H), 6.87 (t, J = 7.8 Hz, 1 H), 6.78 (t, J = 7.8 Hz, 2 H), 4.82 (m, 1 H), 3.42 (s, 1 H), 2.88 (m, 1 H), 2.40 (m, 1 H), 2.22–2.00 (m, 4 H), 1.78 (m, 1 H).
13C NMR (101 MHz, CDCl3): δ = 160.8, 147.9, 138.7, 134.0, 132.0 (2 C), 130.7, 130.2 (2 C), 129.6, 129.1, 127.5 (3 C), 126.9, 126.6 (2 C), 126.5, 125.8, 124.0, 122.9, 122.2, 120.6, 110.6, 110.2, 81.5, 70.0, 71.2, 69.6, 56.1, 43.4, 42.9, 26.5, 25.1, 17.5.
HRMS (ESI): m/z [M+H]+ calcd for C35H28N3: 489.2205; found: 489.2207.
General Procedure B for the One-Pot Three-Component Reaction of 11H-Benzo[4,5]imidazo[1,2-a]indol-11-one, Primary α-Amino Acids and Cyclopropenes
11H-Benzo[4,5]imidazo[1,2-a]indol-11-one (2, 0.3 mmol), primary α-amino acid (3, 0.6 mmol), cyclopropene (1, 0.3 mmol), and anhydrous 1,4-dioxane (4 mL) were added to a screw-capped tube (Schuett-biotec type). The reaction vessel was closed, and the reaction mixture was stirred in a preheated oil bath at 100 °C for 18 h (the reaction progress was monitored by TLC). The mixture was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was subjected to silica gel PTLC using a mixture of hexane̶̶–ethyl acetate as an eluent to give the spiro-adducts 6a−j.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Ethyl-1’,5’,6’-triphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6a)
Prepared according to the general procedure B using cyclopropene 1a (91.5 mg, 0.34 mmol), ketone 2 (75.0 mg, 0.34 mmol), and DL-2-aminobutanoic acid (3b) (70.1 mg, 0.68 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished corresponding cycloadduct (82 mg, 46%) as an inseparable mixture of diastereomers in a ratio of 2.6:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6a as a pure compound (35 mg).
Data for 6a: beige solid; mp 236–238 °C; Rf 0.51 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3057, 2962, 2874, 1606, 1527, 1494, 1470, 1445, 1343, 1220, 1166, 908, 732, 699, 550, 438 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.8 Hz, 1 H), 7.98 (br.d, J = 7.8 Hz, 1 H), 7.63 (d, J = 7.5 Hz, 2 H), 7.47 (d, J = 7.8 Hz, 1 H), 7.40–7.20 (m, 8 H), 7.02 (t, J = 7.5 Hz, 1 H), 6.93 (t, J = 7.5 Hz, 2 H), 6.90–6.50 (br.s, 2 H), 6.73 (t, J = 7.5 Hz, 1 H), 6.59 (br.t, J = 7.5 Hz, 2 H), 6.47 (d, J = 7.5 Hz, 2 H), 4.67 (dd, 4.0, 9.0 Hz, 1 H), 3.85 (s, 1 H), 2.70–2.00 (br.s, 1 H), 1.76–1.60 (m, 2 H), 0.94 (br.t, J = 7.0 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.8, 148.1, 138.4, 137.0, 136.8, 134.3, 133.1 (2 C), 132.3 (2 C), 131.0, 130.9 (2 C), 129.4, 129.2, 127.6 (2 C), 126.6 (2 C), 126.4 (4 C), 125.3, 125.0, 124.2, 123.0, 122.2, 120.7, 110.3, 110.2, 71.2, 68.5, 52.0, 47.4, 29.3, 23.7, 11.1.
HRMS (ESI): m/z [M+H]+ calcd for C38H32N3: 530.2591; found: 530.2595.
(±)-(1’R,2’R,4’R,5’S,6’R)-1’,5’,6’-Triphenyl-4’-propyl-3’-azaspiro[benzo[4,5]imidazo [1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6b)
Prepared according to the general procedure B using cyclopropene 1a (91.5 mg, 0.34 mmol), ketone 2 (75.0 mg, 0.34 mmol), and DL-norvaline (3c) (79.7 mg, 0.68 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (92 mg, 50%) as an inseparable mixture in a ratio of 2.1:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6b as a pure compound (41 mg).
Data for 6b: beige solid; mp 262–264 °C; Rf 0.49 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3372, 3049, 3029, 2955, 2928, 2870, 1608, 1496, 1472, 1446, 1344, 1224, 914, 762, 730, 699, 559, 546 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.8 Hz, 1 H), 7.97 (d, J = 7.8 Hz, 1 H), 7.64 (d, J = 7.5 Hz, 2 H), 7.47 (d, J = 7.7 Hz, 1 H), 7.42–7.18 (m, 8 H), 7.02 (t, J = 7.5 Hz, 1 H), 6.93 (t, J = 7.5 Hz, 2 H), 6.80–6.40 (br.s, 2 H), 6.73 (t, J = 7.5 Hz, 1 H), 6.59 (br.t, J = 7.5 Hz, 2 H), 6.47 (d, J = 7.5 Hz, 2 H), 4.73 (dd, 3.4, 9.5 Hz, 1 H), 3.84 (s, 1 H), 2.80–1.80 (br.s, 1 H), 1.72–1.50 (m, 2 H), 1.50–1.36 (m, 1 H), 1.36–1.21 (m, 1 H), 0.89 (t, J = 7.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.6, 148.1, 138.5, 137.2, 134.4, 133.3 (2 C), 132.4 (2 C), 131.2, 131.0 (3 C), 129.5, 129.3, 127.7 (2 C), 126.7 (2 C), 126.5 (3 C), 125.44, 125.39, 125.2, 124.3, 123.1, 122.3, 120.8, 110.4, 110.3, 71.3, 67.1, 51.9, 47.4, 33.1, 29.3, 20.0, 14.4.
HRMS (ESI): m/z [M+H]+ calcd for C39H34N3: 544.2747; found: 544.2752.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-(2-(Methylthio)ethyl)-1’,5’,6’-triphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6c)
Prepared according to the general procedure B using cyclopropene 1a (91.5 mg, 0.34 mmol), ketone 2 (75.0 mg, 0.34 mmol), and L-methionine (3d) (101.5 mg, 0.68 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (96 mg, 49%) as an inseparable mixture in a ratio of 2.3:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6c as a pure compound (57 mg).
Data for 6c: white solid; mp 245–247 °C; Rf 0.47 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3447, 3309, 3059, 3026, 2915, 2848, 1608, 1521, 1493, 1472, 1445, 1341, 1218, 1169, 1079, 739, 698 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.99 (d, J = 7.8 Hz, 1 H), 7.97 (d, J = 7.8 Hz, 1 H), 7.64 (d, J = 7.5 Hz, 2 H), 7.47 (d, J = 7.7 Hz, 1 H), 7.41–7.21 (m, 8 H), 7.03 (t, J = 7.5 Hz, 1 H), 6.94 (t, J = 7.5 Hz, 2 H), 6.80–6.40 (br.s, 2 H), 6.74 (t, J = 7.5 Hz, 1 H), 6.59 (br.t, J = 7.5 Hz, 2 H), 6.47 (d, J = 7.5 Hz, 2 H), 4.81 (t, 6.0 Hz, 1 H), 3.87 (s, 1 H), 2.95–2.40 (br.s, 1 H), 2.63–2.43 (m, 2 H), 2.03 (s, 3 H), 2.01–1.90 (m, 2 H).
13C NMR (101 MHz, CDCl3): δ = 163.3, 148.0, 138.4, 136.7 (2 C), 133.8, 133.1 (2 C), 132.3 (2 C), 130.9 (3 C), 129.5, 129.2, 127.7 (2 C), 126.8, 126.6 (2 C), 126.4 (3 C), 125.3, 125.2, 124.3, 123.0, 122.3, 120.3, 110.4, 110.2, 71.2, 66.7, 51.7, 47.1, 31.5, 30.5, 29.5, 15.5.
HRMS (ESI): m/z [M+H]+ calcd for C39H34N3S: 576.2468; found: 576.2470.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Butyl-1’,5’,6’-triphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6d)
Prepared according to the general procedure B using cyclopropene 1a (91.5 mg, 0.34 mmol), ketone 2 (75.0 mg, 0.34 mmol), and DL-norleucine (3e) (89.2 mg, 0.68 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (79 mg, 42%) as an inseparable mixture in a ratio of 2.4:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6d as a pure compound (25 mg).
Data for 6d: white solid; mp 267–270 °C; Rf 0.46 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3306, 3031, 2955, 2929, 1608, 1538, 1493, 1473, 1447, 1364, 1226, 1168, 1032, 944, 807, 749, 738, 698, 551 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.98 (d, J = 7.8 Hz, 1 H), 7.92 (d, J = 7.8 Hz, 1 H), 7.55 (d, J = 7.5 Hz, 1 H), 7.41–7.26 (m, 9 H), 7.20 (d, J = 7.5 Hz, 1 H), 6.96 (t, J = 7.5 Hz, 1 H), 6.89 (t, J = 7.5 Hz, 2 H), 6.85–6.40 (br.s, 2 H), 6.76 (t, J = 7.5 Hz, 1 H), 6.62 (br.s, 2 H), 6.53 (d, J = 7.5 Hz, 2 H), 4.28 (dd, 3.4, 9.5 Hz, 1 H), 3.83 (s, 1 H), 3.20–2.60 (br.s, 1 H), 1.76–1.60 (m, 2 H), 1.55–1.43 (m, 1 H), 1.33–1.21 (m, 3 H), 0.83 (t, J = 7.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 160.8, 147.4, 140.8, 137.2, 136.8, 134.2, 132.6 (2 C), 132.0, 131.7 (2 C), 130.9. (2 C), 129.5, 128.7, 127.9 (2 C), 126.9 (2 C), 126.8, 126.3 (2 C), 126.2, 124.9 (2C), 124.4, 123.4, 122.7, 120.6, 110.6, 110.5, 72.8, 71.0, 53.4, 50.2, 30.7, 30.2, 29.3, 22.8, 13.9.
HRMS (ESI): m/z [M+H]+ calcd for C40H36N3: 558.2904; found: 558.2905.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Isobutyl-1’,5’,6’-triphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6e)
Prepared according to the general procedure B using cyclopropene 1a (91.5 mg, 0.34 mmol), ketone 2 (75.0 mg, 0.34 mmol), and L-leucine (3f) (89.2 mg, 0.68 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (101 mg, 53%) as an inseparable mixture in a ratio of 2:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6e as a pure compound (52 mg).
Data for 6e: white solid; mp 255–257 °C; Rf 0.66 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3421, 3056, 3028, 2960, 2914, 2871, 2840, 1609, 1496, 1472, 1446, 1341, 1226, 1170, 763, 728, 699, 563, 546 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.8 Hz, 1 H), 7.97 (d, J = 7.8 Hz, 1 H), 7.79 (d, J = 7.5 Hz, 2 H), 7.47 (d, J = 7.7 Hz, 1 H), 7.41–7.20 (m, 8 H), 7.02 (t, J = 7.5 Hz, 1 H), 6.94 (t, J = 7.5 Hz, 2 H), 6.85–6.45 (br.s, 2 H), 6.73 (t, J = 7.5 Hz, 1 H), 6.59 (t, J = 7.5 Hz, 2 H), 6.48 (d, J = 7.5 Hz, 2 H), 4.80 (dd, 3.4, 9.5 Hz, 1 H), 3.80 (s, 1 H), 2.80–1.80 (br.s, 1 H), 1.75–1.56 (m, 2 H), 1.42–1.32 (m, 1 H), 0.91 (d, J = 6.3 Hz, 3 H), 0.83 (d, J = 6.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.5, 148.2, 138.5, 137.1, 134.2, 133.1 (2 C), 132.3 (2 C), 131.1, 130.9 (2 C), 129.4 (2 C), 129.2, 127.8, 127.6 (2 C), 126.6 (2 C), 126.4 (3 C), 125.2, 125.0, 124.2, 122.9, 122.1, 120.8, 110.3, 110.2, 71.4, 65.2, 51.8, 47.5, 39.7, 29.2, 25.4, 23.9, 21.7.
HRMS (ESI): m/z [M+H]+ calcd for C40H36N3: 558.2904; found: 558.2909.
(±)-(1’R,2’R,4’R,5’S,6’R)-6’-Ethyl-4’-isobutyl-1’,5’-diphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6f)
Prepared according to the general procedure B using cyclopropene 1b (70.0 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-leucine (3f) (83.4 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (79 mg, 48%) as an inseparable mixture in a ratio of 2.7:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6f as a pure compound (31 mg).
Data for 6f: white solid; mp 215–216 °C; Rf 0.60 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3357, 3054, 2951, 2869, 1609, 1495, 1472, 1445, 1338, 1224, 1165, 770, 738, 700 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.98 (d, J = 7.5 Hz, 2 H), 7.94–7.83 (m, 2 H), 7.55–7.40 (m, 4 H), 7.39–7.23 (m, 5 H), 6.84–6.73 (m, 3 H), 6.68 (t, J = 7.5 Hz, 2 H), 4.63 (dd, J = 3.0, 9.0 Hz, 1 H), 2.37 (t, J = 6.5 Hz 1 H), 2.32–1.87 (br.s, 1 H), 1.66–1.46 (m, 3 H), 1.46–1.28 (m, 2 H), 1.20 (t, J = 7.0 Hz, 3 H), 0.88 (d, J = 6.3 Hz, 3 H), 0.83 (d, J = 6.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.9, 148.3, 138.7, 138.0, 137.6, 133.8, 131.8 (2 C), 130.7 (2 C), 129.5, 129.4, 128.1 (2 C), 126.8 (2 C), 126.5, 126.1, 125.1, 124.3, 122.8, 122.1, 120.9, 110.6, 110.2, 70.3, 64.1, 47.2, 44.7, 40.2, 26.4, 25.4, 23.8, 21.9, 20.2, 14.8.
HRMS (ESI): m/z [M+H]+ calcd for C36H36N3: 510.2904; found: 510.2910.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Isobutyl-1’,5’-diphenyl-6’-vinyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6g)
Prepared according to the general procedure B using cyclopropene 1c (69.4 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-leucine (3f) (83.4 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (81 mg, 50%) as an inseparable mixture in a ratio of 3.1:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6g as a pure compound (29 mg).
Data for 6g: white solid; mp 250–251 °C; Rf 0.59 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3330, 3057, 2954, 2923, 2866, 1607, 1525, 1491, 1470, 1447, 1341, 1223, 1167, 996, 896, 771, 742, 700, 634 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.97 (d, J = 7.5 Hz, 1 H), 7.94 (d, J = 7.5 Hz, 2 H), 7.88 (d, J = 7.5 Hz, 1 H), 7.48 (d, J = 7.5 Hz, 1 H), 7.44–7.36 (m, 3 H), 7.35–7.23 (m, 5 H), 6.93 (d, J = 7.5 Hz, 2 H), 6.78 (t, J = 7.5 Hz, 1 H), 6.70 (t, J = 7.5 Hz, 2 H), 5.49 (dd, J = 17.0, 2.5 Hz, 1 H), 5.13 (dt, J = 17.0, 10.0 Hz, 1 H), 5.04 (dd, J = 10.0, 2.5 Hz, 1 H), 4.74 (dd, J = 3.0, 10.0 Hz, 1 H), 3.33 (d, J = 10.0 Hz 1 H), 2.50–1.90 (br.s, 1 H), 1.66–1.53 (m, 2 H), 1.42–1.32 (m, 1 H), 0.90 (d, J = 6.3 Hz, 3 H), 0.84 (d, J = 6.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.5, 148.3, 138.4, 138.2, 136.81, 136.82, 132.5, 132.1 (2 C), 132.0 (2 C), 129.4, 129.3, 127.9 (2 C), 126.7 (2 C), 126.6, 126.4, 125.3, 124.2, 122.9, 122.1, 120.7, 113.1, 110.3, 110.2, 70.8, 64.2, 49.5, 46.1, 39.9, 28.5, 25.4, 23.8, 21.8.
HRMS (ESI): m/z [M+H]+ calcd for C36H34N3: 508.2747; found: 508.2755.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Isobutyl-1’,5’-diphenyl-6’-((trimethylsilyl)ethynyl)-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6h)
Prepared according to the general procedure B using cyclopropene 1d (91.7 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-leucine (3f) (83.4 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (93 mg, 50%) as an inseparable mixture in a ratio of 2.2:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6h as a pure compound (42 mg).
Data for 6h: white solid; mp 182–185 °C; Rf 0.62 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3362, 3050, 2956, 2919, 2161, 1608, 1526, 1492, 1471, 1467, 1343, 1251, 1224, 1168, 844, 730, 702, 696, 577, 474 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.13 (d, J = 7.5 Hz, 2 H), 7.95 (d, J = 7.5 Hz, 1 H), 7.87 (d, J = 7.5 Hz, 1 H), 7.50 (d, J = 7.5 Hz, 1 H), 7.45–7.36 (m, 3 H), 7.34–7.24 (m, 5 H), 6.95 (d, J = 7.5 Hz, 2 H), 6.78 (t, J = 7.5 Hz, 1 H), 6.70 (t, J = 7.5 Hz, 2 H), 4.80 (dd, J = 3.0, 9.0 Hz, 1 H), 3.34 (s, 1 H), 2.45–1.87 (br.s, 1 H), 1.62–1.71 (m, 2 H), 1.49–1.39 (m, 1 H), 0.89 (d, J = 6.3 Hz, 3 H), 0.85 (d, J = 6.3 Hz, 3 H), -0.1 (s, 9 H).
13C NMR (101 MHz, CDCl3): δ = 163.1, 148.1, 138.4, 136.2, 135.1, 131.9 (2 C), 131.6, 131.2 (2 C), 129.5, 129.3, 127.5 (2 C), 126.7, 126.4 (3 C), 125.4, 124.3, 123.0, 122.2, 120.8, 110.4, 110.3, 105.0, 92.0, 69.9, 62.7, 50.9, 46.5, 40.2, 25.4, 23.7, 21.8, 15.7, −0.6 (3 C).
HRMS (ESI): m/z [M+H]+ calcd for C39H40N3Si: 578.2986; found: 578.2994.
(±)-(1’R,2’R,4’R,5’S,6’R)-4’-Isobutyl-N-isopropyl-1’,5’-diphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane]-6’-carboxamide (6i)
Prepared according to the general procedure B using cyclopropene 1f (100.8 mg, 0.36 mmol), ketone 2 (80.0 mg, 0.36 mmol), and L-leucine (3f) (95.3 mg, 0.73 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 2:1) furnished the corresponding cycloadduct (112 mg, 55%) as an inseparable mixture in a ratio of 1.9:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6i with an admixture of the minor 7i (overall 51 mg).
Data for 6i: white solid; mp 214–216 °C; Rf 0.77 (SiO2, hexane–ethyl acetate, 2:1).
IR (KBr): 3422, 3297, 3061, 3025, 2955, 2924, 2868, 1635, 1607, 1535, 1494, 1445, 1348, 1219, 1167, 763, 742, 698 cm−1.
1H NMR (400 MHz, CDCl3): δ = 8.00 (d, J = 7.7 Hz, 1 H), 7.94 (m, 3 H), 7.72 (d, J = 7.7 Hz, 1 H), 7.55–7.40 (m, 5 H), 7.38–7.25 (m, 4 H), 6.98 (d, J = 7.7 Hz, 2 H), 6.84 (t, J = 7.7 Hz, 1 H), 6.74 (t, J = 7.7 Hz, 2 H), 4.63 (d, J = 7.0 Hz, 1 H), 3.71 (m, 1 H), 3.54 (br.s, 1 H), 3.37 (s, 1 H), 2.32 (br.s, 1 H), 1.60 (m, 1 H), 0.88 (d, J = 6.5 Hz, 3 H), 0.80 (d, J = 6.5 Hz, 3 H), 0.59 (d, J = 6.5 Hz, 3 H), 0.29 (d, J = 6.5 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 168.2, 148.1, 138.5, 133.3, 132.6 (2 C), 131.4 (2 C), 131.0, 129.9, 129.8, 129.4, 129.1, 128.9, 128.0 (2 C), 127.6, 127.4, 127.3 (2 C), 125.6, 124.6, 123.0, 122.2, 120.8, 110.3, 110.2, 71.2, 65.2, 50.5, 48.0, 40.8, 39.3, 29.2, 25.3, 23.7, 21.6 (2 C).
HRMS (ESI): m/z [M+Na]+ calcd for C38H38N4ONa: 589.2938; found: 589.2938.
(±)-(1’R,2’R,4’R,5’S)-4’-Isobutyl-1’,5’-diphenyl-3’-azaspiro[benzo[4,5]imidazo[1,2-a]indole-11,2’-bicyclo[3.1.0]hexane] (6j)
Prepared according to the general procedure B using cyclopropene 1h (61.1 mg, 0.32 mmol), ketone 2 (70.0 mg, 0.32 mmol), and L-leucine (3f) (83.4 mg, 0.64 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished the corresponding cycloadduct (81 mg, 53%) as an inseparable mixture in a ratio of 2.1:1, respectively. Crystallization of the mixture from methanol gave the major diastereomer 6j as a pure compound (36 mg).
Data for 6j: white solid; mp 180–183 °C; Rf 0.54 (SiO2, hexane–ethyl acetate, 3:1).
IR (KBr): 3364, 3055, 3023, 2952, 2929, 2862, 2836, 1610, 1529, 1492, 1471, 1447, 1337, 1220, 1169, 1048, 1012, 917, 781, 736, 703, 593, 495 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.96 (d, J = 7.5 Hz, 1 H), 7.85 (d, J = 7.5 Hz, 1 H), 7.67 (d, J = 7.5 Hz, 2 H), 7.51 (d, J = 7.5 Hz, 1 H), 7.42–7.23 (m, 7 H), 7.20 (t, J = 7.5 Hz, 1 H), 6.87 (d, J = 7.5 Hz, 2 H), 6.76 (t, J = 7.5 Hz, 1 H), 6.68 (t, J = 7.5 Hz, 2 H), 5.03–4.98 (m, 1 H), 3.51 (s, 2 H), 2.41 (d, J = 4.0 Hz 1 H), 1.68–1.40 (m, 3 H), 0.92 (d, J = 6.3 Hz, 3 H), 0.90 (d, J = 6.3 Hz, 3 H).
13C NMR (101 MHz, CDCl3): δ = 163.6, 148.2, 138.3, 137.8, 137.1, 135.2, 131.3 (2 C), 129.7 (2 C), 129.4, 129.2, 128.0 (2 C), 126.9 (2 C), 126.3, 125.0, 124.1, 122.9, 122.1, 120.8, 110.4, 110.2, 69.9, 60.9, 50.8, 46.9, 41.6, 40.9, 25.5, 23.7, 22.1, 14.4.
HRMS (ESI): m/z [M+H]+ calcd for C34H32N3: 482.2591; found: 482.2592.
General Procedure C for the One-Pot Three-Component Reaction of 11H-Benzo[4,5]imidazo[1,2-a]indol-11-one, L-Proline and Maleimides
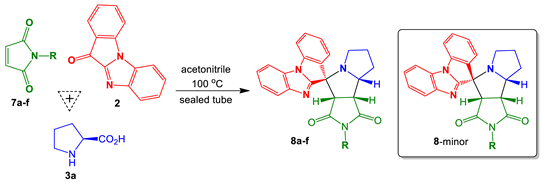
11H-Benzo[4,5]imidazo[1,2-a]indol-11-one (2, 0.25 mmol), L-proline (3a, 0.5 mmol), maleimide (7, 0.25 mmol), and anhydrous acetonitrile (4 mL) were added to a screw-capped tube (Schuett-biotec type). The reaction vessel was closed and the reaction mixture was stirred in a preheated oil bath at 100 °C for 18 h (the reaction progress was monitored using TLC). The mixture was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was subjected to silica gel PTLC using a mixture of hexane−ethyl acetate as an eluent to give the mixtures of spiro-adducts 8a−f and their minor diastereomers.
(±)-(3a’S,4’R,8a’R,8b’R)-3a’,6’,7’,8’,8a’,8b’-Hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8a) and (3a’S,4’S,8a’R,8b’R)-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8a-minor).
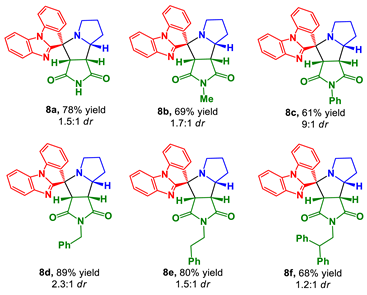
Prepared according to the general procedure C using maleimide 7a (52.5 mg, 0.54 mmol), ketone 2 (85.0 mg, 0.39 mmol), and L-proline (3a) (88.9 mg, 0.77 mmol). Purification of the crude by PTLC (hexane-EtOAc, 1:2) furnished 8a as an inseparable mixture with its minor diastereomer in a ratio of 1.5:1, respectively (113 mg, 78%).
Data for mixture 8a+minor: white solid; mp 244–245 °C.
IR (KBr): 3470, 3152, 3058, 2966, 2765, 1770, 1717, 1609, 1530, 1495, 1474, 1450, 1337, 1194, 743 cm−1.
1H NMR (400 MHz, DMSO-d6): δ = 11.52(8a) (br.s, 1 H), 11.03(minor) (br.s, 1 H), 8.12(8a) (d, J = 7.9 Hz, 1 H), 8.09(minor) (d, J = 7.9 Hz, 1 H), 7.94(minor) (d, J = 7.9 Hz, 1 H), 7.91(8a) (d, J = 7.9 Hz, 1 H), 7.84(minor) (d, J = 7.9 Hz, 1 H), 7.77(8a) (d, J = 7.9 Hz, 1 H), 7.73(minor) (d, J = 7.9 Hz, 1 H), 7.59(minor) (t, J = 7.9 Hz, 1 H), 7.54(8a) (t, J = 7.9 Hz, 1 H), 7.44–7.19(8a+minor) (m, 4 H(8a) + 3 H(minor)), 4.49(minor) (td, J = 7.0, 2.5 Hz, 1 H), 4.39(minor) (d, J = 9.8 Hz, 1 H), 4.20(8a) (q, J = 7.5 Hz, 1 H), 4.08(minor) (q, J = 5.5 Hz, 1 H), 3.78(8a) (d, J = 7.5 Hz, 1 H), 3.59(8a) (t, J = 7.5 Hz, 1 H), 3.56(minor) (dd, J = 10.6, 7.5 Hz, 1 H), 3.17(8a+minor) (d, J = 5.5 Hz, 1 H), 2.98(minor) (m, 1 H), 2.57(minor) (t, J = 7.5 Hz, 1 H), 2.27(8a) (t, J = 7.5 Hz, 1 H), 2.17(minor) (m, 1 H), 2.05–1.60(8a+minor) (m, 4 H(8a) +1 H(epimer)).
13C NMR (101 MHz, DMSO-d6) for 8a: δ = 177.7, 176.1, 160.6, 147.2, 137.7, 132.4, 130.1, 128.8, 127.3, 124.1, 123.7, 122.8, 120.2, 111.1, 65.1, 64.7, 59.8, 53.9, 44.9, 41.5, 26.0, 22.4.
13C NMR (101 MHz, DMSO-d6) for 8a-minor: δ = 179.2, 176.6, 161.0, 147.0, 138.0, 134.3, 130.4, 128.5, 126.9, 124.6, 123.6, 122.6, 120.2, 111.4, 111.3, 69.0, 65.8, 55.1, 48.6, 48.1, 28.9, 24.6.
HRMS (ESI): m/z [M+H]+ calcd for C22H19N4O2: 371.1503; found: 371.1505.
(±)-(3a’S,4’R,8a’R,8b’R)-2’-Methyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8b) and (±)-(3a’S,4’S,8a’R,8b’R)-2’-methyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8b-minor)
Prepared according to the general procedure C using maleimide 7b (57.2 mg, 0.51 mmol), ketone 2 (85.0 mg, 0.39 mmol), and L-proline (3a) (88.9 mg, 0.77 mmol). Purification of the crude by PTLC (hexane–ethyl acetate, 3:1) furnished 8b as an inseparable mixture with its minor diastereomer in a ratio of 1.7:1, respectively (104 mg, 69%). Crystallization of the mixture from methanol gave the major diastereomer 8b as a pure compound (50 mg).
Data for 8b: white solid; mp 238–239 °C; Rf 0.52 (SiO2, hexane–ethyl acetate, 1:2).
IR (KBr): 3064, 2988, 2961, 2949, 2917, 2831, 1774, 1700, 1607, 1471, 1433, 1289, 1222, 1131, 764, 739, 450 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.86 (d, J = 7.9 Hz, 1 H), 7.78 (d, J = 7.9 Hz, 1 H), 7.57 (d, J = 7.9 Hz, 1 H), 7.51 (t, J = 7.9 Hz, 1 H), 7.43 (t, J = 7.9 Hz, 1 H), 7.37 (t, J = 7.9 Hz, 1 H), 7.25 (t, J = 7.9 Hz, 1 H), 7.14 (d, J = 7.9 Hz, 1 H), 4.38 (q, J = 6.8 Hz, 1 H), 3.92 (d, J = 7.5 Hz, 1 H), 3.63 (t, J = 7.5 Hz, 1 H), 3.14 (s, 3 H), 2.37 (m, 1 H), 2.14–1.90 (m, 5 H).
13C NMR (101 MHz, CDCl3): δ = 176.0, 175.0, 160.4, 147.4, 138.3, 132.3, 130.1, 129.2, 127.3, 124.2, 123.9, 123.0, 120.6, 110.8, 110.7, 65.9, 65.3, 58.8, 43.9, 41.5, 26.3, 25.0, 22.6.
HRMS (ESI): m/z [M+H]+ calcd for C23H21N4O2: 385.1659; found: 385.1662.
(±)-(3a’S,4’R,8a’R,8b’R)-2’-Phenyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8c) and (±)-(3a’S,4’S,8a’R,8b’R)-2’-phenyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8c-minor)
Prepared according to the general procedure C using maleimide 7c (42.5 mg, 0.23 mmol), ketone 2 (54.0 mg, 0.25 mmol), and L-proline (3a) (56.5 mg, 0.49 mmol). Purification of the crude by PTLC (hexane-EtOAc, 1:2) furnished 8c as an inseparable mixture with its minor diastereomer in a ratio of 9:1, respectively (68.2 mg, 61%).
Data for mixture 8c+minor: light pink color; mp 198–200 °C.
IR (KBr): 3054, 2961, 2924, 2829, 1777, 1709, 1609, 1525, 1494, 1472, 1390, 1371, 1221, 1185, 744, 692, 616 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.89(8c) (d, J = 7.9 Hz, 1 H), 7.79(8c) (d, J = 7.9 Hz, 1 H), 7.60–7.35(8c+minor) (m, 9 H), 7.28(8c) (d, J = 7.9 Hz, 1 H), 7.22(8c) (t, J = 7.9 Hz, 1 H), 4.52(8c) (q, J = 6.5 Hz, 1 H), 4.36(minor) (br.s, 1 H), 4.16(minor) (d, J = 8.5 Hz, 1 H), 4.08 (8c) (d, J = 7.5 Hz, 1 H), 4.00(minor) (br.s, 1 H), 3.81(8c) (t, J = 7.5 Hz, 1 H), 2.77(minor) (br.m, 1 H), 2.48(8c+minor) (m, 1 H), 2.18–1.98(8c+minor) (m, 5 H).
13C NMR (101 MHz, CDCl3) for 8c: δ = 175.1, 173.7, 160.5, 138.4, 132.2, 131.8, 130.1, 129.1 (2 C), 128.6, 127.5, 126.3 (2 C), 124.3, 124.0, 123.0, 120.6, 110.9, 110.7, 66.3, 65.9, 58.7, 43.9, 42.0, 26.4, 22.8.
HRMS (ESI): m/z [M+H]+ calcd for C28H23N4O2: 447.1816; found: 447.1820.
(±)-(3a’S,4’R,8a’R,8b’R)-2’-Benzyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8d) and (±)-(3a’S,4’S,8a’R,8b’R)-2’-benzyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8d-minor)
Prepared according to the general procedure C using maleimide 7d (45.9 mg, 0.25 mmol), ketone 2 (54.0 mg, 0.25 mmol), and L-proline (3a) (56.5 mg, 0.49 mmol). Purification of the crude by PTLC (hexane-EtOAc, 1:2) furnished 8d as an inseparable mixture with its minor diastereomer in a ratio of 2.3:1, respectively (103 mg, 89%).
Data for mixture 8d+minor: white solid; mp 164–166 °C.
IR (KBr): 3062, 2961, 2829, 1770, 1702, 1698, 1608, 1494, 1474, 1398, 1343, 1222, 1173, 757, 737, 709, 618 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.85(8d) (d, J = 7.9 Hz, 1 H), 7.75(8d) (d, J = 7.9 Hz, 1 H), 7.70(minor) (d, J = 7.9 Hz, 1 H), 7.67(minor) (d, J = 7.9 Hz, 1 H), 7.62(minor) (br.d, J = 7.9 Hz, 1 H), 7.56(minor) (t, J = 7.9 Hz, 1 H), 7.52(8d+minor) (t, J = 7.5 Hz, 2 H), 7.43(8d+minor) (t, J = 7.5 Hz, 1 H), 7.52(8d+minor) (d, J = 7.5 Hz, 2 H), 7.38–7.22(8d+minor) (m, 4 H), 6.97(8d) (t, J = 7.9 Hz, 1 H), 6.55(8d) (d, J = 7.9 Hz, 1 H), 4.87(8d) (d, J = 13.6 Hz, 1 H), 4.75(minor) (br.m, 1 H), 4.68(8d) (d, J = 13.6 Hz, 1 H), 4.66(minor) (d, J = 14.3 Hz, 1 H), 4.43(8d) (q, J = 6.8 Hz, 1 H), 4.38(minor) (d, J = 14.3 Hz, 1 H), 4.23(minor) (br.d, J = 9.6 Hz, 1 H), 3.87 (8d) (d, J = 7.5 Hz, 1 H), 3.66(8d) (t, J = 7.5 Hz, 1 H), 3.48(minor) (m, 1 H), 2.87(minor) (q, J = 7.5 Hz, 1 H), 2.76(minor) (br.t, J = 7.5 Hz, 1 H), 2.39(minor) (m, 1 H), 2.27(8d) (m, 1 H), 2.15–1.90(8d+ minor) (m, 5 H(8d) +3 H(minor)).
13C NMR (101 MHz, CDCl3) for 8d: δ = 175.7, 174.4, 160.5, 147.4, 138.2, 135.6, 132.1, 129.1 (2 C), 128.8, 128.5 (2 C), 128.3, 128.0, 127.8, 124.0, 123.9, 122.9, 120.6, 110.7, 110.6, 66.0, 65.7, 58.4, 44.1, 42.6, 42.0, 26.2, 22.7.
HRMS (ESI): m/z [M+H]+ calcd for C29H25N4O2: 461.1972; found: 461.1978.
(±)-(3a’S,4’R,8a’R,8b’R)-2’-Phenethyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8e) and (±)-(3a’S,4’S,8a’R,8b’R)-2’-phenethyl-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8e-minor)
Prepared according to the general procedure C using maleimide 7e (49.3 mg, 0.25 mmol), ketone 2 (54.0 mg, 0.25 mmol), and L-proline (3a) (56.5 mg, 0.49 mmol). Purification of the crude by PTLC (hexane-EtOAc, 1:2) furnished 8e as an inseparable mixture with its minor diastereomer in a ratio of 1.5:1, respectively (95.4 mg, 80%).
Data for mixture 8e+minor: white solid; mp 162–164 °C.
IR (KBr): 3060, 3027, 2956, 2930, 2817, 1772, 1702, 1609, 1496, 1473, 1449, 1400, 1363, 1168, 767, 747, 738, 698 cm−1.
1H NMR (400 MHz, CDCl3): δ = 7.86(8e) (d, J = 7.9 Hz, 1 H), 7.77(8e) (d, J = 7.9 Hz, 1 H), 7.72(minor) (d, J = 7.9 Hz, 1 H), 7.70(minor) (d, J = 7.9 Hz, 1 H), 7.64(minor) (br.d, J = 7.9 Hz, 1 H), 7.60–7.52(8e+minor) (m, 1 H(8e) + 2 H(minor)), 7.48(8e) (t, J = 7.5 Hz, 1 H), 7.43(8e) (t, J = 7.5 Hz, 1 H), 7.39–7.17(8e+minor) (m, 6 H(8e) + 8 H(minor)), 7.14(8e) (t, J = 7.5 Hz, 1 H), 6.73(8e) (d, J = 7.5 Hz, 1 H), 4.79(minor) (br.m, 1 H), 4.40(8e) (m, 1 H), 4.20(8e) (d, J = 9.5 Hz, 1 H), 3.98(8e) (dt, J = 13.4, 8.0 Hz, 1 H), 3.86(8e) (ddd, J = 13.4, 6.0, 8.0 Hz, 1 H), 3.8(8e) (d, J = 7.5 Hz, 1 H), 3.59(8e) (m, 2 H), 3.47(minor) (m, 1 H), 3.13(8e) (dt, J = 13.4, 8.0 Hz, 1 H), 3.02(8e) (ddd, J = 13.4, 6.0, 8.0 Hz, 1 H), 3.0–2.78(minor) (m, 3 H), 2.69(minor) (m, 1 H), 2.43(minor) (m, 1 H), 2.24(8e) (m, 1 H), 2.18–1.88(8e+minor) (m, 4 H(8e) + 5 H(minor)).
13C NMR (101 MHz, CDCl3) for 8e: δ = 176.0, 174.5, 160.4, 147.6, 138.3, 137.6, 131.9, 130.4, 129.9, 129.1 (2 C), 128.4 (2 C), 128.3, 127.7, 126.7, 124.0, 122.9, 120.6, 110.7, 110.6, 65.7, 65.3, 58.3, 43.6, 41.6, 40.3, 33.3, 26.3, 22.8.
HRMS (ESI): m/z [M+H]+ calcd for C30H27N4O2: 475.2129; found: 475.2134.
(±)-(3a’S,4’R,8a’R,8b’R)-2’-(2,2-Diphenylethyl)-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo [4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8f) and (±)-(3a’S,4’S,8a’R,8b’R)-2’-(2,2-diphenylethyl)-3a’,6’,7’,8’,8a’,8b’-hexahydro-1’H-spiro[benzo[4,5]imidazo[1,2-a]indole-11,4’-pyrrolo[3,4-a]pyrrolizine]-1’,3’(2’H)-dione (8f-minor)
Prepared according to the general procedure C using maleimide 7f (69.3 mg, 0.25 mmol), ketone 2 (54.0 mg, 0.25 mmol), and L-proline (3a) (56.5 mg, 0.49 mmol). Purification of the crude by PTLC (hexane-EtOAc, 1:2) furnished 8f as an inseparable mixture with its minor diastereomer in a ratio of 1.2:1, respectively (93.8 mg, 68%).
Data for mixture 8f+minor: white solid; mp 219–221 °C.
IR (KBr): 3053, 3026, 2960, 2945, 2878, 2828, 1773, 1700, 1609, 1492, 1472, 1446, 1398, 1345, 1169, 756, 744, 701 cm−1.
1H NMR (400 MHz, CDCl3) for 8f+minor: δ = 7.84(minor) (d, J = 7.9 Hz, 1 H), 7.76(8f) (d, J = 7.9 Hz, 1 H), 7.74(8f) (d, J = 7.9 Hz, 1 H), 7.70(minor) (d, J = 7.9 Hz, 1 H), 7.60–7.46(8f+minor) (m, 3 H), 7.44–7.32(8f+minor) (m, 11 H), 7.31–7.10(8f+minor) (m, 14 H), 7.52(minor) (d, J = 7.9 Hz, 1 H), 4.83(8f) (t, J = 8.3 Hz, 1 H), 4.69(minor) (br.s, 1 H), 4.59(minor) (t, J = 8.3 Hz, 1 H), 4.38(8f) (br.q, J = 7.5 Hz, 1 H), 4.32(8f) (dd, J = 8.9, 13.5 Hz, 1 H), 4.24(8f) (dd, J = 7.7, 13.5 Hz, 1 H), 4.15(minor) (dd, J = 8.2, 13.2 Hz, 1 H), 4.03(minor) (d, J = 9.1 Hz, 1 H), 3.85(minor) (dd, J = 9.1, 13.2 Hz, 1 H), 3.67(8f) (d, J = 7.5 Hz, 1 H), 3.53(minor) (t, J = 7.5 Hz, 1 H), 3.29(8f) (br.t, J = 8.0 Hz, 1 H), 2.88–2.73(8f+minor) (m, 2 H), 2.28(8f) (br.q, J = 8.5 Hz, 1 H), 2.18 (br.s, 1 H), 2.11–1.80(8f+minor) (m, 8 H).
13C NMR (101 MHz, CDCl3) for 8f+minor: δ = 177.1(8f), 176.4(minor), 174.6(8f), 174.5(minor), 160.6(8f), 150.1(minor), 147.8(8f), 147.5(minor), 141.13(minor), 141.07(8f), 141.05(minor), 140.8(8f), 139.0(minor), 138.3(8f), 131.6(8f+minor), 130.3(minor), 129.9(8f), 129.2(8f), 129.1(minor), 128.48(8f) (2 C), 128.47(minor) (2 C), 128.43(8f) (2 C), 128.35(minor) (2 C), 128.3(8f) (2 C), 128.1(8f), 128.04(minor) (3 C), 128.02(8f) (2 C), 127.96(minor) (2 C), 127.0(minor), 126.8(8f), 126.6(8f+minor), 126.0(minor), 124.2(8f), 123.9(minor), 123.8(8f), 123.6(8f), 122.9(8f), 122.6(minor), 120.9(minor), 120.6(8f), 111.2(minor), 110.6(8f), 110.53(minor), 110.49(8f), 68.9(minor), 66.5(8f), 65.5(8f), 57.7(8f+minor), 54.4(8f), 51.9(minor), 48.1(minor), 47.77(minor), 47.75(8f), 43.79(minor), 43.75(8f), 43.1(minor), 42.0(8f+ minor), 29.4(minor), 26.1(8f), 25.3(minor), 22.9(8f).
HRMS (ESI): m/z [M+H]+ calcd for C36H31N4O2: 551.2442; found: 551.2450.